Abstract
Chronic heart failure (HF) is a clinical syndrome of myocardial dysfunction characterized by inadequate cardiac output or preserved output that can only be achieved by sustaining abnormal loading conditions. Morphologically, HF with reduced left ventricular function results in progressive chamber remodeling, meaning the ventricle dilates, operating at larger end-diastolic and end-systolic volumes, and takes on an abnormal, spherical shape that increases wall stress. Reverse remodeling is the goal of HF-directed therapies and can be achieved by biological means, ie, altering the loading conditions that, at a cellular level, promote myocardial dysfunction, or physical means, ie, directly altering myocardial mass or shape. In this review, we highlight the existing and emerging device-based mechanisms for biologically and physically reverse remodeling the left ventricle in chronic HF.
Keywords: heart failure, reverse remodeling, transcatheter therapies
Central Illustration
Highlights
-
•
Heart failure syndromes are associated with left ventricular remodeling
-
•
Remodeling alters left ventricular size and morphology
-
•
These deleterious changes promote further ventricular dysfunction
-
•
Reverse remodeling is the goal of medical and device therapy for heart failure
-
•
Reverse remodeling can be achieved through biological and physical mechanisms
Introduction
Left ventricular (LV) dysfunction that results in chronic heart failure (HF) syndromes is associated with stereotypical changes in LV chamber geometry and structure. For example, the ventricular cavity assumes a spherical shape and the orientation of key ventricular structures such as the papillary muscles are altered. These changes—referred to as LV remodeling—perpetuate a vicious cycle that precipitates further ventricular dysfunction and, as a consequence, more pronounced changes in ventricular size and structure.
The goal of contemporary HF therapies is to interrupt this cycle by either targeting the biological mechanisms of ventricular dysfunction and dilation or physically altering LV size and geometry or a combination of both strategies. Although contemporary guideline-directed medical therapy (GDMT) can dramatically improve clinical outcomes, critical treatment gaps remain for patients who are unable to tolerate such therapies (ie, limitations in renal function or blood pressure) or only experience a partial response to therapy. Therefore, transcatheter therapies to reverse the LV remodeling process associated with chronic HF syndromes may play a substantial role in future management of patients with HF. In this review, we highlight some of the key devices that have been developed for this purpose.
The physiology of LV remodeling
The pressure-volume (PV) diagram is a useful tool for understanding the hemodynamic consequences of ventricular remodeling in HF (Figure 1). Furthermore, the PV diagram will be used to highlight the key mechanisms of action for many of the transcatheter devices described in greater detail later in this review.
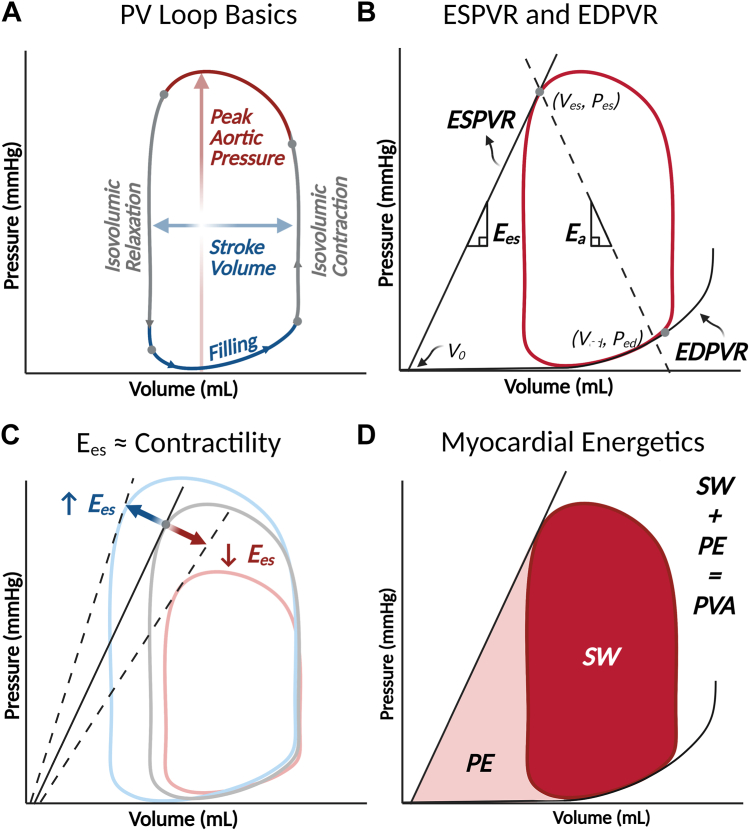
Figure 1.
Basic elements of the left ventricular pressure-volume (PV) diagram. (A) The normal PV loop has 4 discrete phases. Beginning at end-diastole, when the mitral valve closes, in the bottom right hand corner of the loop is isovolumic contraction. During this phase, ventricular pressure increases without any changes in ventricular volume because both the mitral and aortic valves are closed. Then comes the ejection phase when the aortic valve opens as ventricular pressure exceeds diastolic pressure. At the end of ejection, the point of end-systole is reached (top left corner of the loop), and isovolumic relaxation begins. This gives way to the filling phase when ventricular pressure falls below left atrial pressure and the mitral valve opens. (B) The PV loop is bound by 2 fundamental relationships such that the top left corner of the loop (end-systole) is determined by the end-systolic pressure-volume relationship (ESPVR), while the bottom portion of the loop is bound by the nonlinear end-diastolic pressure-volume relationship (EDPVR). (C) The ESPVR’s slope—called end-systolic elastance (Ees), provides a load-independent estimate of contractility. A larger Ees, indicating a steeper ESPVR, implies greater contractility and vice versa. (D) The PV loop is also a helpful tool for visualizing myocardial energetics. The area within the loop, or stroke work (SW), represents the energy exerted during each cardiac cycle to eject blood into the systemic circulation. The potential energy is the energy stored in myofilaments after systolic contraction and is represented by the area bound by the ESPVR and EDPVR, but outside the PV loop. PV area is the sum of potential energy and SW and is linearly related to myocardial oxygen consumption. Figure produced with BioRender.
Recall that the LV PV loop depicts pressure and volume changes in the ventricle during 1 cardiac cycle, proceeding in 4 discrete phases in a counterclockwise fashion from the bottom right corner (end-diastole, closure of the mitral valve): isovolumic contraction, ejection, isovolumic relaxation, and relaxation (Figure 1A). The shape of the normal LV PV loop is roughly rectangular with a domed top, reflecting the rise in pressure during systolic contraction. The LV PV loop is always contained within the end-systolic pressure-volume relationship (ESPVR) and the end-diastolic pressure-volume relationship (EDPVR) (Figure 1B). The ESPVR is roughly linear and connects the point of end-systole with the volume-axis intercept (ie, the unstressed blood volume of the left ventricle), and the slope of the line (end-systolic elastance, Ees) provides a load-independent assessment of LV contractile function (Figure 1C). The EDPVR, unlike the ESPVR, is nonlinear and reflects the extent (but not the rate) of relaxation of the left ventricle during diastole. Figure 2 depicts the characteristic PV loop changes associated with chronic HF with reduced ejection fraction (EF), where systolic function declines, as evidenced by a shallow ESPVR. As the ventricle dilates and end-diastolic volume (EDV) increases, the EDPVR shifts rightward and the ventricle becomes more compliant (ie, larger changes in volume are associated with smaller changes in pressure). These shifts may preserve stroke volume but come at the expense of dramatically increased energy expenditure, which is reflected by PV area (which is the area bound by the ESPVR, EDPVR, and systolic portion of the PV loop) (Figure 1D).
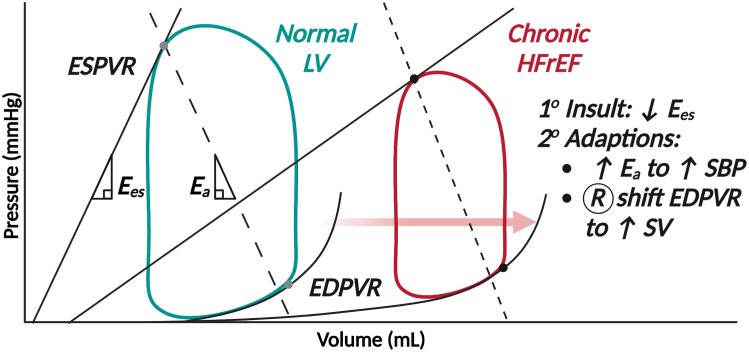
Figure 2.
Pressure-volume (PV) loop changes in chronic heart failure with reduced ejection fraction (HFrEF). In chronic HFrEF, end-systolic elastance (Ees; a surrogate of contractility) declines. To compensate, the PV loop shifts to the right because the end-diastolic pressure-volume relationship (EDPVR) flattens, causing the ventricle to operate at higher volumes. This maintains stroke volume (SV) at the expense of significantly increased PV area and myocardial oxygen consumption. Systolic blood pressure (SBP) also declines as Ees decreases, so afterload (represented by the slope of the effective arterial elastance, Ea, line) may increase (typically as a function of increased adrenergic tone) to maintain perfusion pressure. Figure produced with BioRender.
A complete discussion of the LV PV loop in HF and its subtleties is outside the scope of this discussion. However, a number of excellent resources provide further explanation for readers to take a deeper dive into the subject.1,2 Broadly speaking, however, from a hemodynamic perspective, HF therapies attempt to improve systolic function (increase the slope of and left-shift the ESPVR), reduce afterload (ie, the impediment to forward flow, or effective arterial elastance, Ea, which is highlighted in Figure 1B), and reduce LV dimensions by shrinking ESV and EDV. These hemodynamic changes are the basis for the magnitude of clinical effect of various HF therapies (Table 1).2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16
Table 1.
Summary of HF-focused devices and their effect on left ventricular remodeling and clinical outcomes.
| Device | Description | Remodeling mechanism | Remodeling effect | Clinical outcomes | Pivotal evidence |
|---|---|---|---|---|---|
| Percutaneous valve-based therapies | |||||
| MitraClip | Percutaneous edge-to-edge mitral valve repair | Biologic | LVESV ↓ 6.3 mL vs control, but ↑ 6.5 mL vs baseline | ↓ Mortality and heart failure hospitalizations at 24 mo | COAPT4 |
| Carillon | Reproduces surgical annuloplasty with CS-based device with shaping ribbon | Biologic | LVESV ↓ 6.2 mL; LVEDV ↓ 10.4 mL at 12 mo | ↑ 6MWT time and KCCQ score at 12 mo | REDUCE-FMR,5 TITAN-I/II6,7 |
| ARTO | CS device that tethers the anteroposterior dimension of mitral annulus | Biologic | LVEDVi ↓ 16 mL at 24 mo | ↓ NYHA class and ↓ HF hospitalizations at 24 mo | MAVERICK8 |
| TAVR | Transcatheter aortic valve replacement | Biologic | LVEDVi ↓ 15.3 mL/m2 | ↓ Long-term mortality and HF hospitalizations | Kato et al9 |
| Mechanical heart failure therapies | |||||
| CCM | Nonexcitatory stimulation to enhance left ventricular contractility | Biologic | LVEDV ↓ 7.4 mL LVESV ↓ 11.3 mL |
↑ Peak VO2, ↑ 6MWT, ↓ MLWHFQ, NYHA class, HF hospitalizations | FIX-HF-4,10 FIX-HF-5/5C11,12 |
| Partitioning devices | |||||
| Revivant TC | Minimally invasive device to exclude scarred myocardium from the left ventricle | Physical | LVESVi ↓ 20.0 mL/m2 LVEDVi ↓ 26.0 mL/m2 |
↑ 6MWT and ↓ NYHA class | Klein et al13 |
| Parachute | Percutaneous ventricular partition device | Physical | LVESVi ↓ 13.5 mL/m2 LVEDVi ↓ 18.2 mL/m2 |
↑ 6MWT and ↓ or stable NYHA class in 85% of patients | PARACHUTE III14 |
| Reshaping devices | |||||
| AccuCinch | Subvalvular cinch deployed through anchors into the left ventricle | Physical | LVESVi ↓ 21% at 12 mo | ↑ KCCQ score and ↑ 6MWT | CorCinch-HF15 |
| MIRTH | Intramyocardial catheter based ventricular reshaping | Physical | NA | NA | Bruce et al16 |
Reproduced with permission from Brener et al.2
6MWT, 6-minute walk test; CCM, cardiac contraction modulation; HF, heart failure; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; LVEDVi, indexed LVEDV; LVESV, left ventricular end-systolic volume; LVESVi, indexed LVESV; MIRTH, Myocardial Intramural Remodeling by Transvenous Tether; NA, not available; NYHA, New York Heart Association; TAVR, transcatheter aortic valve replacement.
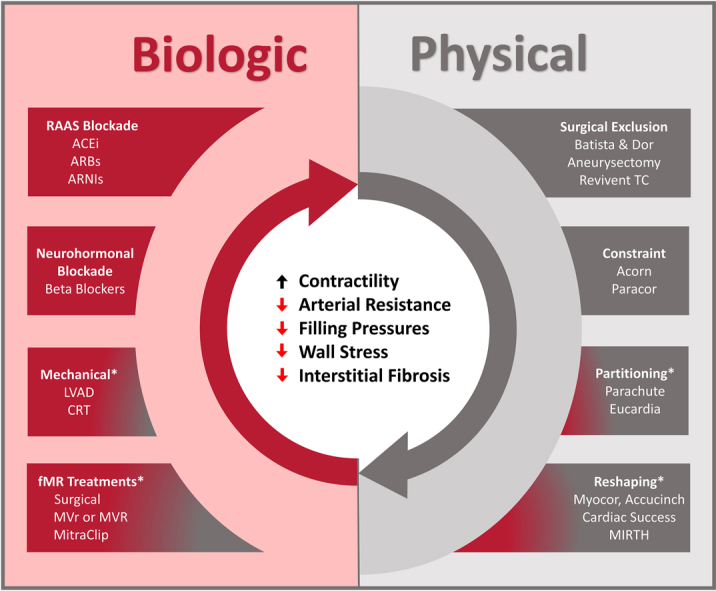
The paradigm of biological and physical reverse remodeling
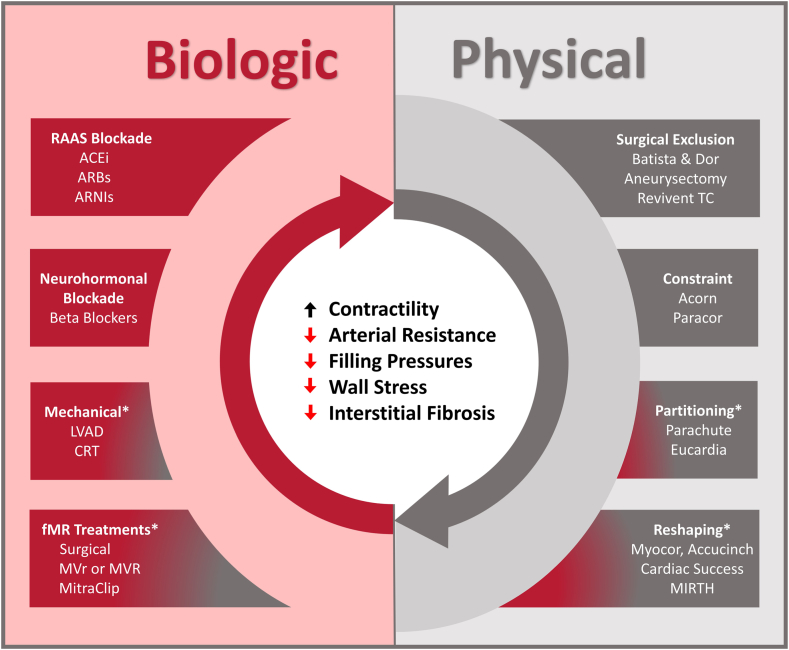
We have previously advocated that LV reverse remodeling can be achieved through 2 fundamental mechanisms: biological and physical means (Central Illustration).2,17 Biological reverse remodeling refers to changes in ventricular structure and function that occur as a byproduct of alterations in loading conditions or neurohormonal activation. The most readily understood form of HF treatments that target biological reverse remodeling are pharmacotherapies that affect ventricular preload (ie, diuretics), afterload (ie, vasodilators), and neurohormonal activity (ie, β-blockers). Device-based therapies can also actuate biological reverse remodeling by modulating loading conditions in a similar fashion, such as LV assist devices, which unload and take over for the work of the heart, aortic valve replacement in the setting of severe aortic stenosis (AS) (which reduces afterload), or mitral valve repair in the context of severe mitral regurgitation (MR) (which can reduce preload). Device-based biological reverse remodeling has been demonstrated in multiple settings to achieve favorable reductions in LV volumes, similar to what has been described with components of contemporary GDMT.
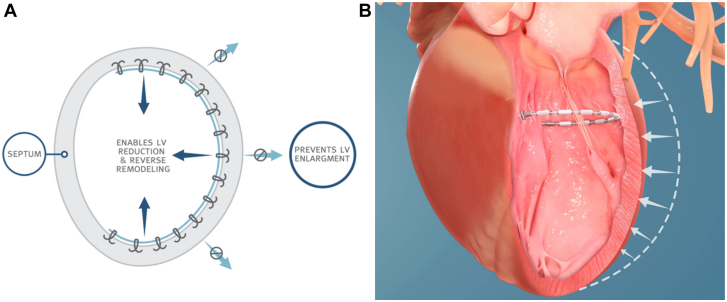
Central Illustration.
Biological and physical mechanisms for reverse remodeling. Left ventricular reverse remodeling can target biological pathways that aberrate ventricular loading conditions and cause cavity enlargement and distortion. Alternatively, it can immediately address changes in left ventricular size and shape, as with physical reverse remodeling. ∗Therapies that have combined biological and physical reverse remodeling mechanisms. Courtesy of Alkhunaizi et al.17 ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; CRT, cardiac resynchronization therapy; fMR, functional mitral regurgitation; LVAD, left ventricular assist device; MIRTH, Myocardial Intramural Remodeling by Transvenous Tether; MVr, mitral valve repair; MVR, mitral valve replacement.
Physical reverse remodeling directly reduces LV mass or alters LV geometry (ie, not as a secondary consequence of therapy). Surgical ventricular restoration with various techniques (ie, the Dor or Batista procedures) constituted the first major attempt to physically remodel the ventricle. Broadly speaking, these procedures restored normal ventricular volumes and geometry by resecting or excluding portions of diseased myocardium.18 However, clinical outcomes with these early techniques demonstrated considerable variability depending on the properties of the myocardium that was removed. Favorable remodeling was achieved only when dyskinetic, aneurysmal tissue was removed (ie, what was achieved with the Dor procedure), whereas excising hypokinetic myocardium (ie, the Batista procedure) precipitated restrictive physiology, which translated into poorer clinical outcomes. Although surgical reverse remodeling has largely been abandoned, a number of emerging transcatheter devices show greater potential and will be the subject of the discussion further. As a general principle, physical reverse remodeling cannot be a stand-alone treatment for HF and must be paired with some form of biological reverse remodeling because the abnormal loading conditions that drive remodeling must be mitigated in some fashion.
It is also important to note that the processes of biological and physical reverse remodeling do not always directly target the left ventricle; rather, they can influence function of other key cardiac structures, which, in turn, improves LV function. The most developed examples of such treatments target the left atrium and left atrial annular function (ie, transcatheter annular restoration devices), which serves a key role in transiting blood to the left ventricle.
Biological reverse remodeling devices
As described earlier, biological reverse remodeling targets the loading conditions that perpetuate the vicious cycle of HF. Although pharmacologic approaches are the first-line means for achieving biological reverse remodeling, a number of devices—many targeted at specific valvular lesions that influence LV loading conditions—have a role alongside contemporary GDMT.
Mitral valve interventions
Functional MR occurs frequently in individuals with HF and is a major driver of disease progression, symptoms, and adverse outcomes.19, 20, 21 The revolutionary effect of mitral transcatheter edge-to-edge repair (mTEER) was affirmed in the Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation (COAPT) trial, which compared mTEER plus GDMT with GDMT alone in individuals with significant MR. mTEER achieved a reduction in the primary end point of HF hospitalizations or mortality at the 24-month follow-up. Despite these obvious clinical benefits, LV volumes actually increased during follow-up for individuals randomized to mTEER, albeit to a far lesser extent than in individuals assigned to GDMT alone.4 Of note, these “beneficial” effects to slow LV remodeling may depend heavily on the patient cohort undergoing mTEER. The complementary MITRA-FR (Percutaneous Repair with the MitraClip Device for Severe Functional/Secondary Mitral Regurgitation) trial, for example, where participants tended to have worse LV function, more advanced LV remodeling, and less severe MR than COAPT trial participants, reported no significant differences in LV reverse remodeling between mTEER and GDMT.22,23
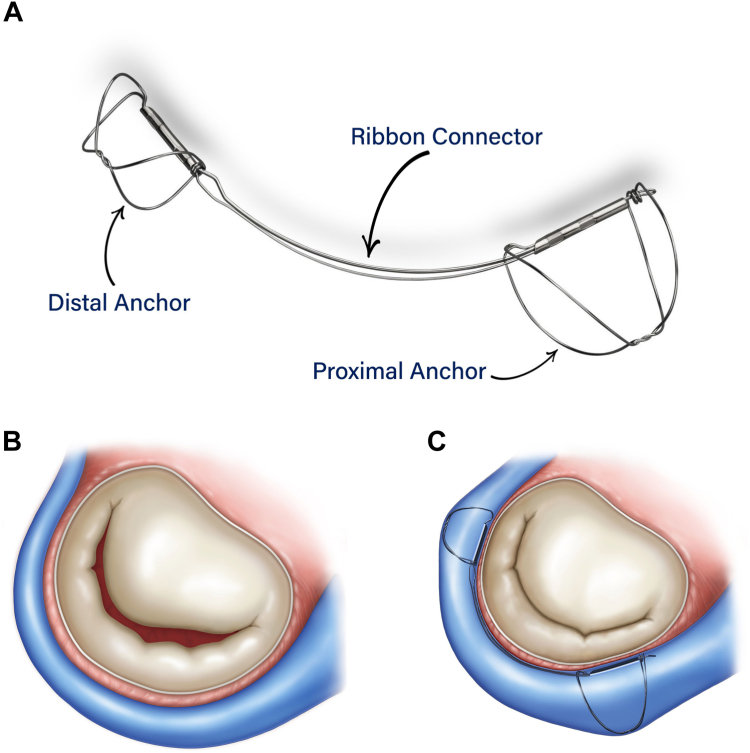
Aside from mTEER, a number of therapies directed at MR reduction have shown promising early results in LV reverse remodeling. The predominant mechanism for MR reduction mimics surgical annuloplasty through devices implanted in the coronary sinus (CS), which wraps around the mitral valve annulus. The Carillon Mitral Contour System (Figure 3) (Cardiac Dimensions) is a nitinol ribbon that is fixed in the CS by 2 anchors at each end of the device. Delivered through a 10F catheter transvenous access, the device’s natural bend reshapes the annulus and decreases its dimension to reduce functional MR. The Carillon device received CE mark in 2011 and has undergone a rigorous clinical trial evaluation in the REDUCE-FMR,5 TITAN-I,6 and TITAN II7 studies. Consequently, it was endorsed in the European Society of Cardiology guidelines for treatment of MR (IIb recommendation) and is currently being evaluated as an adjunct to GDMT in patients with HF and EF of <50% in the randomized EMPOWER trial (NCT03142152). However, a recently published pooled analysis from the 3 published trials evaluating the Carillon device demonstrated significant reductions in LV volumes for individuals with proportionate MR (a median of 20 mL reduction in left ventricular end-diastolic volume [LVEDV]; interquartile range, 7-33 mL; and a median of 18 mL reduction in left ventricular end-systolic volume [LVESV]; interquartile range, 6-30 mL) but not those for individuals with disproportionate MR.24
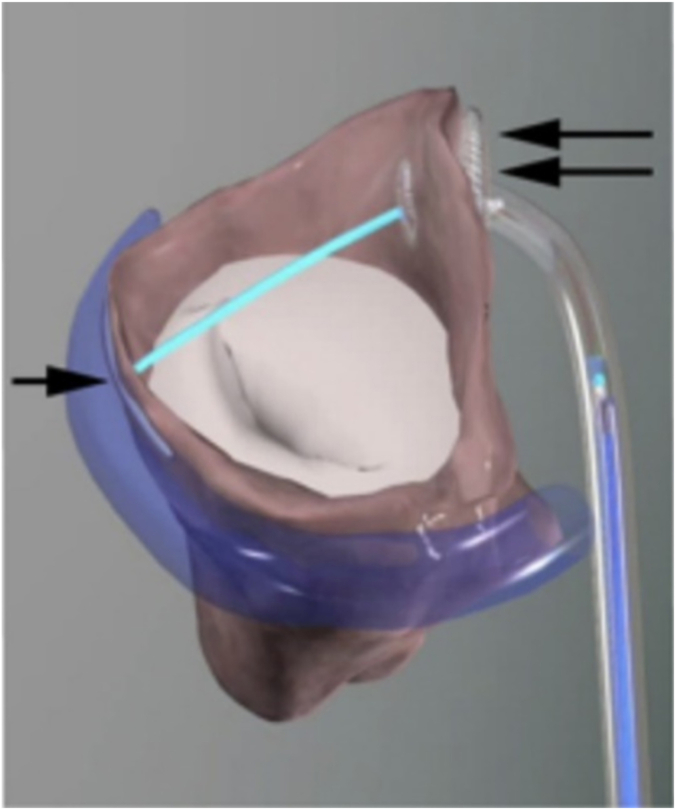
Figure 3.
The CarillonMitralContourSystem. (A) The Carillon Mitral Contour System (Cardiac Dimensions) is a device with 2 anchors at the terminal portion of the device that sit in the coronary sinus. (B, C) The changes in mitral annular dimensions that result with device placement.
The ARTO device (MVRx) is a 12F catheter system that is implanted transvenously and is intended to reduce the minor axis (ie, anteroposterior length) of the mitral annulus with a suture-based method (Figure 4). Similar to the Carillon system, the ARTO device is delivered by cannulating the CS and implanting a “t-bar” into the lateral wall of the heart through the greater cardiac vein. Then, a tether is inserted into the ventricle and grasped through a transseptal implant. The tether is shortened to reduce the degree of MR and has the theoretical advantage of not causing any compression of the circumflex artery by altering the entire CS course. Two-year outcomes from the MAVERICK trial demonstrated the device was safely implanted in 45 patients, with only 2 of the 45 experiencing adverse procedural events at the 30-day mark.8,25 MR reduction was sustained, as was evidence of LV reverse remodeling (indexed left ventricular end-diastolic volume [LVEDVi], 106 ± 26 mL/m2 at baseline to 90 ± 30 mL/m2 at 2-year follow-up).
Figure 4.
The ARTO System. The ARTO System (MVRx) shortens mitral annular dimensions by applying a tether, extending from the coronary sinus (single arrow) through the ventricle (double arrow) to reduce functional mitral regurgitation and reverse remodel the left ventricle.
A myriad of transcatheter mitral valve replacement devices (ie, Tendyne valve, Abbott; Intrepid, Medtronic; and SAPIEN M3, Edwards Lifesciences) and other valvular therapies (ie, Half-Moon posterior mitral leaflet repair system, Half-Moon Medical; and NeoChord, Neochord) are also currently under development and may be a promising transcatheter technology for patients with LV dysfunction. A full review of these devices is discussed in greater detail elsewhere.26
Aortic valve interventions
Transcatheter aortic valve replacement (TAVR) revolutionized the treatment landscape for individuals with aortic valve pathology, providing a minimally invasive means to avoid traditional open heart surgery.27, 28, 29 Multiple elegant cardiac magnetic resonance studies demonstrated a reduction in LVEDVi and indexed left ventricular end-systolic volume (LVESVi) post-TAVR, which is sustained through intermediate follow-up and independent of the specific type of TAVR prosthesis used (ie, balloon vs self-expandable transcatheter valve).30 Furthermore, the cardiac magnetic resonance–based studies demonstrated TAVR has the ability to regress diffuse myocardial fibrosis and reduce myocyte hypertrophy.31 This was further elucidated in the collective experience from the PARTNER (Placement of Aortic Transcatheter Valves) I, II, and S3 trials, which included individuals across the surgical risk spectrum from intermediate to inoperable risk AS and showed a 14.5% decline in LV mass index at 1 year.32 Moreover, the degree of LV mass reduction was associated with less all-cause mortality and HF hospitalization 5 years post-TAVR. Although TAVR provides widespread symptomatic benefit for patients with AS, individuals with unique AS phenotypes, similar to AS that occurs in the context of transthyretin cardiac amyloidosis,33 or individuals post-TAVR who have residual aortic regurgitation,9,34 experience less LV reverse remodeling and may not enjoy similar degrees of clinical benefit.
Although the TAVR experience for pure aortic regurgitation is still in early stages, LV reverse remodeling is a critical end point in this clinical scenario, considering contemporary guidelines use thresholds for LV dimensions to trigger therapy in asymptomatic individuals.35 Data specifically addressing reverse remodeling are lacking, for both currently available and approved prostheses and newer-generation devices, which are designed specifically for aortic regurgitation. The upcoming release of results from the ALIGN-AR (A Study to Assess Safety and Effectiveness of the JenaValve Trilogy Heart Valve System in the Treatment of High Surgical Risk Patients With Symptomatic, Severe Aortic Regurgitation) trial, which evaluated the Trilogy Heart Valve System (JenaValve) will shed light on this important topic.
Cardiac contractility modulation
Cardiac contractility modulation (CCM) is a device-based treatment for HF that enhances native contractility by applying a high-voltage (7.5-V) biphasic signal over a 20-ms period to the right ventricular septum during the absolute refractory period of ventricular excitation (Figure 5A).36 By initially altering calcium cycling within myocytes and during longer-term treatment, inducing beneficial posttranslational modification of key proteins involved in determining contractile force and a shift of myocardial gene expression profile from the fetal genotype typical of chronic HF to a more normal adult genotype,37, 38, 39, 40 CCM treatment has been associated with improved cardiac contractility (Figure 5B). CCM has been studied in the context of 3 randomized trials—FIX-HF-4,10 FIX-HF-5,11 and FIX-HF-5C12—and shown to increase exercise tolerance and improve quality of life and decrease all-cause mortality and HF hospitalizations. In a study using 3-dimensional echocardiography at the 3-month follow-up, LVESV decreased (11.5% ± 10.5%) and EF improved (4.8% ± 3.6%), illustrating the device’s capacity to reverse remodel the left ventricle,41 and longer-term observational studies have shown sustained improvements in LV EF.42
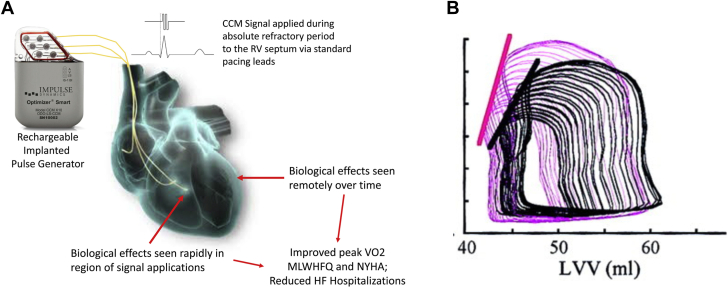
Figure 5.
Cardiac contractility modulation mechanism of action. (A) A pulse generator delivers high-voltage biphasic impulses directly to the right ventricle to enhance contractility. Over time, this alters the local biology of myocardium and eventually effects left ventricular reverse remodeling. (B) A representative example of pressure-volume loops from baseline (black) and after CCM (magenta), illustrating the increase in end-systolic elastance. CCM, cardiac contractility modulation; MLWHFQ, Minnesota Living With Heart Failure questionnaire; NYHA, New York Heart Association. Courtesy of Abraham et al12 and Mohri et al.43
Physical reverse remodeling devices
The original experience with physical reverse remodeling began with adjunctive surgical procedures to improve LV function in the context of surgical revascularization or valve-directed procedures. Broadly speaking, these efforts fall into the following 3 categories: interventions that (1) partition the ventricle and essentially exclude diseased myocardium, (2) reshape the ventricle and attempt to restore the normal elliptical LV form, and (3) constrain the ventricle and attempt to reduce cavity size and wall stress by externally compressing the left ventricle. A variety of transcatheter therapies for partitioning and reshaping the left ventricle have been developed, which will be the focus of the review’s subsequent discussion. Two notable constraint devices—CorCap (Acorn) and HeartNet (ParaCor)—were developed but they were associated with safety and efficacy concerns such that they are no longer under evaluation and, thus, will not be mentioned further.
Partitioning devices
The partial success of surgical ventricular restoration has motivated the development of various devices that can achieve similar results without incurring the risks of traditional, open heart surgery. The most developed technology for this purpose is the Revivant TC System (BioVentrix), which is a transcatheter ventricular enhancement system that is intended to exclude scarred anterior wall myocardium from viable myocardium, thereby reducing LV volume and restoring the normal elliptical shape of the left ventricle (Figure 6).11,43 The device features a pair of polyester-coated titanium microanchors, one that sits in the right ventricle abutting the interventricular septum and another that sits on the exterior surface of the left ventricle. A guide wire is placed through minithoracotomy across the border zone of the infarcted, akinetic myocardium and passed through the interventricular septum into the right ventricle. Then, the guide wire is exteriorized through the internal jugular vein, and one anchor is inserted into the right ventricle while another anchor is inserted along the exterior surface of the left ventricle. Furthermore, the anchors are brought together, plicating the left ventricle and excluding the desired area of myocardium. The procedure can be repeated multiple times to extend the area of excluded myocardium. Transesophageal echocardiographic guidance during insertion of the guide wire and anchors is critical, as is preprocedural cross-sectional imaging.
Figure 6.
The Revivant TCSystem. (A) The BioVentrix Revivent TC myocardial anchoring system (B) attaches to the exterior surface of the left ventricle and the right ventricular side of the interventricular septum to plicate the diseased myocardium. Courtesy of Brener et al.2
The technical feasibility of the procedure was evaluated in an ovine (n = 8) preclinical model with anterior wall infarction where Cheng et al44 described a 40% reduction in LVEDV and a 17% increase in LVEF. Afterward, the procedure—branded Less-Invasive Ventricular Enhancement—was performed in vivo.13,45,46 The largest series reported successful implantation in 86 of the 89 (96.6%) patients, resulting in significant LV reverse remodeling at 1 year (LVESVi declined from 74 ± 28 to 54 ± 23 mL/m2, and LVEDVi declined from 106 ± 33 to 80 ± 26 mL/m2). Patient-reported outcome measures were also favorable; the average New York Heart Association (NYHA) HF class symptoms decreased from 2.6 ± 0.5 to 1.9 ± 0.8 (P < .001) and the Minnesota Living with Heart Failure Questionnaire score declined from 39 ± 21 to 26 ± 22 (P < .001). There were 3 procedure-related deaths (n = 1 each for myocardial necrosis, LV injury, and pulmonary artery injury), but, overall, 1 year survival was 90.6%.13 An investigation device exemption was granted by the US Food and Drug Administration-based on these results, and a pivotal study—the American Less-Invasive Ventricular Enhancement trial—is ongoing. The trial is projected to recruit 126 patients with NYHA class III/IVa symptoms, LVEF of ≤45%, and LVESVi of ≥50 mL/m2 to treatment with the device vs GDMT (NCT02931240). A similar study, REVIVE-HF (Randomized Evaluation and Verification of Ventricular Enhancement), is underway in Europe with a planned enrollment of 180 patients with similar inclusion and exclusion criteria (NCT03845127).
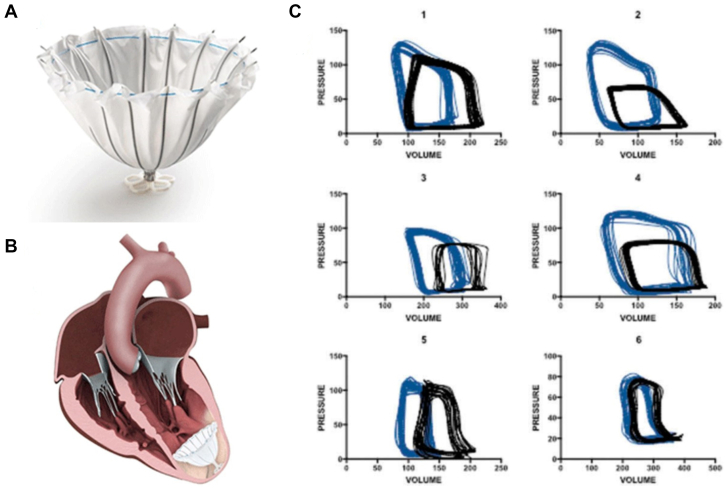
The Parachute device (CardioKinetix) is an entirely percutaneous mechanism that is delivered to the LV apex and is intended to exclude akinetic or aneurysmal apical tissue (Figure 7). The device consists of a polytetrafluoroethylene membrane draped on a self-expanding nitinol frame. The device is implanted through a 14F or 16F catheter transfemoral access point and is manufactured in 4 sizes (diameter ranging from 65.0-95.0 mm). The initial pilot study reported a technical success rate of 83% (15/18 patients; the device was explanted in 1 patient) and improvements in LVESV, LVEDV, LV EF, and clinical parameters, such as NYHA class and 6-minute walk test (6MWT) distance at 12 months.47 A mechanistic study confirmed that when applied to patients with true aneurysms, this devices resulted in leftward shifts of the PV loop and improved overall pump function (Figure 7C).48 Mazzaferri et al14 conducted the single-arm PARACHUTE study in 39 individuals with anteroapical myocardial infarction, LV EF of <40%, and NYHA II-IV HF symptoms, where the device was implanted in 31 subjects (79%; n = 5 implantation not attempted, n = 3 with unsuccessful implants). NYHA class improved (2.5 ± 0.6 to 1.3 ± 0.6; P < .001) but 6MWT distance was unchanged at the 6-month follow-up. The subsequent PARACHUTE III trial, which enrolled 100 individuals, reported a 97% successful implantation rate and significant reductions in LV volumes at the 12-month follow-up (LVESVi 84.0 ± 24.2 to 70.5 ± 24.5 mL/m2; LVEDVi 117.3 ± 26.4 to 99.1 ± 27.3 mL/m2; both P < .0001).49 A subset of 6 patients underwent LV PV analysis before and after device implantation, illustrating many of the aforementioned changes (Figure 7C). However, the 3-year follow-up analysis of the original PARACHUTE study showed that the favorable changes in LV size were not sustained (LVESVi was 89.6 mL/m2 at baseline and 77.1, 76.7, 76.7, and 87.0 mL/m2 at 6, 12, 24, and 36 months, respectively; LVEDVi was 125.7 mL/m2 at baseline and 109.4, 108.9, 108.9, and 114.4 mL/m2 at 6, 12, 24, and 36 months).50 This finding, in addition to safety concerns with device implantation, halted a pivotal study.51
Figure 7.
The Parachute device. (A) The Parachute device (B) as it is placed in the left ventricular apex. (C) Pressure-volume loops from 6 patients illustrate favorable leftward shifting of the pressure-volume loop and changes in the end-systolic pressure-volume relationship. Courtesy Brener et al.2
The Heart Damper (Eucardia) is currently under development and features a percutaneously delivered device that partitions the apex from the rest of the LV cavity but does so with a flexible diaphragm that flattens during diastole and contracts during systole to propel blood out of the left ventricle. In addition to physically remodeling the ventricle, the device also uses energy transferred from ventricular contraction to potentially augment stroke volume and increase cardiac output. Additional studies are currently underway to test the safety and feasibility of the device.
Reshaping devices
The other predominant percutaneous strategy for physical reverse remodeling involves ventricular reshaping to optimize chamber performance. The genesis for developing these devices was the original Coapsys device (Myocor), which was a surgically implanted subvalvular tether that reduced myocardial wall stress by helping the ventricle resume its normal elliptical shape.52 Although the device was associated with a 1-year survival advantage over the standard of care in the RESTOR-MV (Randomized Evaluation of a Surgical Treatment for Off-Pump Repair of the Mitral Valve) study,53 the need for surgical implantation and various financial considerations prompted the discontinuation of the device (even the second iteration of the device, which was a transcatheter system54) and the search for other fully percutaneous approaches.
The AccuCinch ventricular restoration device (Ancora) addresses this treatment gap (Figure 8). The device features a polyethelene cable, which is inserted into the LV retrograde (transaortic) through a 20F transfemoral system and is deployed 1.0 to 2.0 cm below the mitral valve annulus and affixed to the endocardial surface by 12-16 anchors. Once the device is attached to the endocardial surface by the anchors, the cable is tightened to “cinch” the left ventricle and reduce its basal-to-midfree wall circumference. This reduces mitral annular dimensions to attenuate the amount of functional MR and reduces the radius of curvature of the left ventricle to decrease wall stress.55 The device has completed preclinical and early feasibility testing56 and is being evaluated in the pivotal randomized CorCinch-HF (Clinical Evaluation of the AccuCinch Ventricular Restoration System in Patients Who Present With Symptomatic Heart Failure With Reduced Ejection Fraction) trial (NCT04331769), where 400 participants with symptomatic dilated cardiomyopathy with EF between 20% and 40%, LV end-diastolic diameter of ≥55.0 mm, and MR of ≤2+ will be randomized in a 1:1 fashion to GDMT vs GDMT plus AccuCinch implantation.
Figure 8.
The AccuCinch device. (A) Short-axis and (B) long-axis views of the AccuCinch device after it is implanted in the subvalvular position. Courtesy Brener et al.2
An initial report at Technology and Heart Failure Therapeutics 2023 by Hamid et al15 from 51 trial participants from 3 US early feasibility studies (CorCinch-HF with reduced EF, NCT03533517; CorCinch-FMR NCT02806570; CorCinch-PMVI NCT03560167) and 1 European pivotal trial (CorCinch-EU, NCT NCT03183895) demonstrated promising early results. The median time for device implantation was 131 minutes, and an average of 13 anchors were successfully deployed. There was an immediate reduction in LVEDV (−11.2 ± 2.7 mL) after implantation, which was not only sustained but progressed out to 12 months’ follow-up (−33.6 ± 5.4 mL; n = 41/51), implying the device actuated an acute physical reverse remodeling effect, compounded by a later biological reverse remodeling effect. There were similarly favorable improvements in HF symptoms (change in Kansas City Cardiomyopathy Questionnaire score from baseline to 12 months = 16.4 ± 2.7) and 6MWT (+45.9 ± 12.7 feet), with 65% of participants reporting a ≥1+ improvement in NYHA class.15
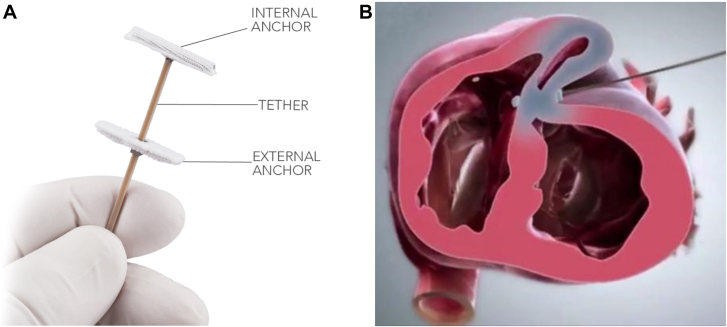
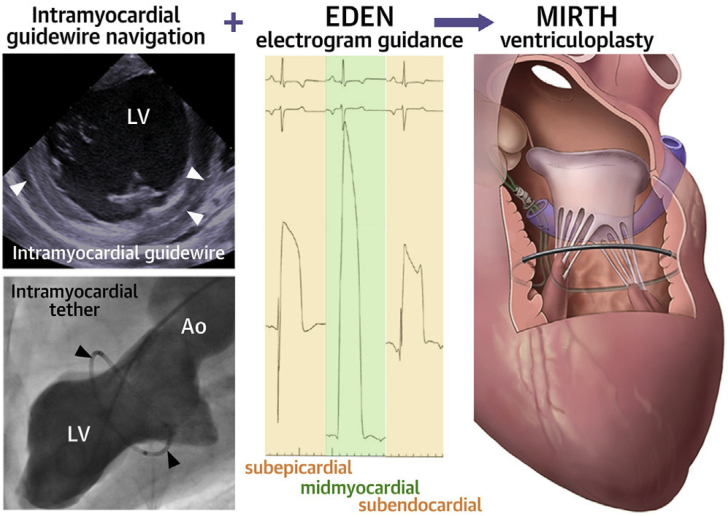
A new procedure— myocardial intramural remodeling by transvenous tether (MIRTH)—uses many of the same principles as the AccuCinch device to reshape the ventricle.16 MIRTH involves the percutaneous implantation of a cable circumferentially within the LV wall, which is then tightened to reduce LV diameter (Figure 9). The procedure is executed by placing a stiff guide wire (Astato XS), originally purposed for penetrating chronic total occlusions in the coronary arteries, into the CS and using it to pierce the myocardium. To be successful, the wire must be placed in the mid-myocardial layer, and this is achieved by connecting it to a novel navigational system called Electrocardiogram radial Depth Navigation (EDEN). EDEN produces a unipolar intramyocardial electrogram that generates a unique signature when the guide wire is in the mid-myocardial layer. This allows the operator to redirect the wire away from the endocardial and epicardial surfaces and traverse the LV circumference within the mid-myocardial layer. Then, the guide wire is externalized after reentering the ventricle and exchanged for an ultra–high-molecular-weight polyethylene-braided suture through a standard, coaxial coronary microcatheter. Tension is subsequently applied to the tether to decrease the LV radius of curvature and perimeter.
Figure 9.
Myocardial intramural remodeling by transvenous tether (MIRTH) ventriculoplasty procedural details. Myocardial Intramural Remodeling by Transvenous Tether (MIRTH) involves placement of a coronary guide wire into the mid-myocardium, navigating it using a novel technique around the left ventricular circumference and replacing the guide wire with a cable that can be cinched to perform ventriculoplasty and reduce left ventricular dimensions. EDEN, electrocardiographic radial depth navigation. Courtesy of Bruce et al.16
Bruce et al16 performed MIRTH in a preclinical model with 12 healthy swine and 13 swine with a fibrotic myocardium from an iatrogenic myocardial infarction. MIRTH was successfully executed in 11 of the 12 healthy swine. Septolateral and anteroposterior end-diastolic and end-systolic diameters decreased after MIRTH and were sustained out to 90 days’ follow-up (7/11 swine), but changes in EDV index and end-systolic volume index were not sustained. The authors also conducted an elegant LV PV study to determine the optimal degree of tension on the cable to achieve favorable hemodynamic responses (defined by the LVEDP). This revealed an inflection point at 21% shortening, suggesting that overshortening may produce some degree of systolic and diastolic dysfunction. Although this technique appears promising, it requires considerably more refinement, especially for safety and feasibility.17
Finally, a percutaneous ventricular reshaping device that encircles the papillary muscles, called the V-sling (Cardiac Success), attempts to recapitulate many of the favorable effects reported with surgical papillary muscle approximation.57, 58, 59 The V-Sling is delivered through 14F catheter transfemoral access to the left ventricle and deployed around the papillary muscles using a steerable sheath. The device brings the papillary muscles together to reduce ventricular dimensions and has a favorable effect on the degree of functional MR in preclinical models. Preliminary data from the first-in-human experience with V-sling were presented by Sievert et al60 at THT 2023, demonstrating feasibility of implantation.
Conclusion
HF in the setting of reduced EF is a clinical syndrome characterized by changes in LV structure and geometry, which promote further myocardial dysfunction and dilation over time. These morphologic changes are the treatment target for HF-directed therapies, which either seek to directly alter LV size and shape, that is, physically reverse remodel the left ventricle, or secondarily change LV size and shape by affecting loading conditions, that is, biologically reverse remodel the left ventricle. Both reverse remodeling strategies have roles to play in the treatment armamentarium for HF, but additional research is required to determine which patients will benefit the most from these interventions and how to integrate such interventions into the evolving landscape of GDMT.
Acknowledgments
Declaration of competing interests
Michael Brener is supported by institutional grants from Abiomed and Abbott and has received honoraria from Artract and Osprey Medical. Samir Kapadia reports no conflicts. Daniel Burkhoff reports institutional grant support from Axon Therapeutics, Ancora Heart, Edwards Lifesciences and ZOLL/TherOx; support for travel from Abiomed (Johnson & Johnson); consulting fees from AquaPass, Axon Therapeutics, Corvia Medical, Cordio, Edwards Lifesciences, Impulse Dynamics, Orchestra Biomed, PVLoops LLC, and ZOLL/TherOx; and equity in Orchestra Biomed.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or non-for-profit sectors.
References
- 1.Bastos M.B., Burkhoff D., Maly J., et al. Invasive left ventricle pressure-volume analysis: overview and practical clinical implications. Eur Heart J. 2020;41(12):1286–1297. doi: 10.1093/eurheartj/ehz552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brener M.I., Uriel N., Burkhoff D. Left ventricular volume reduction and reshaping as a treatment option for heart failure. Struct Heart. 2020;4(4):264–283. doi: 10.1080/24748706.2020.1777359. [DOI] [Google Scholar]
- 3.Konstam M.A., Kramer D.G., Patel A.R., Maron M.S., Udelson J.E. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. J Am Coll Cardiol Img. 2011;4(1):98–108. doi: 10.1016/j.jcmg.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Stone G.W., Lindenfeld J., Abraham W.T., et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. 2018;379(24):2307–2318. doi: 10.1056/NEJMoa1806640. [DOI] [PubMed] [Google Scholar]
- 5.Witte K.K., Lipiecki J., Siminiak T., et al. The REDUCE FMR trial: a randomized sham-controlled study of percutaneous mitral annuloplasty in functional mitral regurgitation. J Am Coll Cardiol HF. 2019;7(11):945–955. doi: 10.1016/j.jchf.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Siminiak T., Wu J.C., Haude M., et al. Treatment of functional mitral regurgitation by percutaneous annuloplasty: results of the TITAN Trial. Eur J Heart Fail. 2012;14(8):931–938. doi: 10.1093/eurjhf/hfs076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipiecki J., Siminiak T., Sievert H., et al. Coronary sinus-based percutaneous annuloplasty as treatment for functional mitral regurgitation: the TITAN II trial. Open Heart. 2016;3(2) doi: 10.1136/openhrt-2016-000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patterson T., Gregson J., Erglis A., et al. Two-year outcomes from the MitrAl ValvE RepaIr Clinical (MAVERIC) trial: a novel percutaneous treatment of functional mitral regurgitation. Eur J Heart Fail. 2021;23(10):1775–1783. doi: 10.1002/ejhf.2321. [DOI] [PubMed] [Google Scholar]
- 9.Sato K., Kumar A., Jones B.M., et al. Reversibility of cardiac function predicts outcome after transcatheter aortic valve replacement in patients with severe aortic stenosis. J Am Heart Assoc. 2017;6(7) doi: 10.1161/JAHA.117.005798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borggrefe M.M., Lawo T., Butter C., et al. Randomized, double blind study of non-excitatory, cardiac contractility modulation electrical impulses for symptomatic heart failure. Eur Heart J. 2008;29(8):1019–1028. doi: 10.1093/eurheartj/ehn020. [DOI] [PubMed] [Google Scholar]
- 11.Kadish A., Nademanee K., Volosin K., et al. A randomized controlled trial evaluating the safety and efficacy of cardiac contractility modulation in advanced heart failure. Am Heart J. 2011;161(2):329–337.e322. doi: 10.1016/j.ahj.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 12.Abraham W.T., Kuck K.-H., Goldsmith R.L., et al. A randomized controlled trial to evaluate the safety and efficacy of cardiac contractility modulation. J Am Coll Cardiol HF. 2018;6(10):874–883. doi: 10.1016/j.jchf.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Klein P., Anker S.D., Wechsler A., et al. Less invasive ventricular reconstruction for ischaemic heart failure. Eur J Heart Fail. 2019;21(12):1638–1650. doi: 10.1002/ejhf.1669. [DOI] [PubMed] [Google Scholar]
- 14.Mazzaferri E.L., Jr., Gradinac S., Sagic D., et al. Percutaneous left ventricular partitioning in patients with chronic heart failure and a prior anterior myocardial infarction: results of the PercutAneous Ventricular RestorAtion in Chronic Heart failUre PaTiEnts Trial. Am Heart J. 2012;163(5):812–820.e1. doi: 10.1016/j.ahj.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Hamid N., Jorde U.P., Reisman M., et al. Transcatheter left ventricular restoration in patients with heart failure. J Card Fail. 2023;29(7):1046–1055. doi: 10.1016/j.cardfail.2023.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Bruce C.G., Khan J.M., Rogers T., et al. Reshaping the ventricle from within: MIRTH (Myocardial Intramural Remodeling by Transvenous Tether) Ventriculoplasty in Swine. J Am Coll Cardiol Basic Trans Sci. 2023;8(1):37–50. doi: 10.1016/j.jacbts.2022.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alkhunaizi F.A., Brener M.I., Burkhoff D. Device-based ventricular reverse remodeling: a multimechanistic therapeutic strategy. J Am Coll Cardiol Basic Transl Sci. 2023;8(1):51–54. doi: 10.1016/j.jacbts.2022.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Artrip J.H., Oz M.C., Burkhoff D. Left ventricular volume reduction surgery for heart failure: a physiologic perspective. J Thorac Cardiovasc Surg. 2001;122(4):775–782. doi: 10.1067/mtc.2001.116208. [DOI] [PubMed] [Google Scholar]
- 19.Cioffi G., Tarantini L., De Feo S., et al. Functional mitral regurgitation predicts 1-year mortality in elderly patients with systolic chronic heart failure. Eur J Heart Fail. 2005;7(7):1112–1117. doi: 10.1016/j.ejheart.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Grigioni F., Enriquez-Sarano M., Zehr K.J., Bailey K.R., Tajik A.J. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001;103(13):1759–1764. doi: 10.1161/01.cir.103.13.1759. [DOI] [PubMed] [Google Scholar]
- 21.Arnold S.V., Stone G.W., Jain S.S., et al. Prognostic importance of health status versus functional status in heart failure and secondary mitral regurgitation. J Am Coll Cardiol HF. 2021;9(9):684–692. doi: 10.1016/j.jchf.2021.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Obadia J.F., Messika-Zeitoun D., Leurent G., et al. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med. 2018;379(24):2297–2306. doi: 10.1056/NEJMoa1805374. [DOI] [PubMed] [Google Scholar]
- 23.Pibarot P., Delgado V., Bax J.J. MITRA-FR vs. COAPT: lessons from two trials with diametrically opposed results. Eur Heart J Cardiovasc Imaging. 2019;20(6):620–624. doi: 10.1093/ehjci/jez073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kałmucki P., Lipiecki J., Witte K.K., Goldberg S.L., Baszko A., Siminiak T. Percutaneous mitral annuloplasty with the Carillon device: outcomes in proportionate and disproportionate functional mitral regurgitation. Am Heart J. 2023;265:137–142. doi: 10.1016/j.ahj.2023.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Rogers J.H., Thomas M., Morice M.C., et al. Treatment of heart failure with associated functional mitral regurgitation using the ARTO system: initial results of the first-in-human MAVERIC trial (mitral valve repair clinical trial) J Am Coll Cardiol Intv. 2015;8(8):1095–1104. doi: 10.1016/j.jcin.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Lander M.M., Brener M.I., Goel K., et al. Mitral interventions in heart failure. J Am Coll Cardiol HF. 2023;11(8 Pt 2):1055–1069. doi: 10.1016/j.jchf.2023.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leon M.B., Smith C.R., Mack M., et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 28.Leon M.B., Smith C.R., Mack M.J., et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374(17):1609–1620. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 29.Mack M.J., Leon M.B., Thourani V.H., et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380(18):1695–1705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 30.Mehdipoor G., Chen S., Chatterjee S., et al. Cardiac structural changes after transcatheter aortic valve replacement: systematic review and meta-analysis of cardiovascular magnetic resonance studies. J Cardiovasc Magn Reson. 2020;22(1):41. doi: 10.1186/s12968-020-00629-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Treibel T.A., Kozor R., Schofield R., et al. Reverse myocardial remodeling following valve replacement in patients with aortic stenosis. J Am Coll Cardiol. 2018;71(8):860–871. doi: 10.1016/j.jacc.2017.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chau K.H., Douglas P.S., Pibarot P., et al. Regression of left ventricular mass after transcatheter aortic valve replacement: the PARTNER trials and registries. J Am Coll Cardiol. 2020;75(19):2446–2458. doi: 10.1016/j.jacc.2020.03.042. [DOI] [PubMed] [Google Scholar]
- 33.Nitsche C., Koschutnik M., Donà C., et al. Reverse remodeling following valve replacement in coexisting aortic stenosis and transthyretin cardiac amyloidosis. Circ Cardiovasc Imaging. 2022;15(7) doi: 10.1161/CIRCIMAGING.122.014115. [DOI] [PubMed] [Google Scholar]
- 34.Merten C., Beurich H.-W., Zachow D., et al. Aortic regurgitation and left ventricular remodeling after transcatheter aortic valve implantation: a serial cardiac magnetic resonance imaging study. Circ Cardiovasc Interv. 2013;6(4):476–483. doi: 10.1161/CIRCINTERVENTIONS.112.000115. [DOI] [PubMed] [Google Scholar]
- 35.Otto C.M., Nishimura R.A., Bonow R.O., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2021;143(5):e72–e227. doi: 10.1161/CIR.0000000000000923. [DOI] [PubMed] [Google Scholar]
- 36.Borggrefe M., Mann D.L. Cardiac contractility modulation in 2018. Circulation. 2018;138(24):2738–2740. doi: 10.1161/CIRCULATIONAHA.118.036460. [DOI] [PubMed] [Google Scholar]
- 37.Imai M., Rastogi S., Gupta R.C., et al. Therapy with cardiac contractility modulation electrical signals improves left ventricular function and remodeling in dogs with chronic heart failure. J Am Coll Cardiol. 2007;49(21):2120–2128. doi: 10.1016/j.jacc.2006.10.082. [DOI] [PubMed] [Google Scholar]
- 38.Butter C., Rastogi S., Minden H.H., Meyhöfer J., Burkhoff D., Sabbah H.N. Cardiac contractility modulation electrical signals improve myocardial gene expression in patients with heart failure. J Am Coll Cardiol. 2008;51(18):1784–1789. doi: 10.1016/j.jacc.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 39.Lyon A.R., Samara M.A., Feldman D.S. Cardiac contractility modulation therapy in advanced systolic heart failure. Nat Rev Cardiol. 2013;10(10):584–598. doi: 10.1038/nrcardio.2013.114. [DOI] [PubMed] [Google Scholar]
- 40.Tschöpe C., Kherad B., Klein O., et al. Cardiac contractility modulation: mechanisms of action in heart failure with reduced ejection fraction and beyond. Eur J Heart Fail. 2019;21(1):14–22. doi: 10.1002/ejhf.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu C.-M., Chan J.Y.-S., Zhang Q., et al. Impact of cardiac contractility modulation on left ventricular global and regional function and remodeling. J Am Coll Cardiol Img. 2009;2(12):1341–1349. doi: 10.1016/j.jcmg.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 42.Kuschyk J., Falk P., Demming T., et al. Long-term clinical experience with cardiac contractility modulation therapy delivered by the Optimizer Smart system. Eur J Heart Fail. 2021;23(7):1160–1169. doi: 10.1002/ejhf.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohri S., He K.-L., Dickstein M., et al. Cardiac contractility modulation by electric currents applied during the refractory period. Am J Physiol Heart Circ Physiol. 2002;282(5):H1642–H1647. doi: 10.1152/ajpheart.00959.2001. [DOI] [PubMed] [Google Scholar]
- 44.Cheng Y., Aboodi M.S., Annest L.S., et al. Off-pump epicardial ventricular reconstruction restores left ventricular twist and reverses remodeling in an ovine anteroapical aneurysm model. J Thorac Cardiovasc Surg. 2014;148(1):225–231. doi: 10.1016/j.jtcvs.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 45.Loforte A., Alfonsi J., Gliozzi G., et al. Less invasive ventricular enhancement (LIVE) as potential therapy for ischaemic cardiomyopathy end-stage heart failure. J Thorac Dis. 2019;11(suppl 6):S921–S928. doi: 10.21037/jtd.2019.02.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klein P., Agostoni P., van Boven W.J., de Winter R.J., Swaans M.J. Transcatheter and minimally invasive surgical left ventricular reconstruction for the treatment of ischaemic cardiomyopathy: preliminary results. Interact Cardiovasc Thorac Surg. 2019;28(3):441–446. doi: 10.1093/icvts/ivy259. [DOI] [PubMed] [Google Scholar]
- 47.Sagic D., Otasevic P., Sievert H., Elsasser A., Mitrovic V., Gradinac S. Percutaneous implantation of the left ventricular partitioning device for chronic heart failure: a pilot study with 1-year follow-up. Eur J Heart Fail. 2010;12(6):600–606. doi: 10.1093/eurjhf/hfq051. [DOI] [PubMed] [Google Scholar]
- 48.Patterson T, Schreuder J, Burkhoff D, et al. Percutaneous ventricular restoration using the parachute device: the parachute III pressure-volume loop sub-study. Struct Heart. 201;1(1-2):65-74.
- 49.Thomas M., Nienaber C.A., Ince H., et al. Percutaneous ventricular restoration (PVR) therapy using the Parachute device in 100 subjects with ischaemic dilated heart failure: one-year primary endpoint results of PARACHUTE III, a European trial. EuroIntervention. 2015;11(6):710–717. doi: 10.4244/eijv11i6a143. [DOI] [PubMed] [Google Scholar]
- 50.Costa M.A., Mazzaferri E.L., Jr., Sievert H., Abraham W.T. Percutaneous ventricular restoration using the parachute device in patients with ischemic heart failure: three-year outcomes of the PARACHUTE first-in-human study. Circ Heart Fail. 2014;7(5):752–758. doi: 10.1161/circheartfailure.114.001127. [DOI] [PubMed] [Google Scholar]
- 51.Costa M.A., Pencina M., Nikolic S., Engels T., Templin B., Abraham W.T. The PARACHUTE IV trial design and rationale: percutaneous ventricular restoration using the parachute device in patients with ischemic heart failure and dilated left ventricles. Am Heart J. 2013;165(4):531–536. doi: 10.1016/j.ahj.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 52.Mishra Y.K., Mittal S., Jaguri P., Trehan N. Coapsys mitral annuloplasty for chronic functional ischemic mitral regurgitation: 1-year results. Ann Thorac Surg. 2006;81(1):42–46. doi: 10.1016/j.athoracsur.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 53.Grossi E.A., Patel N., Woo Y.J., et al. Outcomes of the RESTOR-MV trial (randomized evaluation of a surgical treatment for off-pump repair of the mitral valve) J Am Coll Cardiol. 2010;56(24):1984–1993. doi: 10.1016/j.jacc.2010.06.051. [DOI] [PubMed] [Google Scholar]
- 54.Pedersen W.R., Block P., Leon M., et al. iCoapsys mitral valve repair system: percutaneous implantation in an animal model. Catheter Cardiovasc Interv. 2008;72(1):125–131. doi: 10.1002/ccd.21551. [DOI] [PubMed] [Google Scholar]
- 55.Gooley R.P., Meredith I.T. The Accucinch transcatheter direct mitral valve annuloplasty system. EuroIntervention. 2015;11(Suppl W):W60–W61. doi: 10.4244/eijv11swa16. [DOI] [PubMed] [Google Scholar]
- 56.Tenorio C., González N., Jaramillo J.S., et al. Device-based therapy for mitral regurgitation and ventricular reshaping. Struct Heart. 2017;1(3-4):195–198. doi: 10.1080/24748706.2017.1362608. [DOI] [Google Scholar]
- 57.Mihos C.G., Capoulade R., Yucel E., Melnitchouk S., Hung J. Combined papillary muscle sling and ring annuloplasty for moderate-to-severe secondary mitral regurgitation. J Card Surg. 2016;31(11):664–671. doi: 10.1111/jocs.12843. [DOI] [PubMed] [Google Scholar]
- 58.Mihos C.G., Capoulade R., Yucel E., et al. Mitral valve and subvalvular repair for secondary mitral regurgitation: rationale and clinical outcomes of the papillary muscle sling. Cardiol Rev. 2018;26(1):22–28. doi: 10.1097/CRD.0000000000000168. [DOI] [PubMed] [Google Scholar]
- 59.Hvass U., Tapia M., Baron F., Pouzet B., Shafy A. Papillary muscle sling: a new functional approach to mitral repair in patients with ischemic left ventricular dysfunction and functional mitral regurgitation. Ann Thorac Surg. 2003;75(3):809–811. doi: 10.1016/s0003-4975(02)04678-7. [DOI] [PubMed] [Google Scholar]
- 60.Sievert H, Sievert K, Bertog S, et al. CardiacSuccess / V-Sling. Presented at THT 2023; March 21, 2023; Boston, MA. https://www.tctmd.com/slide/cardiacsuccess-v-sling Accessed October 19, 2023.