Abstract
As of this writing, there have been approximately 24 randomized controlled trial publications, 32 meta-analyses, and 85 registries comparing intravascular ultrasound (IVUS) or optical coherence tomography (OCT) versus angiography-guided drug-eluting stent implantation (or IVUS versus OCT guidance). Although in specific clinical scenarios IVUS or OCT may be preferred, in most drug-eluting stent implantation procedures, either intravascular ultrasound or OCT can be used safely, efficiently, effectively, and interchangeably and will improve patient outcomes compared with stent implantation procedures performed just with angiography guidance.
Keywords: Angiography, intravascular imaging, intravascular ultrasound, optical coherence tomography, outcomes, percutaneous coronary intervention
Central Illustration
Highlights
-
•
More than 140 publications (randomized controlled trials, meta-analyses, and registries) have assessed intravascular imaging–guided percutaneous coronary intervention.
-
•
Intravascular imaging–guided percutaneous coronary intervention reduces all-cause and cardiovascular mortality.
-
•
Intravascular imaging guidance should be routinely incorporated into most percutaneous coronary intervention procedures.
As of this writing, there have been approximately 24 randomized controlled trial publications, 32 meta-analyses, and 85 registries comparing intravascular imaging (IVI) versus angiography-guided drug-eluting stent (DES) implantation. This review article will summarize the evidence that IVI-guided DES implantation improves patient outcomes compared with angiography guidance alone. The intended audience includes interventional cardiologists who are not familiar with this material, IVI advocates who want an easily accessed compilation of data, and members of the guideline committees who make recommendations while ignoring the wealth of available evidence. Conversely, this review article is not intended to be another how-to guide on the use of IVI; there are many such articles in the literature.
Stent sizing and optimization
An early in vitro study reported that intravascular ultrasound (IVUS) overestimated measurements compared with a phantom, whereas optical coherence tomography (OCT) measurements were similar to the phantom.1 However, more recent studies show little or no difference between measurements made by OCT versus high-definition IVUS.2,3 This has led to a universal approach to stent sizing and optimization. For example, in early studies, a lumen-based stent sizing protocol by OCT resulted in smaller stent expansion and lumen gain than an external elastic lamina stent sizing by IVUS4,5; however, more recent studies revealed that an external elastic lamina-based stent sizing by OCT resulted in final minimum stent areas and stent expansions noninferior to those obtained using IVUS guidance.6,7
Reference lumen diameters are typically larger by IVUS or OCT than by angiography, especially in smaller vessels. For stent sizing, both IVUS and OCT use the outer boundary of the muscular media, variably called the external elastic lamina or external elastic membrane and because of accumulated plaque, the external elastic lamina or external elastic membrane is always larger than the lumen. OCT can visualize the external elastic lamina in the presence of a normal vessel or fibrotic plaque while lipid or a necrotic core obscures the external elastic lamina. Lipid causes much less IVUS attenuation, except in the presence of large necrotic core-containing thin-cap fibroatheroma (attenuated plaque), whereas calcium causes shadowing of the vessel wall.
Overall, <10% of angiographically normal reference segments are disease-free; the plaque burden seen in angiographically normal reference segments averages 50%. Stent lengths are based on the distance between proximal and distal landing zones as close to normal as possible: largest lumens with a plaque burden of no more than 50% (by IVUS) or visualization of at least half of the external elastic lamina circumference (by OCT). Stents should be implanted so that edges avoid lipidic plaque, especially a thin-cap fibroatheroma or attenuated plaque (that can cause distal embolization), significant superficial calcium (that can cause a dissection), or a muscle bridge (that can cause stent compression). Distal reference stent sizing should be employed when healthy landing zones or predominantly fibrous plaques are present. The postdilation balloon should be similarly sized to the proximal reference. A lumen-based strategy is used if the external elastic lamina or external elastic membrane cannot be visualized (either IVUS or OCT).
Stent underexpansion is the most consistent predictor of stent thrombosis or restenosis, and coronary calcification is the major cause of stent underexpansion. OCT can measure the arc, thickness, length, area, and volume of calcium but will miss calcium behind lipid. IVUS can measure the arc and length of calcium but cannot measure calcium thickness because ultrasound does not penetrate calcium. Nevertheless, both IVUS and OCT are more accurate than angiography, differentiate superficial from deep calcium, and detect nodular calcium, as seen in one-fourth to one-half of the severely calcified lesions. An OCT-based calcium score predicts stent underexpansion and the need for prestent calcium modification in the presence of a calcium arc of >180° plus thickness of >0.5 mm plus length of >5 mm.8 A similar score has been developed for IVUS based on a >270° arc of calcium that is of >5 mm in length, vessel size of <3.5 mm (media-to-media), and presence of either circumferential calcium or a calcified nodule.9 Stent edge problems—geographic miss, large edge dissections, or intramural hematomas—are second only to stent underexpansion as predictors of events.10 However, even in randomized clinical trials, optimal stent implantation is seen in only half of the IVI-guided procedures.
There is little or no evidence suggesting that acute stent malapposition—whether detected by IVUS or OCT and irrespective of size—is associated with adverse events; the only exception is at the proximal stent edge of the stent, where malapposition should be minimized to avoid a guide wire getting behind stent struts during a subsequent procedure. Tissue protrusion does not require treatment, especially in patients presenting with stable coronary artery disease; additional percutaneous coronary intervention (PCI) should be reserved for a reduced effective lumen area or in the setting of an acute coronary syndrome. There is no clinical impact of stent asymmetry or eccentricity.
Although there are specific clinical scenarios in which IVUS or OCT may be preferred, in most DES implantation procedures, either IVUS or OCT can be used safely, efficiently, effectively, and interchangeably.11
Randomized controlled trial publications
IVUS versus angiographic guidance
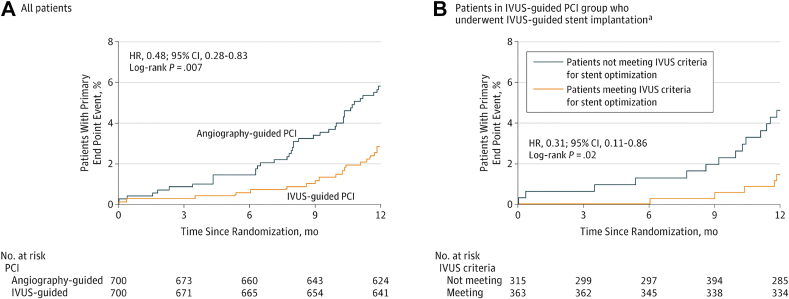
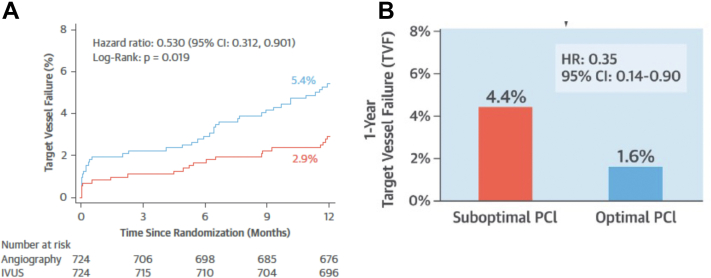
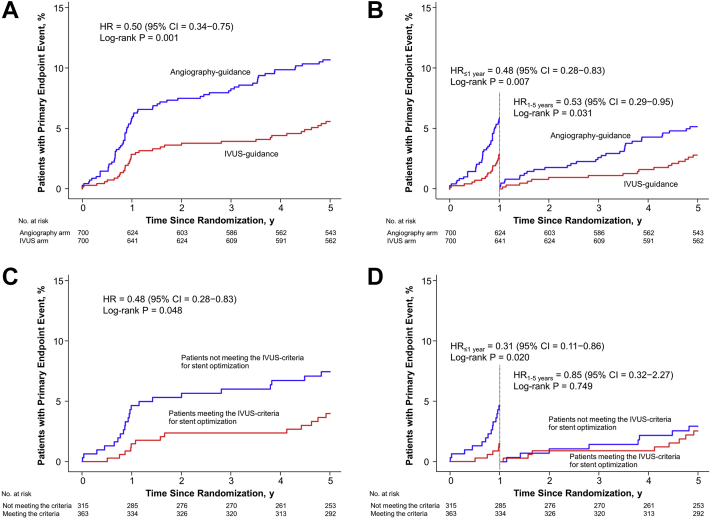
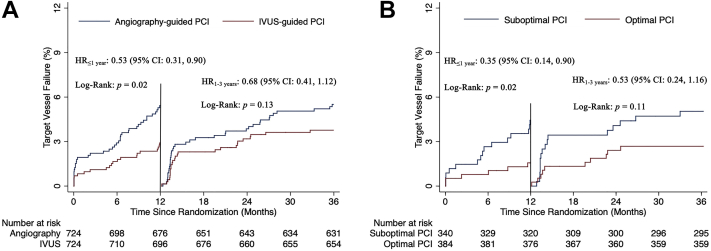
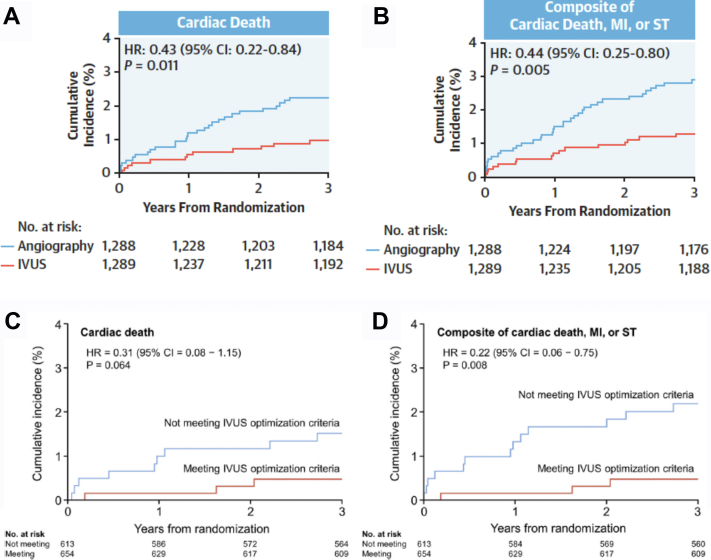
The 2 largest published randomized controlled trials—1400-patient IVUS-XPL (Impact of Intravascular Ultrasound Guidance on Outcomes of Xience Prime Stents in Long Lesions)12 and 1448-patient ULTIMATE (Intravascular Ultrasound Guided Drug Eluting Stents Implantation in “All-Comers” Coronary Lesions)13—showed a reduction in primary end points at 1-year follow-up: major adverse cardiac events (MACE: cardiac death, target lesion-related myocardial infarction [MI], or ischemia-driven target lesion revascularization [TLR]) from 5.8% to 2.9% in IVUS-XPL trial (Figure 1) and target vessel failure (TVF: cardiac death, target vessel MI, and clinically driven TLR) from 5.4% to 2.9% in ULTIMATE trial (Figure 2). The reduction in events was maintained at 5 years in IVUS-XPL trial (10.7% vs 6.6%; Figure 3)14 and 3 years in the ULTIMATE trial (10.7% vs 6.6%; Figure 4).15 The estimated number-needed-to-treat to prevent 1 MACE in the IVUS-XPL trial is 36 at 1 year and 21 at 5 years and 41 and 25 to prevent 1 TVF in ULTIMATE at 1 and 3 years, respectively. An analysis integrating patient-level data from these 2 studies reported a mortality reduction of 50% at 3 years with IVUS guidance; the annual mortality fell to 0.15% when an optimal IVUS-guided result was obtained (Figure 5).16 In this pooled analysis, there was a remarkable number-needed-to-treat of 81 to prevent 1 cardiac death at 3 years.
Figure 1.
Kaplan-Meier estimates of occurrence of the primary end point for all patients and patients who underwent IVUS-guided stent implantation in IVUS-XPL. Cumulative incidence curves are for the primary end point of cardiac death, target lesion–related myocardial infarction, and target lesion revascularization. (A) Comparison of patients randomized to IVUS versus angiographic guidance. (B) Patients in the IVUS-guidance group, comparing those with versus those without stent optimization. HR, hazard ratio; IVUS, intravascular ultrasound; IVUS-XPL, Impact of Intravascular Ultrasound Guidance on Outcomes of Xience Prime Stents in Long Lesions; PCI, percutaneous coronary intervention. Reprinted with permission from Hong SJ, Kim BK, Shin DH, et al. Effect of intravascular ultrasound-guided vs angiography-guided everolimus-eluting stent implantation: the IVUS-XPL randomized clinical trial. aThere were 30 patients in the angiography-guided PCI group who underwent IVUS-guided PCI but they are not included in this analysis. JAMA. 2015;314(20):2155-2163.
Figure 2.
Kaplan-Meier estimates of occurrence of the primary end point for all patients and for patients who underwent IVUS-guided stent implantation in ULTIMATE. Cumulative incidence curves are for the primary end points of cardiac death, target vessel myocardial infarction, and clinically driven TLR. (A) Comparison of patients randomized to IVUS versus angiographic guidance. (B) Patients in the IVUS-guidance group, comparing those with versus those without stent optimization. HR, hazard ratio; IVUS, intravascular ultrasound; PCI, percutaneous coronary intervention; TLR, target vessel revascularization. Reprinted with permission from Zhang J, Gao X, Kan J, et al. Intravascular ultrasound versus angiography-guided drug-eluting stent implantation: the ULTIMATE trial. J Am Coll Cardiol. 2018;72(24):3126-3137.
Figure 3.
Five-year data from IVUS-XPL. Cumulative incidence curves for the primary endpoint of cardiac death, target lesion–related myocardial infarction, and target lesion revascularization. (A) All patients. (B) Landmark analyses for all patients. (C) Patients in the IVUS-guided percutaneous coronary intervention group who underwent IVUS-guided stent implantation comparing those with versus those without stent optimization. (D) Landmark analyses for the patients in the IVUS-guided PCI group who underwent IVUS-guided stent implantation comparing those with versus those without stent optimization. HR, hazard ratio; IVUS, intravascular ultrasound; IVUS-XPL, Impact of Intravascular Ultrasound Guidance on Outcomes of Xience Prime Stents in Long Lesions; PCI, percutaneous coronary intervention. Reprinted with permission from Hong SJ, Mintz GS, Ahn CM, et al. Effect of intravascular ultrasound-guided drug-eluting stent implantation: 5-year follow-up of the IVUS-XPL randomized trial. JACC Cardiovasc Interv. 2020;13(1):62-71.
Figure 4.
Three-year data from ULTIMATE. (A) Landmark analyses for all patients. (B) Landmark analyses for patients in the IVUS guidance group comparing those with those versus without stent optimization. HR, hazard ratio; IVUS, intravascular ultrasound; PCI, percutaneous coronary intervention. Adapted from Gao XF, Ge Z, Kong XQ, et al. 3-year outcomes of the ULTIMATE trial comparing intravascular ultrasound versus angiography-guided drug-eluting stent implantation. JACC Cardiovasc Interv. 2021;14(3):247-257.
Figure 5.
Pooled patient-level data from IVUS-XPL and ULTIMATE showing improved 3-year Cardiac survival after IVUS-guided drug-eluting stent implantation. (A) Cumulative incidence of cardiac death comparing patients randomized to IVUS versus angiographic guidance. (B) Cumulative incidence of the composite of cardiac death, MI, or ST death comparing patients randomized to IVUS versus angiographic guidance. (C) Cumulative incidence of cardiac death comparing patients in IVUS-guided percutaneous coronary intervention group who underwent IVUS-guided stent implantation comparing those with versus those without stent optimization. (D) Cumulative incidence of the composite of cardiac death, myocardial infarction, or stent thrombosis death comparing patients in IVUS-guided percutaneous coronary intervention group who underwent IVUS-guided stent implantation comparing those with versus those without stent optimization. HR, hazard ratio; IVUS, intravascular ultrasound; IVUS-XPL, Impact of Intravascular Ultrasound Guidance on Outcomes of Xience Prime Stents in Long Lesions; MI, myocardial infarction; ST, stent thrombosis. Reprinted with permission from Hong SJ, Zhang JJ, Mintz GS, et al. Improved 3-year cardiac survival after IVUS-guided long DES implantation: a patient-level analysis from 2 randomized trials. JACC Cardiovasc Interv. 2022;15(2):208-216.
Two randomized controlled trials reported mortality advantages associated with IVUS-guided left main coronary artery (LMCA) DES implantation, the largest, enrolling 336 patients (Figure 6).17 After 1-year of follow-up, the MACE rate was significantly lower in the IVUS-guided than in the angiography-guided group (13.2% vs 21.9%; P = .031), mainly from a reduction in cardiac death (1.8% vs 5.9%; P = .048) with a trend toward a reduction in target vessel revascularization (TVR) (4.2% vs 8.9%; P = .068). The number-needed-to-treat to prevent one TLR is 57 in PCI of LMCA stenoses.
Figure 6.
Freedom from adverse events in the IVUS-guided group versus the control (angiography-guided) group in a randomized controlled trial of left main coronary artery disease treated with drug-eluting stent implantation. (A) Freedom from MACE. (B) Freedom from CD. CD, cardiac death; IVUS, intravascular ultrasound; MACE, major adverse cardiac event. Adapted from Liu XM, Yang ZM, Liu XK, et al. Intravascular ultrasound-guided drug-eluting stent implantation for patients with unprotected left main coronary artery lesions: a single-center randomized trial. Anatol J Cardiol. 2019;21(2):83-90.
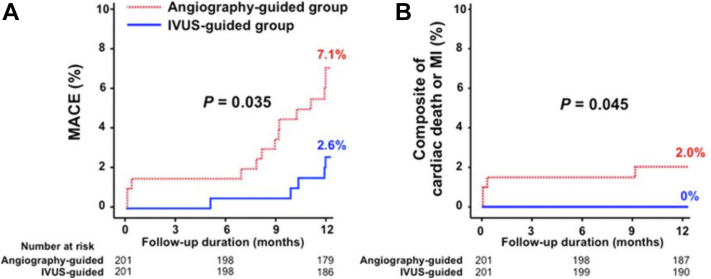
In the Chronic Total Occlusion–IVUS randomized controlled trial, there was a reduction in the composite of death/MI with IVUS-guided stent optimization after guide wire crossing (Figure 7). At 12 months of follow-up, MACE rates were significantly lower in the IVUS-guided than in the angiography-guided group (2.6% vs 7.1%; P = .035), as was the occurrence of the composite of cardiac death or MI (0% vs 2.0%; P = .045).18 The number-needed-to-treat to prevent 1 TLR is 67 in chronic total occlusion PCI.
Figure 7.
IVUS-CTO randomized controlled trial. Clinical impact of IVUS-guided CTO intervention. (A) Occurrence of MACE. (B) Composite of CD or MI. CD, cardiac death; CTO, chronic total occlusion; IVUS, intravascular ultrasound; MACE, major adverse cardiac event; MI, myocardial infarction. Reprinted with permission from Kim BK, Shin DH, Hong MK, et al. Clinical impact of intravascular ultrasound-guided chronic total occlusion intervention with zotarolimus-eluting versus Biolimus-eluting stent implantation: randomized study. Circ Cardiovasc Interv. 2015;8(7):e002592.
Findings associated with periprocedure MI or slow/no-reflow are grayscale IVUS attenuated plaque, radiofrequency-IVUS necrotic core, radiofrequency-IVUS or OCT thin-cap fibroatheroma, OCT or near-infrared spectroscopy lipid-rich plaque, or IVUS or OCT plaque rupture. The positive predictive value of these findings is relatively low, but the absence of these findings is associated with low probability of a periprocedure MI or slow/no-reflow. The VAMPIRE-3 (Assessment of Distal Protection Device in Patients at High Risk for Distal Embolism in Acute Coronary Syndrome) trial reported a reduction in distal embolization when distal protection was used in lesions with grayscale IVUS attenuated plaque of >180° in circumference and >5 mm in length. The primary end point (no-reflow) occurred in 26.5% of the distal protection versus 41.7% in the conventional treatment group (P = .026), and the composite incidence of cardiac death, cardiac arrest, cardiogenic shock after revascularization requiring defibrillation, cardiopulmonary resuscitation, or extracorporeal membrane oxygenation was significantly lower in the distal protection than in the conventional treatment group (0% vs 5.2%; P = .028).19 At 1 year, MACE occurred in 12.2% of the distal protection vs 3.1% of the conventional treatment group (P = .029), driven by a higher risk of TVR (11.2% vs 2.1%; P = .018).20
OCT versus angiographic or IVUS guidance
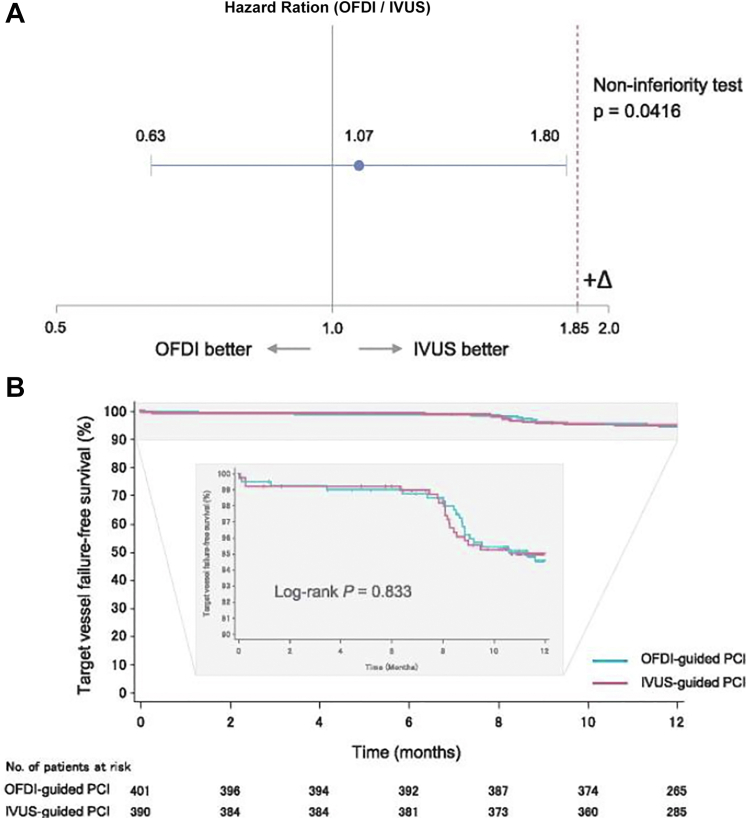
There is a paucity of randomized controlled trial data comparing OCT guidance with either IVUS or angiographic guidance. The OPtical frequency domain imaging versus INtravascular ultrasound in the percutaneous coronary InterventiON (OPINION) trial was powered to evaluate the noninferiority of OCT-guided DES implantation compared with IVUS-guided DES implantation. The primary end point—TVF, a composite of cardiac death, target vessel related MI, and ischemia-driven TVR at 12 months—occurred in 5.2% of patients treated with OCT guidance versus 4.9% of patients treated with IVUS guidance, along with comparable in-stent (1.6% vs 1.6%; P = 1.000) and in-segment (6.2% vs 6.0%; P = 1.000) restenosis (Figure 8).5
Figure 8.
One-year angiographic and clinical results from the OPINION Trial: OCT versus IVUS in percutaneous coronary intervention. (A) Noninferiority test for target vessel failure (primary end point) and (B) target vessel failure-free survival curves through the 12-month follow-up. IVUS, intravascular ultrasound; OCT, optical coherence tomography; OFDI, optical frequency domain imaging; PCI, percutaneous coronary intervention. Reprinted with permission from Kubo T, Shinke T, Okamura T, et al. Optical frequency domain imaging vs. intravascular ultrasound in percutaneous coronary intervention (OPINION trial): one-year angiographic and clinical results. Eur Heart J. 2017;38(42):3139-3147.
In ILUMIEN III, there was no significant difference among OCT, IVUS, and angiography guidance in rates of MACE (9.8% for OCT, 9.1% for IVUS, and 7.9% for angiography), TLR (2.0% for OCT, 3.7% for IVUS, and 1.4% for angiography), or any of the individual components of these outcomes.21
Other randomized controlled trial publications
Additional primary and secondary randomized controlled trial publications are listed in the references.22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36
Meta-analyses
From 2012 to 2022, 32 meta-analyses reported the prognostic impact of IVI (IVUS and/or OCT) to guide PCI (Table 1).10,37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67 Ten meta-analyses included only randomized controlled trials, and the remaining included a mixture of randomized controlled trials and observational studies. In total, 95 unique publications arose from these analyses.
Table 1.
Meta-analyses reporting clinical outcomes (risk reduction and 95% confidence intervals) associated with intravascular imaging–guided drug-eluting stent implantation.
| Reference, year | Study populationa | All-cause mortality | Cardiovascular mortality | MACE | Myocardial infarction | Stent thrombosis | TVR | TLR |
|---|---|---|---|---|---|---|---|---|
| IVUS vs angiography | ||||||||
| Zhang et al,37 2012 | 11 studies (1 RCT) 19,619 patients |
0.59 (0.48-0.73) | N/R | 0.87 (0.78-0.96) | 0.82 (0.63-1.06) | 0.58 (0.44-0.77) | 0.90 (0.77-1.05) | 0.90 (0.73-1.11) |
| Propensity score–matched | 5300 patients | 0.73 (0.54-0.99) | N/R | N/R | 0.63 (0.43-0.91) | 0.57 (0.39-0.84) | N/R | N/R |
| Zhang et al,38 2013 | 14 studies (3 RCTs) 29,029 patients |
0.66 (0.55-0.78) | N/R | 0.86 (0.77-0.95) | 0.74 (0.62-0.90) | 0.57 (0.44-0.73) | N/R | 0.82 (0.68-0.97) |
| Klersy et al,39 2013 | 12 studies (3 RCTs) 18,707 patients |
0.60 (0.48-0.74) | N/R | 0.80 (0.71-0.89) | 0.59 (0.44-0.80) | 0.50 (0.32-0.80) | 0.95 (0.82-1.09) | N/R |
| Jang et al,40 2014 | 15 studies (3 RCTs) 24,849 patients |
0.64 (0.51-0.81) | N/R | 0.79 (0.69-0.91) | 0.57 (0.42-0.78) | 0.59 (0.42-0.82) | 0.81 (0.68-0.95) | 0.76 (0.62-0.94) |
| Propensity score–matched | 13,545 patients | 0.58 (0.42-0.81) | N/R | 0.79 (0.66-0.95) | 0.56 (0.33-0.97) | 0.52 (0.34-0.82) | 0.93 (0.79-1.09) | 0.85 (0.64-1.13) |
| Ahn et al,41 2014 | 17 studies (3 RCTs) 26,503 patients |
0.61 (0.48-0.79) | N/R | 0.74 (0.64-0.85) | 0.57 (0.44-0.75) | 0.59 (0.47-0.75) | 0.82 (0.70-0.97) | 0.81 (0.66-1.00) |
| Zhang et al,42 2015 | 20 studies (3 RCTs) 29,068 patients |
0.62 (0.54-0.71) | N/R | 0.77 (0.71-0.83) | 0.64 (0.55-0.75) | 0.59 (0.47-0.73) | 0.86 (0.77-0.97) | 0.81 (0.69-0.94) |
| Propensity score–matched | 8331 patients | 0.64 (0.52-0.79) | N/R | 0.79 (0.70-0.88) | 0.69 (0.56-0.85) | 0.55 (0.39-0.78) | 0.82 (0.68-0.98) | 0.92 (0.76-1.11) |
| Complex lesions or patients with acute coronary syndrome | 6393 patients | 0.52 (0.40-0.67) | N/R | 0.69 (0.60-0.79) | N/R | 0.41 (0.25-0.69) | N/R | N/R |
| Steinvil et al,43 2016 | 25 studies (7 RCTs) 31,283 patients |
0.62 (0.54-0.72) | N/R | 0.76 (0.70-0.82) | 0.67 (0.56-0.80) | 0.58 (0.47-0.73) | 0.85 (0.76-0.95) | 0.77 (0.67-0.89) |
| Complex lesions (LMCA, bifurcations, CTO) | 13 studies | 0.52 (0.41-0.67) | N/R | 0.70 (0.61-0.80) | 0.72 (0.57-0.92) | 0.32 (0.18-0.57) | 0.62 (0.45-0.84) | 0.76 (0.57-1.01) |
| Propensity score–matched and RCT reports | 16 studies 10,486 patients |
0.65 (0.53-0.80) | N/R | 0.76 (0.68-0.85) | 0.75 (0.62-0.90) | 0.55 (0.39-0.76) | 0.80 (0.67-0.94) | 0.84 (0.70-1.00) |
| Randomized controlled trials | 7 RCTs 3192 patients |
0.81 (0.44-1.47) | N/R | 0.66 (0.52-0.84) | 1.04 (0.68-1.59) | 0.71 (0.28-1.78) | 0.61 (0.41-0.90) | 0.61 (0.43-0.87) |
| Elgendy et al,44 2016 | 7 RCTs 3275 patients |
N/R | 0.46 (0.21-1.00) | 0.60 (0.46-0.77) | 0.52 (0.26-1.02) | 0.49 (0.24-0.99) | 0.61 (0.41-0.91) | 0.60 (0.43-0.84) |
| Shin et al,45 2016 | 3 RCTs 2345 patients |
N/R | 0.38 (0.10-1.42) | 0.36 (0.13-0.99) | N/R | 0.50 (0.13-2.01) | N/R | 0.61 (0.40-0.93) |
| Nerlekar et al,46 2017 | 15 (6 RCTs) 9313 patients |
N/R | 0.55 (0.36-0.83) | 0.73 (0.64-0.85) | 0.67 (0.50-0.90) | 0.52 (0.38-0.72) | 0.79 (0.64-0.98) | 0.66 (0.52-0.84) |
| First generation DES | 11 studies (6156 patients) | N/R | 0.64 (0.39-1.04) | 0.79 (0.67-0.92) | 0.63 (0.45-0.89) | 0.56 (0.40-0.79) | 0.88 (0.70-1.11) | 0.70 (0.51-0.95) |
| Second-generation DES | 6 studies (3157 patients) | N/R | 0.33 (0.14-0.78) | 0.57 (0.43-0.77) | 0.82 (0.45-1.49) | 0.31 (0.12-0.78) | 0.47 (0.28-0.79) | 0.61 (0.42-0.90) |
| Fan et al,47 2017 | 15 studies (6 RCTs) 8084 patients |
0.52 (0.40-0.67) | N/R | 0.63 (0.53-0.73) | 0.70 (0.56-0.86) | 0.31 (0.20-0.50) | 0.53 (0.40-0.70) | 0.69 (0.50-0.94) |
| Propensity-matched studies and RCTs | 7 propensity-matched studies; 6 RCTs 6573 patients |
0.49 (0.37-0.65) | N/R | 0.67 (0.57-0.78) | 0.76 (0.60-0.97) | 0.34 (0.20-0.58) | 0.57 (0.42-0.77) | 0.79 (0.61-1.01) |
| Bavishi et al,48 2017 | 8 RCTs 3276 patients |
1.00 (0.48-2.09) | 0.51 (0.23-1.12) | 0.64 (0.51-0.80) | 0.90 (0.58-1.41) | 0.57 (0.26-1.23) | 0.60 (0.42-0.87) | 0.62 (0.45-0.86) |
| Buccheri et al,49 2017 | Bayesian network meta-analysis of 31 studies and 17,882 patients | 0.74 (0.58-0.98) | 0.47 (0.32-0.66) | 0.79 (0.67-0.91) | 0.72 (0.52-0.93) | 0.42 (0.20-0.72) | N/R | 0.74 (0.58-0.90) |
| Räber et al,10 2018 | 8 RCTs 3276 patients |
N/R | 0.51 (0.23-1.12) | 0.64 (0.51-0.80) | 0.61 (0.32-1.16) | N/R | N/R | 0.60 (0.43-0.83) |
| di Mario et al,50 2018 | 9 RCTs 4724 patients |
N/R | 0.51 (0.27-0.96) | 0.62 (0.51-0.77) | 0.62 (0.36-1.05) | N/R | N/R | 0.58 (0.43-0.78) |
| Gao et al,51 2019 | 9 RCTs 4724 patients |
0.78 (0.46-1.31) | 0.49 (0.26-0.92) | 0.61 (0.49-0.74) | 0.79 (0.54-1.17) | 0.45 (0.23-0.87) | 0.58 (0.42-0.80) | 0.59 (0.44-0.80) |
| Elgendy et al,52 2019 | 10 RCTs 5060 patients |
N/R | 0.44 (0.26-0.75) | N/R | 0.55 (0.32-0.94) | 0.44 (0.24-0.79) | N/R | 0.57 (0.42-0.77) |
| Kumar et al,53 2019 | 11 RCTs 5352 patients |
N/R | 0.45 (0.25-0.80) | N/R | 0.83 (0.54-1.28) | 0.47 (0.24-0.94) | N/R | 0.56 (0.41-0.77) |
| Malik et al,54 2020 | 10 RCTs 5007 patients |
N/R | 0.51 (0.27-0.96) | 0.63 (0.51-0.77) | 0.86 (0.58-1.29) | 0.50 (0.24-1.04) | 0.59 (0.43-0.81) | 0.59 (0.44-0.80) |
| Darmoch et al,55 2020 | 19 (6 RCTs)b 27637 patients |
N/R | 0.63 (0.54-0.73) | N/R | 0.71 (0.58-0.86) | 0.57 (0.41-0.79) | N/R | 0.81 (0.70-0.94) |
| Pang et al,56 2020 | Bayesian network meta-analysis of 18 studies (62,197 patients) | 1.7∗e−9 (9.9e−25 to 0.24) | N/R | 0.45 (0.20-0.97) | 1.0 (0.09-11.0) | N/R | N/R | N/R |
| Zhang et al,57 2021 | 20 studies (3 RCTs) 24,783 |
0.79 (0.63-0.98) | 0.62 (0.47-0.82) | 0.65 (0.58-0.73) | 0.68 (0.57-0.80) | 0.47 (0.33-0.67) | 0.74 (0.65-0.85) | 0.67 (0.56-0.80) |
| Studies with 2-year follow-up | 5 studies | N/R | 0.62 (0.32-1.23) | 0.51 (0.36-0.71) | 0.57 (0.37-0.87) | 0.28 (0.10-0.80) | 0.79 (0.58-1.07) | 0.66 (0.39-1.12) |
| Studies with 3-year follow-up | 6 studies | 0.54 (0.36-0.81) | 0.41 (0.24-0.69) | 0.45 (0.31-0.65) | 0.64 (0.49-0.83) | 0.84 (0.43-1.61) | 0.95 (0.67-1.34) | 0.89 (0.58-1.37) |
| Groenland et al,58 2022 | 9 studies (1 RCT) 838,902 patients with myocardial infarction |
0.70 (0.59-0.82) | 0.62 (0.29-1.33) | 0.86 (0.74-0.99) | N/R | N/R | 0.83 (0.73-0.95) | N/R |
| OCT vs angiography | ||||||||
| Buccheri et al,49 2017 | Bayesian network meta-analysis of 31 studies and 17,882 patients | 0.59 (0.29-1.20) | 0.31 (0.13-0.66) | 0.68 (0.49-0.97) | 0.79 (0.44-1.40) | 0.39 (0.10-1.20) | N/R | 0.66 (0.35-1.20) |
| Kuku et al,59 2018 | 6 studies and 2781 patients (OCT vs Angio-guidance: 1753 patients) | N/R | 0.40 (0.18-0.90) | 0.70 (0.49-1.00) | 0.70 (0.42-1.16) | 1.17 (0.40-3.43) | N/R | 1.07 (0.48-2.38) |
| Sharma et al,60 2019 | 5 RCTs 931 patients |
3.03 (0.12-75.0) | N/R | N/R | 2.21 (0.39-12.49) | 0.70 (0.11-4.51) | 1.36 (0.40-4.40) | N/R |
| Pang et al,56 2020 | Bayesian network meta-analysis of 18 studies (62,197 patients) | 0.88 (0.24-2.80) | N/R | 0.89 (0.45-1.50) | 0.87 (0.28-2.00) | N/R | N/R | N/R |
| LMCA intervention (IVUS vs angiography) | ||||||||
| Ye et al,61 2017 | 10 studies (1 RCT) 6480 patients |
0.60 (0.47-0.75) | 0.47 (0.33-0.66) | N/R | 0.80 (0.61-1.06) | 0.28 (0.12-0.67) | 0.89 (0.66-1.20) | 0.43 (0.25-0.73) |
| Wang et al,62 2018 | 7 studies (1 RCT) 4592 patients |
0.55 (0.42-0.71) | 0.45 (0.32-0.62) | 0.61 (0.53-0.70) | 0.66 (0.55-0.80) | 0.48 (0.27-0.84) | 0.64 (0.26-1.56) | 0.60 (0.31-1.18) |
| Elgendy et al,63 2019 | 9 studies (2 RCTs) 4971 patients |
0.53 (0.40-0.70) | 0.40 (0.28-0.59) | N/R | 0.69 (0.50-0.96) | 0.47 (0.32-0.70) | N/R | 0.76 (0.50-1.16) |
| Elgendy et al,64 2020 | 11 studies (2 RCTs) 15,083 patients |
0.59 (0.53-0.66) | 0.39 (0.27-0.58) | N/R | 0.66 (0.48-0.90) | 0.38 (0.26-0.56) | N/R | 0.51 (0.39-0.68) |
| Saleem et al,65 2021 | 14 studies (2 RCTs) 18,944 patients |
0.57 (0.46-0.70) | 0.37 (0.26-0.54) | N/R | 0.80 (0.66-0.97) | 0.57 (0.31-1.05) | N/R | 0.63 (0.45-0.89) |
| OCT vs IVUS | ||||||||
| Buccheri et al,49 2017 | Bayesian network meta-analysis of 31 studies and 17,882 patients | 0.81 (0.41-1.60) | 0.66 (0.28-1.50) | 0.87 (0.61-1.30) | 1.10 (0.61-2.10) | 0.93 (0.23-3.60) | N/R | 0.89 (0.47-1.70) |
| Kuku et al,59 2018 | 6 studies and 2781 patients (OCT vs IVUS guidance: 1028 patients) | N/R | 0.56 (0.12-2.70) | 0.89 (0.46-1.73) | 0.56 (0.12-2.70) | 0.43 (0.06-2.95) | N/R | 0.99 (0.45-2.18) |
| Pang et al,56 2020 | Bayesian network meta-analysis of 18 studies (62,197 patients) | 1.30 (0.43-4.00) | N/R | 0.91 (0.49-1.70) | 1.60 (0.41-6.60) | N/R | N/R | N/R |
| Salesh et al,66 2021 | 4 RCTs and 3 propensity-matched observational studies (13,995 patients) | 1.05 (0.59-1.87) | N/R | 1.10 (0.69-1.74) | 1.22 (0.53-2.82) | 0.69 (0.13-3.61) | N/R | 0.88 (0.46-1.68) |
| Sattar et al,67 2021 | 7 studies (4 RCTs) 5917 patients |
0.74 (0.39-1.39) | 0.97 (0.27-3.46) | 0.78 (0.57-1.09) | 1.27 (0.52-3.07) | 0.70 (0.13-3.61) | N/R | 1.09 (0.53-2.25) |
CTO, chronic total occlusion; DES, drug-eluting stent; IVUS, intravascular ultrasound; LMCA, left main coronary artery; MACE, major adverse cardiac events; N/R, not reported; OCT, optical coherence tomography; RCT, randomized controlled trial; TLR, target lesion revascularization; TVR, target vessel revascularization.
Presented as the total number of studies (randomized controlled trials).
From the 19 included studies, 16 (84.2%) studies used DES; however, the clinical outcomes results are presented for the overall population only, with no separation for bare metal stent and DES.
IVUS-guided versus angiography-guided PCI
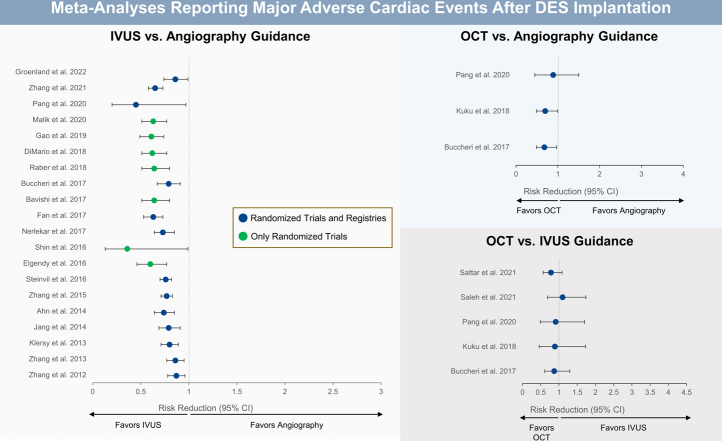
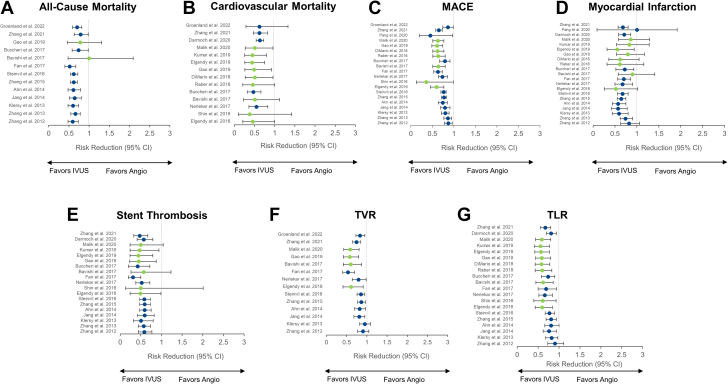
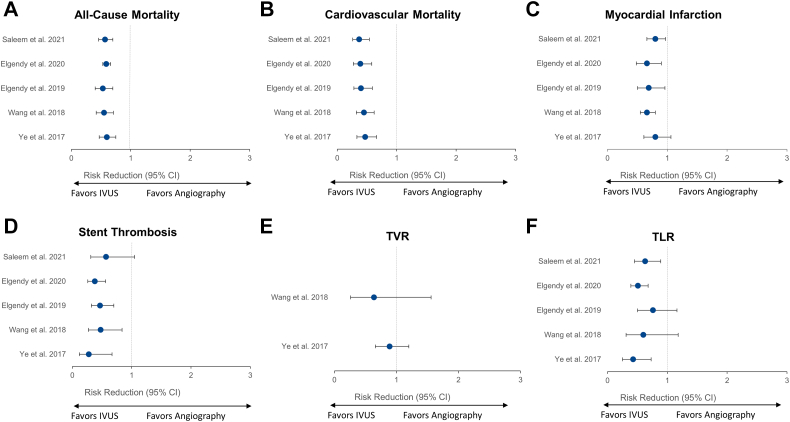
Twenty-three meta-analyses compared the clinical outcomes of IVUS-guided PCI versus angiography-guided PCI, 9 (39.1%) of which included only randomized controlled trials (Figure 9). The benefit of IVUS guidance was greater in meta-analyses restricted to randomized controlled trials, where the reduction in MACE ranged from 36%10,48 to 64%.45
Figure 9.
Twenty-three meta-analyses comparing the clinical outcomes of IVUS-guided versus angiography-guided PCI. Risk reduction (95% CI) for the meta-analyses that reported on the comparison of IVUS- versus angiography-guided PCI for (A) all-cause mortality, (B) cardiovascular mortality, (C) MACE, (D) myocardial infarction, (E) stent thrombosis, (F) TVR, and (G) TLR. Point estimates in green indicate meta-analyses with randomized controlled trials only. Point estimates in blue represent meta-analyses of randomized controlled trials and observational studies. IVUS, intravascular ultrasound; MACE, major adverse cardiac event; PCI, percutaneous coronary intervention; TLR, target lesion revascularization; TVR, target vessel revascularization.
Cardiovascular mortality was reported in 14 meta-analyses (9 restricted to randomized controlled trials). Except for 4 meta-analyses,10,44,45,59 IVUS guidance provided a significant reduction in cardiovascular mortality. Again, this benefit was more pronounced in meta-analyses of randomized controlled trials, in which the effects of IVUS in reducing cardiovascular mortality ranged from 49%48,50,54 to 62%.45
Myocardial infarction was reported in 21 meta-analyses (8 of which were restricted to randomized controlled trials). The point estimates show a reduction in MI with IVUS guidance in all, but 1 meta-analysis.56
Stent thrombosis was reported in 19 meta-analyses, 7 restricted to randomized controlled trials. Except for 3 meta-analysis,45,48,54 IVUS guidance was associated with a significant reduction in stent thrombosis, with similar effects between meta-analyses that included only randomized controlled trials or a combination of randomized controlled trials and observational studies.
Target vessel revascularization and TLR were reported in 14 and 20 meta-analyses, of which 4 and 9 included only randomized controlled trials, respectively. IVUS guidance reduced the occurrence of TVR and TLR in all meta-analyses. The magnitude of reduction was significant in all, but 2 older meta-analyses for TVR37,39 and TLR.37,41
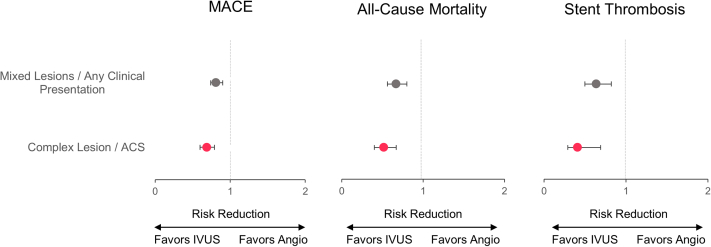
Two meta-analyses42,43 performed an additional evaluation of patients with complex lesions. The effects of IVUS guidance were more pronounced in this higher-risk subgroup than in the overall population included in each meta-analysis. The meta-analysis by Zhang et al42 included 29,068 patients from 20 studies (3 randomized controlled trials). Of these, 13 studies included 6393 patients with complex lesions or presenting with the acute coronary syndrome. IVUS guidance was associated with lower risk of MACE (odds ratios [OR], 0.69; 95% CI, 0.60-0.79; P < .001), all-cause mortality (OR, 0.52; 95% CI, 0.40-0.67; P < .001) and stent thrombosis (OR, 0.41; 95% CI, 0.25-0.69; P = .001) in the subgroup of patients with complex lesions or acute coronary syndrome than patients with unselected lesions or any clinical presentation (MACE: OR, 0.81; 95% CI, 0.74-0.90; P < .001; all-cause mortality: OR, 0.67; 95% CI, 0.56-0.80; P < .001; stent thrombosis: OR, 0.64; 95% CI, 0.50-0.82; P < .001) (Figure 10). In the meta-analysis by Steinvil et al,43 a subanalysis of 13 studies addressing the use of IVUS for complex lesions (LMCA lesions, bifurcation lesions, or chronic total occlusion) showed similar results in the angiography and IVUS-guided groups, with a marginal benefit after the use of IVUS for TLR.
Figure 10.
Meta-analysis of complex lesions or patients presenting with the acute coronary syndrome. Subanalysis of the meta-analysis by Zhang et al42 presenting the risk reductions (95% CI) of clinical outcomes for the comparison of IVUS- versus angiography-guided PCI in the subgroup of patients with acute coronary syndrome and complex lesions (red) versus unselected patients and lesions (gray). ACS, acute coronary syndrome; IVUS, intravascular ultrasound; PCI, percutaneous coronary intervention.
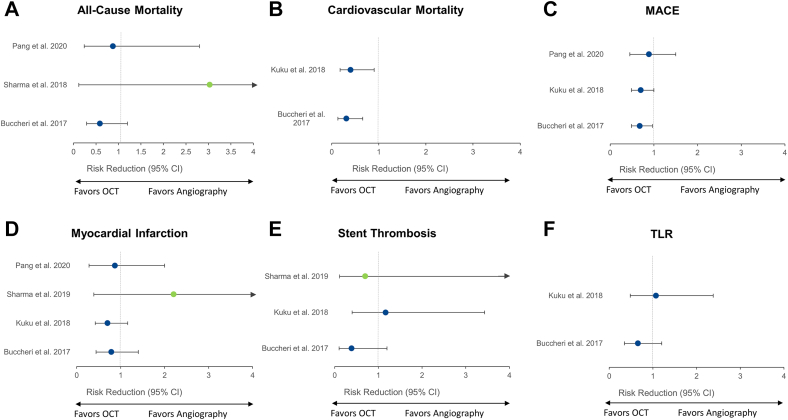
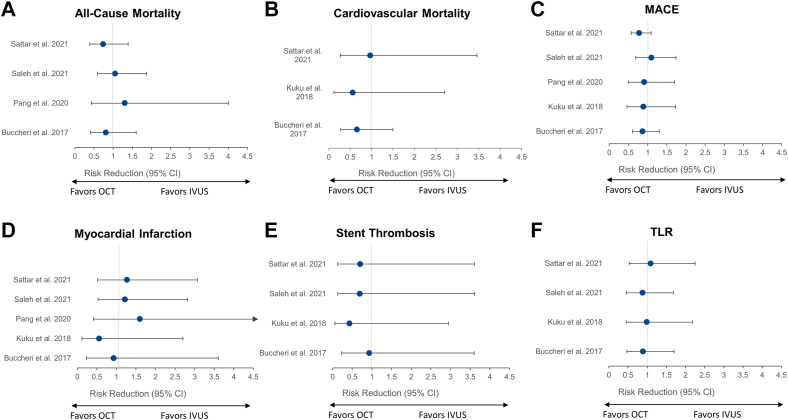
OCT-guided versus angiography-guided PCI
Four meta-analyses49,56,59,60 compared the clinical outcomes of OCT-guided and angiography-guided PCI (Figure 11). Of these, 1 meta-analysis60 included only randomized controlled trials.
Figure 11.
Meta-analyses comparing OCT-guided versus angiography-guided PCI. Risk reduction (95% CI) for the meta-analyses that reported on the comparison of OCT- versus angiography-guided PCI for (A) all-cause mortality, (B) cardiovascular mortality, (C) MACE, (D) myocardial infarction, (E) stent thrombosis, and (F) TLR. Point estimates in green indicate meta-analyses with randomized controlled trials only. Point estimates in blue represent meta-analyses of randomized controlled trials and observational studies. MACE, major adverse cardiac event; OCT, optical coherence tomography; PCI, percutaneous coronary intervention; TLR, target lesion revascularization.
Optical coherence tomography guidance reduced MACE in 249,59 of the 349,56,59 meta-analyses that assessed this outcome. Cardiovascular mortality was reported in 2 meta-analyses,49,59 and both showed significant benefits of OCT guidance over angiographic guidance. The effects of OCT guidance on the occurrence of all-cause mortality, MI, stent thrombosis, and TLR were neutral. Nonetheless, the wide confidence intervals indicate uncertainty, reflected by the insufficient number of patients included in such analyses.
IVUS-guided versus angiography-guided PCI of LMCA lesions
Five meta-analyses evaluated the effects of IVUS guidance in the treatment of LMCA lesions compared with angiographic guidance alone.61, 62, 63, 64, 65 IVUS guidance significantly reduced all-cause and cardiovascular mortality in all 5 meta-analyses (Figure 12). Reductions in all-cause and cardiovascular death ranged from 40% to 47%,61,63 and from 53% to 61%, respectively. All but 161 meta-analyses demonstrated significant reductions in the risk of MI with IVUS guidance. Stent thrombosis was also significantly reduced with the use of IVUS in 4 meta-analyses, with the meta-analysis by Saleem et al65 showing a strong trend toward significance. TLR was significantly reduced with IVUS guidance in 361,64,65 of the 5 meta-analyses. TVR was evaluated in only 2 meta-analyses61,62 that reported a neutral effect of IVUS guidance on this outcome.
Figure 12.
Meta-analyses evaluating the effects of IVUS guidance in the treatment of LMCA disease. Risk reduction (95% CI) for the meta-analyses that reported on the comparisons of IVUS- versus angiography-guided PCI in patients with left main coronary artery lesions for (A) all-cause mortality, (B) cardiovascular mortality, (C) myocardial infarction, (D) stent thrombosis, (E) TVR, and (F) TLR. All meta-analyses included randomized controlled trials and observational studies. IVUS, intravascular ultrasound; LMCA, left main coronary artery; PCI, percutaneous coronary intervention; TLR, target lesion revascularization; TVR, target vessel revascularization.
OCT-guided versus IVUS-guided PCI
The impact of OCT versus IVUS guidance on clinical outcomes has been evaluated in 5 meta-analyses (Figure 13).49,56,59,66,67 In all, there was no benefit of 1 imaging modality over the other regarding any of the clinical outcomes evaluated.
Figure 13.
Meta-analyses of OCT versus IVUS guidance. Risk reduction (95% CI) for the meta-analyses that reported on the comparisons of OCT- versus IVUS-guided PCI for (A) all-cause mortality, (B) cardiovascular mortality, (C) MACE, (D) myocardial infarction, (E) stent thrombosis, and (F) TLR. All meta-analyses included randomized controlled trials and observational studies. IVUS, intravascular ultrasound; MACE, major adverse cardiac event; OCT, optical coherence tomography; PCI, percutaneous coronary intervention; TLR, target lesion revascularization.
Registries
The advantage of registry studies is that they often address a specific patient, procedure, or lesion subset. However, a consistent limitation of these studies is the imbalance in the baseline demographic characteristics between groups, the absence of information about lesion anatomy and complexity, the lack of a standardized protocol for IVI use and PCI guidance (as well as angiography guidance), the lack of recommendation for optimal stent implantation in the IVI and angiography-guided groups (often), the lack of quantitative information (either angiography or IVI), and unadjudicated outcomes. The following is a selection of these many registry studies.
ADAPT-DES and iOPEN
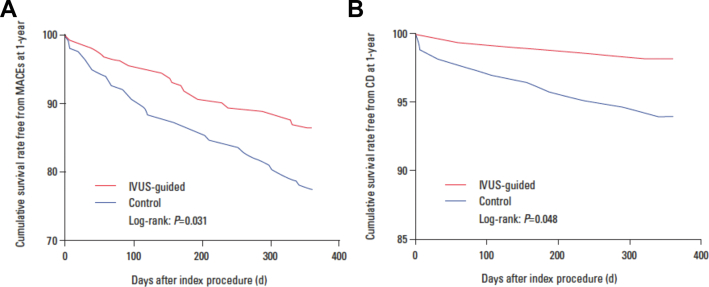
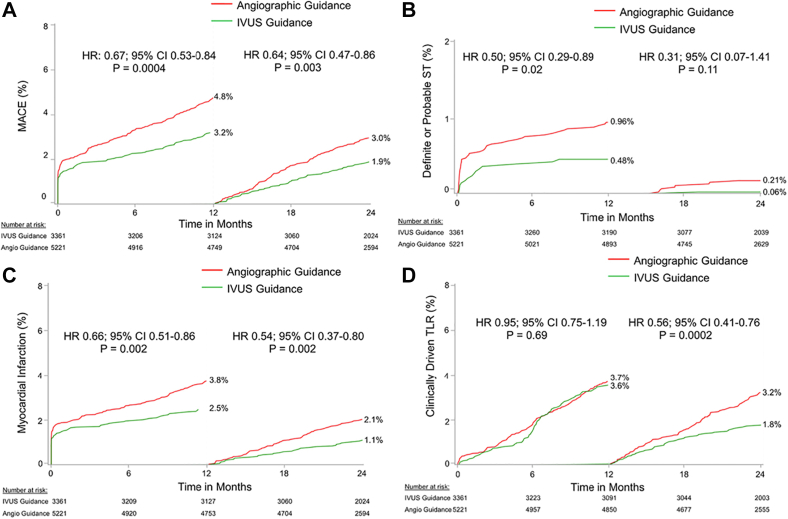
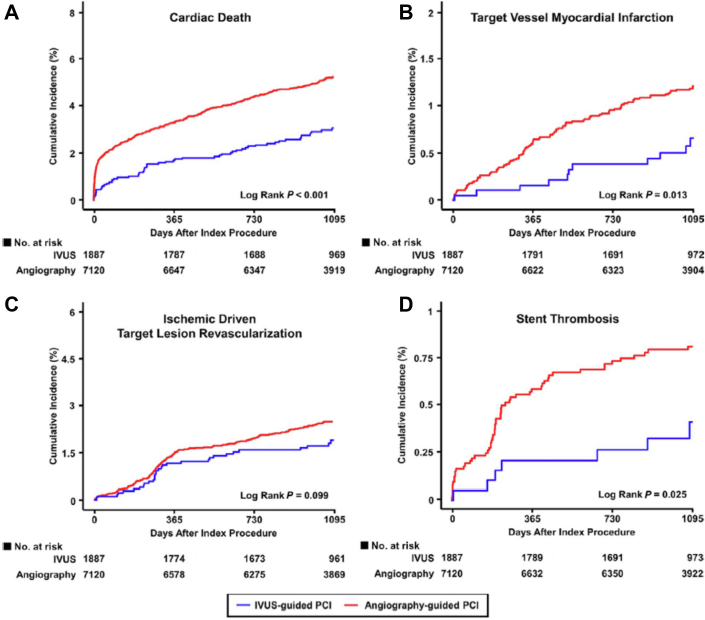
The Assessment of Dual Antiplatelet Therapy With Drug-Eluting Stents (ADAPT-DES) study highlighted the prognostic implications of IVUS in PCI planning with a second-generation DES.68 The primary end point of the study was definite or probable stent thrombosis; additional end points included all-cause mortality, MI, revascularization, and bleeding. In total, 8665 patients were enrolled; 39% of these patients had IVUS-guided and 61% had angiography-guided PCI. IVUS guidance was associated with a lower incidence of stent thrombosis (0.6% vs 1.0%; P = .02), target vessel MI (1.7% vs 2.9%; P < .001), TLR (1.5% vs 2.4%; P = .007), and TVR (2.4% vs 4.0%; P < .001) at 1 year, whereas there was no difference between the 2 groups in mortality (1.8% vs 2.0%; P = .40).
Results were consistent at 2 years. IVUS guidance was associated with a lower incidence of stent thrombosis, target vessel MI, TVR, and TLR, and improved all-cause mortality.69 Landmark analysis showed a lower MACE rate (defined as the combined end point of cardiac death or definite or probable stent thrombosis or MI) and lower MI and TLR rates between 1 and 2 years, but there was no difference between groups in the incidence of stent thrombosis after the first year of follow-up (Figure 14). The benefits of IVUS were especially evident in patients with acute coronary syndrome and complex lesions, although significant reductions in MACE were present in all patient subgroups.
Figure 14.
ADAPT-DES. Landmark analysis with time to events curves within 1 year and between 1 and 2 years for (A) MACE which consists of the combined end point cardiac death, stent thrombosis, and MI, (B) the incidence of definite or probable stent thrombosis, (C) MI, and (D) clinically driven TLR in the IVUS-guided and angiography-guided groups. ADAPT, Assessment of Dual Antiplatelet Therapy With Drug-Eluting Stents; DES, drug-eluting stent; IVUS, intravascular ultrasound; MACE, major adverse cardiac event; MI, myocardial infarction; TLR, target lesion revascularization. Reprinted with permission from Maehara A, Mintz GS, Witzenbichler B, et al. Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents. Circ Cardiovasc Interv. 2018;11(11):e006243.
In contrast to the ADAPT-DES registry, the impact of Intravascular ultrasound on Outcomes following PErcutaneous coronary interventioN (iOPEN) complex registry included only patients who had complex lesions.70 Overall, 6855 patients had IVUS (67.3%) or angiography-guided PCI (32.7%). At 1 year, IVUS-guided revascularization was associated with a statistically lower MACE rate, defined as the combined end point of all-cause death, Q-wave MI, or TVR, compared with angiography guidance (13.4% vs 18.3%; P < .001), a finding that persisted after an inverse probability of treatment weighing adjusted analysis. These findings were in line with the results of a large single-center retrospective study of 6005 patients with complex lesions undergoing PCI under angiography or IVUS guidance that reported lower all-cause mortality (10.2% vs 16.9%; P < .001), target vessel MI, stent thrombosis, or TLR during 64 months (median) of follow-up.71
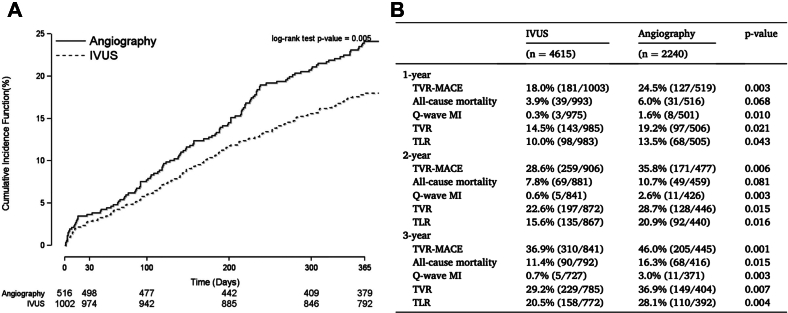
A second analysis of the iOPEN group addressed PCI for in-stent restenosis.72 This study included 1522 patients in whom IVUS imaging was used in two-thirds while angiography alone was used in one-third. IVUS guidance was associated with more frequent implantation of an additional stent. Patients treated with IVUS imaging had a lower MACE rate (defined as the combined end point of all case death, Q-wave MI, or TVR: 18% vs 24.5%; P = .003) that was attributed to a lower rate of Q-wave MI (0.3% vs 1.6%; P = .010) and TVR (14.5% vs 19.2%; P = .021). The prognostic benefit of IVUS imaging persisted at the 3-year follow-up (MACE rate: 36.9% vs 46.0%; P = .001) where there was also a lower incidence of all-cause mortality (11.4% vs 16.3%; P = .015) (Figure 15).
Figure 15.
iOPEN PCI for in-stent restenosis. (A)One-year MACE estimated event rates. (B) Clinical outcomes, patient level. iOPEN, impact of Intravascular ultrasound on Outcomes following PErcutaneous coronary interventioN; IVUS, intravascular ultrasound; MACE, major adverse cardiac events; PCI, percutaneous coronary intervention; TLR, target lesion revascularization; TVR, target vessel revascularization. Reprinted with permission from Shlofmitz E, Torguson R, Zhang C, et al. Impact of intravascular ultrasound on Outcomes following PErcutaneous coronary interventioN for in-stent restenosis (iOPEN-ISR study). Int J Cardiol. 2021;340:17-21.
LMCA intervention
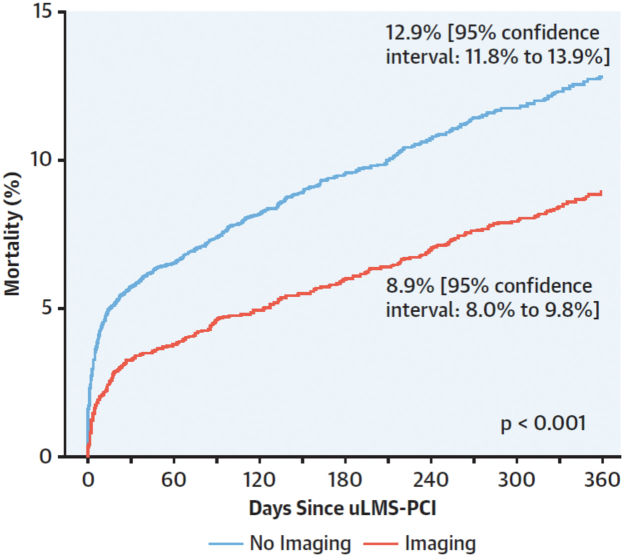
Kinnaird et al73 used the data of the British Cardiovascular Intervention Society to examine the prognostic value of IVUS guidance in unprotected LMCA PCI. In this analysis of 11,264 patients, 45% had IVUS-guided and 55% angiography-guided revascularization. The in-hospital major adverse cardiovascular and cerebrovascular event rates, as well as the mortality at 1 year, were lower in the imaging-guided group than in the angiography-guided group (Figure 16). The results were not different in the propensity match analysis performed to address differences in the baseline demographic characteristics between groups. In the multivariate analysis, IVUS imaging was an independent predictor of lower mortality at 12 months (OR, 0.770; 95% CI, 0.690-0.860; P < .001). Of note, the mortality reduction of 34% in the British Cardiovascular Intervention Society increased to 60% in the quartile of operators with the greatest LMCA PCI experience. There were similar findings in a multicenter registry from Spain.74 ROCK-II showed no difference between IVUS and OCT.75
Figure 16.
BCIS registry data examining the prognostic value of IVUS guidance in unprotected LMCA PCI. Kaplan-Meier curves showed a 34% mortality reduction. BCIS, British Cardiovascular Intervention Society; IVUS, intravascular ultrasound; LMCA, left main coronary artery; PCI, percutaneous coronary intervention; uLMS, unprotected left main stem. Reprinted with permission from Kinnaird T, Johnson T, Anderson R, et al. Intravascular imaging and 12-month mortality after unprotected left main stem PCI: an analysis from the British Cardiovascular Intervention Society database. JACC Cardiovasc Interv. 2020;13(3):346-357.
The mortality reduction with IVI guidance can be explained by the mortality associated with LMCA in-stent restenosis. In a patient-level pooled analysis of the randomized ISAR-LEFT-MAIN (Drug-Eluting-Stents for Unprotected Left Main Stem Disease) and ISAR-LEFT-MAIN-2 (DESs to Treat Unprotected Coronary Left Main Disease) trials, the 5-year mortality rate associated with clinical restenosis and its treatment was 30.2% vs 17.3% in a patient without clinical restenosis (P < .001).76
Acute coronary syndrome
In the first report of the Korea Acute Myocardial Infarction Registry (KAMIR), IVUS imaging did not appear to improve prognosis in this cohort.77 Similar findings were also reported in the CREDO-Kyoto AMI registry that included 3028 patients admitted with an ST-elevation MI.78
In the second publication of the Korea Acute Myocardial Infarction Registry in 2019, the investigators reported a larger cohort of 11,731 patients.79 Patient-oriented composite end points were defined as all-cause death, any infarction, and any revascularization; the device-oriented composite end point was defined as cardiac death, target vessel reinfarction, and TLR. Overall, IVUS was used in 2333 (19.9%) and OCT was used in 277 (2.4%). In the unmatched cohort, patient-oriented composite end point (5.4 vs 8.5%; adjusted hazard ratio [HR], 0.75; 95% CI, 0.61-0.93; P = .008) and device-oriented composite end point (4.6 vs. 7.4%; adjusted HR, 0.77; 95% CI, 0.61-0.97; P = .028) were significantly lower in IVI-guided PCI than in angiography-guided PCI. Differences were mainly driven by reduced all-cause mortality (P < .001) and cardiac mortality (P < .001), respectively.
The Cardiovascular Risk and Identification of Potential High-Risk Population in Acute Myocardial Infarction registry that included 9846 patients admitted with an acute coronary syndrome reported better outcomes in the IVUS-guided group at 4 years of follow-up. The results were consistent for all end points of the study (ie, MACE, all-cause and cardiac mortality, MI, and TLR) and did not change after propensity score matching analysis.80
Similar results were also reported by the Korea Acute Myocardial Infarction National Institutes of Health (KAMIR-NIH) registry that included 13,104 patients admitted with an acute coronary syndrome treated with IVUS or angiography guidance using the second-generation DES. The authors reported a lower incidence of the composite end point of target lesion failure (cardiac death, target vessel MI, or TLR) at 3 years of follow-up (4.8% vs 8.0%; P < .001) that was attributed to a lower incidence of cardiac death (3.1% vs 5.5%; P < .001), target vessel MI (0.6% vs 1.2%; P = .015), and stent thrombosis (0.4% vs 0.8%; P = .030) (Figure 17).81
Figure 17.
KAMIR-NIH registry. Kaplan-Meier analyses showing event rates in patients treated using IVUS and angiography alone guidance for the end points (A) cardiac death, (B) target vessel MI, (C) ischemic driven TLR, and (D) stent thrombosis. KAMIR-NIH, Korea Acute Myocardial Infarction National Institutes of Health; IVUS, intravascular ultrasound; MI, myocardial infarction; TLR, target lesion revascularization. Adapted from Kim Y, Bae S, Johnson TW, et al. Role of intravascular ultrasound-guided percutaneous coronary intervention in optimizing outcomes in acute myocardial infarction. J Am Heart Assoc. 2022;11(5):e023481.
Zero contrast PCI
Contrast-induced nephropathy in patients with underlying chronic kidney disease is related to the amount of contrast and is associated with increased mortality. IVUS-guided stenting can be performed without any contrast, even in complex interventions.82, 83, 84, 85, 86 This minimizes contrast-induced nephropathy and the need for renal replacement therapy. A similar approach is useful for patients with severe contrast allergies.
Conclusions
As shown in the randomized clinical trials, meta-analyses (Central Illustration), and registries, IVI-guided PCI is associated with a reduction in MACE or TVF, TLR, and/or TVR, especially the hard end points of all-cause and cardiovascular mortality, MI, and stent thrombosis. Because of the overwhelming evidence that IVI guidance improves patient outcomes, we believe that it should be routinely incorporated into PCI procedures, especially in high-risk and complex patients and lesions.
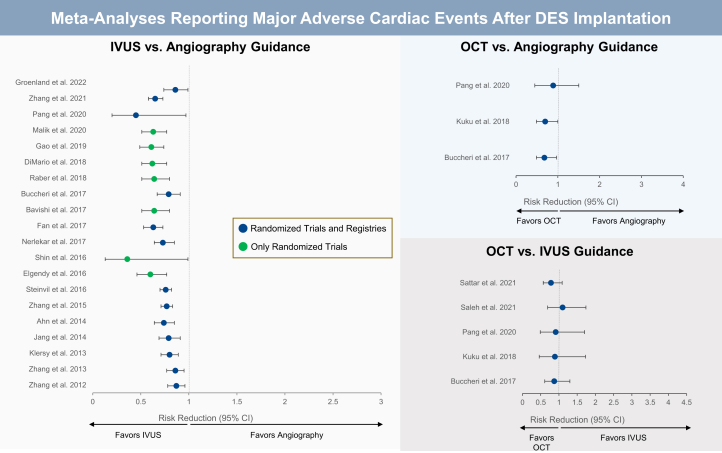
Central Illustration.
Meta-analyses comparing major adverse cardiac events after IVUS versus angiography guidance, OCT versus angiography guidance, and OCT versus IVUS guidance when implanting a DES. DES, drug-eluting stent; IVUS, intravascular imaging; OCT, optical coherence tomography.
Acknowledgments
Declaration of competing interest
Dr Mintz is a consultant and/or received honoraria from Boston Scientific, Medtronic, and Abiomed. Drs Bourantas and Chamié reported no financial interests.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics statement
This review article was based entirely on published literature.
References
- 1.Kubo T., Akasaka T., Shite J., et al. OCT compared with IVUS in a coronary lesion assessment: the OPUS-CLASS study. JACC Cardiovasc Imaging. 2013;6(10):1095–1104. doi: 10.1016/j.jcmg.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Guimaraes M., Antuña P., De la Cuerda F., et al. High-definition IVUS versus OCT to assess coronary artery disease and results of stent implantation. JACC Cardiovasc Imaging. 2020;13(2 Pt 1):519–521. doi: 10.1016/j.jcmg.2019.08.019. [DOI] [PubMed] [Google Scholar]
- 3.Nishi T., Imura S., Kitahara H., et al. Head-to-head comparison of quantitative measurements between intravascular imaging systems: an in vitro phantom study. Int J Cardiol Heart Vasc. 2021;36 doi: 10.1016/j.ijcha.2021.100867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Habara M., Nasu K., Terashima M., et al. Impact of frequency-domain optical coherence tomography guidance for optimal coronary stent implantation in comparison with intravascular ultrasound guidance. Circ Cardiovasc Interv. 2012;5(2):193–201. doi: 10.1161/CIRCINTERVENTIONS.111.965111. [DOI] [PubMed] [Google Scholar]
- 5.Kubo T., Shinke T., Okamura T., et al. Optical frequency domain imaging vs. intravascular ultrasound in percutaneous coronary intervention (OPINION trial): one-year angiographic and clinical results. Eur Heart J. 2017;38(42):3139–3147. doi: 10.1093/eurheartj/ehx351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ali Z.A., Maehara A., Généreux P., et al. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomised controlled trial. Lancet. 2016;388(10060):2618–2628. doi: 10.1016/S0140-6736(16)31922-5. [DOI] [PubMed] [Google Scholar]
- 7.Chamié D., Costa J.R., Damiani L.P., et al. Optical coherence tomography versus intravascular ultrasound and angiography to guide percutaneous coronary interventions: the iSIGHT randomized trial. Circ Cardiovasc Interv. 2021;14(3) doi: 10.1161/CIRCINTERVENTIONS.120.009452. [DOI] [PubMed] [Google Scholar]
- 8.Fujino A., Mintz G.S., Matsumura M., et al. A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. EuroIntervention. 2018;13(18):e2182–e2189. doi: 10.4244/EIJ-D-17-00962. [DOI] [PubMed] [Google Scholar]
- 9.Zhang M., Matsumura M., Usui E., et al. Intravascular ultrasound-derived calcium score to predict stent expansion in severely calcified lesions. Circ Cardiovasc Interv. 2021;14(10) doi: 10.1161/CIRCINTERVENTIONS.120.010296. [DOI] [PubMed] [Google Scholar]
- 10.Räber L., Mintz G.S., Koskinas K.C., et al. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J. 2018;39(35):3281–3300. doi: 10.1093/eurheartj/ehy285. [DOI] [PubMed] [Google Scholar]
- 11.Mintz GS, Matsumura M, Ali Z, Maehara A. Utility of intravascular imaging to guide percutaneous coronary intervention – past, present, and future. JACC Cardiovasc Imaging. In press. [DOI] [PubMed]
- 12.Hong S.J., Kim B.K., Shin D.H., et al. Effect of intravascular ultrasound-guided vs angiography-guided everolimus-eluting stent implantation: the IVUS-XPL randomized clinical trial. JAMA. 2015;314(20):2155–2163. doi: 10.1001/jama.2015.15454. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J., Gao X., Kan J., et al. Intravascular ultrasound versus angiography-guided drug-eluting stent implantation: the ULTIMATE trial. J Am Coll Cardiol. 2018;72(24):3126–3137. doi: 10.1016/j.jacc.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Hong S.J., Mintz G.S., Ahn C.M., et al. Effect of intravascular ultrasound-guided drug-eluting stent implantation: 5-year follow-up of the IVUS-XPL randomized trial. JACC Cardiovasc Interv. 2020;13(1):62–71. doi: 10.1016/j.jcin.2019.09.033. [DOI] [PubMed] [Google Scholar]
- 15.Gao X.F., Ge Z., Kong X.Q., et al. 3-year outcomes of the ULTIMATE trial comparing intravascular ultrasound versus angiography-guided drug-eluting stent implantation. JACC Cardiovasc Interv. 2021;14(3):247–257. doi: 10.1016/j.jcin.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Hong S.J., Zhang J.J., Mintz G.S., et al. Improved 3-year cardiac survival after IVUS-guided long DES implantation: a patient-level analysis from 2 randomized trials. JACC Cardiovasc Interv. 2022;15(2):208–216. doi: 10.1016/j.jcin.2021.10.020. [DOI] [PubMed] [Google Scholar]
- 17.Liu X.M., Yang Z.M., Liu X.K., et al. Intravascular ultrasound-guided drug-eluting stent implantation for patients with unprotected left main coronary artery lesions: a single-center randomized trial. Anatol J Cardiol. 2019;21(2):83–90. doi: 10.14744/AnatolJCardiol.2018.21447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim B.K., Shin D.H., Hong M.K., et al. Clinical impact of intravascular ultrasound-guided chronic total occlusion intervention with zotarolimus-eluting versus Biolimus-eluting stent implantation: randomized study. Circ Cardiovasc Interv. 2015;8(7) doi: 10.1161/CIRCINTERVENTIONS.115.002592. [DOI] [PubMed] [Google Scholar]
- 19.Hibi K., Kozuma K., Sonoda S., et al. A randomized study of distal filter protection versus conventional treatment during percutaneous coronary intervention in patients with attenuated plaque identified by intravascular ultrasound. JACC Cardiovasc Interv. 2018;11(16):1545–1555. doi: 10.1016/j.jcin.2018.03.021. [DOI] [PubMed] [Google Scholar]
- 20.Hibi K., Kozuma K., Maejima N., et al. Long-term clinical outcomes after filter protection during percutaneous coronary intervention in patients with attenuated plaque-1-year follow up of the VAMPIRE 3 (Vacuum Aspiration Thrombus Reemoval 3) trial. Circ J. 2020;85(1):44–49. doi: 10.1253/circj.CJ-20-0449. [DOI] [PubMed] [Google Scholar]
- 21.Ali Z.A., Karimi Galougahi K., Maehara A., et al. Outcomes of optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation: one-year results from the ILUMIEN III: OPTIMIZE PCI trial. EuroIntervention. 2021;16(13):1085–1091. doi: 10.4244/EIJ-D-20-00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jakabcin J., Spacek R., Bystron M., et al. Long-term health outcome and mortality evaluation after invasive coronary treatment using drug eluting stents with or without the IVUS guidance. Randomized control trial. HOME DES IVUS. Catheter Cardiovasc Interv. 2010;75(4):578–583. doi: 10.1002/ccd.22244. [DOI] [PubMed] [Google Scholar]
- 23.Chieffo A., Latib A., Caussin C., et al. A prospective, randomized trial of intravascular-ultrasound guided compared to angiography guided stent implantation in complex coronary lesions: the AVIO trial. Am Heart J. 2013;165(1):65–72. doi: 10.1016/j.ahj.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 24.Kim J.S., Kang T.S., Mintz G.S., et al. Randomized comparison of clinical outcomes between intravascular ultrasound and angiography-guided drug-eluting stent implantation for long coronary artery stenoses. JACC Cardiovasc Interv. 2013;6(4):369–376. doi: 10.1016/j.jcin.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Tian N.L., Gami S.K., Ye F., et al. Angiographic and clinical comparisons of intravascular ultrasound- versus angiography-guided drug-eluting stent implantation for patients with chronic total occlusion lesions: two-year results from a randomised AIR-CTO study. EuroIntervention. 2015;10(12):1409–1417. doi: 10.4244/EIJV10I12A245. [DOI] [PubMed] [Google Scholar]
- 26.Tan Q., Wang Q., Liu D., Zhang S., Zhang Y., Li Y. Intravascular ultrasound–guided unprotected left main coronary artery stenting in the elderly. Saudi Med J. 2015;36(5):549–553. doi: 10.15537/smj.2015.5.11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J.Q., Shi R., Pang W., et al. Application of intravascular ultrasound in stent implantation for small coronary arteries. J Clin Invasive Cardiol. 2016;3(1):2–8. [Google Scholar]
- 28.Mariani J., Jr., Guedes C., Soares P., et al. Intravascular ultrasound guidance to minimize the use of iodine contrast in percutaneous coronary intervention: the MOZART (Minimizing cOntrast utiliZation With IVUS Guidance in coRonary angioplasty) randomized controlled trial. JACC Cardiovasc Interv. 2014;7(11):1287–1293. doi: 10.1016/j.jcin.2014.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim J.S., Shin D.H., Kim B.K., et al. Randomized comparison of stent strut coverage following angiography- or optical coherence tomography-guided percutaneous coronary intervention. Rev Esp Cardiol (Engl Ed) 2015;68(3):190–197. doi: 10.1016/j.rec.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 30.Meneveau N., Souteyrand G., Motreff P., et al. Optical coherence tomography to optimize results of percutaneous coronary intervention in patients with non-ST-elevation acute coronary syndrome: results of the multicenter, randomized DOCTORS study (does optical coherence tomography optimize results of stenting) Circulation. 2016;134(13):906–917. doi: 10.1161/CIRCULATIONAHA.116.024393. [DOI] [PubMed] [Google Scholar]
- 31.Lee S.Y., Kim J.S., Yoon H.J., et al. Early strut coverage in patients receiving drug-eluting stents and its implications for dual antiplatelet therapy: a randomized trial. JACC Cardiovasc Imaging. 2018;11(12):1810–1819. doi: 10.1016/j.jcmg.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 32.Kala P., Cervinka P., Jakl M., et al. OCT guidance during stent implantation in primary PCI: a randomized multicenter study with nine months of optical coherence tomography follow-up. Int J Cardiol. 2018;250:98–103. doi: 10.1016/j.ijcard.2017.10.059. [DOI] [PubMed] [Google Scholar]
- 33.Antonsen L., Thayssen P., Maehara A., et al. Optical coherence tomography guided percutaneous coronary intervention with nobori stent implantation in patients with non-ST-segment-elevation myocardial infarction (OCTACS) trial: difference in strut coverage and dynamic malapposition patterns at 6 months. Circ Cardiovasc Interv. 2015;8(8) doi: 10.1161/CIRCINTERVENTIONS.114.002446. [DOI] [PubMed] [Google Scholar]
- 34.Otake H., Kubo T., Takahashi H., et al. Optical frequency domain imaging versus intravascular ultrasound in percutaneous coronary intervention (OPINION trial): results from the OPINION imaging study. JACC Cardiovasc Imaging. 2018;11(1):111–123. doi: 10.1016/j.jcmg.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 35.Lee Y.J., Zhang J.J., Mintz G.S., et al. Is routine postdilation during angiography-guided stent implantation as good as intravascular ultrasound guidance?: an analysis using data from IVUS-XPL and ULTIMATE. Circ Cardiovasc Interv. 2022;15(1) doi: 10.1161/CIRCINTERVENTIONS.121.011366. [DOI] [PubMed] [Google Scholar]
- 36.Lee Y.J., Zhang J.J., Mintz G.S., et al. Impact of intravascular ultrasound-guided optimal stent expansion on 3-year hard clinical outcomes. Circ Cardiovasc Interv. 2021;14(10) doi: 10.1161/CIRCINTERVENTIONS.121.011124. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y., Farooq V., Garcia-Garcia H.M., et al. Comparison of intravascular ultrasound versus angiography-guided drug-eluting stent implantation: a meta-analysis of one randomised trial and ten observational studies involving 19,619 patients. EuroIntervention. 2012;8(7):855–865. doi: 10.4244/EIJV8I7A129. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y.J., Garcia-Garcia H.M., Farooq V., Bourantas C.V., Serruys P.W., Chen S.L. Revisiting: “Comparison of intravascular ultrasound versus angiography-guided drug-eluting stent implantation: a meta-analysis of one randomised trial and ten observational studies involving 19,619 patients”. EuroIntervention. 2013;9(7):891–892. doi: 10.4244/EIJV9I7A148. [DOI] [PubMed] [Google Scholar]
- 39.Klersy C., Ferlini M., Raisaro A., et al. Use of IVUS guided coronary stenting with drug eluting stent: a systematic review and meta-analysis of randomized controlled clinical trials and high quality observational studies. Int J Cardiol. 2013;170(1):54–63. doi: 10.1016/j.ijcard.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Jang J.S., Song Y.J., Kang W., et al. Intravascular ultrasound-guided implantation of drug-eluting stents to improve outcome: a meta-analysis. JACC Cardiovasc Interv. 2014;7(3):233–243. doi: 10.1016/j.jcin.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 41.Ahn J.M., Kang S.J., Yoon S.H., et al. Meta-analysis of outcomes after intravascular ultrasound-guided versus angiography-guided drug-eluting stent implantation in 26,503 patients enrolled in three randomized trials and 14 observational studies. Am J Cardiol. 2014;113(8):1338–1347. doi: 10.1016/j.amjcard.2013.12.043. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y.J., Pang S., Chen X.Y., et al. Comparison of intravascular ultrasound guided versus angiography guided drug eluting stent implantation: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2015;15:153. doi: 10.1186/s12872-015-0144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steinvil A., Zhang Y.J., Lee S.Y., et al. Intravascular ultrasound-guided drug-eluting stent implantation: an updated meta-analysis of randomized control trials and observational studies. Int J Cardiol. 2016;216:133–139. doi: 10.1016/j.ijcard.2016.04.154. [DOI] [PubMed] [Google Scholar]
- 44.Elgendy I.Y., Mahmoud A.N., Elgendy A.Y., Bavry A.A. Outcomes with intravascular ultrasound-guided stent implantation: a meta-analysis of randomized trials in the era of drug-eluting stents. Circ Cardiovasc Interv. 2016;9(4) doi: 10.1161/CIRCINTERVENTIONS.116.003700. [DOI] [PubMed] [Google Scholar]
- 45.Shin D.H., Hong S.J., Mintz G.S., et al. Effects of intravascular ultrasound-guided versus angiography-guided new-generation drug-eluting stent implantation: meta-analysis with individual patient-level data from 2,345 randomized patients. JACC Cardiovasc Interv. 2016;9(21):2232–2239. doi: 10.1016/j.jcin.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 46.Nerlekar N., Cheshire C.J., Verma K.P., et al. Intravascular ultrasound guidance improves clinical outcomes during implantation of both first- and second-generation drug-eluting stents: a meta-analysis. EuroIntervention. 2017;12(13):1632–1642. doi: 10.4244/EIJ-D-16-00769. [DOI] [PubMed] [Google Scholar]
- 47.Fan Z.G., Gao X.F., Li X.B., et al. The outcomes of intravascular ultrasound-guided drug-eluting stent implantation among patients with complex coronary lesions: a comprehensive meta-analysis of 15 clinical trials and 8,084 patients. Anatol J Cardiol. 2017;17(4):258–268. doi: 10.14744/AnatolJCardiol.2016.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bavishi C., Sardar P., Chatterjee S., et al. Intravascular ultrasound-guided vs angiography-guided drug-eluting stent implantation in complex coronary lesions: meta-analysis of randomized trials. Am Heart J. 2017;185:26–34. doi: 10.1016/j.ahj.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 49.Buccheri S., Franchina G., Romano S., et al. Clinical outcomes following intravascular imaging-guided versus coronary angiography-guided percutaneous coronary intervention with stent implantation: a systematic review and bayesian network meta-analysis of 31 studies and 17,882 patients. JACC Cardiovasc Interv. 2017;10(24):2488–2498. doi: 10.1016/j.jcin.2017.08.051. [DOI] [PubMed] [Google Scholar]
- 50.di Mario C., Koskinas K.C., Räber L. Clinical benefit of IVUS guidance for coronary stenting: the ULTIMATE step toward definitive evidence? J Am Coll Cardiol. 2018;72(24):3138–3141. doi: 10.1016/j.jacc.2018.10.029. [DOI] [PubMed] [Google Scholar]
- 51.Gao X.F., Wang Z.M., Wang F., et al. Intravascular ultrasound guidance reduces cardiac death and coronary revascularization in patients undergoing drug-eluting stent implantation: results from a meta-analysis of 9 randomized trials and 4724 patients. Int J Cardiovasc Imaging. 2019;35(2):239–247. doi: 10.1007/s10554-019-01555-3. [DOI] [PubMed] [Google Scholar]
- 52.Elgendy I.Y., Mahmoud A.N., Elgendy A.Y., Mintz G.S. Intravascular ultrasound-guidance is associated with lower cardiovascular mortality and myocardial infarction for drug-eluting stent implantation- insights from an updated meta-analysis of randomized trials. Circ J. 2019;83(6):1410–1413. doi: 10.1253/circj.CJ-19-0209. [DOI] [PubMed] [Google Scholar]
- 53.Kumar A., Shariff M., Adalja D., Doshi R. Intravascular ultrasound versus angiogram guided drug eluting stent implantation. A systematic review and updated meta-analysis with trial sequential analysis. Int J Cardiol Heart Vasc. 2019;25 doi: 10.1016/j.ijcha.2019.100419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malik A.H., Yandrapalli S., Aronow W.S., Panza J.A., Cooper H.A. Intravascular ultrasound-guided stent implantation reduces cardiovascular mortality – updated meta-analysis of randomized controlled trials. Int J Cardiol. 2020;299:100–105. doi: 10.1016/j.ijcard.2019.07.033. [DOI] [PubMed] [Google Scholar]
- 55.Darmoch F., Alraies M.C., Al-Khadra Y., Moussa Pacha H., Pinto D.S., Osborn E.A. Intravascular ultrasound imaging-guided versus coronary angiography-guided percutaneous coronary intervention: a systematic review and meta-analysis. J Am Heart Assoc. 2020;9(5) doi: 10.1161/JAHA.119.013678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pang J., Ye L., Chen Q. How to guide PCI?: a network meta-analysis. Medicine (Baltimore) 2020;99(20) doi: 10.1097/MD.0000000000020168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Q., Wang B., Han Y., Sun S., Lv R., Wei S. Short- and long-term prognosis of intravascular ultrasound-versus angiography-guided percutaneous coronary intervention: a meta-analysis involving 24,783 patients. J Interv Cardiol. 2021;2021 doi: 10.1155/2021/6082581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Groenland F.T.W., Neleman T., Kakar H., et al. Intravascular ultrasound-guided versus coronary angiography-guided percutaneous coronary intervention in patients with acute myocardial infarction: a systematic review and meta-analysis. Int J Cardiol. 2022;353:35–42. doi: 10.1016/j.ijcard.2022.01.021. [DOI] [PubMed] [Google Scholar]
- 59.Kuku K.O., Ekanem E., Azizi V., et al. Optical coherence tomography-guided percutaneous coronary intervention compared with other imaging guidance: a meta-analysis. Int J Cardiovasc Imaging. 2018;34(4):503–513. doi: 10.1007/s10554-017-1272-2. [DOI] [PubMed] [Google Scholar]
- 60.Sharma S.P., Rijal J., Dahal K. Optical coherence tomography guidance in percutaneous coronary intervention: a meta-analysis of randomized controlled trials. Cardiovasc Interv Ther. 2019;34(2):113–121. doi: 10.1007/s12928-018-0529-6. [DOI] [PubMed] [Google Scholar]
- 61.Ye Y., Yang M., Zhang S., Zeng Y. Percutaneous coronary intervention in left main coronary artery disease with or without intravascular ultrasound: a meta-analysis. PLoS One. 2017;12(6) doi: 10.1371/journal.pone.0179756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y., Mintz G.S., Gu Z., et al. Meta-analysis and systematic review of intravascular ultrasound versus angiography-guided drug eluting stent implantation in left main coronary disease in 4592 patients. BMC Cardiovasc Disord. 2018;18(1):115. doi: 10.1186/s12872-018-0843-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elgendy I.Y., Gad M., Jain A., Mahmoud A.N., Mintz G.S. Outcomes with intravascular ultrasound-guided drug eluting stent implantation for unprotected left main coronary lesions: a meta-analysis. Am J Cardiol. 2019;124(10):1652–1653. doi: 10.1016/j.amjcard.2019.08.023. [DOI] [PubMed] [Google Scholar]
- 64.Elgendy I.Y., Gad M., Mintz G.S. Meta-analysis of intravascular ultrasound-guided drug-eluting stent implantation for left main coronary disease. Am J Cardiol. 2020;128:92–93. doi: 10.1016/j.amjcard.2020.05.013. [DOI] [PubMed] [Google Scholar]
- 65.Saleem S., Ullah W., Mukhtar M., et al. Angiographic-only or intravascular ultrasound-guided approach for left-main coronary artery intervention: a systematic review and meta-analysis. Expert Rev Cardiovasc Ther. 2021;19(11):1029–1035. doi: 10.1080/14779072.2021.2004122. [DOI] [PubMed] [Google Scholar]
- 66.Salesh Y., Al-abcha A., Abdelkarim O., et al. Clinical outcomes of optical coherence tomography-guided compared with intravascular ultrasound-guided percutaneous coronary intervention: a meta-analysis. J Am Coll Cardiol. 2021;78:B37–B38. [Google Scholar]
- 67.Sattar Y., Abdul Razzack A., Kompella R., et al. Outcomes of intravascular ultrasound versus optical coherence tomography guided percutaneous coronary angiography: a meta regression-based analysis. Catheter Cardiovasc Interv. 2022;99(1):E1–E11. doi: 10.1002/ccd.29976. [DOI] [PubMed] [Google Scholar]
- 68.Witzenbichler B., Maehara A., Weisz G., et al. Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents: the assessment of dual antiplatelet therapy with drug-eluting stents (ADAPT-DES) study. Circulation. 2014;129(4):463–470. doi: 10.1161/CIRCULATIONAHA.113.003942. [DOI] [PubMed] [Google Scholar]
- 69.Maehara A., Mintz G.S., Witzenbichler B., et al. Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents. Circ Cardiovasc Interv. 2018;11(11) doi: 10.1161/CIRCINTERVENTIONS.117.006243. [DOI] [PubMed] [Google Scholar]
- 70.Shlofmitz E., Torguson R., Zhang C., et al. Impact of intravascular ultrasound on outcomes following PErcutaneous coronary InterventioN in complex lesions (iOPEN complex) Am Heart J. 2020;221:74–83. doi: 10.1016/j.ahj.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 71.Choi K.H., Song Y.B., Lee J.M., et al. Impact of intravascular ultrasound-guided percutaneous coronary intervention on long-term clinical outcomes in patients undergoing complex procedures. JACC Cardiovasc Interv. 2019;12(7):607–620. doi: 10.1016/j.jcin.2019.01.227. [DOI] [PubMed] [Google Scholar]
- 72.Shlofmitz E., Torguson R., Zhang C., et al. Impact of intravascular ultrasound on Outcomes following PErcutaneous coronary interventioN for in-stent restenosis (iOPEN-ISR study) Int J Cardiol. 2021;340:17–21. doi: 10.1016/j.ijcard.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 73.Kinnaird T., Johnson T., Anderson R., et al. Intravascular imaging and 12-month mortality after unprotected left main stem PCI: an analysis from the British Cardiovascular Intervention Society database. JACC Cardiovasc Interv. 2020;13(3):346–357. doi: 10.1016/j.jcin.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 74.de la Torre Hernandez J.M., Baz Alonso J.A., Gómez Hospital J.A., et al. Clinical impact of intravascular ultrasound guidance in drug-eluting stent implantation for unprotected left main coronary disease: pooled analysis at the patient-level of 4 registries. JACC Cardiovasc Interv. 2014;7(3):244–254. doi: 10.1016/j.jcin.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 75.Cortese B., de la Torre Hernandez J.M., Lanocha M., et al. Optical coherence tomography, intravascular ultrasound or angiography guidance for distal left main coronary stenting. The ROCK cohort II study. Catheter Cardiovasc Interv. 2022;99(3):664–673. doi: 10.1002/ccd.29959. [DOI] [PubMed] [Google Scholar]
- 76.Wiebe J., Kuna C., Ibrahim T., et al. Long-term prognostic impact of restenosis of the unprotected left main coronary artery requiring repeat revascularization. JACC Cardiovasc Interv. 2020;13(19):2266–2274. doi: 10.1016/j.jcin.2020.07.017. [DOI] [PubMed] [Google Scholar]
- 77.Ahmed K., Jeong M.H., Chakraborty R., et al. Role of intravascular ultrasound in patients with acute myocardial infarction undergoing percutaneous coronary intervention. Am J Cardiol. 2011;108(1):8–14. doi: 10.1016/j.amjcard.2011.02.339. [DOI] [PubMed] [Google Scholar]
- 78.Nakatsuma K., Shiomi H., Morimoto T., et al. Intravascular ultrasound Guidance vs. angiographic guidance in primary percutaneous coronary intervention for ST-segment elevation myocardial infarction – long-term clinical outcomes from the CREDO-Kyoto AMI Registry. Circ J. 2016;80(2):477–484. doi: 10.1253/circj.CJ-15-0870. [DOI] [PubMed] [Google Scholar]
- 79.Kim N., Lee J.H., Jang S.Y., et al. Intravascular modality-guided versus angiography-guided percutaneous coronary intervention in acute myocardial infarction. Catheter Cardiovasc Interv. 2020;95(4):696–703. doi: 10.1002/ccd.28359. [DOI] [PubMed] [Google Scholar]
- 80.Choi I.J., Lim S., Choo E.H., et al. Impact of intravascular ultrasound on long-term clinical outcomes in patients with acute myocardial infarction. JACC Cardiovasc Interv. 2021;14(22):2431–2443. doi: 10.1016/j.jcin.2021.08.021. [DOI] [PubMed] [Google Scholar]
- 81.Kim Y., Bae S., Johnson T.W., et al. Role of intravascular ultrasound-guided percutaneous coronary intervention in optimizing outcomes in acute myocardial infarction. J Am Heart Assoc. 2022;11(5) doi: 10.1161/JAHA.121.023481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ali Z.A., Karimi Galougahi K., Nazif T., et al. Imaging- and physiology-guided percutaneous coronary intervention without contrast administration in advanced renal failure: a feasibility, safety, and outcome study. Eur Heart J. 2016;37(40):3090–3095. doi: 10.1093/eurheartj/ehw078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sacha J., Gierlotka M., Lipski P., Feusette P., Dudek D. Zero-contrast percutaneous coronary interventions to preserve kidney function in patients with severe renal impairment and hemodialysis subjects. Postepy Kardiol Interwencyjnej. 2019;15(2):137–142. doi: 10.5114/aic.2019.86008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shibata K., Wakabayashi K., Ishinaga T., et al. Feasibility, safety, and long-term outcomes of zero-contrast percutaneous coronary intervention in patients with chronic kidney disease. Circ J. 2022;86(5):787–796. doi: 10.1253/circj.CJ-21-0905. [DOI] [PubMed] [Google Scholar]
- 85.Nandhakumar V., Pakshirajan B., Chopra A., et al. Safety and feasibility of intravascular ultrasound guided zero-contrast percutaneous coronary intervention-a prospective study. Int J Cardiol. 2022;353:22–28. doi: 10.1016/j.ijcard.2022.01.034. [DOI] [PubMed] [Google Scholar]
- 86.Burlacu A., Tinica G., Brinza C., Crisan-Dabija R., Popa I.V., Covic A. Safety and efficacy of minimum- or zero-contrast IVUS-guided percutaneous coronary interventions in chronic kidney disease patients: a systematic review. J Clin Med. 2021;10(9):1996–1997. doi: 10.3390/jcm10091996. [DOI] [PMC free article] [PubMed] [Google Scholar]