Abstract
Eosinophilic esophagitis (EoE) is a chronic type 2 inflammatory disease characterized by an eosinophilic inflammatory infiltrate in the esophagus, leading to remodeling, stricture formation, and fibrosis. Triggered by food and aeroallergens, type 2 cytokines interleukin (IL)-4, IL-13, IL-5 produced by CD4+ T helper 2 cells (Th2), eosinophils, mast cells, basophils, and type 2 innate lymphoid cells alter the esophageal epithelial barrier and increase inflammatory cell tissue infiltration. Clustering analysis based on the expression of type 2 inflammatory genes demonstrated the diversity of EoE endotypes. Despite the availability of treatment options for patients with EoE, which include dietary restriction, proton pump inhibitors, swallowed topical steroids, and esophageal dilation, there are still no Food and Drug Administration–approved medications for this disease; as such, there are clear unmet medical needs for these patients. A number of novel biologic therapies currently in clinical trials represent a promising avenue for targeted therapeutic approaches in EoE. This review summarizes our current knowledge on the role of type 2 inflammatory cells and mediators in EoE disease pathogenesis, as well as the future treatment landscape targeting underlying inflammation in EoE.
Keywords: Eosinophilic Esophagitis, Eosinophils, Endotypes, Type 2 Inflammation
Introduction
Eosinophilic esophagitis (EoE) is a chronic type 2 inflammatory disease characterized by eosinophilic inflammation of the esophagus, epithelial barrier dysfunction, and esophageal subepithelial fibrosis.1, 2, 3 In addition to symptoms related to esophageal dysfunction, a diagnosis of EoE requires the presence of ≥15 eosinophils/high-power field in the esophageal mucosa in the absence of other potential causes of esophageal eosinophilia.1
Although eosinophils are the histologic diagnostic feature of EoE, multiple immune cell types likely contribute to the complex mechanisms underlying EoE pathophysiology. EoE is characterized by epithelial barrier dysfunction in response to food antigens, leading to immune dysfunction and inflammation involving the type 2 inflammatory cytokines interleukin (IL)-4, IL-5, and IL-13.4 This response is initiated by the processing and presentation of foreign (food) antigens by antigen-presenting cells (APCs) to adaptive immune cells such as naïve Th cells, leading to their polarization to effector cells. Dendritic cells are a major type of APC, and Langerhans cells, a type of dendritic cell found in squamous epithelia, appear to play a role in driving the type 2 inflammation characteristic of EoE. The downstream release of type 2 cytokines drives a positive feedback loop, promoting further inflammation and epithelial barrier dysfunction, ultimately resulting in persistent esophageal inflammation and the characteristic pathophysiologic features of EoE (such as esophageal fibrosis and tissue remodeling), as well as clinical symptoms of EoE (such as dysphagia, food impaction, and chest pain in adults and reflux, failure to thrive, and food refusal in children).5, 6, 7, 8, 9, 10, 11, 12 Persistent symptoms associated with EoE have a substantial impact on patient quality of life, and current treatment options are characterized by variable success rates.13, 14, 15
Although EoE is predominantly triggered by a type 2 response, other non–type 2 inflammatory factors (including interferon and tumor necrosis factor family member signaling) have also been implicated in EoE.16,17 Given its predominance in EoE pathogenesis, this review will focus on the underlying mechanisms associated with type 2 inflammation in EoE.
With new targeted therapies in development for the treatment of EoE, better understanding of the underlying disease pathophysiology and the phenotypes and endotypes associated with type 2 inflammation offers the potential for precision medicine approaches in EoE. In this review, we summarize our current understanding of the roles of various type 2 inflammatory cells and cytokines in the pathophysiology of EoE and review the prospective treatment landscape targeting type 2 inflammation in EoE.
Type 2 Inflammation in EoE
The immune system is comprised of 2 parts (innate and adaptive) in which the innate immune system acts as a rapid, nonspecific first line of defense against pathogens and environmental insults, followed temporally by the adaptive system, which acts with specificity to provide a more robust and targeted response. The type 1 vs type 2 immunity paradigm was first described in the context of cytokine production from the adaptive T helper 1 (Th1) and T helper 2 (Th2) cell subsets.18 It is now appreciated that the type 1/type 2 paradigm is larger than T cell subsets: type 1 immunity involves interferon-γ production from both innate and adaptive immune cells; type 2 inflammation is driven by the activity of key type 2 cytokines IL-4, IL-5, and IL-13, which are produced by the adaptive and innate arms of the immune system, including Th2 cells, type 2 innate lymphoid cells (ILC2s), mast cells, basophils, and eosinophils, through shared cellular and gene transcriptional activation domains such as GATA-3 transcription factor.19, 20, 21, 22, 23, 24, 25, 26, 27, 28
Type 2 inflammation in patients with EoE is characterized by elevated levels of type 2 cytokines IL-4, IL-5, and IL-1329,30 and chemokines such as eotaxin-3 (Table 1).29,46 In addition to the presence of eosinophils, elevated levels of Th2, ILC2s, basophils, and mast cells have also been detected in esophageal biopsies from patients with EoE.26,30,36,46, 47, 48 Furthermore, locally elevated levels of immunoglobulin (Ig) E and IgG4 to shared antigens have also been found in EoE patients.49,50 Despite the antigen-driven process associated with EoE that includes IgE-mediated allergic comorbidities, non-IgE-mediated type 2 inflammatory pathways are now recognized as playing pivotal roles in EoE pathogenesis.2,3,51
Table 1.
Key Type 2 Cytokines and Chemokines and Their Role in EoE
| Inflammatory mediator | Proposed role in EoE | Citation |
|---|---|---|
| IL-4 | Differentiation of Th2 cells; secretion of eotaxin-3; B cell class switching to IgE; proliferation and activation of mast cells | Dunn 202031; Swain 199032; Cheng 201333; Moore 200234; McLeod 201535; Noti 201336 |
| IL-5 | Involved in eosinophil development, activation, survival, and recruitment; promotes tissue remodeling | Kouro 200937; Mishra 200838 |
| IL-13 | Promotes Th2 effector responses; involved in B cell class switching to IgE; eosinophil recruitment; mediates impaired epithelial architecture and barrier dysfunction | Cheng 201333; Muir 20199; Aceves 20105; Blanchard 200739; Ryu 202040 |
| IL-25 | Produced by epithelial cells in response to environmental trigger; activates ILC2s | Camelo 201741 |
| IL-33 | Produced by epithelial cells in response to environmental trigger; activates ILC2s | Camelo 201741 |
| Periostin | Promotion of eosinophil chemotaxis | Blanchard 200842 |
| Eotaxin-3/CCL26 | Eosinophil chemoattractant | Blanchard 201129 |
| TSLP | Produced by epithelial cells in response to environmental trigger; activates ILC2s; mediates basophil response | Camelo 201741 |
| Eosinophils | Present in esophageal mucosa in EoE; release of eosinophil-associated proteins, including IL-4, IL-13, IL-5, TGF-β, T NFα, and IL-1β amphiregulin and osteopontin; potentially contribute to fibrosis | Straumann 200543; Doyle 202044 |
| Mast cells | Release inflammatory mediators, including type 2 cytokines, TGF-β, histamines, and proteases; contribute to smooth muscle dysfunction | Aceves 20105; McLeod 201535; Ryu 202040 |
| Th2 cells | Produce of type 2 inflammatory cytokines IL-4, IL-5, IL-13 | Wen 201945; Cianferoni 201830 |
| Type 2 innate lymphoid cells (ILC2s) | Produce high levels of L-5 and IL-13; potential role in steroid resistance | Doherty 201526 |
CCL26, C-C motif chemokine ligand 26; EoE, eosinophilic esophagitis; Ig, immunoglobulin; IL, interleukin; TGF-β, transforming growth factor beta; Th2, T helper cell type 2; TSLP, thymic stromal lymphopoietin.
Characterization of Endotypes in EoE
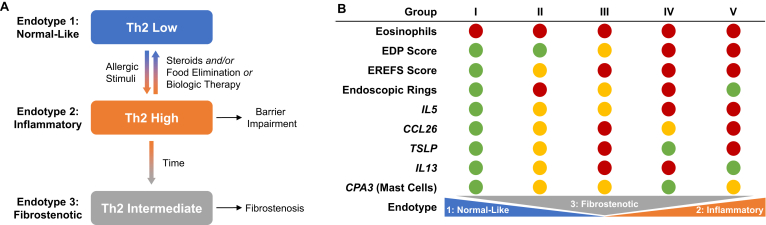
Three distinct endotypes of EoE, with differing levels of type 2 inflammation, have been described in a multisite cross-sectional study of differential gene expression patterns in esophageal biopsies using the esophagitis diagnostic panel.52 Endotype 1 is characterized by a normal endoscopic appearance, usually steroid sensitive, and has normal levels of type 2 inflammation hallmarks, classified as Th2 low; endotype 2 is seen primarily in pediatric patients and is associated with atopy, lack of a steroid response, and upregulation of pro-inflammatory cytokines (eg, IL-4 and thymic stromal lymphopoietin [TSLP]), classified as Th2 high; endotype 3 is seen primarily in adults, is non-atopic, is associated with fibrostenosis and narrow-caliber esophagus, and is associated with low expression of genes controlling epithelial differentiation, classified as Th2 intermediate (Figure). In a separate study, 5 subgroups of patients with active EoE were identified by unsupervised clustering based on the expression of the type 2 inflammatory genes IL4, IL5, IL13, C-C motif ligand (CCL)26, TSLP, Charcot-Leyden crystal, C-C motif chemokine receptor 3, and carboxypeptidase A3.31 Group V patients had the highest expression of IL5, TSLP, CCL26, and genes associated with tissue remodeling; IL5 and IL13 were highly expressed in group IV; groups II and III had intermediate expression of IL5 and carboxypeptidase A3, with high TSLP and IL13 in group III.31 The 5 groups varied not only by the expression of type 2 inflammatory genes but also in terms of membership in EoE endotypes 1–3 (Figure). Interestingly, the 3 endotypes had similar levels of esophageal eosinophils, indicating an apparent disconnect between the level of type 2 inflammation and number of eosinophils infiltrating the esophagus31; this represents a challenge to the clinician in terms of personalizing and optimizing treatment. As these endotypes suggest, heterogenous type 2 gene expression is observed in patients with EoE as a whole; however, the degree of type 2 gene overexpression is not directly correlated with the severity of disease features as defined by peak esophageal eosinophil count (Figure).31 These findings may drive the variable responses to currently available therapies and to targeted biologic therapy, emphasizing the clinical importance of understanding the endotypes associated with the type 2 inflammatory pathophysiology of EoE.
Figure.
Endotypes of eosinophilic esophagitis and their characteristic features. (A) Model depicting patient progression from Th2-low phenotype (endotype 1) to a Th2-high phenotype (endotype 2) following allergic or inflammatory insult. On steroid treatment, food elimination, or biologic therapy the Th2-gene expression decreases and patients either resolve inflammation by reverting to a Th2-low phenotype or develop a fibrostenotic (endotype 3) signature. (B) Five subgroups of patients with active EoE were identified based on a variety of criteria, including expression of IL5, IL13, CCL26, TSLP, and CPA3. Relative levels of each criterion are reported in red (high), yellow (intermediate), or green (low). The 5 groups differed in the EoE endotypes spanned, but not in eosinophil levels, which were universally high. Group V patients had the highest expression of IL5, TSLP, CCL26, and genes associated with tissue remodeling. Groups II and III (which exhibited intermediate expression of IL5 and CPA3) were differentiated by high TSLP and IL13 in group III. CCL26, C-C motif chemokine ligand 26; EDP, eosinophilic esophagitis diagnostic panel; EREFS, endoscopic reference score; Th2, T helper cell type 2; TSLP, thymic stromal lymphopoietin (Adapted from J Allergy Clin Immunol: 2020;145:1629–1640.e4.).31
There are several lines of evidence to support the paradigm that these endotypes and their associated clinical phenotypes may reflect the natural history of EoE, although further understanding is required to determine how these may be positioned within EoE classification. Distinct differences in clinical presentation and endoscopic findings are seen in pediatric vs adult EoE patients.53,54 One study revealed that endoscopically defined inflammatory, fibrostenotic, and mixed EoE phenotypes were associated with distinct clinical characteristics and symptomatology and that for every 10-year increase in age, the odds of having a fibrostenotic phenotype more than doubles.55 It is noted, however, that interpretation of this study is limited, given its retrospective nature, which by design only included patients who followed up. The association of fibrostenosis with age suggests that the natural history of EoE may be a progression from an inflammatory to a fibrostenotic disease. However, not all patients may progress at the same rate, and this progression may be related more to the duration of untreated disease than age,56 underscoring the potential importance of rapidly and efficaciously bringing inflammation under control. This concept remains to be further investigated with prospective longitudinal studies.
Role of Key Type 2 Effector Cells and Inflammatory Mediators in the Pathophysiological Features of EoE
Eosinophils and Mast Cells
Eosinophils and mast cells are type 2 effector cells that are similar yet distinct classes of granulocytes, serving critical roles in allergic inflammation.20 Eosinophils and mast cells are both found in the esophageal epithelium of patients with EoE (Table 1) and can persist in some patients despite clinical remission.43,57,58 When activated, both eosinophils and mast cells degranulate to release pro-inflammatory mediators (particularly type 2 cytokines) that can contribute to inflammation, remodeling, and fibrosis when dysregulated (Table 1).40,44,59
The critical role of eosinophils as a mediator of EoE pathophysiology has been demonstrated by several transgenic murine models. Notably, greater basal layer thickening and increased collagen deposition were observed in the epithelial mucosa and lamina propria of mice with experimental EoE compared with experimental controls. Conversely, eosinophil-deficient mice have significantly reduced the thickening of the basal layer and lamina propria collagen and do not develop esophageal strictures.38,60 Further support for the role played by eosinophils comes from models of egg-induced EoE. These have demonstrated that inhibition of AMCase, an innate immune modulator, or sialic acid–binding immunoglobulin-like lectin (Siglec), a receptor highly expressed by eosinophils, reduces eosinophilic inflammation and esophagus remodeling.61,62 In addition, evidence from an allergen-induced mouse model of EoE demonstrated that mast cells increase under inflammatory conditions, but esophageal eosinophil recruitment was not dependent on the presence of mast cells.63 In addition, mice genetically deficient in mast cells were protected from smooth muscle cell hyperplasia in this model, suggesting that mast cells may impact peristaltic function in EoE.63
Although eosinophils are a defining feature of EoE histopathology,1,3 peripheral blood eosinophils do not correlate with esophageal eosinophil counts or disease activity. Furthermore, there is a well-described discrepancy between peak esophageal eosinophil counts and symptom severity in adults,64,65 although this may not be the case in children.66 Other measures of disease severity, such as the level of esophageal fibrosis, have been correlated with the extent of esophageal eosinophilic degranulation rather than eosinophil count.67 These observations are important for the clinician who is looking for objective measures to guide clinical decision-making. In addition, given the lack of association between eosinophils and symptomatology, other factors clearly contribute to this observation.
Type 2 Innate Lymphoid Cells and T Helper 2 Cells
The type 2 inflammatory cascade in EoE is thought to begin with environmental triggers such as food and/or aero antigens, resulting in epithelial release of the inflammatory alarmin molecules TSLP, IL-25, and IL-33; these in turn activate ILC2s and promote Th2 cell differentiation via effects on APCs, resulting in IL-4, IL-13, and IL-5 production (Table 1).26,39,68,69
Th2 cells are the dominant population of T cells in EoE pathology, expressing high levels of IL-13, IL-4, and IL-5 (Table 1).45 ILC2s express high levels of IL-13 and IL-5 (Table 1), are highly enriched in biopsies of patients with active EoE, and are positively correlated with esophageal eosinophil counts.26 Interestingly, the production of IL-5 and IL-13 from ILC2s is not sensitive to steroid inhibition and may provide mechanistic insight into steroid resistance in some EoE patients.23 These Th2- and ILC2-derived type 2 inflammatory cytokines drive a positive feedback loop to promote further inflammation and epithelial barrier dysfunction (Table 1).11,59
IL-4 and IL-13
IL-4 and IL-13 are key and central mediators of type 2 inflammation affecting a range of inflammatory cells and downstream mediators (Table 1).4,5,9,20,21,26,35,36,41,47,70, 71, 72, 73, 74, 75 IL-4 and IL-13 share some overlapping features because of shared receptor signaling. The type I heterodimeric IL-4 receptor is comprised of the IL-4Rα subunit paired with the common γ chain, expressed largely on hematopoietic cells. However, IL-4, as well as IL-13, can signal through the type II heterodimeric receptor, the IL-4Rα subunit paired with IL-13Rα1, expressed on nonhematopoietic cells.76 A second receptor for IL-13 is IL-13Rα2, previously thought to be a decoy receptor.77 Multiple cell types express IL-4Rα, including mast cells, eosinophils, macrophages, lymphocytes, and epithelial cells. IL-4 and 13 signaling through IL-4Rα activates signal transducer and activator of transcription 6 (STAT6), contributing to Th2 effector function and the production of type 2 cytokines IL-4, IL-5, and IL-13 through GATA3.19,32,78
Both IL-4 and IL-13 upregulate the expression of chemokines, such as eotaxin-3 and periostin, which promote migration and trafficking of inflammatory cells, including eosinophils, to the site of inflammation, contributing to additional inflammatory infiltrate, cytokine production, and tissue remodeling and fibrosis in the context of EoE (Table 1).4,20,23,79 IL-4 and IL-13 also contribute to B cell class switching to IgE,49 leading to mast cell and basophil degranulation and the resulting release of pro-inflammatory mediators.4,20,35,36,47,72, 73, 74 In addition, IL-4 directly activates mast cells leading to their enhanced proliferation and survival, increased type 2 cytokine production, and enhanced mast cell degranulation (Table 1).35,36 There may also be a role for basophil-derived IL-4 in eosinophil infiltration into tissue.79 Elevated IL-4 is observed in blood, in esophageal biopsies, and in esophageal T cells from patients with EoE,31,39,45,59 highlighting the potential role of this cytokine in EoE pathogenesis, and suggesting IL-4-targeted therapies may be promising for treatment of EoE.
IL-13 plays a critical role in tissue remodeling, fibrosis, and smooth muscle contractility in EoE, mediated largely through effects on epithelial cells, including the induced expression of proteases and matrix proteins (Table 1).4,5,9,33 IL-13 contributes to impaired epithelial architecture and barrier dysfunction by inducing calpain 14 (CAPN14), an intracellular calcium-activated protease, more than 100-fold in esophageal epithelial cells.4 CAPN14 overexpression is associated with impaired epithelial architecture and barrier dysfunction,80 and CAPN14 genetic variants are implicated in very early onset EoE.81 In fibroblasts, IL-13 induces the expression of matrix proteins, including collagen, matrix metalloproteases, and periostin.33 IL-13 may also mediate barrier function by downregulating DSG-1, filaggrin, and involucrin genes important for epithelial integrity.40 Finally, IL-13 promotes epithelial mesenchymal transition (EMT), a process in which polarized epithelial cells transition to a mesenchymal cell phenotype, via transforming growth factor beta (TGF-β), contributing to tissue fibrosis in the context of chronic inflammation.9,33 The process of EMT contributes to the subepithelial fibrosis characteristic of EoE, and in esophageal biopsies from EoE patients, EMT is correlated with the presence of eosinophils and eosinophil peroxidase, TGF-β, and fibrosis.82
In vivo data support IL-13 as a promising therapeutic target in EoE. IL-13 antibody blockade reduces esophageal eosinophilia in these models.83, 84, 85, 86 Intratracheal delivery of recombinant IL-13 induces epithelial hyperplasia in the esophagus in a manner dependent on both STAT6 and IL-5.86 In mouse models of allergen-induced EoE, mice genetically deficient in IL-13, IL-5, or STAT6 were at least partially protected from disease.83
Real-world evidence also supports a role for IL-13 in EoE pathogenesis. IL-13 messenger RNA (mRNA) levels are increased in esophageal biopsies from EoE patients compared with healthy controls,39 and IL-13 expression is significantly elevated in activated eosinophils in the esophagus and intestine of patients with EoE.43 Experimentally, IL-13 stimulation of esophageal epithelial cells in vitro induces an EoE-specific esophageal transcriptome very similar to that observed in biopsies from EoE patients, suggesting that IL-13 is a fundamental regulator of EoE.39 Both increased IL-13 levels and the EoE transcriptome are largely reversible after steroid treatment in vivo, which acts as a global, nonspecific suppressor of inflammation.39
Taken together, the evidence presented here implicates IL-13 as an important driver of epithelial barrier dysfunction and fibrosis, driving esophageal dysfunction and food impaction, respectively.
Interleukin-5
Early in vitro studies indicated a role for IL-5 as an eosinophil-specific differentiation factor.87 IL-5 is now appreciated as a critical factor in the maturation, differentiation, and survival of eosinophils.37 IL-5 signals through a heterodimeric receptor, consisting of the IL-5Rα chain and the common β chain, to activate Janus kinase-STAT as well as phosphoinositide 3-kinase (extracellular signal-regulated kinase signaling pathways).88 In humans, the IL-5Rα chain is expressed by eosinophils and basophils (Table 1).37
Mouse models support a role for IL-5 in EoE (Table 1).38,89, 90, 91 Experimentally, overexpression of IL-5 in the esophagus leads to elevated local levels of IL-13 and eotaxin-1.89 Transgenic mice overexpressing IL-5 in T cells have an eosinophilic infiltrate in the esophagus that recapitulates features of human disease such as strictures, supporting a role for IL-5 in fibrostenosis.90,91 In an allergen-induced mouse model of EoE, IL-5-mediated eosinophilia promoted tissue remodeling of the esophagus, including collagen deposition in the mucosa and lamina propria, and thickening of the basal layer.38
Therefore, IL-5 presents another attractive therapeutic target. Data on the role of IL-5 in EoE in humans come largely from clinical trials, in which monoclonal antibodies were used to target IL-5 and its receptor. These monoclonal antibodies reduced total esophageal eosinophil counts, but clinical improvement was not consistently noted. However, these were studies undertaken before the development of validated patient report outcome measures.92, 93, 94, 95 The role of IL-5 as a therapeutic target is currently being investigated (NCT03656380).
Role of Other Inflammatory Mediators on the Pathophysiologic Features of EoE
Other mediators have been implicated in the type 2 inflammatory pathways contributing to the pathogenesis of EoE, including periostin, eotaxin-3, IgE, and the alarmin TSLP.
Periostin is an extracellular matrix protein largely produced by fibroblasts and epithelial cells, which can be induced by TGF-β and IL-13 (Table 1).42 As a result of its interaction with extracellular matrix proteins, it may play a role in promoting eosinophil trafficking and adhesion.59 In a mouse model of allergen-induced esophageal eosinophilia, mice genetically deficient in periostin had increased blood eosinophils levels and decreased eosinophils in the esophagus compared with controls, directly implicating periostin in eosinophil chemotaxis.42 Although serum periostin levels are only slightly elevated in EoE patients compared with controls,96 periostin expression is increased in the esophageal mucosa of active EoE patients.97
Eotaxin-3 (CCL26) is a potent eosinophil and mast cell chemoattractant (Table 1). In vitro, IL-4 and IL-13 can induce eotaxin-3 expression via STAT6 in esophageal epithelial cells.33 Eotaxin-3 not only attracts eosinophils but also induces eosinophil activation and degranulation via MAP kinase activation.98 Eotaxin signaling is required for the development of EoE in an experimental mouse model.99 Eotaxin-3 is the most highly expressed gene in esophageal tissue in patients with EoE relative to controls, and mRNA and protein levels of eotaxin-3 correlate with the number of eosinophils and mast cells in tissue.99 Highlighting the importance of this protein, a single nucleotide polymorphism in the untranslated region of the eotaxin-3 gene is associated with susceptibility to EoE.99 Esophageal eotaxin-3 mRNA level alone has an 89% sensitivity in distinguishing patients with and without EoE, and circulating eotaxin-3 levels correlate with esophageal eotaxin-3 expression, suggesting its potential utility as a diagnostic biomarker.29,100
The high concurrence of comorbid atopic conditions in EoE suggests a role for IgE in EoE pathophysiology.101 However, experimental models indicate that EoE can develop in an IgE-independent manner, dependent instead on TSLP-elicited basophils.36 The anti-IgE antibody omalizumab had limited clinical or histologic effects in EoE patients, further supporting only a peripheral role for IgE in directly impacting disease pathogenesis.50
TSLP is a cytokine expressed by epithelial cells and keratinocytes. TSLP can induce activated dendritic cells to express the co-stimulatory molecule OX-40 ligand, a member of the tumor necrosis factor superfamily, which is involved in interactions between dendritic cells and T cells. OX-40 ligand is thought to drive the subsequent polarization of naïve Th cells toward a type 2 phenotype.102 TSLP also activates ILC2 cells, which also serve as important sources of IL-5 and IL-13 in the type 2 immune cascade (Table 1). TSLP can also induce basophils to express IL-4, particularly in the context of non-IgE type 2 inflammation.59 Experimentally, TSLP mRNA expression can be induced in primary esophageal epithelial cells in response to toll-like receptor signaling, suggesting the esophageal epithelium is an important source of TSLP in EoE.69 In a mouse model of food antigen-driven EoE, TSLP was required for the development of disease in a manner that was also dependent on basophils, suggesting a role for the TSLP-basophil axis in EoE development.36 In this model, neutralizing antibodies to TSLP were also effective in treating established EoE.36 TSLP levels are elevated in esophageal tissue from patients with EoE compared with controls,36,103,104 and TSLP is highly expressed in esophageal epithelium in areas infiltrated by basophils.105 TSLP therefore contributes to EoE pathophysiology by acting as an upstream regulator of type 2 inflammation and may also have potential as a biomarker.
Current Therapeutic Options for EoE
Current treatment options for EoE are limited and have variable rates of remission induction and long-term maintenance therapy.13, 14, 15 Although treatment recommendations for eosinophilic esophagitis continue to evolve, first-line approaches in patients with EoE include dietary therapy, proton pump inhibitors (PPIs), and swallowed topical corticosteroids.1,14,106 Endoscopic dilation of the esophagus is often performed on patients with advanced disease resulting in esophageal strictures or narrow-caliber esophagus. Dilation is used for stricturing disease, but it does not treat the underlying type 2 inflammation; therefore, repeat dilations may be required because of restricturing.107 Understanding how each treatment impacts EoE pathophysiology is key to tailoring therapy to EoE patients of different phenotypes or endotypes to increase the chance of therapeutic success.
Dietary Therapy
Three main categories of dietary therapy have been described: elemental diets, consisting of exclusive feeding with nonallergenic, amino acid–based formulas14,108; empiric diets, based on removal of the 1, 2, 4, or 6 common food antigens (cow’s milk, wheat, egg, soy, peanut/tree nuts, fish/shellfish)109, 110, 111, 112; and specific food allergy test–directed elimination diets.106 Specific food allergy testing using standard allergy tests for IgE-mediated reactions is poorly predictive of food triggers in EoE, making test-directed elimination diets the least effective dietary therapy option.106 Dietary therapy can eliminate or prevent the need for chronic medication by identifying and removing trigger foods and reducing inflammation systemically rather than locally.109 Although empiric dietary therapy options are generally effective in achieving and maintaining EoE remission, they can be challenging to maintain.
PPI Therapy
PPI therapy is effective in a subgroup of EoE patients that are clinically indistinguishable from non-PPI responsive patients,113 with a reported pooled histologic response rate of 42%.14 In responsive patients, PPI therapy is associated with reduced Th2 inflammation, gene expression,114 and endoscopic features of fibrosis.115 PPIs have been shown to inhibit Th2-induced eotaxin-3 mRNA and protein expression in esophageal epithelial cells in a mechanism dependent on STAT6,33,116 highlighting a novel role for PPIs independent of gastric acid reduction.
Topical Corticosteroid Therapy
As in many chronic inflammatory conditions, steroids are a common and effective treatment for many EoE patients.117 Swallowed topical corticosteroids induce histologic remission in approximately two-thirds of patients14 and are generally well tolerated in both short- and long-term studies.14,118,119 A meta-analysis demonstrated no clear trends in symptom reduction with topical steroids.117 However, more recent studies report an improvement in dysphagia symptoms.14,120 Topical corticosteroids may reverse remodeling due to edema or fibrosis; however, alleviation of established fibrosis may require mechanical dilation.107,121
EoE is a progressive disease, with long-term challenges that impact patients’ health-related quality of life across multiple parameters. Repeated endoscopies, including those used to establish diagnosis and monitor response to therapy, involve considerable cost.122 Although each of the currently available therapies is effective to a certain extent, or in a subgroup of patients, significant unmet needs remain, including the ability to predict who will respond to a given therapy, the role and safety of chronic maintenance therapy, and how best to approach patients who fail to respond to a given class of therapy. Importantly, many patients are unable to control their disease with currently available therapies, and significant variation in adherence to guidelines regarding treatment choice and assessment of response have been documented.123, 124, 125, 126
Novel Targeted Therapies
A variety of novel therapies that target the underlying type 2 inflammatory pathophysiology of EoE detailed previously are now in various stages of development (Tables 1 and 2).
Table 2.
Novel Targeted Therapies for EoE
| Drug | Target | MOA | Reference |
|---|---|---|---|
| Omalizumab | IgE | Lowers free IgE levels | Clayton 201450 |
| Mepolizumab | IL-5 | Stops IL-5 from binding to its receptor on the surface of eosinophils and decreases eosinophil accumulation | Straumann 201092; Assa’ad 201193; Otani 201395 |
| Reslizumab | IL-5 | Blocks IL-5 signaling on eosinophils and eosinophil accumulation | Spergel 201294; Markowitz 2018127 |
| Benralizumab | IL-5 receptor alpha | Blocks IL-5 signaling; induces eosinophil apoptosis via NK cell–mediated antibody-dependent cellular cytotoxicity | Bleecker 2016128; Kolbeck 2010129 |
| Cendakimab (RPC4046) | IL-13 | Binds to the IL-13 ligand, inhibits binding to IL-13Rα1 and IL-13Rα2 subunits | Hirano 2019130; Dellon 2021119 |
| Dupilumab | IL-4 receptor alpha | Blocks signaling of IL-4 and IL-13 that contribute to type 2 inflammation in EoE | Hirano 202014 |
| Lirentelimab (AK002) | Siglec8 | Blocks Siglec8 signaling, leading to eosinophil depletion and mast cell inhibition | Dellon 2020131 |
| Etrasimod | S1P receptor | Modulates S1P receptor signaling to block lymphocyte trafficking to sites of inflammation |
S1P, sphingosine 1 phosphate; Siglec8, sialic acid–binding Ig-like lectin 8.
Clinical trials investigating the IL-5-targeting agents mepolizumab and reslizumab, commonly prescribed for asthma, support a role for IL-5 in terms of eosinophil accumulation in the epithelium, including in pediatric patients. However, only a small group of patients achieved complete histologic remission, and clinical improvement was inconsistent. Although mepolizumab and reslizumab were generally well tolerated, small patient numbers and a lack of control groups (only 2 studies were placebo controlled) preclude any definitive conclusions.92, 93, 94, 95 A randomized, placebo-controlled, phase 2 study of mepolizumab is currently ongoing (NCT03656380).
Benralizumab is a humanized anti-IL-5 receptor alpha monoclonal antibody used for the treatment of eosinophilic asthma.128 Benralizumab acts by blocking IL-5 signaling and also by inducing eosinophil apoptosis via natural killercell–mediated antibody-dependent cellular cytotoxicity.129 A randomized, placebo-controlled phase 3 trial of benralizumab in adults and adolescents with EoE without esophageal strictures preventing passage of a standard adult endoscope is currently ongoing (NCT04543409).
Given the important role of IL-13 in mediating tissue remodeling, fibrosis, and smooth muscle contractility (as discussed in the prior section), several studies have examined the targeting of IL-13 signaling.14,119,130,132 In a placebo-controlled phase 2 trial, the anti-IL-13 antibody cendakimab (formerly RPC4046) reduced esophageal eosinophil counts and endoscopic and histologic scores at week 16 and week 52 in a long-term open-label extension analysis of adult patients with EoE without esophageal stricture preventing passage of a standard adult endoscope.119,130 Importantly, comparable responses were observed in both nonsteroid-refractory and steroid-refractory subgroups, suggesting this biologic therapy may be beneficial to this group of patients who currently have no suitable pharmacological options. However, cendakimab did not reduce dysphagia symptom severity and frequency, although the study was not powered to assess these outcomes. Furthermore, the study is potentially limited by the inability of approximately 25% of patients to complete the 52-week long-term extension study. Cendakimab was well tolerated with a consistent safety profile across the induction and long-term extension periods. Most adverse events were mild or moderate, with upper respiratory tract infection (21%) and nasopharyngitis (14%) most commonly reported through week 52.119,130 A randomized, double-blind, placebo-controlled phase 3 trial of this drug is currently ongoing (NCT04753697).
As both IL-4 and IL-13 upregulate the expression of chemokines that promote eosinophil migration to the site of inflammation, several studies have investigated the targeting of IL-4/13 signaling in patients with EoE.14,133 In a randomized, placebo-controlled phase 2 trial dupilumab, a fully human monoclonal antibody that inhibits the signaling of the shared receptor component for IL-4 and IL-13, reduced dysphagia, histologic features of disease including esophageal eosinophils, and abnormal esophageal endoscopic features compared with placebo in adults with active EoE and without esophageal stricture preventing passage of a standard endoscope.14 Dupilumab also increased esophageal distensibility, with reduced distensibility being associated with food impaction and need for dilation, and was generally well tolerated. The most common adverse events reported by patients who received dupilumab or placebo during the 12-week study period were nonserious injection-site erythema (35% and 8%, respectively) and nasopharyngitis (17% and 4%).14,133 Data from the phase 3 LIBERTY-EoE-TREET study (NCT03633617) demonstrate that patients who received dupilumab achieved clinically meaningful improvements in histologic, symptomatic, and endoscopic aspects of EoE at week 24, which were sustained to week 52, with an acceptable safety profile.134 Placebo-treated patients who switched to dupilumab experienced similar outcomes to dupilumab-treated patients. These findings were confirmed in a larger sample size of adolescents and adults with EoE.135,136 In addition, a separate randomized, placebo-controlled phase 3 study in pediatric patients with active EoE (NCT04394351) is ongoing.
Lirentelimab, (AK002), is an investigational, monoclonal antibody targeting Siglec8, a cell-surface protein expressed exclusively on eosinophils and mast cells131 that leads to eosinophil depletion and mast cell stabilization. In a recent phase 2 placebo-controlled trial of AK002 in adult patients with eosinophilic gastritis and/or eosinophilic duodenitis, a subset of patients had concurrent EoE that improved with therapy. Lirentelimab was well tolerated, although higher rates of mild-to-moderate infusion-related reactions were reported in the lirentelimab vs placebo group (60% vs 23%, respectively). Other common adverse events were flushing, feeling of warmth, and headache.131 A randomized, placebo-controlled phase 2/3 trial evaluating lirentelimab in adults and adolescents with EoE is currently underway (NCT04322708).
Etrasimod is an investigational, oral selective sphingosine 1 phosphate receptor modulator being developed for the treatment of inflammatory and immune-mediated disease and acts, in part, by blocking lymphocyte trafficking to sites of inflammation. Etrasimod is being evaluated in a randomized, placebo-controlled phase 2 trial of adults with EoE (NCT04682639).
Evidence from clinical trials interrogating pathways of type 2 inflammation suggest that histologic improvement alone, that is, reduction in esophageal-associated eosinophils, may be insufficient to address the complex underlying pathophysiology of EoE and reduce clinical symptoms.92, 93, 94, 95 This may reflect differences in eosinophil homing vs activation vs differentiation.
Conclusion
EoE is a complex disease, with multiple phenotypes and endotypes emerging that are associated with type 2 inflammation and that may reflect the natural history of EoE disease progression. Although eosinophils are a hallmark feature of the disease, their roles in disease activity and progression remain unclear. Current treatment options, while effective for some patients, leave many with incompletely controlled disease and persistent symptoms. With promising therapeutic options in the pipeline, there is a need for a greater understanding of the underlying inflammatory pathophysiology of EoE and endotypes to enable precision medicine approaches and better tailor treatment for this complex and burdensome disease.
Acknowledgments:
The authors would like to thank Linda Williams from Regeneron Pharmaceuticals Inc, and Melissa Auclair, Ledia Goga, and Lila Glotfelty from Sanofi. Research was sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. Medical writing/editorial assistance was provided by Joseph Worrall, PhD, of Excerpta Medica, and was funded by Sanofi and Regeneron Pharmaceuticals, Inc, according to the Good Publication Practice guideline.
Authors' Contributions:
Mirna Chehade, Gary W. Falk, Jason K. Lee, Vinay Mehta, John Leung, Seema Aceves, Brad Shumel, Juby A. Jacob-Nara, Yamo Deniz, Paul J. Rowe, Danen Cunoosamy, and Angela Khodzhayev contributed to the concept and design of this review, interpreted the literature, provided critical feedback on the manuscript, approved the final manuscript for submission, and were accountable for the accuracy and integrity of the article.
Footnotes
Conflicts of Interest: These authors disclose the following: M.C. is a consultant for Adare Pharma Solutions, Allakos, AstraZeneca, BMS, Ellodi Pharmaceuticals, Phathom, Regeneron Pharmaceuticals, Inc, Sanofi, Shire, Takeda; and has received research funding from Adare Pharma Solutions, Allakos, AstraZeneca, Danone, Ellodi Pharmaceuticals, Regeneron Pharmaceuticals Inc, Shire, Takeda. G.W.F. reports grant support from Adare Pharma Solutions, Allakos, Arena Pharmaceuticals, BMS, Celgene, Ellodi Pharmaceuticals, Lucid; Regeneron Pharmaceuticals, Inc, Shire, Takeda; and is a consultant for Adare Pharma Solutions, Allakos, BMS, Celgene, Ellodi Pharmaceuticals, Lucid, Regeneron Pharmaceuticals, Inc, Shire, Takeda. J.K.L. discloses speaking fees from ALK, AstraZeneca, Bausch Health, CSL, Genentech, GSK, Medexus, Miravo, Novartis, Regeneron Pharmaceuticals, Inc, Sanofi, Takeda; research funding and clinical trials from AstraZeneca, Genentech, GSK, Novartis, Regeneron Pharmaceuticals, Inc, Sanofi, Takeda. V.M. reports inclusion in speaker’s bureau for AstraZeneca, GSK, Sanofi, Regeneron; clinical research support from AstraZeneca, Ellodi Pharmaceuticals, Regeneron Pharmaceuticals, Inc, and Sanofi. J.L. is a consultant for Huron Consulting Group, Ribon Therapeutics, Takeda. S.A. is a consultant for AstraZeneca, Aimmune Therapeutics; and co-inventor of oral viscous budesonide (UCSD patent, Shire-Takeda licensed). B.S., Y.D., and A.K. are employees and shareholders of Regeneron Pharmaceuticals, Inc. J.A.J.-N., P.J.R., and D.C. are employees of Sanofi and may hold stock and/or stock options in the company.
Funding: This work was funded by Sanofi and Regeneron Pharmaceuticals, Inc.
Ethical Statement: The study did not require the approval of an institutional review board.
Writing Assistance: Medical writing/editorial assistance was provided by Joseph Worrall, PhD, of Excerpta Medica, and was funded by Sanofi and Regeneron Pharmaceuticals, Inc, according to the Good Publication Practice guideline.
References
- 1.Dellon E.S., Liacouras C.A., Molina-Infante J., et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: proceedings of the AGREE conference. Gastroenterology. 2018;155:1022–1033.e10. doi: 10.1053/j.gastro.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greuter T., Hirano I., Dellon E.S. Emerging therapies for eosinophilic esophagitis. J Allergy Clin Immunol. 2020;145:38–45. doi: 10.1016/j.jaci.2019.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonsalves N.P., Aceves S.S. Diagnosis and treatment of eosinophilic esophagitis. J Allergy Clin Immunol. 2020;145:1–7. doi: 10.1016/j.jaci.2019.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis B.P., Rothenberg M.E. Mechanisms of disease of eosinophilic esophagitis. Annu Rev Pathol. 2016;11:365–393. doi: 10.1146/annurev-pathol-012615-044241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aceves S.S., Chen D., Newbury R.O., et al. Mast cells infiltrate the esophageal smooth muscle in patients with eosinophilic esophagitis, express TGF-β1, and increase esophageal smooth muscle contraction. J Allergy Clin Immunol. 2010;126:1198–1204.e4. doi: 10.1016/j.jaci.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 6.Croese J., Fairley S.K., Masson J.W., et al. Clinical and endoscopic features of eosinophilic esophagitis in adults. Gastrointest Endosc. 2003;58:516–522. doi: 10.1067/s0016-5107(03)01870-4. [DOI] [PubMed] [Google Scholar]
- 7.Kapel R.C., Miller J.K., Torres C., et al. Eosinophilic esophagitis: a prevalent disease in the United States that affects all age groups. Gastroenterology. 2008;134:1316–1321. doi: 10.1053/j.gastro.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 8.Kim H.P., Vance R.B., Shaheen N.J., et al. The prevalence and diagnostic utility of endoscopic features of eosinophilic esophagitis: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:988–996.e5. doi: 10.1016/j.cgh.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muir A.B., Wang J.X., Nakagawa H. Epithelial-stromal crosstalk and fibrosis in eosinophilic esophagitis. J Gastroenterol. 2019;54:10–18. doi: 10.1007/s00535-018-1498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sgouros S.N., Bergele C., Mantides A. Eosinophilic esophagitis in adults: a systematic review. Eur J Gastroenterol Hepatol. 2006;18:211–217. doi: 10.1097/00042737-200602000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Sherrill J.D., Kc K., Wu D., et al. Desmoglein-1 regulates esophageal epithelial barrier function and immune responses in eosinophilic esophagitis. Mucosal Immunol. 2014;7:718–729. doi: 10.1038/mi.2013.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Visaggi P., Savarino E., Sciume G., et al. Eosinophilic esophagitis: clinical, endoscopic, histologic and therapeutic differences and similarities between children and adults. Therap Adv Gastroenterol. 2021;14 doi: 10.1177/1756284820980860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeBrosse C.W., Franciosi J.P., King E.C., et al. Long-term outcomes in pediatric-onset esophageal eosinophilia. J Allergy Clin Immunol. 2011;128:132–138. doi: 10.1016/j.jaci.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirano I., Dellon E.S., Hamilton J.D., et al. Efficacy of dupilumab in a phase 2 randomized trial of adults with active eosinophilic esophagitis. Gastroenterology. 2020;158:111–122.e10. doi: 10.1053/j.gastro.2019.09.042. [DOI] [PubMed] [Google Scholar]
- 15.Laserna-Mendieta E.J., Casabona S., Savarino E., et al. Efficacy of therapy for eosinophilic esophagitis in real-world practice. Clin Gastroenterol Hepatol. 2020;18:2903–2911.e4. doi: 10.1016/j.cgh.2020.01.024. [DOI] [PubMed] [Google Scholar]
- 16.Manresa M.C., Chiang A.W.T., Kurten R.C., et al. Increased production of LIGHT by T cells in eosinophilic esophagitis promotes differentiation of esophageal fibroblasts toward an inflammatory phenotype. Gastroenterology. 2020;159:1778–1792.e13. doi: 10.1053/j.gastro.2020.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruffner M.A., Hu A., Dilollo J., et al. Conserved IFN signature between adult and pediatric eosinophilic esophagitis. J Immunol. 2021;206:1361–1371. doi: 10.4049/jimmunol.2000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosmann T.R., Cherwinski H., Bond M.W., et al. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 19.Gandhi N.A., Pirozzi G., Graham N.M.H. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev Clin Immunol. 2017;13:425–437. doi: 10.1080/1744666X.2017.1298443. [DOI] [PubMed] [Google Scholar]
- 20.Stone K.D., Prussin C., Metcalfe D.D. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S73–S80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wechsler J.B., Bryce P.J. Allergic mechanisms in eosinophilic esophagitis. Gastroenterol Clin North Am. 2014;43:281–296. doi: 10.1016/j.gtc.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smithgall M.D., Comeau M.R., Yoon B.R., et al. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol. 2008;20:1019–1030. doi: 10.1093/intimm/dxn060. [DOI] [PubMed] [Google Scholar]
- 23.Lianto P., Zhang Y., Che H. Signals from the various immune cells in promoting food allergy-induced eosinophilic esophagitis like disease. Asia Pac Allergy. 2019;9:e28. doi: 10.5415/apallergy.2019.9.e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Annunziato F., Romagnani C., Romagnani S. The 3 major types of innate and adaptive cell-mediated effector immunity. J Allergy Clin Immunol. 2015;135:626–635. doi: 10.1016/j.jaci.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Butcher M.J., Zhu J. Recent advances in understanding the Th1/Th2 effector choice. Fac Rev. 2021;10:30. doi: 10.12703/r/10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doherty T.A., Baum R., Newbury R.O., et al. Group 2 innate lymphocytes (ILC2) are enriched in active eosinophilic esophagitis. J Allergy Clin Immunol. 2015;136:792–794.e3. doi: 10.1016/j.jaci.2015.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gieseck R.L., 3rd, Wilson M.S., Wynn T.A. Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol. 2018;18:62–76. doi: 10.1038/nri.2017.90. [DOI] [PubMed] [Google Scholar]
- 28.Zhu X., Zhu J. CD4 T helper cell subsets and related human immunological disorders. Int J Mol Sci. 2020;21:8011. doi: 10.3390/ijms21218011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blanchard C., Stucke E.M., Rodriguez-Jimenez B., et al. A striking local esophageal cytokine expression profile in eosinophilic esophagitis. J Allergy Clin Immunol. 2011;127:208–217.e7. doi: 10.1016/j.jaci.2010.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cianferoni A., Ruffner M.A., Guzek R., et al. Elevated expression of activated TH2 cells and milk-specific TH2 cells in milk-induced eosinophilic esophagitis. Ann Allergy Asthma Immunol. 2018;120:177–183.e2. doi: 10.1016/j.anai.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunn J.L.M., Shoda T., Caldwell J.M., et al. Esophageal type 2 cytokine expression heterogeneity in eosinophilic esophagitis in a multisite cohort. J Allergy Clin Immunol. 2020;145:1629–1640.e4. doi: 10.1016/j.jaci.2020.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swain S.L., Weinberg A.D., English M., et al. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990;145:3796–3806. [PubMed] [Google Scholar]
- 33.Cheng E., Zhang X., Huo X., et al. Omeprazole blocks eotaxin-3 expression by oesophageal squamous cells from patients with eosinophilic oesophagitis and GORD. Gut. 2013;62:824–832. doi: 10.1136/gutjnl-2012-302250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore P.E., Church T.L., Chism D.D., et al. IL-13 and IL-4 cause eotaxin release in human airway smooth muscle cells: a role for ERK. Am J Physiol Lung Cell Mol Physiol. 2002;282:L847–L853. doi: 10.1152/ajplung.00245.2001. [DOI] [PubMed] [Google Scholar]
- 35.McLeod J.J., Baker B., Ryan J.J. Mast cell production and response to IL-4 and IL-13. Cytokine. 2015;75:57–61. doi: 10.1016/j.cyto.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noti M., Wojno E.D., Kim B.S., et al. Thymic stromal lymphopoietin-elicited basophil responses promote eosinophilic esophagitis. Nat Med. 2013;19:1005–1013. doi: 10.1038/nm.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kouro T., Takatsu K. IL-5- and eosinophil-mediated inflammation: from discovery to therapy. Int Immunol. 2009;21:1303–1309. doi: 10.1093/intimm/dxp102. [DOI] [PubMed] [Google Scholar]
- 38.Mishra A., Wang M., Pemmaraju V.R., et al. Esophageal remodeling develops as a consequence of tissue specific IL-5-induced eosinophilia. Gastroenterology. 2008;134:204–214. doi: 10.1053/j.gastro.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blanchard C., Mingler M.K., Vicario M., et al. IL-13 involvement in eosinophilic esophagitis: transcriptome analysis and reversibility with glucocorticoids. J Allergy Clin Immunol. 2007;120:1292–1300. doi: 10.1016/j.jaci.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 40.Ryu S., Lee K.H., Tizaoui K., et al. Pathogenesis of eosinophilic esophagitis: a comprehensive review of the genetic and molecular aspects. Int J Mol Sci. 2020;21:7253. doi: 10.3390/ijms21197253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Camelo A., Rosignoli G., Ohne Y., et al. IL-33, IL-25, and TSLP induce a distinct phenotypic and activation profile in human type 2 innate lymphoid cells. Blood Adv. 2017;1:577–589. doi: 10.1182/bloodadvances.2016002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blanchard C., Mingler M.K., McBride M., et al. Periostin facilitates eosinophil tissue infiltration in allergic lung and esophageal responses. Mucosal Immunol. 2008;1:289–296. doi: 10.1038/mi.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Straumann A., Kristl J., Conus S., et al. Cytokine expression in healthy and inflamed mucosa: probing the role of eosinophils in the digestive tract. Inflamm Bowel Dis. 2005;11:720–726. doi: 10.1097/01.mib.0000172557.39767.53. [DOI] [PubMed] [Google Scholar]
- 44.Doyle A.D., Masuda M.Y., Kita H., et al. Eosinophils in eosinophilic esophagitis: the road to fibrostenosis is paved with good intentions. Front Immunol. 2020;11:603295. doi: 10.3389/fimmu.2020.603295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wen T., Aronow B.J., Rochman Y., et al. Single cell RNA sequencing identifies inflammatory tissue T cells in eosinophilic esophagitis. J Clin Invest. 2019;129:2014–2028. doi: 10.1172/JCI125917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Straumann A., Bauer M., Fischer B., et al. Idiopathic eosinophilic esophagitis is associated with a T(H)2-type allergic inflammatory response. J Allergy Clin Immunol. 2001;108:954–961. doi: 10.1067/mai.2001.119917. [DOI] [PubMed] [Google Scholar]
- 47.Abonia J.P., Blanchard C., Butz B.B., et al. Involvement of mast cells in eosinophilic esophagitis. J Allergy Clin Immunol. 2010;126:140–149. doi: 10.1016/j.jaci.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Straumann A., Conus S., Degen L., et al. Long-term budesonide maintenance treatment is partially effective for patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2011;9:400–409.e1. doi: 10.1016/j.cgh.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 49.Vicario M., Blanchard C., Stringer K.F., et al. Local B cells and IgE production in the oesophageal mucosa in eosinophilic oesophagitis. Gut. 2010;59:12–20. doi: 10.1136/gut.2009.178020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clayton F., Fang J.C., Gleich G.J., et al. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology. 2014;147:602–609. doi: 10.1053/j.gastro.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 51.Kottyan L.C., Parameswaran S., Weirauch M.T., et al. The genetic etiology of eosinophilic esophagitis. J Allergy Clin Immunol. 2020;145:9–15. doi: 10.1016/j.jaci.2019.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shoda T., Wen T., Aceves S.S., et al. Eosinophilic oesophagitis endotype classification by molecular, clinical, and histopathological analyses: a cross-sectional study. Lancet Gastroenterol Hepatol. 2018;3:477–488. doi: 10.1016/S2468-1253(18)30096-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gonsalves N. Distinct features in the clinical presentations of eosinophilic esophagitis in children and adults: is this the same disease? Dig Dis. 2014;32:89–92. doi: 10.1159/000357078. [DOI] [PubMed] [Google Scholar]
- 54.Straumann A., Aceves S.S., Blanchard C., et al. Pediatric and adult eosinophilic esophagitis: similarities and differences. Allergy. 2012;67:477–490. doi: 10.1111/j.1398-9995.2012.02787.x. [DOI] [PubMed] [Google Scholar]
- 55.Dellon E.S., Kim H.P., Sperry S.L., et al. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc. 2014;79:577–585.e4. doi: 10.1016/j.gie.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schoepfer A.M., Safroneeva E., Bussmann C., et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013;145:1230–1236.e2. doi: 10.1053/j.gastro.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 57.Strasser D.S., Seger S., Bussmann C., et al. Eosinophilic oesophagitis: relevance of mast cell infiltration. Histopathology. 2018;73:454–463. doi: 10.1111/his.13653. [DOI] [PubMed] [Google Scholar]
- 58.Bolton S.M., Kagalwalla A.F., Arva N.C., et al. Mast cell infiltration is associated with persistent symptoms and endoscopic abnormalities despite resolution of eosinophilia in pediatric eosinophilic esophagitis. Am J Gastroenterol. 2020;115:224–233. doi: 10.14309/ajg.0000000000000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hill D.A., Spergel J.M. The immunologic mechanisms of eosinophilic esophagitis. Curr Allergy Asthma Rep. 2016;16:9. doi: 10.1007/s11882-015-0592-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mavi P., Rajavelu P., Rayapudi M., et al. Esophageal functional impairments in experimental eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1347–G1355. doi: 10.1152/ajpgi.00013.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cho J.Y., Rosenthal P., Miller M., et al. Targeting AMCase reduces esophageal eosinophilic inflammation and remodeling in a mouse model of egg induced eosinophilic esophagitis. Int Immunopharmacol. 2014;18:35–42. doi: 10.1016/j.intimp.2013.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rubinstein E., Cho J.Y., Rosenthal P., et al. Siglec-F inhibition reduces esophageal eosinophilia and angiogenesis in a mouse model of eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2011;53:409–416. doi: 10.1097/MPG.0b013e3182182ff8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Niranjan R., Mavi P., Rayapudi M., et al. Pathogenic role of mast cells in experimental eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol. 2013;304:G1087–G1094. doi: 10.1152/ajpgi.00070.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Safroneeva E., Straumann A., Coslovsky M., et al. Symptoms have modest accuracy in detecting endoscopic and histologic remission in adults with eosinophilic esophagitis. Gastroenterology. 2016;150:581–590.e4. doi: 10.1053/j.gastro.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pentiuk S., Putnam P.E., Collins M.H., et al. Dissociation between symptoms and histological severity in pediatric eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2009;48:152–160. doi: 10.1097/MPG.0b013e31817f0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aceves S.S., King E., Collins M.H., et al. Alignment of parent- and child-reported outcomes and histology in eosinophilic esophagitis across multiple CEGIR sites. J Allergy Clin Immunol. 2018;142:130–138.e1. doi: 10.1016/j.jaci.2018.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chehade M., Sampson H.A., Morotti R.A., et al. Esophageal subepithelial fibrosis in children with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2007;45:319–328. doi: 10.1097/MPG.0b013e31806ab384. [DOI] [PubMed] [Google Scholar]
- 68.Mjösberg J.M., Trifari S., Crellin N.K., et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 69.Sherrill J.D., Gao P.S., Stucke E.M., et al. Variants of thymic stromal lymphopoietin and its receptor associate with eosinophilic esophagitis. J Allergy Clin Immunol. 2010;126:160–165.e3. doi: 10.1016/j.jaci.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gause W.C., Wynn T.A., Allen J.E. Type 2 immunity and wound healing: evolutionary refinement of adaptive immunity by helminths. Nat Rev Immunol. 2013;13:607–614. doi: 10.1038/nri3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miller D.E., Forney C., Rochman M., et al. Genetic, inflammatory, and epithelial cell differentiation factors control expression of human calpain-14. G3 (Bethesda) 2019;9:729–736. doi: 10.1534/g3.118.200901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ozdemir C., Akdis M., Akdis C.A. T-cell response to allergens. Chem Immunol Allergy. 2010;95:22–44. doi: 10.1159/000315936. [DOI] [PubMed] [Google Scholar]
- 73.Cocks B.G., de Waal Malefyt R., Galizzi J.P., et al. IL-13 induces proliferation and differentiation of human B cells activated by the CD40 ligand. Int Immunol. 1993;5:657–663. doi: 10.1093/intimm/5.6.657. [DOI] [PubMed] [Google Scholar]
- 74.Nilsson G., Nilsson K. Effects of interleukin (IL)-13 on immediate-early response gene expression, phenotype and differentiation of human mast cells. Comparison with IL-4. Eur J Immunol. 1995;25:870–873. doi: 10.1002/eji.1830250337. [DOI] [PubMed] [Google Scholar]
- 75.Roufousse F. Targeting the interleukin-5 pathway for treatment of eosinophilic conditions other than asthma. Front Med (Lausanne) 2018;5:49. doi: 10.3389/fmed.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.LaPorte S.L., Juo Z.S., Vaclavikova J., et al. Molecular and structural basis of cytokine receptor pleiotropy in the interleukin-4/13 system. Cell. 2008;132:259–272. doi: 10.1016/j.cell.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arima K., Sato K., Tanaka G., et al. Characterization of the interaction between interleukin-13 and interleukin-13 receptors. J Biol Chem. 2005;280:24915–24922. doi: 10.1074/jbc.M502571200. [DOI] [PubMed] [Google Scholar]
- 78.Zhu J., Guo L., Watson C.J., et al. Stat6 is necessary and sufficient for IL-4’s role in Th2 differentiation and cell expansion. J Immunol. 2001;166:7276–7281. doi: 10.4049/jimmunol.166.12.7276. [DOI] [PubMed] [Google Scholar]
- 79.Miyake K., Karasuyama H. Emerging roles of basophils in allergic inflammation. Allergol Int. 2017;66:382–391. doi: 10.1016/j.alit.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 80.Davis B.P., Stucke E.M., Khorki M.E., et al. Eosinophilic esophagitis-linked calpain 14 is an IL-13-induced protease that mediates esophageal epithelial barrier impairment. JCI Insight. 2016;1:e86355. doi: 10.1172/jci.insight.86355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lyles J.L., Martin L.J., Shoda T., et al. Very early onset eosinophilic esophagitis is common, responds to standard therapy, and demonstrates enrichment for CAPN14 genetic variants. J Allergy Clin Immunol. 2021;147:244–254.e6. doi: 10.1016/j.jaci.2020.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kagalwalla A.F., Akhtar N., Woodruff S.A., et al. Eosinophilic esophagitis: epithelial mesenchymal transition contributes to esophageal remodeling and reverses with treatment. J Allergy Clin Immunol. 2012;129:1387–1396.e7. doi: 10.1016/j.jaci.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Akei H.S., Mishra A., Blanchard C., et al. Epicutaneous antigen exposure primes for experimental eosinophilic esophagitis in mice. Gastroenterology. 2005;129:985–994. doi: 10.1053/j.gastro.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 84.Akei H.S., Brandt E.B., Mishra A., et al. Epicutaneous aeroallergen exposure induces systemic TH2 immunity that predisposes to allergic nasal responses. J Allergy Clin Immunol. 2006;118:62–69. doi: 10.1016/j.jaci.2006.04.046. [DOI] [PubMed] [Google Scholar]
- 85.Blanchard C., Mishra A., Saito-Akei H., et al. Inhibition of human interleukin-13-induced respiratory and oesophageal inflammation by anti-human-interleukin-13 antibody (CAT-354) Clin Exp Allergy. 2005;35:1096–1103. doi: 10.1111/j.1365-2222.2005.02299.x. [DOI] [PubMed] [Google Scholar]
- 86.Mishra A., Rothenberg M.E. Intratracheal IL-13 induces eosinophilic esophagitis by an IL-5, eotaxin-1, and STAT6-dependent mechanism. Gastroenterology. 2003;125:1419–1427. doi: 10.1016/j.gastro.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 87.Yamaguchi Y., Suda T., Suda J., et al. Purified interleukin 5 supports the terminal differentiation and proliferation of murine eosinophilic precursors. J Exp Med. 1988;167:43–56. doi: 10.1084/jem.167.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dougan M., Dranoff G., Dougan S.K. GM-CSF, IL-3, and IL-5 family of cytokines: regulators of inflammation. Immunity. 2019;50:796–811. doi: 10.1016/j.immuni.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 89.Masterson J.C., McNamee E.N., Hosford L., et al. Local hypersensitivity reaction in transgenic mice with squamous epithelial IL-5 overexpression provides a novel model of eosinophilic oesophagitis. Gut. 2014;63:43–53. doi: 10.1136/gutjnl-2012-303631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mishra A., Hogan S.P., Brandt E.B., et al. IL-5 promotes eosinophil trafficking to the esophagus. J Immunol. 2002;168:2464–2469. doi: 10.4049/jimmunol.168.5.2464. [DOI] [PubMed] [Google Scholar]
- 91.Mishra A. Significance of mouse models in dissecting the mechanism of human eosinophilic gastrointestinal diseases (EGID) J Gastroenterol Hepatol Res. 2013;2:845–853. doi: 10.6051/j.issn2224-3992.2013.02.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Straumann A., Conus S., Grzonka P., et al. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomised, placebo-controlled, double-blind trial. Gut. 2010;59:21–30. doi: 10.1136/gut.2009.178558. [DOI] [PubMed] [Google Scholar]
- 93.Assa’ad A.H., Gupta S.K., Collins M.H., et al. An antibody against IL-5 reduces numbers of oesophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology. 2011;141:1593–1604. doi: 10.1053/j.gastro.2011.07.044. [DOI] [PubMed] [Google Scholar]
- 94.Spergel J.M., Rothenberg M.E., Collins M.H., et al. Reslizumab in children and adolescents with eosinophilic esophagitis: results of a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2012;129:456–463.e3. doi: 10.1016/j.jaci.2011.11.044. [DOI] [PubMed] [Google Scholar]
- 95.Otani I.M., Anilkumar A.A., Newbury R.O., et al. Anti-IL-5 therapy reduces mast cell and IL-9 cell numbers in pediatric patients with eosinophilic esophagitis. J Allergy Clin Immunol. 2013;131:1576–1582. doi: 10.1016/j.jaci.2013.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dellon E.S., Higgins L.L., Beitia R., et al. Prospective assessment of serum periostin as a biomarker for diagnosis and monitoring of eosinophilic oesophagitis. Aliment Pharmacol Ther. 2016;44:189–197. doi: 10.1111/apt.13672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Politi E., Angelakopoulou A., Grapsa D., et al. Filaggrin and periostin expression is altered in eosinophilic esophagitis and normalized with treatment. J Pediatr Gastroenterol Nutr. 2017;65:47–52. doi: 10.1097/MPG.0000000000001419. [DOI] [PubMed] [Google Scholar]
- 98.Kampen G.T., Stafford S., Adachi T., et al. Eotaxin induces degranulation and chemotaxis of eosinophils through the activation of ERK2 and p38 mitogen-activated protein kinases. Blood. 2000;95:1911–1917. [PubMed] [Google Scholar]
- 99.Blanchard C., Wang N., Stringer K.F., et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest. 2006;116:536–547. doi: 10.1172/JCI26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shoda T., Wen T., Caldwell J.M., et al. Molecular, endoscopic, histologic, and circulating biomarker-based diagnosis of eosinophilic gastritis: multi-site study. J Allergy Clin Immunol. 2020;145:255–269. doi: 10.1016/j.jaci.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Simon D., Marti H., Heer P., et al. Eosinophilic esophagitis is frequently associated with IgE-mediated allergic airway diseases. J Allergy Clin Immunol. 2005;115:1090–1092. doi: 10.1016/j.jaci.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 102.Ito T., Wang Y.H., Duramad O., et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rothenberg M.E., Spergel J.M., Sherrill J.D., et al. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat Genet. 2010;42:289–291. doi: 10.1038/ng.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Simon D., Radonjic-Hösli S., Straumann A., et al. Active eosinophilic esophagitis is characterized by epithelial barrier defects and eosinophil extracellular trap formation. Allergy. 2015;70:443–452. doi: 10.1111/all.12570. [DOI] [PubMed] [Google Scholar]
- 105.Iwakura N., Fujiwara Y., Tanaka F., et al. Basophil infiltration in eosinophilic oesophagitis and proton pump inhibitor-responsive oesophageal eosinophilia. Aliment Pharmacol Ther. 2015;41:776–784. doi: 10.1111/apt.13141. [DOI] [PubMed] [Google Scholar]
- 106.Lucendo A.J., Molina-Infante J., Arias A., et al. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United Eur Gastroenterol J. 2017;5:335–358. doi: 10.1177/2050640616689525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Arias Á., Lucendo A.J. Molecular basis and cellular mechanisms of eosinophilic esophagitis for the clinical practice. Expert Rev Gastroenterol Hepatol. 2019;13:99–117. doi: 10.1080/17474124.2019.1546120. [DOI] [PubMed] [Google Scholar]
- 108.Arias A., González-Cervera J., Tenias J.M., et al. Efficacy of dietary interventions for inducing histologic remission in patients with eosinophilic esophagitis: a systematic review and meta-analysis. Gastroenterology. 2014;146:1639–1648. doi: 10.1053/j.gastro.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 109.Chehade M., Brown S. Elimination diets for eosinophilic esophagitis: making the best choice. Expert Rev Clin Immunol. 2020;16:679–687. doi: 10.1080/1744666X.2020.1801419. [DOI] [PubMed] [Google Scholar]
- 110.De Vlieger L., Smolders L., Nuyttens L., et al. A clinical perspective on the dietary therapies for pediatric eosinophilic esophagitis: the gap between research and daily practice. Front Immunol. 2021;12:677859. doi: 10.3389/fimmu.2021.677859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Molina-Infante J., Arias Á., Alcedo J., et al. Step-up empiric elimination diet for pediatric and adult eosinophilic esophagitis: the 2-4-6 study. J Allergy Clin Immunol. 2018;141:1365–1372. doi: 10.1016/j.jaci.2017.08.038. [DOI] [PubMed] [Google Scholar]
- 112.Wechsler J.B., Schwartz S., Arva N.C., et al. A single food milk elimination diet is effective for treatment of eosinophilic esophagitis in children. Clin Gastroenterol Hepatol. 2021 doi: 10.1016/j.cgh.2021.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lucendo A.J., Arias Á., Molina-Infante J. Efficacy of proton pump inhibitor drugs for inducing clinical and histologic remission in patients with symptomatic esophageal eosinophilia: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2016;14:13–22.e1. doi: 10.1016/j.cgh.2015.07.041. [DOI] [PubMed] [Google Scholar]
- 114.Cavalli E., Brusaferro A., Pieri E.S., et al. Eosinophilic esophagitis in children: doubts and future perspectives. J Transl Med. 2019;17:262. doi: 10.1186/s12967-019-2014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Navarro P., Laserna-Mendieta E.J., Guagnozzi D., et al. Proton pump inhibitor therapy reverses endoscopic features of fibrosis in eosinophilic esophagitis. Dig Liver Dis. 2021;53:1479–1485. doi: 10.1016/j.dld.2021.05.025. [DOI] [PubMed] [Google Scholar]
- 116.Zhang X., Cheng E., Huo X., et al. Omeprazole blocks STAT6 binding to the eotaxin-3 promoter in eosinophilic esophagitis cells. PLoS One. 2012;7:e50037. doi: 10.1371/journal.pone.0050037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chuang M.Y., Chinnaratha M.A., Hancock D.G., et al. Topical steroid therapy for the treatment of eosinophilic esophagitis (EoE): a systematic review and meta-analysis. Clin Transl Gastroenterol. 2015;6:e82. doi: 10.1038/ctg.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Andreae D.A., Hanna M.G., Magid M.S., et al. Swallowed fluticasone propionate is an effective long-term maintenance therapy for children with eosinophilic esophagitis. Am J Gastroenterol. 2016;111:1187–1197. doi: 10.1038/ajg.2016.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dellon E.S., Collins M.H., Rothenberg M.E., et al. Long-term efficacy and tolerability of RPC4046 in an open-label extension trial of patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2021;19:473–483.e17. doi: 10.1016/j.cgh.2020.03.036. [DOI] [PubMed] [Google Scholar]
- 120.Dellon E.S., Collins M.H., Katzka D.A., et al. Long-term treatment of eosinophilic esophagitis with budesonide oral suspension. Clin Gastroenterol Hepatol. 2021 doi: 10.1016/j.cgh.2021.06.020. [DOI] [PubMed] [Google Scholar]
- 121.Lieberman J.A., Morotti R.A., Konstantinou G.N., et al. Dietary therapy can reverse esophageal subepithelial fibrosis in patients with eosinophilic esophagitis: a historical cohort. Allergy. 2012;67:1299–1307. doi: 10.1111/j.1398-9995.2012.02881.x. [DOI] [PubMed] [Google Scholar]
- 122.Watts A., Alexander J.A., Gupta S.K. Eosinophilic esophagitis: search for noninvasive techniques for long-term monitoring. Gastrointest Endosc. 2016;83:307–308. doi: 10.1016/j.gie.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Peery A.F., Shaheen N.J., Dellon E.S. Practice patterns for the evaluation and treatment of eosinophilic oesophagitis. Aliment Pharmacol Ther. 2010;32:1373–1382. doi: 10.1111/j.1365-2036.2010.04476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Spergel J.M., Book W.M., Mays E., et al. Variation in prevalence, diagnostic criteria, and initial management options for eosinophilic gastrointestinal diseases in the United States. J Pediatr Gastroenterol Nutr. 2011;52:300–306. doi: 10.1097/MPG.0b013e3181eb5a9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Huang K.Z., Jensen E.T., Chen H.X., et al. Practice pattern variation in pediatric eosinophilic esophagitis in the Carolinas EoE Collaborative: a research model in Community and Academic Practices. South Med J. 2018;111:328–332. doi: 10.14423/SMJ.0000000000000817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vermeulen B.D., Bogte A., Verhagen M.A., et al. Management of eosinophilic esophagitis in daily clinical practice. Dis Esophagus. 2018;31 doi: 10.1093/dote/dox119. [DOI] [PubMed] [Google Scholar]
- 127.Markowitz J.E., Jobe L., Miller M., et al. Safety and efficacy of reslizumab for children and adolescents with eosinophilic esophagitis treated for 9 years. J Pediatr Gastroenterol Nutr. 2018;66:893–897. doi: 10.1097/MPG.0000000000001840. [DOI] [PubMed] [Google Scholar]
- 128.Bleecker E.R., FitzGerald J.M., Chanez P., et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388:2115–2127. doi: 10.1016/S0140-6736(16)31324-1. [DOI] [PubMed] [Google Scholar]
- 129.Kolbeck R., Kozhich A., Koike M., et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125:1344–1353.e2. doi: 10.1016/j.jaci.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 130.Hirano I., Collins M.H., Assouline-Dayan Y., et al. RPC4046, a monoclonal antibody against IL13, reduces histologic and endoscopic activity in patients with eosinophilic esophagitis. Gastroenterology. 2019;156:592–603.e10. doi: 10.1053/j.gastro.2018.10.051. [DOI] [PubMed] [Google Scholar]
- 131.Dellon E.S., Peterson K.A., Murray J.A., et al. Anti-Siglec-8 antibody for eosinophilic gastritis and duodenitis. N Engl J Med. 2020;383:1624–1634. doi: 10.1056/NEJMoa2012047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rothenberg M.E., Wen T., Greenberg A., et al. Intravenous anti-IL-13 mAb QAX576 for the treatment of eosinophilic esophagitis. J Allergy Clin Immunol. 2015;135:500–507. doi: 10.1016/j.jaci.2014.07.049. [DOI] [PubMed] [Google Scholar]
- 133.Nicodème F., Hirano I., Chen J., et al. Esophageal distensibility as a measure of disease severity in patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2013;11:1101–1107.e1. doi: 10.1016/j.cgh.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dellon E.S., Rothernberg M.E., Collins M.H., et al. Dupilumab efficacy and safety up to 52 weeks in adult and adolescent patients with eosinophilic esophagitis: results from part a and c of a randomized, placebo-controlled, three-part, phase 3 liberty eoe treet study. United Eur Gastroenterol J. 2021;9:1204–1207. [Google Scholar]
- 135.Rothenberg M, Dellon E, Bredenoord A, et al. Dupilumab improves clinical and histologic aspects of disease in adult and adolescent patients with eosinophilic esophagitis at week 24: results from part B of the 3-part LIBERTY EoE TREET study. Presented at: AAAAI 2022 Annual Meeting; February 25-28, 2022; Phoenix, Arizona. Abstract L02.
- 136.Hirano I, Dellon E, Collins M, et al. Dupilumab reduces biomarkers of type 2 inflammation in adult and adolescent patients with eosinophilic esophagitis: results from parts A and C of a three-part, phase 3 LIBERTY EoE TREET study. Presented at: AAAAI 2022 Annual Meeting; February 25-28, 2022; Phoenix, Arizona. Abstract 633.