Abstract
Background
Catheter-based interventions have emerged for both acute and chronic pulmonary thromboembolic disease. With this development and the need for segmental cannulation, anatomic understanding of pulmonary arterial segmental branch origination is important. We aim to describe the prevalence of different pulmonary arterial segmental branch origination patterns.
Methods
This study included 179 consecutive patients who underwent bilateral nonselective invasive pulmonary angiography for the evaluation of chronic thromboembolic pulmonary hypertension.
Results
In our study population (age, 59.0 ± 14.8 years, 55.3% female, 71% White), we found several anatomic variations of branches to the different lobes. These included 7 branching patterns in the right upper lobe, 3 in the right middle lobe, and 10 in the right lower lobe (4 patterns for the origin of the superior segmental artery and 6 for the origin of the basilar segmental arteries). On the left side, we found 8 patterns in the left upper lobe, with 5 involving lingular branches, and 9 in the left lower lobe (5 for the origin of the superior segmental artery and 4 for the basilar segmental pulmonary arteries). Although there were many variations, only 2-3 variations for each individual lobe accounted for >90% of the angiograms.
Conclusions
Up to 3 anatomic branching patterns per lobe were noted to account for >90% of pulmonary artery branching variations in this study. This knowledge is not only useful for the interventionalist performing catheter-directed therapies but also for future research efforts that aim to standardize reporting of pulmonary angiographic findings.
Keywords: chronic thromboembolic pulmonary hypertension, invasive pulmonary angiography, pulmonary thromboembolic disease, segmental pulmonary artery branch origin
Central Illustration
Highlights
-
•
This report describes anatomic variations in the pulmonary arterial segment origins.
-
•
We found up to 3 patterns accounted for >90% of each lobe's angiogram.
-
•
This information is useful for selective pulmonary angiography and interventions.
Introduction
Treatment of pulmonary thromboembolic disease has advanced significantly over the past decade. Historically, anticoagulation and systemic thrombolysis have been the cornerstone of therapy in patients with acute pulmonary embolism and surgical thromboendarterectomy has been the mainstay for patients with chronic thromboembolic pulmonary disease. More recently, catheter-based interventions have shown promise in the treatment of both acute and chronic thromboembolic disease.1,2
All catheter-based therapies require a deep understanding of the anatomy of the targeted vascular bed; pulmonary vascular interventions are no exception to this rule. Whether it is catheter-based thrombus removal for acute intermediate high–risk pulmonary embolism or balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension (CTEPH), operators must be able to safely cannulate segmental branches. This requires an understanding of the many anatomical variations that exist within pulmonary vasculature.
Pulmonary arterial interventions can be particularly challenging, given the high degree of anatomical variability.3 Segmental pulmonary arteries can vary in their number, relative size, and location of origin; they can arise as common trunks or as separate arteries. Catheter-based intervention necessitates identification and selective cannulation of both segmental and subsegmental branches. Therefore, it is critical for the interventionalist to understand where these segmental branches originate and which territories they supply. Distortions and alterations in orientation resulting from chronic pulmonary disease and CTEPH further underscores the importance of delineation of the pulmonary vasculature and understanding the variations. This knowledge is a prerequisite for safe segmental and subsegmental cannulation, angiography, and intervention.
Although there are previous descriptions of pulmonary vascular anatomy and variants that are based on evaluation of anatomical specimens,3,4 a contemporary systematic imaging study describing the type and prevalence of variations in pulmonary segmental arterial origination is not available. In this study, we aimed to define the most common variations in the segmental branch originations of the pulmonary arteries in a contemporary cohort of patients undergoing invasive pulmonary angiography for the evaluation of CTEPH.
Methods
Study population
Between September 26, 2018, and October 5, 2021, 179 patients underwent invasive pulmonary angiography at the University of Michigan Frankel Cardiovascular Center for the evaluation of CTEPH. This study was approved by the University of Michigan institutional review board (HUM00208936).
Nonselective invasive pulmonary angiography technique
All pulmonary angiograms were performed using a standardized protocol. Vascular access was through the internal jugular vein or common femoral vein. Pulmonary angiography was performed through an 8F Berman catheter or a 7F high-flow angled pigtail catheter. Orthogonal images were obtained in each lung using digital subtraction and power injections of iodinated contrast. For the right lung, 2 projections were taken, including a right anterior oblique (RAO) of 20°-30° view and a left anterior oblique (LAO) of 30°-60° view. For the left lung, 2 projections were similarly taken, including an LAO of 20°-30° view and an RAO of 40°-60° view. A contrast dose of 20 mL per second for 2 seconds was used per injection.
Pulmonary angiogram review
Two trained observers (E.M. and M.P.) at the Michigan Pulmonary Angiography and Core Evaluation (Mi-PACE) laboratory independently reviewed the pulmonary angiograms and identified the pulmonary arterial segmental branch origins. Per protocol, when the 2 observers did not come to the same conclusion regarding the segmental branch origination, a third trained observer (V.A.) independently reviewed the pulmonary angiograms to resolve conflicts. Overall, 1074 different lung lobes in 179 unique patients were reviewed, and anatomical patterns were independently interpreted by observer 1 (E.M.) and observer 2 (M.P.). Disagreement between observers 1 and 2 was noted in 325 of the 1074 (30.2%) lung lobes. Observer 3 (V.A.) reviewed and adjudicated each disagreement. He agreed with either observer 1 or 2 in each case where a disagreement was noted.
The pulmonary arterial segmental branch origin patterns were identified for each lung lobe. All trained observers used the same 3-step methodology for determining the segmental branching pattern as follows:
Step 1: For each lobe, the perfusion zones were first identified in the frontal (anteroposterior) and lateral projections (Figure 1).
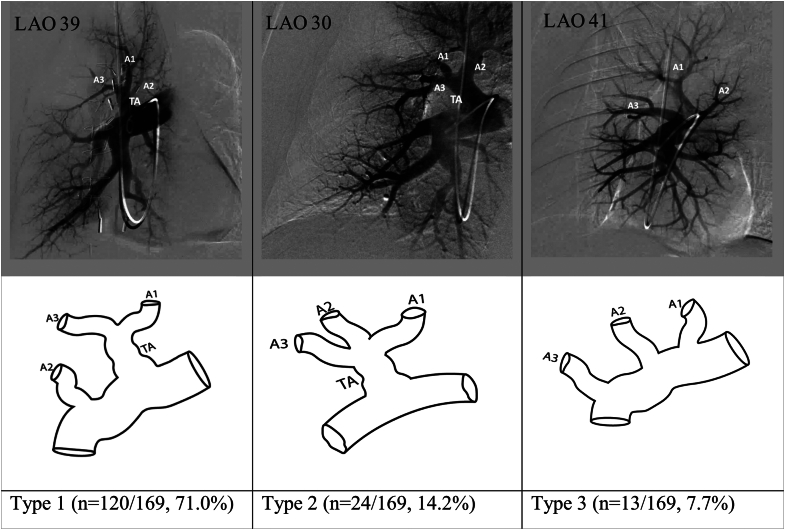
Figure 1.
Most common anatomic variations in the origins of the segmental pulmonary arteries of the right upper lobe. LAO, left anterior oblique; TA, truncus arteriosus.
Step 2: Then, for each perfusion zone, the major blood vessel supplying that zone was identified.
Step 3: Finally, the major blood vessels were traced back to their origin to determine the branching pattern.
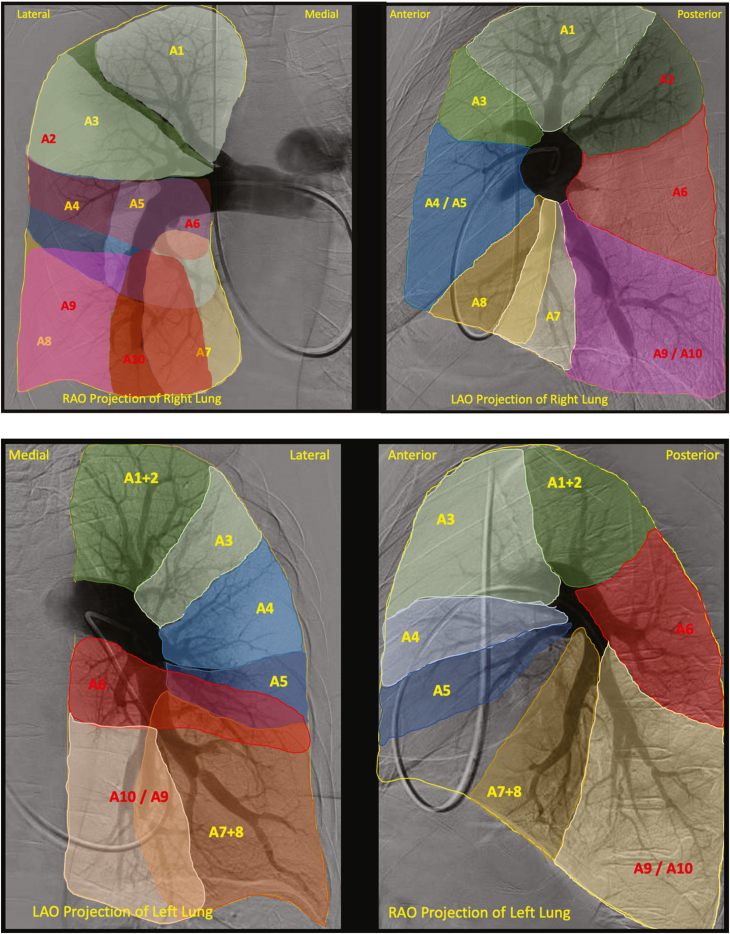
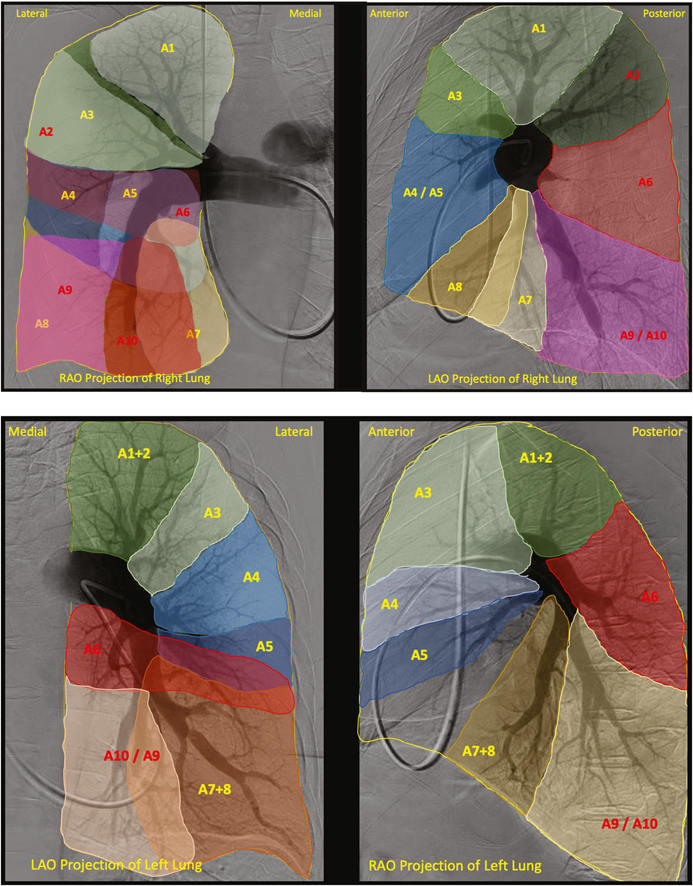
Examples of the perfusion zones for the right and left lungs can be seen in Central Illustration. When severe proximal thromboembolic occlusive disease in a particular lung lobe precluded us from identifying segmental branch origins, those lobes were excluded from the analysis. The most common segmental branch origination patterns for each lobe are reported in this article.
Central Illustration.
Perfusion map of the right lung (top) and left lung (bottom) in orthogonal RAO and LAO projections with each bronchopulmonary segment labeled. Anterior segments are in yellow and posterior segments are in red. In the right lung, the bronchopulmonary segments include the apical (A1), posterior (A2), anterior (A3), lateral (A4), medial (A5), superior (A6) medial basal (A7), anterior basal (A8), lateral basal (A9), and posterior basal (A10) segments. In the left lung, the bronchopulmonary segments include the apicoposterior (A1+2), anterior (A3), superior lingular (A4), inferior lingular (A5), superior (A6), anteromedial basal (A7+8), lateral basal (A9), and posterior basal (A10) segments. LAO, left anterior oblique; RAO, right anterior oblique.
Results
The mean age of our cohort was 59.0 ± 14.8 years (range, 19-86 years), 99 of the 179 (55.3%) were female, and 128 of the 179 (71%) were White (Table 1). The pulmonary arterial segmental branch origination patterns for each lung lobe are reported further.
Table 1.
Patient population demographic characteristic data.
| Demographic Characteristic | Value |
|---|---|
| Age, y | 59.0 ± 14.8 |
| Female sex | 99/179 (55.3) |
| Race | |
| White/Caucasian | 128/179 (71.5) |
| Black/African American | 39/179 (21.8) |
| American Indian | 3/179 (1.7) |
| Asian | 3/179 (1.7) |
| Native Hawaiian | 1/179 (0.6) |
| Other | 5/179 (2.8) |
Values are given as n/N (%) or mean ± SD.
Right upper lobe
The right upper lobe comprises 3 bronchopulmonary segments: apical, posterior, and anterior. Reliable assessment of segmental branch origins for the right upper lobe was possible in 169 of the 179 angiograms. Overall, 7 different pulmonary arterial segmental branch origination patterns were identified, of which 3 patterns (reported as types 1, 2, and 3 in Figure 1) accounted for 92.9% of all the right upper lobe pulmonary angiograms.
In type 1, the truncus anterior (TA) is the first major branch off the right pulmonary artery and bifurcates into the apical (A1) and anterior (A3) segmental arteries. The posterior segmental artery (A2) originates from the interlobar artery just distal to the origin of the TA. This variation was the most common and was found in 120 of the 169 angiograms (71.0%). In type 2, all 3 segmental arteries (A1, A2, and A3) originate from a single truncus. This variation was seen in 24 of the 169 angiograms (14.2%). In type 3, there is no major TA. All 3 segmental arteries (A1, A2, and A3) arise as separate branches from the interlobar artery. This variation was seen in 13 of the 169 angiograms (7.7%).
The remaining 4 types of right upper lobe arterial patterns were relatively rare and together accounted for 12 of the 169 angiograms (7.1%).
Right middle lobe
The right middle lobe comprises 2 bronchopulmonary segments—lateral and medial segments. Reliable assessment was possible for segmental branch origins for the right middle lobe in 167 of the 179 angiograms. Three pulmonary arterial segmental branch origination patterns were found in the right middle lobe, with 2 of these (reported as types 1 and 2 in Figure 2) accounting for 99.4% of the angiograms reviewed.
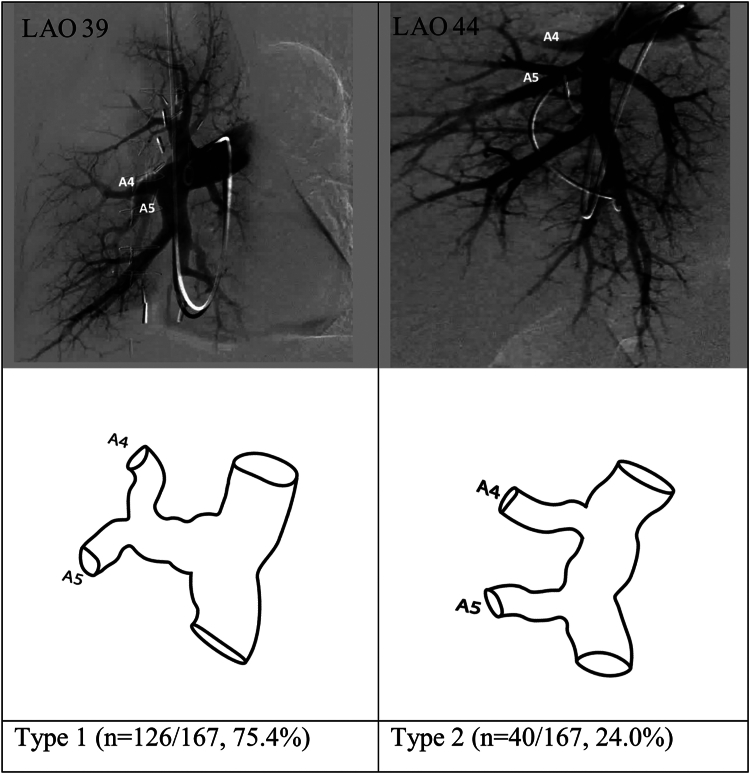
Figure 2.
Most common anatomic variations in the origins of the segmental pulmonary arteries of the right middle lobe. LAO, left anterior oblique.
In type 1, lateral (A4) and medial (A5) segmental arteries arise as a common trunk off the interlobar artery before dividing. This variation was the most common and was seen in 126 of the 167 angiograms (75.4%). In type 2, A4 and A5 have separate origins arising from the interlobar artery. This was seen in 40 of the 167 angiograms (24.0%).
Right lower lobe
The right lower lobe comprises 5 bronchopulmonary segments including the superior segment and 4 basal segments (medial basal, anterior basal, lateral basal, and posterior basal segments). Reliable assessment of the superior segmental branch origin (A6) was possible in 157 of the 179 angiograms. Overall, 4 different pulmonary arterial segmental branch origination patterns were identified for A6. Of these, 2 (reported as types 1a and 2a in Figure 3) accounted for 93% of the angiograms reviewed.
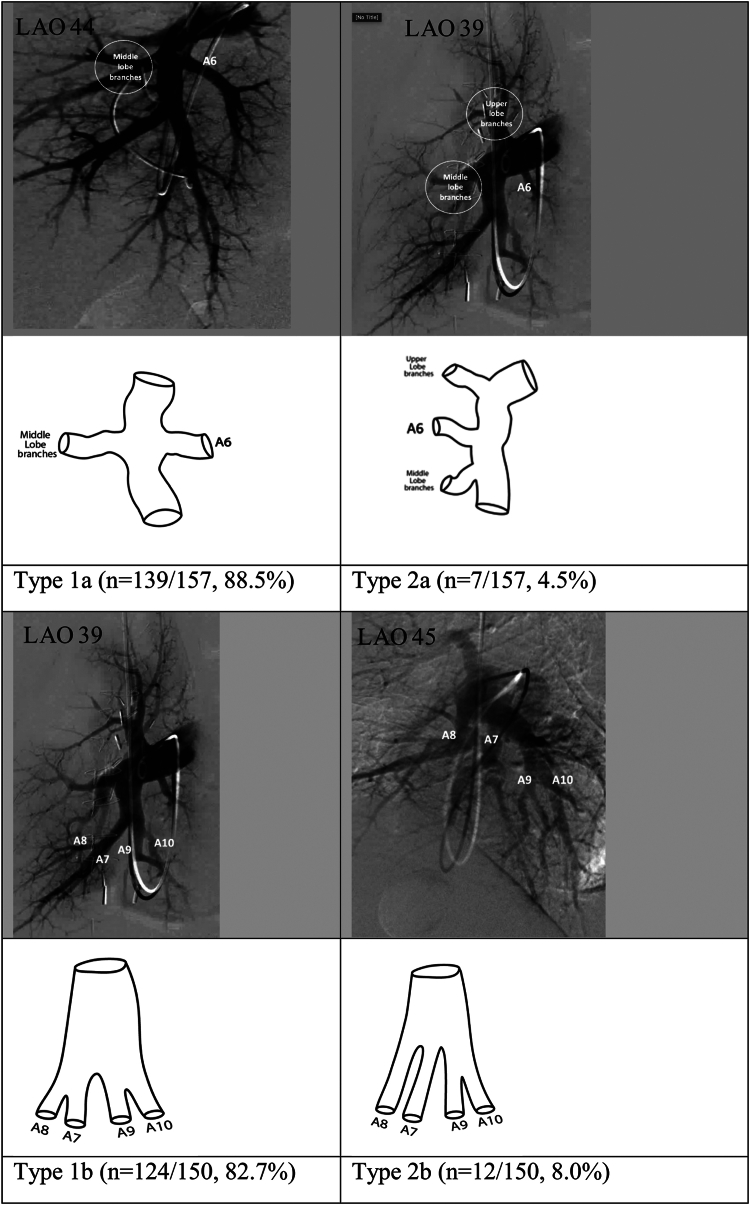
Figure 3.
Most common anatomic variations in the origins of the superior segmental artery (A6) of the right lower lobe and the origins of the basilar segmental pulmonary arteries of the right lower lobe. LAO, left anterior oblique.
In type 1a, the origin of A6 is at the same level as the origin of the right middle lobe arteries but oriented posteriorly from the interlobar artery. This was the most common variation and was seen in 139 of the 157 angiograms (88.5%). In type 2a, the origin of A6 was proximal to the middle lobe arteries and was seen in 7of the 157 angiograms (4.5%).
The medial basal (A7), anterior basal (A8), lateral basal (A9), and posterior basal (A10) segmental arteries supply the basilar bronchopulmonary segments. Reliable assessment of segmental branch origins for the basilar right lower lobe was possible in 150 of the 179 angiograms. Overall, 6 different pulmonary arterial segmental branch origination patterns were identified in the basilar bronchopulmonary segments. Two patterns (reported as type 1b and 2b in Figure 4) accounted for 90.7% of the angiograms reviewed.
Figure 4.
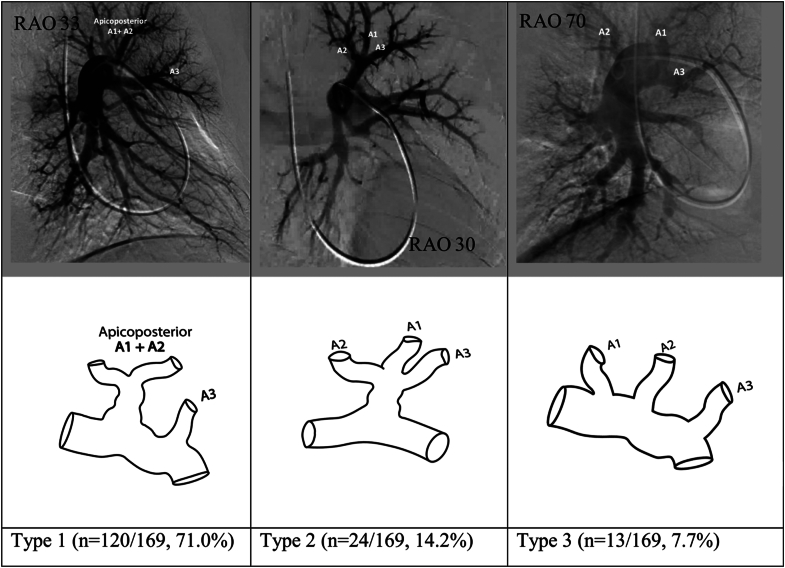
Most common anatomic variations in the origins of the segmental pulmonary arteries of the left upper lobe. RAO, right anterior oblique.
In type 1b, the interlobar artery branches into 2 major trunks. The first of those trunks later divides and terminates in the A7 and A8 segmental arteries, and the second trunk terminates in the A9 and A10 segmental arteries. This variation was the most common and was seen in 124 of the 150 angiograms (82.7%). In type 2b, there are 3 major branches supplying the basilar bronchopulmonary segments. These include separate trunks for the A7 segmental artery and for the A8 segmental artery and a third trunk that later terminates in the A9 and A10 segmental arteries. This variation was seen in 12 of the 150 angiograms (8.0%).
Left upper lobe
The left upper lobe comprises a single apicoposterior segment (A1-2), an anterior segment (A3), and 2 lingular segments—the superior (A4) and inferior (A5) lingular segments. The origin of the arteries supplying the lingular segments of the left upper lobe have been reported separately.
Reliable assessment for segmental branch origins for the apicoposterior (A1-2) and anterior (A3) segments was possible in 169 of the 179 angiograms reviewed. Eight different pulmonary arterial segmental branch origination patterns were identified in the pulmonary arterial anatomy supplying these segments. Three patterns (reported as types 1, 2, and 3 in Figure 4) accounted for 92.9% of the angiograms reviewed.
In type 1, 2 main branches arise from the proximal left pulmonary artery. The first is a single apicoposteror segmental artery (A1-2), which can later divide into multiple subsegmental branches, and the second is an anterior segmental artery (A3). This variation was the most common and was found in 120 of the 169 angiograms (71%). In type 2, both the apicoposterior (A1-2) and anterior (A3) segmental arteries originate from a single branch off the proximal left pulmonary artery. This variation was seen in 24 of the 169 angiograms (14.2%). In type 3, there are 3 segmental arteries (A1, A2, and A3), with each originating as a separate branch from the proximal left pulmonary artery. This variation was seen in 13 of the 169 angiograms (7.7%).
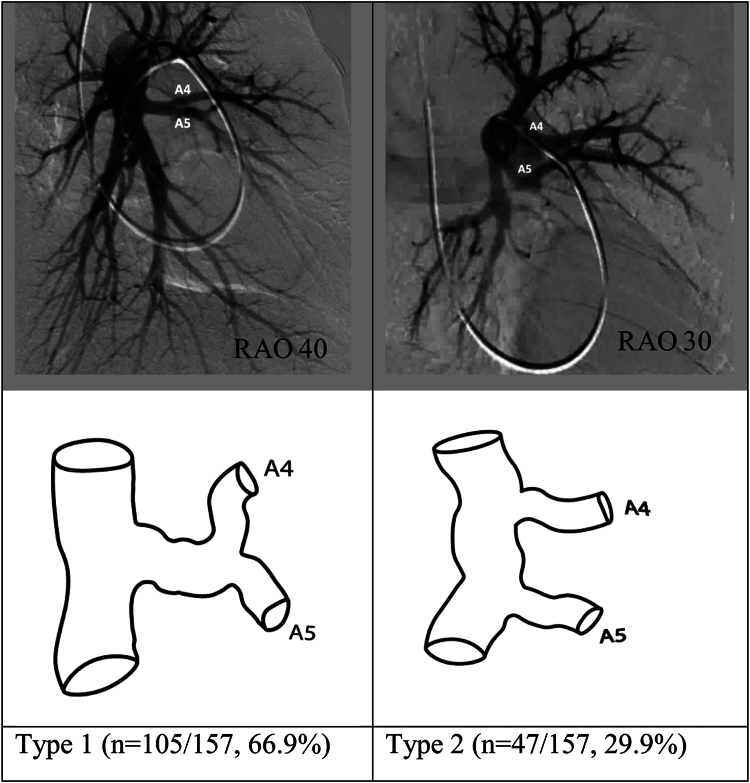
Reliable assessment was possible for segmental branch origins for the lingular segments in 157 of the 179 angiograms reviewed. There were 5 pulmonary arterial segmental branch origination patterns of the pulmonary arteries supplying the superior (A4) and inferior (A5) lingular segments. Of the 5 variations, 2 (reported as types 1 and 2 in Figure 5) accounted for 96.8% of the angiograms reviewed.
Figure 5.
Most common anatomic variations in the origins of the lingular segmental pulmonary arteries of the left upper lobe. RAO, right anterior oblique.
Left lower lobe
The left lower lobe comprises 4 bronchopulmonary segments—the superior (A6), anteromedial basal (A7-8), lateral basal (A9), and posterior basal (A10) segments.
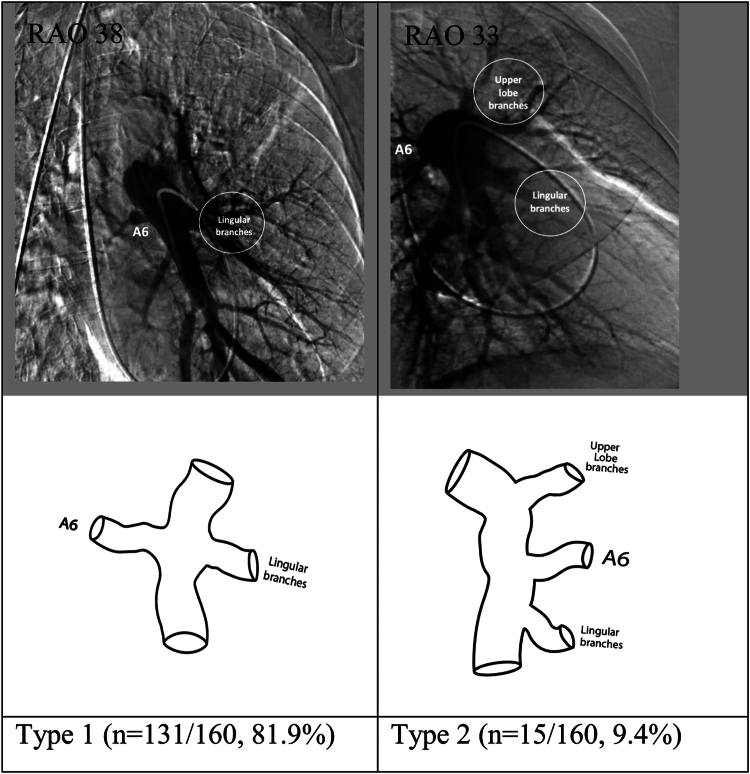
Reliable assessment of the origin of A6 was possible in 160 of the 179 left lower lobe angiograms. There were 5 pulmonary arterial segmental branch origination patterns for A6. Of these, 2 (reported as types 1 and 2 in Figure 6) accounted for >90% of the angiograms reviewed. In type 1, the origin of A6 is at the same level as the lingular branches of the left upper lobe, just oriented posteriorly off the interlobar artery. This was the most common variation and was seen in 131 of the 160 angiograms (81.9%). In type 2, the origin of A6 was proximal to the lingular branches of the left upper lobe and was seen in 15 of the 160 angiograms (9.4%).
Figure 6.
Most common anatomic variations in the origins of the superior segmental artery (A6) of the left lower lobe. RAO, right anterior oblique.
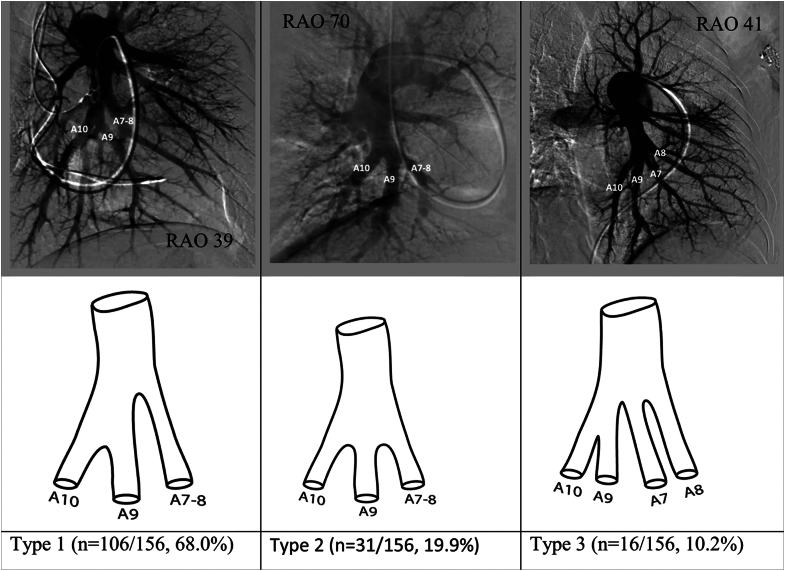
The anteromedial basal (A7-8), lateral basal (A9) and posterior basal (A10) segmental arteries supply the basilar bronchopulmonary segments of the left lower lobe. Reliable assessment of the segmental branch origins for the basilar branches of the left lower lobe was possible in 156 of the 179 angiograms reviewed. Overall, 4 different pulmonary arterial segmental branch origination patterns of the pulmonary arteries supplying basilar bronchopulmonary segments were identified, of which 3 (reported as types 1, 2, and 3 in Figure 7) accounted for >90% of the angiograms reviewed. In type 1, the interlobar branches into 2 major trunks, the first of which is the anteromedial basal (A7-8) segmental artery. The second trunk terminates in the A9 and A10 segmental arteries. This variation was the most common and was seen in 106 of the 156 angiograms (68.0%). In type 2, there are 3 major branches supplying the basilar bronchopulmonary segments. There are separate originations for the A7-8 segmental artery, the A9 segmental artery, and the A10 segmental artery. This variation was seen in 31 of the 156 angiograms (19.9%). In type 3, the interlobar again branches into 2 major trunks. However, in this case, the first trunk gives rise to the anteromedial basal (A7-8) and the lateral basal (A9) segmental arteries, whereas the second trunk supplies the posterior basal (A10) segment alone. This variation was seen in 16 of the 156 angiograms (10.2%).
Figure 7.
Most common anatomic variations in the origins of the basilar segmental pulmonary arteries of the left lower lobe. RAO, right anterior oblique.
Discussion
This report is the first in literature to describe pulmonary arterial segmental branch origination patterns based on the systematic analysis of invasive nonselective pulmonary angiograms. Importantly, although we found several different patterns for each lobe, up to 3 patterns alone accounted for >90% of the angiograms for each lobe. This information is vital for the growing field of pulmonary vascular interventions.
Segmental and subselective pulmonary angiography, with proper visualization of the obstructive lesions, is the foundation for successful pulmonary vascular interventions. Navigation into these segmental branches requires both a high level of skill and knowledge of the unique anatomy in each patient. Our findings delineate the various anatomical configurations that are predominant and their relative frequency in these patients. This vital information will enhance understanding among practicing physicians, both by defining the accurate location of obstructions and by directing the selective angiography and actual interventions. Although computed tomography pulmonary angiogram was previously the dominant imaging modality for patients with CTEPH, the emergence of catheter-based pulmonary vascular interventions has led to the reemergence of nonselective invasive pulmonary angiography as an important diagnostic and procedural planning tool.5 Delineating the anatomical patterns that are most commonly present promises to facilitate procedural planning, enhance intraprocedural navigation, reduce procedural time, and improve the safety and effectiveness of interventional therapies in the pulmonary vasculature.
In addition to enhancing understanding of pulmonary artery anatomy, this information can be useful for development of specialty catheters and other devices tailored for use in the pulmonary artery. Much as coronary guide catheters (which incidentally are currently used for PA interventions such as balloon pulmonary angioplasty) are configured to “fit” various coronary ostial positions, a set of catheters might be developed that are designed for specific pulmonary artery configurations.
Finally, the patterns that we have defined for each pulmonary segment can potentially serve as a backbone for data collection, in both clinical trials and registries designed to provide a robust evidence base for PA interventions. Standardization of vessel location and nomenclature, and lung territory supplied, will be essential to expanding this evidence base.
The primary limitation of this study is the relatively small sample size. Therefore, how these anatomic patterns correlate with individual demographic and clinical characteristics cannot be ascertained in this analysis. In addition, all patients in this study underwent invasive nonselective pulmonary angiography for the evaluation of CTEPH. It is unclear whether there are certain anatomic variations that place patients at a higher risk of developing CTEPH, so the true incidence of variations in the segmental branch origins in the pulmonary arterial vasculature may be different in a general population. In addition, the orthogonal angles selected for defining the anatomical origin and configuration of vessels in our patients were predefined in LAO and RAO views. It is conceivable that our findings may have been altered where we to have included straight anteroposterior and 90° lateral views. Finally, the findings in this study will need to be validated in other patient populations to further confirm (and further enhance) our understanding of this anatomy.
Conclusion
This analysis revealed several different anatomic patterns for each pulmonary arterial segmental branch origin. For any given segment, there were no >3 variations that accounted for >90% of all pulmonary lobes. Knowledge of the most common types of configurations in the pulmonary vascular bed is useful for the interventionalist planning and performing catheter-directed therapies for acute and chronic pulmonary thromboembolic disease.
Acknowledgments
Declaration of competing interest
Jay Giri has served on advisory boards and received research funds to the institution from Abiomed, Boston Scientific, Abbott Vascular, Inari Medical, ReCor Medical, and AstraZeneca. Vallerie M. McLaughlin received funding being provided to the University of Michigan to perform research from Acceleron Pharma and Jansen. Kenneth Rosenfield is a consultant for or a member of scientific advisory board of Abbott Vascular, Althea Medical, Angiodynamics, Auxetics, Becton-Dickinson, Boston Scientific, Contego, Crossliner, Innova Vascular, InspireMD, Janssen/Johnson & Johnson, Magneto, Mayo Clinic, MedAlliance, Neptune Medical, Penumbra, Philips, Surmodics, Terumo, Thrombolex, Truvic, Vasorum, Vumedi; receives institutional grants/research from the NIH, Abiomed, Boston Scientific, Novo Nordisk, Penumbra, Getinge-Atrium; holds equity interest in Accolade, Access Vascular, Aerami, Althea Medical, Auxetics, Contego, Crossliner, Cruzar Systems, Embolitech, Endospan, Imperative Care/Truvic, Innova Vascular, InspireMD, JanaCare, Magneto, MedAlliance, Neptune Medical, Orchestra, PQ Bypass, Prosomnus, Shockwave, Skydance, Summa Therapeutics, Thrombolex, Valcare, Vasorum, Vumedi; and is a board member and founder of the National PERT Consortium. Riyaz Bashir is the coinventor of the Bashir endovascular catheter; has equity interest in Thrombolex; and is supported by an NHLBI grant under the Small Business Innovation Research grant mechanism. All other authors have no relevant relationships to disclose.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics statement and patient consent
This research was conducted according to the ethical standard of the University of Michigan institutional review board (HUM00208936).
References
- 1.Xue X., Sista A.K. Catheter-directed thrombolysis for pulmonary embolism: the state of practice. Tech Vasc Interv Radiol. 2018;21(2):78–84. doi: 10.1053/j.tvir.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Kataoka M., Inami T., Kawakami T., Fukuda K., Satoh T. Balloon pulmonary angioplasty (percutaneous transluminal pulmonary angioplasty) for chronic thromboembolic pulmonary hypertension: a Japanese perspective. J Am Coll Cardiol Intv. 2019;12(14):1382–1388. doi: 10.1016/j.jcin.2019.01.237. [DOI] [PubMed] [Google Scholar]
- 3.Kandathil A., Chamarthy M. Pulmonary vascular anatomy & anatomical variants. Cardiovasc Diagn Ther. 2018;8(3):201–207. doi: 10.21037/cdt.2018.01.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cory R.A., Valentine E.J. Varying patterns of the lobar branches of the pulmonary artery. A study of 524 lungs and lobes seen at operation of 426 patients. Thorax. 1959;14(4):267–280. doi: 10.1136/thx.14.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yandrapalli S., Tariq S., Kumar J., et al. Chronic thromboembolic pulmonary hypertension: epidemiology, diagnosis, and management. Cardiol Rev. 2018;26(2):62–72. doi: 10.1097/CRD.0000000000000164. [DOI] [PubMed] [Google Scholar]