Abstract
Background and Aims
Cachexia is a metabolic syndrome defined by a loss of more than 5% of body weight in patients with chronic diseases. The goal of this study was to investigate the link between cirrhotic cachexia and hospital mortality and the 30-day risk of all-cause readmission.
Methods
The study utilized Nationwide Readmission Database for the years 2016–2019 in which all patients older than 18 year old with a primary diagnosis of cirrhosis were included. We excluded patients with a concurrent diagnosis of Human Immunodeficiency Virus, chronic lung disease, end-stage renal disease, malignancy, heart failure, and certain neurological diseases. We compared baseline characteristics and outcomes between those who were cachectic and those who were not. Survey multivariate logistic regression was used to analyze the independent impact of cachexia on categorical outcomes.
Results
The study cohort was 342,030 cases. Cachexia was identified in approximately 17% of the study population (58,509 discharges). The mean age was 56 years. Slightly more female patients noted in cachexia group (41% vs 38%). Inpatient mortality during index hospitalization were higher in patients with cirrhotic cachexia (6.7% vs 3%, P < .01). Inpatient mortality during first all-cause readmission within 30 days of index discharge was also higher in cachexia group (8.6% vs 6.5%, P < .01).
Conclusion
Cachexia is an adverse prognosticator for inpatient outcomes in patients with cirrhosis. It is associated with greater readmission rates, inpatient mortality, and prolonged hospital admissions.
Keywords: Cachexia, Inpatient Mortality, Cirrhosis
Introduction
Cachexia is a metabolic syndrome defined by a loss of more than 5% of body weight in patients with chronic diseases, such as cancer, chronic kidney disease, heart failure,1 and chronic obstructive pulmonary disease.2, 3, 4, 5 It is characterized by muscle mass loss with or without loss of fat mass, as well as reduced food intake, inflammation, insulin resistance, and increased muscle protein breakdown.3 Cachexia is more prevalent in patients with cirrhosis and has been linked to increased morbidity and mortality.6, 7, 8, 9 Although cirrhosis-associated cachexia has been investigated previously, larger national data are needed. This article aims to study the relationship between cachexia and inpatient outcomes in patients with cirrhosis at a national level in the United States. Understanding this relationship could help clinicians identify high-risk patients.
Methods
Data Source
The Agency for Health-care Research and Quality initiated a project to evaluate health-care cost and utilization. The project contains the largest longitudinal hospital care data collection in the United States. All discharge information in each state is collected by state authorities. From this data, the State Inpatient Database is a database created with up to 49 states contributing to the health-care cost and utilization project. Nationwide Readmission Database (NRD) is produced by State Inpatient Database to help evaluate inpatient outcomes and readmission across all hospitals within the States. It contains weighting variables to adjust for the complex design of the study. Also, it includes a verified patient linkage variable (NRD_visitLink) that can be used to track patient admissions every year in any state. Strict privacy rules are applied during this process with the race and ethnicity of patients removed. Data available includes diagnosis and procedure reported using the International Classification of Diseases 10th edition (ICD-10) and outcomes such as inpatient mortality and length of hospitalization. The database is deidentified and publicly available. Hence, the study was exempted by the institutional review board.
Study Design and Inclusion Criteria
For this study, we utilized the NRD for 2016–2019. We followed analysis guidelines according to the Agency for Health-care Research and Quality. We included all adult patients (>18 year old) with a primary admission diagnosis of cirrhosis. Based on our clinical experience, we believe that cachexia is underevaluated and underreported and may be reported in general terms such as failure to thrive. There is only one ICD-10 code for cachexia (R64). We aimed to capture as many cases as possible. Since the data do not contain anthropometric measurements, we defined a case with cirrhotic cachexia when a primary diagnosis of cirrhosis was associated with a diagnosis of cachexia or another weight loss-related diagnosis (See Table A1). The criteria for cachexia incorporated ICD codes representing “unspecified severe protein-energy malnutrition,” “moderate protein-calorie malnutrition,” “sequelae of protein-calorie malnutrition,” “unspecified protein-energy malnutrition,” “mild protein-calorie malnutrition,” “adult failure to thrive,” “abnormal weight loss,” “underweight,” and “body mass index (BMI) 19 or less, adult”. This broader definition was adopted to enhance the study's sensitivity in capturing all cirrhotic-associated cachexia. To validate our point, we did a sensitivity analysis to see if our conclusion about inpatient mortality (for example) is different when we use the only single ICD-10 code for cachexia. The results were robust. We excluded all patients with a concurrent diagnosis of Human Immunodeficiency Virus, chronic lung disease, end-stage renal disease, malignancy, heart failure, and certain neurological conditions. These exclusion criteria were meant to increase the specificity of our target population to be more representative of cirrhotic-associated cachexia rather than cachexia (or weight loss) related to other etiologies. We additionally excluded patients who were discharged in December; this is important to be able to assess 30-day readmissions accurately. We also excluded patients with missing values for length of stay (LOS) or event time (NRD_DaysToEvent) for the same purpose.
Statistical Analysis
NRD has a complex survey design. We utilized discharge weight (DISCWT), clustering (HOSP_NRD), and stratum (NRD_STRATUM, YEAR) provided health-care cost and utilization project to obtain national estimates using survey procedures in statistical analysis software (SAS). Categorical variables were reported as frequencies and percentages, and continuous variables were reported as means and standard deviations. We compared baseline characteristics and outcomes in those who were cachectic vs those who were not. Differences in means and frequencies were assessed using the least-squares means and Chi-square test. Survey logistic regression was used to analyze the independent impact of cachexia on categorical outcomes (ie, inpatient mortality). Inpatient mortality was adjusted for age category (reference group <55 year old), gender, hypertension, diabetes mellitus, chronic kidney disease, alcoholism, dementia, coagulopathy, pulmonary hypertension, blood loss anemia, and peptic ulcer disease.
The statistical cutoff for significance was considered as P value < .05. All analyses were performed using SAS Enterprise Guide version 8.3 (© 2019–2020, SAS Institute Inc., Cary, NC, USA).
Results
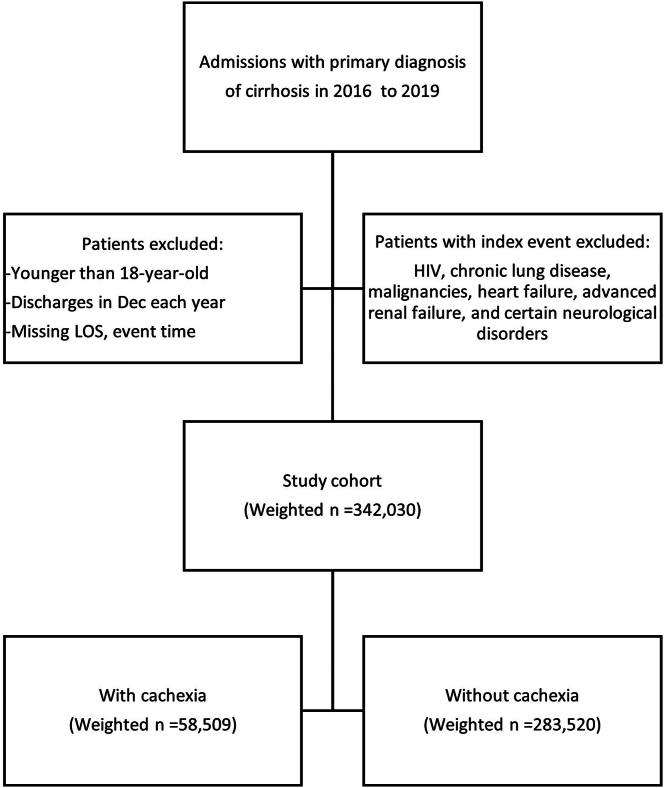
A total of 342,030 cases met our inclusion criteria. Cachexia, as defined in the methodology section, was identified in approximately 17% of the study population (58,509 discharges). See Figure 1.
Figure 1.
Methodology flow chart.
Demographic Characteristics of the Study Cohort
Table 1 demonstrates the baseline characteristics of the study cohort, including demographics, comorbidities, and hospitalization details. The mean age was 56 years, similar for patients with or without cirrhotic cachexia. More female patients were noted in cachexia group (41% vs 38%; P < .0001). Patients with cirrhotic cachexia had a significantly higher prevalence of alcohol abuse, dementia, depression, deficiency anemia, and chronic kidney disease. Most patients were admitted to large metropolitan hospitals without significant clinical differences between the 2 groups. Socioeconomic status distribution was similar as well.
Table 1.
Baseline Characteristics of Cohort Study
| Variable | Total N = 342,030 |
With cachexia n = 58,509 | Without cachexia n = 283,521 | P value |
|---|---|---|---|---|
| Age (y), mean ± SD | 56.3 ± 16.0 | 56.4 ± 16.2 | 56.2 ± 16.0 | .19 |
| Female gender, n (%) | 131,218 (38.4%) | 24,010 (41.0%) | 107,208 (37.8%) | <.0001 |
| Comorbidities, n (%) | ||||
| Alcohol abuse | 173,019 (50.6%) | 32,733 (55.9%) | 140,286 (49.5%) | <.0001 |
| Autoimmune disease | 6234 (1.8%) | 1113 (1.9%) | 5121 (1.8%) | .35 |
| Dementia | 8626 (2.5%) | 1828 (3.1%) | 6798 (2.4%) | <.0001 |
| Depression | 48,422 (14.2%) | 9111 (15.6%) | 39,311 (13.9%) | <.0001 |
| Diabetes mellitus | 101,684 (29.7%) | 13,684 (23.4%) | 87,999 (31.0%) | <.0001 |
| Drug abuse | 21,664 (6.3%) | 3793 (6.5%) | 17,871 (6.3%) | .30 |
| Hypertension | 155,191 (45.4%) | 23,613 (40.4%) | 131,578 (46.4%) | <.0001 |
| Obesity | 44,769 (13.1%) | 8089 (13.8%) | 36,680 (12.9%) | .003 |
| PVD | 12,047 (3.5%) | 2681 (4.6%) | 9366 (3.3%) | <.0001 |
| Deficiency anemia | 123,882 (36.2%) | 27,299 (46.7%) | 96,584 (34.1%) | <.0001 |
| Blood loss | 10,329 (3.0%) | 1886 (3.2%) | 8443 (3.0%) | .03 |
| Coagulopathy | 190,397 (55.7%) | 35,502 (60.7%) | 154,895 (54.6%) | <.0001 |
| Movement disorder | 4659 (1.4%) | 715 (1.2%) | 3944 (1.4%) | .03 |
| Seizure | 15,334 (4.5%) | 2822 (4.8%) | 12,511 (4.4%) | .002 |
| Paralysis | 3408 (1.0%) | 665 (1.1%) | 2743 (1.0%) | .01 |
| Psychosis | 15,061 (4.4%) | 2415 (4.1%) | 12,647 (4.5%) | .02 |
| Pulmonary HTN | 5263 (1.5%) | 1008 (1.7%) | 4254 (1.5%) | .01 |
| CKD | 41,474 (12.1%) | 8219 (14.0%) | 33,255 (11.7%) | <.0001 |
| PUD | 12,518 (3.7%) | 2292 (3.9%) | 10,226 (3.6%) | .01 |
| CBVD (POA) | 2025 (0.6%) | 470 (0.8%) | 1555 (0.5%) | <.0001 |
| CBVD sequelae | 2542 (0.7%) | 422 (0.7%) | 2120 (0.7%) | .66 |
| Valvular disease | 7732 (2.3%) | 1328 (2.3%) | 6404 (2.3%) | .90 |
| Hospital location, n (%) | .0002 | |||
| Central metropolitan | 88,845 (26.0%) | 16,295 (27.8%) | 72,550 (25.6%) | |
| Fringe metropolitan | 83,670 (24.5%) | 13,839 (23.7%) | 69,831 (24.6%) | |
| Medium metropolitan | 78,223 (22.9%) | 12,900 (22.0%) | 65,323 (23.0%) | |
| Small metropolitan | 34,370 (10.0%) | 5907 (10.1%) | 28,464 (10.0%) | |
| Micropolitan counties | 29,406 (8.6%) | 4913 (8.4%) | 24,493 (8.6%) | |
| Other | 24,200 (7.1%) | 4057 (6.9%) | 20,143 (7.1%) | |
| Socioeconomic status, n (%) | <.0001 | |||
| Low status | 110,349 (32.3%) | 17,996 (30.8%) | 92,353 (32.6%) | |
| Median status | 94,376 (27.6%) | 16,036 (27.4%) | 78,340 (27.6%) | |
| 50–75 percentile | 79,936 (23.4%) | 13,791 (23.6%) | 66,145 (23.3%) | |
| 75–100 percentile | 51,774 (15.1%) | 9804 (16.8%) | 41,970 (14.8%) | |
CBVD, cerebrovascular disease; CKD, chronic kidney disease; HTN, hypertension; POA, present on admission; PUD, peptic ulcer disease; PVD, peripheral vascular disease; SD, standard deviation.
Patient Outcomes With and Without Cachexia
Table 2 represents inpatient outcomes for patients with cirrhotic cachexia in comparison to patients without documentation of cachexia. Inpatient mortality during index hospitalization was higher in patients with cirrhotic cachexia (6.7% vs 3%, P value less than .0001). They also had almost double the LOS (8.3 days vs 4.8 days, P value less than .0001). Hence, average hospitalization charges were almost doubled for patients with cirrhotic cachexia compared to patients without cachexia. A smaller percentage of patients with cirrhotic cachexia were discharged home independently as a higher percentage required to be discharged to a facility or with a home health agency. Cachectic patients had higher rates of 30-day readmissions compared to those without cachexia (32.3% vs 30.7%, P value less than .0001). Inpatient mortality during first all-cause readmission within 30 days of index discharge was also higher in the cachexia group (8.6% vs 6.5%, P value less than .0001).
Table 2.
Inpatient Outcomes for Patients With Cirrhotic Cachexia in Comparison to Patients Without Documentation of Cachexia
| Outcome | Total N = 342,030 | With cachexia n = 58,509 | Without cachexia n = 283,521 | P value |
|---|---|---|---|---|
| Index admission mortality, n (%) | 12,824 (3.7%) | 3908 (6.7%) | 8916 (3.1%) | <.0001 |
| LOS (d), mean ± SD | 5.4 ± 8.9 | 8.3 ± 13.5 | 4.8 ± 7.3 | <.0001 |
| Total charges ($), mean ± SD | 60,717.6 ± 151,446.0 | 98,957.6 ± 265,973.3 | 52,854.7 ± 111,936.0 | <.0001 |
| Discharge disposition, n (%) | <.0001 | |||
| Discharged to home | 222,926 (65.2%) | 29,482 (50.4%) | 193,444 (68.2%) | |
| Transferred | 4987 (1.5%) | 989 (1.7%) | 3999 (1.4%) | |
| Discharged to a facility | 41,066 (12.0%) | 11,241 (19.2%) | 29,825 (10.5%) | |
| Home health care | 49,959 (14.6%) | 11,541 (19.7%) | 38,418 (13.6%) | |
| 30-d readmission, n (%) | 106,088 (31.0%) | 18,951 (32.4%) | 87,137 (30.7%) | <.0001 |
| Days to readmission, mean ± SD | 12.5 ± 11.0 | 12.3 ± 10.9 | 12.5 ± 11.0 | <.0001 |
| Mortality during first 30-d readmission, n (%) | 7273 (6.9%) | 1644 (8.6%) | 5628 (6.5%) | <.0001 |
| Readmission LOS (d), mean ± SD | 6.4 ± 11.6 | 7.3 ± 14.0 | 6.2 ± 11.0 | <.0001 |
LOS, length of stay; SD, standard deviation.
Risk Factors for Inpatient Mortality
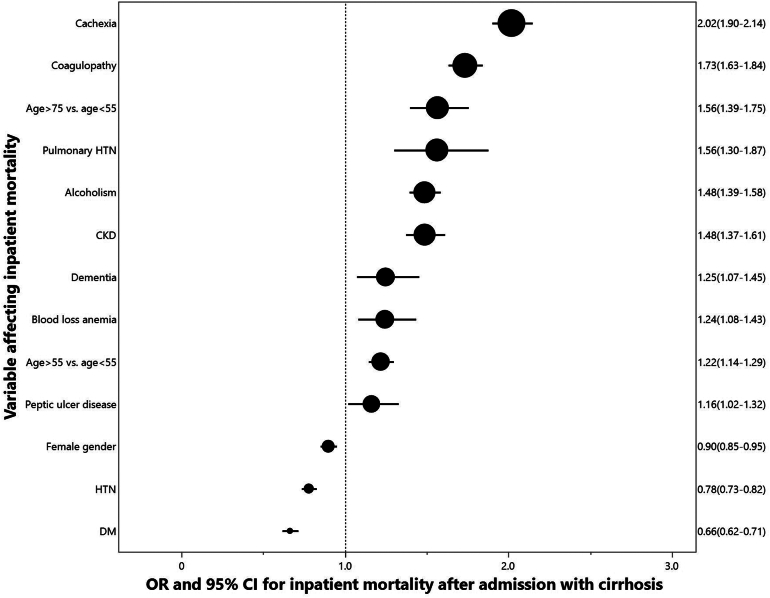
Using multivariate logistic regression, cachexia was an independent risk factor for inpatient mortality (adjusted OR 2.02; 95% confidence interval [CI] 1.90–2.14) even after adjustment for coagulopathy, age group, pulmonary hypertension, alcoholism, chronic kidney disease, dementia, blood loss anemia, peptic ulcer disease, female gender, hypertension, and diabetes mellitus. See Figure 2.
Figure 2.
Predictors of inpatient mortality.
Discussion
This study aimed to investigate the link between cirrhotic cachexia and hospital mortality and the 30-day risk of all-cause readmission at national level. Our research included 342,030 discharges with a primary diagnosis of cirrhosis, with 17% having cachexia. After adjusting for demographics, and comorbidities, our findings revealed that cirrhotic cachexia was associated with higher odds of hospital mortality (adjusted odds ratio [OR] 2.02 [1.90–2.14], P = .001). Patients with cirrhotic cachexia also had longer hospitalizations compared to those without cirrhotic cachexia.
Our findings indicate that cachexia is an adverse prognosticator for inpatient outcomes. This could be related to various factors, including systemic inflammation, hormone resistance, and increased muscle protein degradation in addition to decreased dietary consumption.10 Evans et al. (2008) conducted a study to find the cachexia link between patient readmission and outcome. They found that weight loss in adults (adjusted for fluid retention) or failure of growth in children (without endocrine problems) are the main clinical manifestations of cachexia. Cachexia is typically accompanied by anorexia, inflammation, insulin resistance, and accelerated muscle protein breakdown.3 According to a 2015 study by Kalafateli et al.11 muscle wasting has a negative effect on patients with cirrhosis' ability to survive. It is, therefore, unclear whether nutritional changes and exercise would reduce muscle wasting and increase survival in this situation.12 Treatment options may include reducing inflammation, boosting diet, and encouraging physical exercise.13 These factors may aggravate cirrhotic patients' liver function and result in a worse prognosis.
Our findings are in line with prior studies conducted by Alberino et al. in 2001, Tandon et al. in 2012, Dasarathy & Merly in 2016, and Merli & Lucidi et al. in 2013, with findings that cachexia is a significant factor in cirrhotic patients’ illness and death.14, 15, 16, 17 However, these studies were international and not based in the United States, comprised of a small sample size or did not focus on cachexia. Cachexia in cirrhotic individuals must be addressed because it substantially affects the patients’ general health and prognosis.16,17
The study by Alberino et al. in 2001 did a 2-year follow-up in 212 hospitalized patients with a primary diagnosis of liver cirrhosis to evaluate the effects of malnutrition on survival outcomes. It was found that patients who were significantly and moderately undernourished had poorer survival rates than patients who were adequately nourished. Using Cox's regression analysis, it was discovered that substantial loss of muscle mass and body fat were independently associated indicators of survival. In individuals with hepatocellular carcinoma (HCC), the prevalence of cancer-related weight loss appears to be an early, independent indicator of less favorable outcomes.18 Using body cell mass (BCM) data collected from bioimpedance, it was demonstrated that patients of cachexia with liver cirrhosis getting a liver transplant had a worse prognosis than those with an improved BCM and less elevated metabolism.19,20 A worse outcome was also seen in nontransplanted patients with liver cirrhosis who lost muscle or BCM.11,21
Previous research has found that cirrhotic patients with cachexia are more likely to develop complications, consume more health-care resources, and have a poorer survival rate than those without cachexia.22, 23, 24, 25 Our study found that cirrhotic cachexia was associated with higher odds of hospital mortality (adjusted OR 2.02 [1.90–2.14], P = .001). The results are similar to the study by Rich et al. in 2022. They found that patients with cachexia had lower survival compared to those with precachexia or stable weight (11.3 vs 20.4 vs 23.5 months, respectively; P .001) and patients had a lower probability to undergo HCC treatment (OR, 0.38; 95% CI, 0.21–0.71). Cachexia continued to be negatively correlated with survival (hazard ratio, 1.43; 95% confidence range, 1.11–1.84); it affects around 1 in 4 individuals with HCC. However, unlike our study, data was taken from 2 health systems in the United States. Further, it was conducted in patients with a primary diagnosis of HCC, and did not evaluate the effects of cachexia on hospitalization length and readmission rates among the patients.18
Readmission of patients with cirrhosis has been examined in several studies. Berman et al. in 2011 and Volk et al. in 2012 conducted studies regarding hospital readmission for patients with liver cirrhosis and showed that the model for chronic liver failure scores at discharge were independently linked to readmission within 30 days (OR, 1.06; 95% CI, 1.02–1.09; P = .002), diabetes (OR, 1.78; 95% CI, 1.07–2.95; P = .027), and male sex (OR, 1.73; 95% CI, 1.03–2.89; P = .038). Patients admitted again to the hospital within 30 days had a substantially higher 90-day death rate compared to patients who were not (26.8% vs 9.8%; OR, 2.6; 95% CI, 1.36–5.02; P = .004).22,23,26,27and higher readmission rates, lengthier hospital stays, and increased health-care utilization in cirrhotic patients with cachexia.25,28 These studies, however, were completed in a relatively small number of patients, and did not evaluate in-hospital outcomes, such as LOS. Moreover, the effects of cachexia or malnutrition on the survival outcomes of the patients with liver cirrhosis were also not identified. Our study findings have demonstrated longer hospital stays (8.3 days vs 4.8) and readmissions (7 days vs 6 days) that indicate that patients with cirrhotic cachexia require more care and therapy for complications, resulting in higher health-care expenses and more resource use. Prolonged hospital stay and mortality rates are clinically significant findings of the present study. These findings are in line with a previous nationwide analysis that included 162,694 patients with cirrhosis, with 11.2% having a diagnosis of muscle loss phenotype.29 Their findings showed that muscle loss was associated with higher mortality rates (19.3% vs 8.2%) and LOS (14.2 ± 15.8 vs 4.6 ± 6.9 days). Furthermore, multivariate regression analysis revealed that muscle loss increased mortality by 130% and LOS by 80%.
The subtle differences in readmissions rates between the group with cachexia and patients without cachexia could be at least partially explained by the higher inpatient mortality rate for patients with cachexia. Despite this competing risk, the difference is still statistically significant. Also, the findings translate into a considerable burden on health-care resources that is evident by a stark difference in mean total charges between cachexia and noncachexia patients (98,957.6 ± 265,973.3 vs 52,854.7 ± 111,936.0) in the present study.
Our study has several limitations. First, because the research was conducted retrospectively, we could not establish a causal relationship between cirrhotic cachexia and the results observed. Also, the worse outcomes noted in patients with cachexia could be related to other confounders that we did not account for such as severity of cirrhosis/ portal hypertension or related complications. Second, the NRD database does not contain laboratory or imaging reports, which could affect our results. Furthermore, coding errors can happen, which may affect results. More interventional research is needed.
In the future, prospective studies should establish the causative relationships between cirrhotic cachexia and recurrence rates, hospital mortality, and health-care resource utilization. The discovery of indicators and clinical indications that can forecast when cachexia occurs and how it can progress in cirrhotic patients may also aid in early intervention and therapy. Investigating whether the efficacy of various treatments, such as dietary assistance, medication regimens, and exercise programs, would help improve the care and results of cirrhotic individuals with cachexia.
Conclusion
Cachexia is an adverse prognosticator for inpatient outcomes in patients with cirrhosis. Patients with cirrhotic cachexia had a higher prevalence of alcohol abuse, dementia, depression, deficiency anemia, and chronic kidney disease. Cirrhotic cachexia is associated with greater readmission rates, inpatient mortality, and prolonged hospital admissions. Identification of cachexia in cirrhotic patients can add prognostic value.
Acknowledgments
Authors' Contributions:
Mohammad Alabbas: project administration, literature review, writing- original writing, review & editing; Abdelkader Chaar: writing, review & editing; Cheryl A. Gibson: writing, review & editing; Mohamad Alhoda Mohamad Alahmad: conceptualization, investigation, methodology, formal analysis, data curation, visualization, and Supervision.
Footnotes
Conflicts of Interest: The authors disclose no conflicts.
Funding: The authors report no funding.
Ethical Statement: The database used is deidentified and publicly available. Hence, the study was exempted by the Institutional Review Board.
Data Transparency Statement: The data, analytic methods, and study materials will not be made available to other researchers. The data that support the findings of this study are publicly available.
Reporting Guidelines: STROBE, SAGER.
Material associated with this article can be found in the online version at https://doi.org/10.1016/j.gastha.2023.11.017.
Supplementary Materials
References
- 1.Alahmad M.A., Acharya P., Gibson C.A., et al. Cachexia is associated with adverse outcomes in patients admitted with heart failure. Am J Cardiol. 2023;186:30–35. doi: 10.1016/j.amjcard.2022.10.017. [DOI] [PubMed] [Google Scholar]
- 2.Alahmad M.A.M., Gibson C.A. The impact of pulmonary cachexia on inpatient outcomes: a national study. Ann Thorac Med. 2023;18:156–161. doi: 10.4103/atm.atm_31_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans W.J., Morley J.E., Argilés J., et al. Cachexia: a new definition. Clin Nutr. 2008;27:793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Von Haehling S., Anker S.D. Prevalence, incidence and clinical impact of cachexia: facts and numbers-update 2014. J Cachexia Sarcopenia Muscle. 2014;5:261–263. doi: 10.1007/s13539-014-0164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tisdale M.J. Cachexia in cancer patients. Nat Rev Cancer. 2002;2:862–871. doi: 10.1038/nrc927. [DOI] [PubMed] [Google Scholar]
- 6.Montano-Loza A.J., Meza-Junco J., Prado C.M., et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10:166–173. doi: 10.1016/j.cgh.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 7.Shah R., Haydek C., Mulki R., et al. Incidence and predictors of 30-day readmissions in patients hospitalized with chronic pancreatitis: a nationwide analysis. Pancreatology. 2018;18:386–393. doi: 10.1016/j.pan.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Mohamad Alahmad M.A., Acharya P., Gibson C.A., Wiley M., Hockstad E., Gupta K. Cachexia is associated with adverse outcomes in patients admitted with heart. Am J Cardiol. 2023;186:30–35. doi: 10.1016/j.amjcard.2022.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Mukhtar O., Shrestha B., Khalid M., et al. Characteristics of 30-day readmission in spontaneous pneumothorax in the United States: a nationwide retrospective study. J Community Hosp Intern Med Perspect. 2019;9:215–220. doi: 10.1080/20009666.2019.1618135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma B., John S. StatPearls Publishing; Treasure Island (FL): 2023. Hepatic cirrhosis. [Updated 2022 Oct 31]. In: StatPearls [Internet] [PubMed] [Google Scholar]
- 11.Kalafateli M., Konstantakis C., Thomopoulos K., et al. Impact of muscle wasting on survival in patients with liver cirrhosis. World J Gastroenterol. 2015;21:7357–7361. doi: 10.3748/wjg.v21.i24.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Brien A., Williams R. Nutrition in end-stage liver disease: principles and practice. Gastroenterology. 2008;134:1729–1740. doi: 10.1053/j.gastro.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Tsien C.D., McCullough A.J., Dasarathy S. Late evening snack: exploiting a period of anabolic opportunity in cirrhosis. J Gastroenterol Hepatol. 2012;27:430–441. doi: 10.1111/j.1440-1746.2011.06951.x. [DOI] [PubMed] [Google Scholar]
- 14.Alberino F., Gatta A., Amodio P., et al. Nutrition and survival in patients with liver cirrhosis. Nutrition. 2001;17:445–450. doi: 10.1016/s0899-9007(01)00521-4. [DOI] [PubMed] [Google Scholar]
- 15.Tandon P., Ney M., Irwin I., et al. Severe muscle depletion in patients on the liver transplant wait list: its prevalence and independent prognostic value. Liver Transpl. 2012;18:1209–1216. doi: 10.1002/lt.23495. [DOI] [PubMed] [Google Scholar]
- 16.Dasarathy S., Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol. 2016;65:1232–1244. doi: 10.1016/j.jhep.2016.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merli M., Giusto M., Lucidi C., et al. Muscle depletion increases the risk of overt and minimal hepatic encephalopathy: results of a prospective study. Metab Brain Dis. 2013;28:281–284. doi: 10.1007/s11011-012-9365-z. [DOI] [PubMed] [Google Scholar]
- 18.Rich N.E., Phen S., Desai N., et al. Cachexia is prevalent in patients with hepatocellular carcinoma and associated with worse prognosis. Clin Gastroenterol Hepatol. 2022;20:e1157–e1169. doi: 10.1016/j.cgh.2021.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller M.J., Lautz H.U., Plogmann B., et al. Energy expenditure and substrate oxidation in patients with cirrhosis:the impact of cause, clinical staging and nutritional state. Hepatology. 1992;15:782–794. doi: 10.1002/hep.1840150507. [DOI] [PubMed] [Google Scholar]
- 20.Selberg O., Bottcher J., Tusch G., et al. Identification of high- and low risk patients before liver transplantation: a prospective cohort study of nutritional and metabolic parameters in 150 patients. Hepatology. 1997;25:652–657. doi: 10.1002/hep.510250327. [DOI] [PubMed] [Google Scholar]
- 21.Selberg O., Bottcher J., Pirlich M., et al. Clinical significance and correlates of whole body potassium status in patients with liver cirrhosis. Hepatol Res. 1999;16:36–48. [Google Scholar]
- 22.Charlton M.R., Burns J.M., Pedersen R.A., et al. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249–1253. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 23.D'Amico G., Garcia-Tsao G., Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Tsao G., Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. 2010;362:823–832. doi: 10.1056/NEJMra0901512. [DOI] [PubMed] [Google Scholar]
- 25.Berman K., Tandra S., Forssell K., et al. Incidence and predictors of 30-day readmission among patients hospitalized for advanced liver disease. Clin Gastroenterol Hepatol. 2011;9:254–259. doi: 10.1016/j.cgh.2010.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alahmad M.A. Pulmonary cachexia is associated with worse patient outcomes. Chest. 2022;162:A1933. [Google Scholar]
- 27.Bataller R., Brenner D.A. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Volk M.L., Tocco R.S., Bazick J., et al. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107:247–252. doi: 10.1038/ajg.2011.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vural A., Attaway A., Welch N., et al. Skeletal muscle loss phenotype in cirrhosis: a nationwide analysis of hospitalized patients. Clin Nutr. 2020;39(12):3711–3720. doi: 10.1016/j.clnu.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.