Abstract
Host-virus interactions control disease progression in human immunodeficiency virus-infected human beings and in nonhuman primates infected with simian or simian/human immunodeficiency viruses (SHIV). These interactions evolve rapidly during acute infection and are key to the mechanisms of viral persistence and AIDS. SHIV89.6PD infection in rhesus macaques can deplete CD4+ T cells from the peripheral blood, spleen, and lymph nodes within 2 weeks after exposure and is a model for virulent, acute infection. Lymphocytes isolated from blood and tissues during the interval of acute SHIV89.6PD infection have lost the capacity to proliferate in response to phytohemagglutinin (PHA). T-cell unresponsiveness to mitogen occurred within 1 week after mucosal inoculation yet prior to massive CD4+ T-cell depletion and extensive virus dissemination. The lack of mitogen response was due to apoptosis in vitro, and increased activation marker expression on circulating T cells in vivo coincided with the appearance of PHA-induced apoptosis in vitro. Inappropriately high immune stimulation associated with rapid loss of mature CD4+ T cells suggested that activation-induced cell death is a mechanism for helper T-cell depletion in the brief period before widespread virus dissemination. Elevated levels of lymphocyte activation likely enhance SHIV89.6PD replication, thus increasing the loss of CD4+ T cells and diminishing the levels of virus-specific immunity that remain after acute infection. The level of surviving immunity may dictate the capacity to control virus replication and disease progression. We describe this level of immune competence as the host set point to show its pivotal role in AIDS pathogenesis.
Primary infection with human immunodeficiency virus type 1 (HIV-1) can elicit an acute retroviral syndrome characterized by fever, pharyngitis, lymphadenopathy, myalgia, rash, and headache (2). Although estimates vary, as many as half of HIV-1-infected persons in the United States experience some symptoms of this acute retroviral syndrome between 2 and 6 weeks after exposure (21, 32). The variable response to HIV-1 infection may be linked to host genetics, coincident infections with viral or nonviral agents, previous immunological experience, or particulars of the infecting virus strain. At present, there is little understanding of the events during acute infection and we have no explanations for variation within a population or for how differences in the initial host-virus interactions influence the course of disease progression.
Studies of acute HIV-1 syndrome noted transiently high levels of plasma viremia that were not correlated directly with the severity of symptoms (2, 6). Elevated plasma HIV-1 RNA levels during the initial 4 months were not associated subsequently with higher rates of CD4+ T-cell depletion (26), although faster progression to AIDS may occur among individuals who experience more severe acute infection symptoms (23, 26). At the end of the acute infection interval, virus-specific cellular and humoral immune responses arise (21) and plasma viral RNA levels stabilize to provide a prognostic marker for the risk of AIDS (12, 26). Our studies seek to understand host-virus interactions during acute infection, to explain mechanisms accounting for variation within a population of infected individuals, and to show how events of early infection influence subsequent disease progression.
The rhesus macaque model is well suited for studies of acute virus infection. This outbred animal population retains sufficiently complex host genetics to reveal populational variation, and we can control for virus type, along with the time, dose, and route of exposure (33). Use of the pathogenic simian/human immunodeficiency virus (SHIV) strain SHIV89.6PD (25) and intrarectal (i.r.) inoculation (29) provides a model for infection with acute CD4+ T-cell loss. In this system, rapid disease progression was associated with greater than 90% CD4+ T-cell loss during the first month after infection whereas prolonged survival was correlated with moderate CD4+ T-cell losses, increases in circulating B cells, and production of virus-binding antibody (29).
In the present study, we first used a cohort of six macaques that were infected with SHIV89.6PD by the intravenous (i.v.), i.r., or intravaginal (i.vag.) route. These animals were necropsied between days 4 and 15 after infection to assess patterns and kinetics of virus dissemination. A second cohort of three macaques was infected with SHIV89.6PD by the i.r. route for a detailed study of changes in lymphocyte phenotype and the kinetics of cell depletion, and we obtained baseline data from a number of virus-naive animals to show the range of lymphocyte counts and expression of activation markers. We observed that CD4+ T cells are highly activated during acute infection and are unusually susceptible to activation-induced cell death (AICD). Overall levels of lymphocyte activation marker expression were maximal between weeks 2 and 4 and coincided with increased CD4+ T-cell susceptibility to apoptosis and peak viremia at week 2. Substantial lymphocyte activation appeared to increase the level of SHIV replication and promote cell death by apoptosis. The combined effect of these mechanisms may be to reduce the levels of antiviral immunity and increase subsequent rates of disease progression.
MATERIALS AND METHODS
Animals and virus infections.
Eighteen captive-bred rhesus macaques (Macaca mulatta) were housed at the Wisconsin Regional Primate Research Center (WRPRC) and used in these studies. The WRPRC is accredited by the American Academy of Laboratory Animal Care. All animal research protocols were approved by the Institutional Animal Care and Use Committee. Animals were confirmed negative for antibodies to simian immunodeficiency virus and type D simian retroviruses and were negative by standard coculture assays prior to these studies. Macaques were restrained with ketamine hydrochloride (10 mg/kg of body weight) before all virus inoculations and blood collections. General Medical Laboratories (Madison, Wis.) performed automated complete blood counts (CBC) on all samples.
We used SHIV89.6PD for these infection studies. Prior to these experiments, we had characterized this virus in more than 35 rhesus macaques and published detailed studies on the outcome of i.r. inoculation (29) and a comparison of multiple routes of inoculation (18). In our experience, i.r. doses of 2,500 tissue culture-infective doses (TCID) or higher produce a persistent infection in >95% of animals and we have not detected any outcomes consistent with transient viremia (29, 31).
Two macaques were infected i.v. with 25 TCID of SHIV89.6PD (provided by Yichen Lu, Avant Immunotherapeutics, Inc., Cambridge, Mass.; reference 18), two macaques were infected i.r. with 2,500 TCID, and two macaques were infected i.vag. with 25,000 TCID. The i.v. infected animals were euthanized on days 4 and 8 after infection. One animal from each of the mucosally infected groups was euthanized on days 8 and 15 after infection. Our previous experience showed that mucosal infections progress slower than i.v. infections, and we selected these time points to provide comparable data for all infection routes.
To investigate the expression of activation markers on peripheral blood mononuclear cells (PBMC) during and after acute infection, four additional macaques (AQ73, AQ80, AQ93, and AR08) were inoculated i.r. with 2,500 TCID of SHIV89.6PD. Blood samples were obtained from these animals on days 0, 7, 14, 28, 56, and 89 after inoculation. In addition, we obtained baseline data from a group of eight virus-naive animals.
Virus isolation and plasma antigenemia.
Peripheral blood was collected in heparinized blood tubes. Mononuclear cells were isolated by Ficoll-Hypaque (Pharmacia Biotech, Piscataway, N.J.) gradient centrifugation. Starting with 106 PBMC, duplicate serial 10-fold dilutions of cells were stimulated overnight with 0.5 μg of phytohemagglutinin (PHA; Murex Diagnostics Inc., Dartford, United Kingdom) per ml in complete RPMI medium (Gibco Bethesda Research Laboratories, Grand Island, N.Y.) with 10% fetal bovine serum (Harlan, Indianapolis, Ind.), 2 mM l-glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin (Sigma, St. Louis, Mo.) per ml. The next day, medium was removed and cells were cocultured with 2.5 × 105 CEM×174 cells in 2 ml of complete RPMI medium. Cultures were split twice weekly until positive virus isolation was determined by the appearance of a cytopathic effect or the cultures were scored as virus isolation negative after 1 month. Cocultures of mononuclear cells from tissues were performed as described above, with the exception that as many as 5 × 106 cells were seeded per well as the upper limit. Plasma samples were assayed for p27 by enzyme-linked immunosorbent assay ELISA (Coulter, Miami, Fla.).
Tissue collection and processing.
Venous blood was collected from animals anesthetized with ketamine hydrochloride. Euthanasia was performed by i.v. administration of 0.3 ml of Beuthanasia (pentobarbital sodium and phenytoin sodium; Schering-Plough Animal Health, Kenilworth, N.J.) per kg of body weight. At necropsy, the lymph nodes (LN), spleen, thymus, ileum, cecum, and rectum were harvested. Portions of each tissue type were embedded in paraffin for histopathologic analysis and in situ hybridization. Single-cell suspensions prepared from LN, spleen, and thymus tissues were subjected to Ficoll-Hypaque gradient centrifugation. Mononuclear cells isolated from the blood, LN, spleen, and thymus were used for flow cytometry, proliferation assays, and virus isolation.
In situ hybridization for viral RNA.
In situ hybridization used a pool of digoxigenin-labeled RNA probes generated by Sp6 or T7 polymerase transcription from the entire genome of SIVmac239 and HIV-1bh10 (Lofstrand, Gaithersburg, Md.). The method was described previously in detail (13). Formalin-fixed, paraffin-embedded tissue sections (4- to 5-μm thickness) were placed on diethylpyrocarbonate water–N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid-coated glass slides. They were dried overnight and treated as previously described (13). Slides were prehybridized in buffer (50% formamide, 4× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 1× Denhardt’s solution, 4 mM NaPO4, 0.1% sodium dodecyl sulfate, 5% dextran sulfate, 250 μg of tRNA per ml, 250 μg of salmon sperm DNA per ml in diethylpyrocarbonate water) in a preheated humidity chamber for 15 min. Slides were hybridized with 1.75 ng of riboprobes per ml at 52°C overnight, washed in 2× SSC–50% formamide solution and then in 2× SSC and incubated in an RNase solution (RNase T1 and RNase A in 2× SSC) for 30 min at 37°C. The slides were blocked with a buffer containing 2% horse serum, 2% sheep serum, 150 mM NaCl, 100 mM Tris (pH 7.4), and 12 mg of levamisole per ml for 1 h. Slides were incubated for 1 h with sheep anti-digoxigenin–alkaline phosphatase conjugate (Boehringer Mannheim, Indianapolis, Ind.) at a 1:500 dilution, rinsed in 0.1 M Tris buffer (pH 7.4) and then in 0.1 M Tris buffer (pH 9.5), and incubated overnight at room temperature with nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate (Vector, Burlingame, Calif.) substrate in the dark. The stained specimens were rinsed in water, counterstained with nuclear fast red, dehydrated, and overlaid with glass coverslips. Controls included sense probes hybridized on SHIV-infected tissues and antisense probes with uninfected tissues.
Flow cytometry analysis of blood and tissue lymphocyte subsets.
Mononuclear cells (2 × 105) were stained for 30 min at 4°C with the fluorescein isothiocyanate (FITC)-conjugated monoclonal antibody (MAb) against CD2 (Antigenix, Franklin Square, N.Y.) and either MAb CD4-phycoerythrin (PE) or CD8-PE (Antigenix) or CD20-PE (Becton Dickinson, Mountain View, Calif.). To assess activation marker expression on lymphocyte subsets, mononuclear cells were stained with MAb CD4-PE, CD8-PE, or CD20-PE and MAb CD25-FITC (Endogen, Woburn, Mass.), HLA-DR-FITC, or CD69-FITC (Becton Dickinson). Samples stained with the appropriate isotype controls and compensation controls were included. Cells were washed and fixed with 1% paraformaldehyde in phosphate-buffered saline (PBS). Ten thousand events per sample were acquired by a FACSCalibur flow cytometer, and data were analyzed by Cell Quest software (Becton Dickinson).
Proliferation assays.
Mononuclear cells (105) from the blood, LN, or spleen were plated in triplicate in flat-bottom 96-well tissue culture plates in complete RPMI medium with or without 0.5 μg of PHA per ml or 5 μg of p27 per ml. The recombinant p27 was prepared as described previously (30). Plates were incubated in a humidified atmosphere of 5% CO2 in air at 37°C. Samples containing PHA were pulsed with 1 μCi of tritiated thymidine per well on day 3 and harvested on day 4. Samples including p27 were pulsed with 1 μCi of tritiated thymidine per well on day 6 and harvested on day 7. Samples were harvested onto glass fiber filters and allowed to dry. Tritiated-thymidine incorporation was measured by liquid scintillation counting. Mean numbers of counts per minute of triplicate samples were determined and reported as such or as a stimulation index (SI). The SI was determined by dividing the mean number of counts per minute incorporated in the presence of PHA or p27 with the mean number of counts per minute incorporated in the presence of medium alone. Replicate plates without addition of tritiated thymidine were set up for live- and dead-cell counts based on eosin dye exclusion.
Apoptosis assay.
The 7-amino actinomycin D (7-AAD) assay was used to determine the percentage of lymphocytes undergoing apoptosis (28). Mononuclear cells were cultured for 24 h in complete RPMI medium with or without 0.5 μg of PHA per ml. Cells were counted and washed with PBS. Cell samples were stained for surface markers with MAb CD4-PE (Antigenix), CD8-FITC (Coulter-Immunotech, Miami, Fla.), CD20-FITC (Becton Dickinson), or the relevant isotype control. Cells were washed and incubated with 20 μg of 7-AAD (Sigma) per ml in PBS for 20 min at 4°C. Cells were washed twice with PBS and then fixed with 1% paraformaldehyde in PBS containing 20 μg of actinomycin D (Sigma) per ml to block any nonspecific uptake of 7-AAD after fixation. Samples were acquired by a FACSCalibur flow cytometer, and data were analyzed by Flow Jo software (Becton Dickinson).
RESULTS
Virus dissemination.
Six rhesus macaques were infected with SHIV89.6PD and euthanized between 4 and 15 days later. Two animals were infected i.v., two were infected i.r., and two were infected i.vag. The i.v. infected macaques (94074 and 94089) were euthanized on days 4 and 8 after infection. For i.r. and i.vag. infected animals, we euthanized one from each group at day 8 and the second animal from each group at day 15 after inoculation. We collected blood and tissue samples that were used to prepare mononuclear cells or fixed and embedded for in situ hybridization studies.
Plasma samples collected on the day of euthanasia were assayed by ELISA for viral core antigen p27. Samples from i.v. infected macaque 94074 (euthanized on day 4 after infection), i.r. infected macaque 94079 (day 8), and i.vag. infected macaque 94069 (day 8) had less than 5 pg/ml in plasma. The i.v. infected macaque 94089 (euthanized on day 8 after infection) had 1.17 ng of p27 per ml of plasma. The i.r. infected macaque 94077 and i.vag. infected macaque 92071 (euthanized on day 15 after infection) had 4.51 and 3.46 ng of p27 per ml of plasma, respectively. These results are consistent with kinetics of antigenemia reported in previous infection studies with SHIV89.6PD (18, 29).
In situ hybridization showed that tissue sections from the LN, spleens, ileums, cecums, and rectums of the day 8 i.v. infected macaque and the day 15 mucosally infected macaques were positive for virus, while samples from the day 4 i.v. infected animal and the day 8 i.r. infected animal were negative (Table 1). A negative sample had no positive cells in five high-power fields. This was not a rigorous effort to detect a low frequency of positive cells and was used mainly to indicate that negative samples are clearly distinct from positive specimens. In addition, we scored all positive signals and did not attempt to discriminate lymphocytes, monocytes, or other cell types.
TABLE 1.
In situ hybridization for SHIV RNA in tissues at necropsya
| Tissue | Result obtained with animal:
|
|||||
|---|---|---|---|---|---|---|
| 94074 | 94089 | 94079 | 94077 | 94069 | 92071 | |
| Spleen | − | +++ | − | + | − | + |
| Axillary LN | − | + | − | ++ | − | + |
| Cervical LN | − | NT | NT | ++ | − | + |
| Ileocecal LN | NT | ++ | − | ++ | − | ++ |
| Iliac LN | NT | + | NT | NT | NT | ++ |
| Inguinal LN | − | NT | − | ++ | − | + |
| Mesenteric LN | NT | ++ | − | ++ | + | + |
| Thymus | NT | ++ | − | +++ | − | +++ |
| Ileum | NT | ++ | NT | + | NT | + |
| Cecum | NT | ++ | NT | ++ | + | + |
| Rectum | NT | + | − | + | NT | + |
In situ hybridization for virus detection was performed on sections of formalin-fixed, paraffin-embedded tissues. Five fields were observed at a final magnification of ×100. With approximately 5,000 cells per field, the following scores were assigned: −, no hybridization observed; +, <500 cells positive for the hybridization signal; ++, between 500 and 1,250 cells positive for the hybridization signal; +++, between 1,250 and 2,500 cells positive for the hybridization signal. Animals were infected as follows: 94074, i.v. for 4 days; 94089, i.v. for 8 days; 94079, i.r. for 8 days; 94077, i.r. for 15 days; 94069, i.vag. for 8 days; 92071, i.vag. for 15 days. NT, not tested.
The mesenteric LN and cecum samples from the day 8 i.vag. infected macaque were positive for viral RNA, while the spleen, thymus, and other LN were negative. Virus was isolated after coculture with 5 × 106 PHA-stimulated mononuclear cells from the blood and mesenteric and inguinal LN of day 8 i.vag. infected animal 94069. Similarly, virus could be cocultured from greater than 106 PBMC or from mononuclear cells in the inguinal LN of day 4 i.v. infected animal 94074. Overall, we observed limited virus dissemination at early time points and noted a high level viral replication by 8 days after i.v. infection or by 15 days after mucosal infection.
CD4+ T-cell depletion and T-cell response to mitogen.
At 15 days after mucosal infection (i.r. or i.vag.), we observed substantial depletion of CD4+ T cells from the peripheral blood, spleen, and LN (Table 2). We reported similar observations of 90% or greater CD4+ T-cell loss from peripheral blood of rapidly progressing SHIV89.6PD i.r. inoculated macaques within 2 weeks after infection (29), and we reported previously (24) that uninfected macaques (n = 5) showed approximately 40% CD4+ T cells in the mononuclear cells from inguinal LN biopsies. The short-term serial sacrifice studies presented here demonstrated that CD4+ T-cell loss after infection was substantial in tissues. In 8 day i.v. infected macaque 94089, CD4+ T-cell depletion was extensive in the spleen and moderate in the LN. Although the percentage of 94089 CD4+ T cells in blood had not changed substantially compared to preinfection values, the absolute number of CD4+ T cells per microliter of blood fell dramatically by 8 days after i.v. infection (Table 2). Lymphocytes from the 15-day mucosally infected animals were predominantly CD8+ CD2+ T cells or CD20+ B cells (Table 2). These two subsets together represent the majority of lymphocytes from blood or tissue in these animals. Interestingly, the percentage of CD4+ CD8+ thymocytes remained high in the infected macaques, despite the high viral load in the thymus by day 15 in mucosally infected animals. Thus, the acute loss was restricted mainly to mature CD4+ T cells.
TABLE 2.
Rhesus macaque blood and tissue lymphocyte subsetsa
| Animal and sampleb | CD4% (absolute CD4+ cell no.)c | CD8% | CD20% | Animal and sampleb | CD4% (absolute CD4+ cell no.)c | CD8% | CD20% | |
|---|---|---|---|---|---|---|---|---|
| 94074 | ||||||||
| Day 0 PBMC | 34 (3,530) | 19 | 36 | |||||
| PBMC | 29 (555) | 16 | 27 | |||||
| Spleen | 18 | 22 | 55 | |||||
| Cervical LN | 49 | 24 | 30 | |||||
| Axillary LN | 54 | 24 | 23 | |||||
| Ileocecal LN | 51 | 24 | 24 | |||||
| Mesenteric LN | 47 | 21 | 38 | |||||
| Inguinal LN | 45 | 21 | 26 | |||||
| Iliac LN | 36 | 17 | 37 | |||||
| Thymus | 80 | 79 | 1 | |||||
| 94079 | ||||||||
| Day 0 PBMC | 29 (770) | 14 | 17 | |||||
| PBMC | 34 (291) | 21 | 34 | |||||
| Spleen | 16 | 17 | 53 | |||||
| Cervical LN | NTd | NT | NT | |||||
| Axillary LN | 50 | 24 | 16 | |||||
| Ileocecal LN | 42 | 22 | 19 | |||||
| Mesenteric LN | 54 | 27 | 9 | |||||
| Inguinal LN | NT | NT | NT | |||||
| Thymus | 73 | 43 | 5 | |||||
| 94069 | ||||||||
| Day 0 PBMC | 25 (1,177) | 17 | 27 | |||||
| PBMC | 21 (718) | 27 | 15 | |||||
| Spleen | 15 | 19 | 53 | |||||
| Cervical LN | 46 | 14 | 36 | |||||
| Axillary LN | 49 | 17 | 20 | |||||
| Ileocecal LN | 60 | 15 | 22 | |||||
| Mesenteric LN | 45 | 15 | 27 | |||||
| Inguinal LN | 45 | 18 | 28 | |||||
| Iliac LN | 39 | 11 | 49 | |||||
| Thymus | 72 | 61 | 3 |
| 94089 | |||
| Day 0 PBMC | 43 (2,932) | 29 | 20 |
| PBMC | 39 (630) | 24 | 8 |
| Spleen | 1 | 3 | 83 |
| Cervical LN | NT | NT | NT |
| Axillary LN | 34 | 15 | 40 |
| Ileocecal LN | 13 | 13 | 56 |
| Mesenteric LN | 22 | 10 | 60 |
| Inguinal LN | 22 | 13 | 39 |
| Iliac LN | 18 | 7 | 70 |
| Thymus | 75 | 78 | 1 |
| 94077 | |||
| Day 0 PBMC | 49 (2,342) | 20 | 22 |
| PBMC | 11 (90) | 70 | 13 |
| Spleen | 14 | 33 | 50 |
| Cervical LN | 9 | 42 | 49 |
| Axillary LN | 11 | 48 | 37 |
| Ileocecal LN | 12 | 45 | 48 |
| Mesenteric LN | 11 | 34 | 58 |
| Inguinal LN | 15 | 55 | 28 |
| Thymus | 79 | 74 | 2 |
| 92071 | |||
| Day 0 PBMC | 28 (1,303) | 26 | 31 |
| PBMC | 4 (74) | 24 | 14 |
| Spleen | 6 | 14 | 83 |
| Cervical LN | 5 | 20 | 71 |
| Axillary LN | 4 | 21 | 63 |
| Ileocecal LN | 2 | 22 | 73 |
| Mesenteric LN | 4 | 25 | 68 |
| Inguinal LN | 5 | 25 | 65 |
| Iliac LN | 3 | 24 | 70 |
| Thymus | 78 | 93 | NT |
SHIV89.6PD-infected rhesus macaques were euthanized on the indicated day after infection (see footnote b). Mononuclear cells from blood and tissues were stained with MAbs CD2-FITC and CD4-PE, MAbs CD2-FITC and CD8-PE, or MAb CD20-PE. The reported CD4+ and CD8+ T-cell percentages are double positive for CD2 expression. Day 0 (before infection) PBMC lymphocyte subset percentages and lymphocyte subset percentages for blood, spleen, LN, and thymus samples on the indicated day after SHIV89.6PD inoculation (day of euthanasia and necropsy) are reported. The absolute number of CD4+ T cells per microliter of blood was determined from the percentage of lymphocytes double positive for CD2 and CD4 and the total number of lymphocytes per microliter of blood as determined by CBC. Values for virus-naive macaque (n = 5) blood samples were 38.8% CD4+, 21.2% CD8+, and 16.6% CD20+ lymphocytes, compared to inguinal lymph node samples with 39.6% CD4+, 16.8% CD8+, and 30.8% CD20+ lymphocytes (27).
Infection routes and sample collection times are as follows: 94074, i.v. inoculation, day 4 necropsy; 94079, i.r. inoculation, day 8 necropsy; 94069, i.vag. inoculation, day 8 necropsy; 94089, i.v. inoculation, day 8 necropsy; 94077, i.r. inoculation, day 15 necropsy; 92071, i.vag. inoculation, day 15 necropsy.
In parentheses are absolute numbers of CD4+ T cells per microliter of blood.
NT, not tested.
Mononuclear cell preparations from the blood, LN, and spleen were tested for proliferative responses to simian immunodeficiency virus capsid protein p27 and to the mitogen PHA. p27-specific proliferative responses were not detected in any macaque samples from these early time points after infection (data not shown) by the same assay that detected antigen-specific lymphoproliferation in immunized macaques (31). It was surprising that proliferative responses to PHA were low or absent in lymphocytes of the blood, LN, and spleen from animal 94089 by 8 days after i.v. infection (Table 3). In contrast, blood and lymphoid tissue lymphocytes from 4 day i.v. infected animal 94074 proliferated in response to PHA with SIs comparable to those of control lymphocytes from uninfected rhesus macaques (range, 40 to 650; data not shown). Freshly isolated mononuclear cells from LN of mucosally infected macaques had poor responses to PHA as early as 8 days after infection (Table 4), and these results were very similar to those obtained with cryopreserved cells. This inability to proliferate in response to the mitogen preceded the bulk depletion of CD4+ T cells from blood and tissues and occurred before extensive virus dissemination. These observations were confirmed in repetitive experiments testing proliferative responses with cryopreserved lymphocytes from blood and tissues. In all cases, cryopreserved cells were more than 90% viable based on vital dye assays and we did not detect systematic differences in the response to mitogen among cryopreserved or freshly isolated cells. Cell counts in replicate plates demonstrated that cells from nonresponsive cultures were dying in response to stimulation (data not shown).
TABLE 3.
Lymphocyte tritiated-thymidine incorporation in response to PHAa
| Sample | 94074b
|
94089c
|
||
|---|---|---|---|---|
| Medium cpm | SI (PHA cpm) | Medium cpm | SI (PHA cpm) | |
| Necropsy PBMC | 119 ± 6 | 220 (26,211 ± 1,432) | 76 ± 21 | 1 (75 ± 17) |
| Spleen | 56 ± 1 | 122 (6,780 ± 506) | 68 ± 15 | 1 (71 ± 28) |
| Mesenteric LN | 38 ± 4 | 40 (1,502 ± 517) | 44 ± 15 | 1 (35 ± 4) |
| Inguinal LN | 31 ± 3 | 78 (2,377 ± 534) | 84 ± 11 | 3 (229 ± 114) |
PBMC or tissue mononuclear cells (105) isolated from euthanized rhesus macaques on the indicated days after infection (see footnotes b and c) were tested for DNA synthesis in response to medium or PHA. Mean counts per minute of triplicate samples ± the standard deviations are reported.
Day 4 after i.v. infection with SHIV89.6PD.
Day 8 after i.v. infection with SHIV89.6PD.
TABLE 4.
Lymphocyte SIs in response to PHAa
| Sample | 94079
|
94077
|
94069
|
92071
|
||||
|---|---|---|---|---|---|---|---|---|
| Medium cpm | SI (PHA cpm) | Medium cpm | SI (PHA cpm) | Medium cpm | SI (PHA cpm) | Medium cpm | SI (PHA cpm) | |
| Axillary LN | 34 ± 9 | 1 (44 ± 16) | 36 ± 2 | 2 (66 ± 13) | 43 ± 26 | 4 (179 ± 64) | 30 ± 7 | 14 (420 ± 105) |
| Ileocecal LN | 32 ± 4 | 5 (160 ± 24) | 61 ± 12 | 2 (112 ± 45) | 29 ± 6 | 1 (33 ± 4) | 32 ± 7 | 1 (30 ± 8) |
| Mesenteric LN | 29 ± 1 | 1 (32 ± 6) | 54 ± 40 | 1 (74 ± 75) | 38 ± 10 | 3 (120 ± 45) | 27 ± 12 | 1 (27 ± 11) |
| Inguinal LN | NTb | NT | 25 ± 7 | 3 (63 ± 20) | 45 ± 12 | 3 (152 ± 45) | 120 ± 113 | 3 (379 ± 124) |
We euthanized i.r. SHIV89.6PD-infected rhesus macaques 94079 and 94077 on days 8 and 15 after infection, respectively, and i.v. infected rhesus macaques 94069 and 92071 on days 8 and 15 after infection, respectively. Mononuclear cells (105) isolated from LN were tested in triplicate samples for DNA synthesis in response to medium or PHA. Mean counts per minute of triplicate samples ± the standard deviations are reported. The mean counts per minute of PBMC isolated from macaques 94069 and 92071 were 1,739 ± 315 and 2,168 ± 176, respectively, in response to PHA before infection.
NT, not tested.
Lymphocyte activation markers.
We chose to explore the possibility that AICD was responsible for loss of in vitro proliferative responses. Initially, we examined lymphocyte surface markers for evidence of polyclonal activation in vivo. We began this study by infecting three additional macaques, AQ93, AQ80, and AR08, by the i.r. route with SHIV89.6PD. A fourth animal, AQ73, received the same inoculum but was negative for infection by all laboratory tests and did not even show transient viremia. By establishing a new infected-animal cohort, we were able to obtain fresh PBMC samples that improved our ability to study activation markers and cellular apoptosis. Infected macaques had detectable p27 antigenemia at 2 weeks after inoculation, and virus could be isolated from as few as 1,000 PBMC. The number of infected cells in the PBMC decreased to 1 in 10,000 or 1 in 100,000 by 4 weeks after infection. These animals experienced significant losses of peripheral blood CD4+ T cells between 2 and 4 weeks after infection (Fig. 1A). AQ80 and AQ93 produced virus-specific antibodies by 4 weeks after infection, and AR08 seroconverted at 8 weeks after infection. All animals developed moderate to high levels of virus-binding antibodies (29) that were assayed at a serum dilution of 1:500 (A405, >0.2) by using a commercial ELISA kit (Sanofi/Pasteur, Chaska, Minn.).
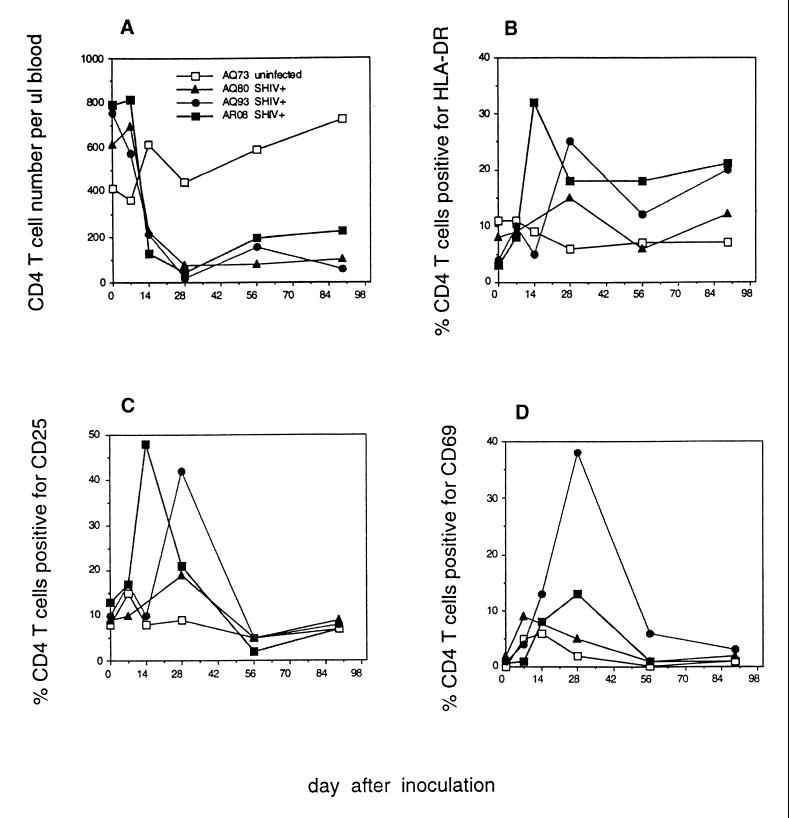
FIG. 1.
CD4+ T-cell numbers and activation marker expression after SHIV89.6PD infection. Rhesus macaques AQ73, AQ80, AQ93, and AR08 were inoculated i.r. with SHIV89.6PD. All except AQ73 were confirmed to be infected on the basis of at least two positive virus isolations, seroconversion, and PCR assay for viral gag sequences as described previously (33). Blood was drawn on weeks 0, 1, 2, 4, 8, and 13 after inoculation. The absolute number of CD4+ T cells per microliter of blood (A) was determined from the percentage of lymphocytes double positive for CD2 and CD4 and the total number of lymphocytes per microliter of blood as determined by CBC. The percentage of CD4+ T cells positive for HLA-DR (B), CD25 (C), or CD69 (D) was determined by flow cytometry.
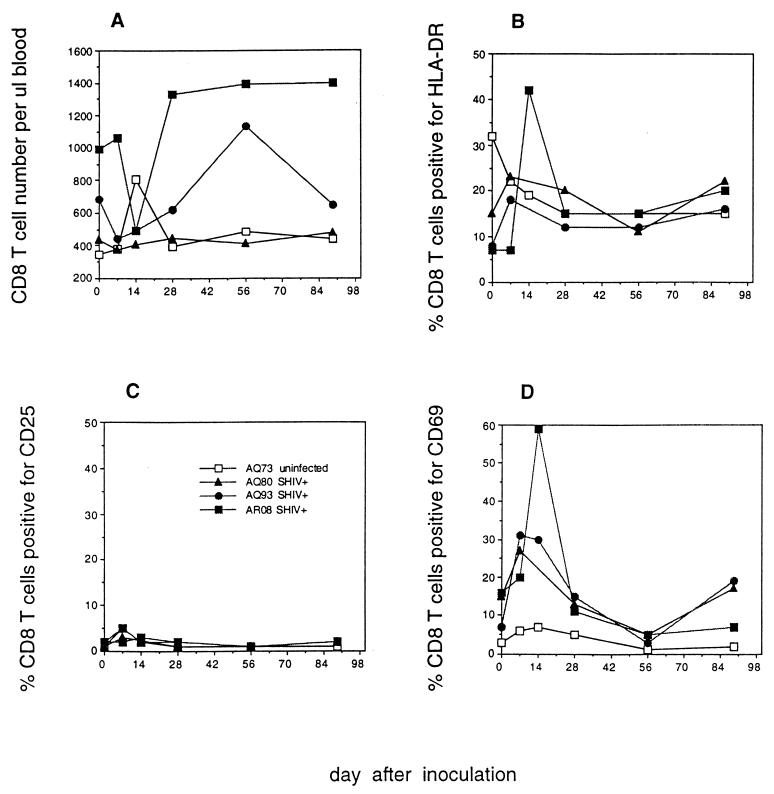
Increased HLA-DR, CD25, and CD69 expression was apparent on CD4+ T cells of SHIV89.6PD-infected macaques AQ93 and AR08 between 14 and 28 days after infection (Fig. 1B to D). We were unable to assess activation marker expression for macaque AQ80 at 2 weeks after infection due to low PBMC counts. However, we detected increased HLA-DR and CD25 expression on AQ80 CD4+ T cells by 28 days after infection (Fig. 1B and C). HLA-DR and CD69 expression increased on CD8+ T cells from AQ93 and AR08 between days 7 and 14 (Fig. 2B and D). CD69 expression increased on CD8+ T cells of AQ80 by day 7 after infection. The kinetics of polyclonal lymphocyte activation coincided with the kinetics of CD4+ T-cell depletion among infected macaques. Increased T-cell activation marker expression during the first month after inoculation was in contrast to low activation marker expression on T cells from uninfected macaque AQ73 (Fig. 1 and 2). By 56 days after infection, increased expression of CD25 and CD69 on T cells from SHIV89.6PD-infected macaques waned to preinfection levels. Additional data for lymphocyte phenotype in virus-naive animals (Table 5) showed low levels of activation marker expression in both CD4+ and CD8+ T-cell populations.
FIG. 2.
CD8+ T-cell number and activation marker expression after SHIV89.6PD infection. Rhesus macaques AQ73, AQ80, AQ93, and AR08 were inoculated with SHIV89.6PD i.r. All except AQ73 were infected. Blood was drawn on weeks 0, 1, 2, 4, 8, and 13 after inoculation. The absolute number of CD8+ T cells per microliter of blood (A) was determined from the percentage of lymphocytes double positive for CD2 and CD8 and the total number of lymphocytes per microliter of blood as determined by CBC. The percentage of CD8+ T cells positive for HLA-DR (B), CD25 (C), or CD69 (D) was determined by flow cytometry.
TABLE 5.
Activation marker expression on lymphocytes from eight virus-naive macaquesa
| Marker expression | Mean (range) % of lymphocytes |
|---|---|
| CD4+/HLA-DR+ | 3.43 (1.69–4.23) |
| CD4+/CD25+ | 6.15 (4.07–9.08) |
| CD4+/CD69+ | 0.49 (0.40–0.61) |
| CD8+/HLA-DR+ | 15.56 (7.29–25.22) |
| CD8+/CD25+ | 0.78 (0.40–1.32) |
| CD8+/CD69+ | 6.43 (1.95–13.02) |
The percentage of CD4+ or CD8+ T cells double positive for HLA-DR, CD25, or CD69 was determined by flow cytometry.
Apoptosis of lymphocytes from SHIV89.6PD-infected macaques.
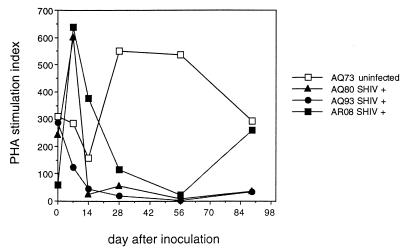
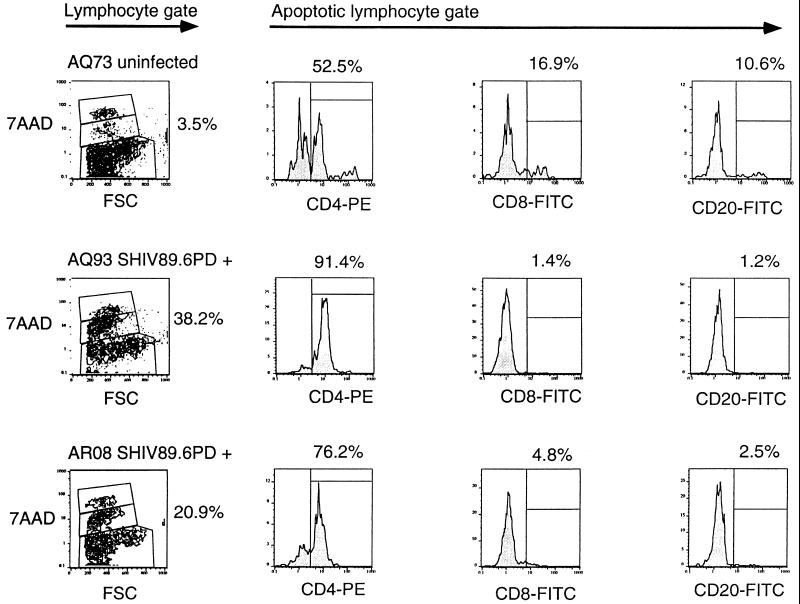
The lack of response to PHA was associated with cell death in vitro. We next wanted to determine whether apoptosis was occurring in these stimulated lymphocyte cultures, and we employed a flow cytometry method utilizing 7-AAD staining in conjunction with cell surface staining for lymphocyte differentiation markers (28). This method gave results similar to those of annexin-V assays and was preferred for obtaining data that could be combined with cell surface marker phenotype studies (15). Moreover, 7-AAD staining easily allowed the identification and exclusion of debris or apoptotic bodies. Cells staining dimly with 7-AAD are entering apoptosis, whereas those which stain brightly are late in apoptosis or already dead. PBMC proliferation in response to PHA was diminished substantially at 2 weeks after infection for AQ80 and AQ93 and at 4 weeks after infection for AR08. SIs for uninfected animal AQ73 remained above 150 at all time points (Fig. 3). We utilized the 7-AAD assay to determine the percentage of cells undergoing apoptosis after 24 h in culture with PHA (Table 6 and Fig. 4). By gating on dimly 7-AAD-staining lymphocytes, we were able to determine the percentage of apoptotic cells. Substantial increases in the percentages of lymphocytes undergoing apoptosis after PHA stimulation were observed in blood from infected animals AQ93 and AR08 by 2 weeks after infection (Table 6). Sufficient PBMC were not available from macaque AQ80 to perform the 7-AAD assay at week 2. However, the percentage of PBMC undergoing apoptosis was increased in this infected animal by 4 weeks after SHIV infection compared to the earlier time points (Table 6). Apoptotic cells from AQ93 and AR08 at 2 weeks were predominantly CD4+ T cells. CD8+ T cells and CD20+ B cells together accounted for less than 8% of the apoptotic cells (Fig. 4). It is possible that some CD8+ T cells and CD20+ B cells made up a proportion of the late-apoptotic or dead-cell population at the time of assay. However, as dead cells often bind surface antibodies nonspecifically, we chose not to analyze the brightly 7-AAD-staining population. The percentage of apoptotic lymphocytes after PHA stimulation remained between 4 and 6% for all blood draws from uninfected macaque AQ73 (Table 6 and Fig. 4). Additional data for the percentage of apoptotic lymphocytes after PHA stimulation from virus-naive macaques were similarly low (Table 6). Consistent with these results, we observed higher numbers of eosin-staining dead cells from cultures with high levels of brightly 7-AAD-staining populations. Overnight culture of PBMC in medium alone did not induce apoptosis in lymphocytes from either infected or uninfected macaques (data not shown).
FIG. 3.
Proliferation of rhesus macaque lymphocytes in response to PHA. Rhesus macaques AQ73, AQ80, AQ93, and AR08 were inoculated with SHIV89.6PD i.r. All except AQ73 were infected. PBMC isolated from the animals on weeks 0, 1, 2, 4, 8, and 13 after inoculation were tested for the ability to proliferate in response to PHA or medium alone. Data are reported as SIs.
TABLE 6.
Apoptosis of lymphocytes after PHA stimulationa
| Animal | Mean % of apoptotic cells in lymphocyte gate ± SD on following day after SHIV89.6PD inoculation:
|
|||
|---|---|---|---|---|
| 0 | 7 | 14 | 28 | |
| AQ73 | 4.1 ± 0.4 | 5.3 ± 0.2 | 4.7 ± 1.4 | 5.9 ± 0.7 |
| AQ80 | 6.6 ± 2.3 | 6.9 ± 1.3 | NTb | 12.2 ± 1.6 |
| AQ93 | 3.7 ± 0.6 | 9.4 ± 0.4 | 36.7 ± 11.4 | 5.3 ± 1.4 |
| AR08 | 4.6 ± 0.1 | 7.1 ± 0.6 | 20.7 ± 0.4 | 1.2 ± 0.2 |
PBMC (106/ml) were stimulated with 0.5 μg of PHA per ml for 24 h. Cells were counted and harvested for staining with MAb CD4-PE, CD8-FITC, or CD20-FITC and reactivity with 7-AAD to assess the percentage of cells undergoing apoptosis. The percentage of dimly 7-AAD-staining cells in the lymphocyte gate was determined for each of triplicate samples. The results are reported for triplicate samples. Samples from eight virus-naive animals were analyzed in the same manner as other samples, and the average percentage of apoptotic cells (± the standard deviation) was 7.8 ± 1.4.
NT, not tested.
FIG. 4.
Apoptosis of CD4+ T cells from macaques. Data are results from one uninfected macaque, AQ73, and two SHIV89.6PD-infected macaques, AQ93 and AR08. PBMC isolated from the animals 2 weeks after inoculation were stimulated by incubation with PHA in complete RPMI medium for 24 h. Cells were counted, and samples were stained with MAbs against surface markers (CD4-PE, CD8-FITC, or CD20-FITC) or appropriate isotype control MAbs. Cells were then stained with 7-AAD as described in Materials and Methods. Data were analyzed by the Flow Jo analysis program. A lymphocyte gate was first set by using forward-side scatter (FSC) on the x axis and side scatter on the y axis. From the defined lymphocyte gate, dot plots with FSC on the x axis and 7-AAD brightness on the y axis are shown. Lymphocytes reacting dimly with 7-AAD in the central region of these plots are undergoing apoptosis. Cells in this apoptotic gate were analyzed for surface marker expression as shown by the histograms.
DISCUSSION
We examined events during acute SHIV89.6PD infection of macaques to characterize changes in host-virus interactions that might dictate disease progression rates. These studies tested the hypothesis that a wave of lymphocyte activation occurs very soon after primary infection and serves to accelerate disease progression by increasing direct (virus infection) and indirect (apoptosis) CD4+ T-cell depletion and limits the magnitude of the subsequent immune responses to virus.
Approximately 2 weeks elapsed between the time of mucosal inoculation and the onset of abundant virus replication in secondary lymphoid tissues. The early spread of virus was accompanied by polyclonal cell activation that was observed as increased expression of specific cell surface markers and increased susceptibility to AICD upon exposure to mitogen in vitro. During the first week after infection, increased expression of early T-cell activation markers on CD4+ T cells was observed in the SHIV89.6PD-infected macaques. Overall levels of CD4+ T-cell activation marker expression were maximal between weeks 2 and 4 after infection. By week 2 after mucosal infection, systemic virus replication reached high levels which correlated with the period of rapid CD4+ T-cell loss from the blood and tissue compartments. During this early interval, remaining CD4+ T cells were highly susceptible to AICD when they were stimulated in vitro with polyclonal mitogen. However, the extent of activation and subsequent AICD greatly exceeded the frequency of infected CD4+ T cells. The wave of polyclonal lymphocyte activation likely contributed to the high levels of SHIV89.6PD replication. Accelerated growth of virus within the population of activated lymphocytes furthers cell depletion and establishes a condition favorable to persistent infection with progressing disease. Declining virus replication was coincident with the onset of host immune responses, and the interval of acute infection ended by 8 weeks after infection.
During the crucial first week after exposure, very low levels of virus initiated widespread changes in uninfected CD4+ T cells and the cells appeared to be at risk for AICD and virus infection. These events are amplified and accelerated in the case of a highly virulent virus such as SHIV89.6PD, and this model provides a convenient system for evaluation of their contributions to pathogenesis. We have used SHIV89.6PD infection because it exaggerates the events of acute infection. Even though this model may only represent the most aggressive HIV infections, we hope to uncover general mechanisms that can be tested in a broad range of infection examples.
T-cell unresponsiveness to mitogenic stimulation was associated with cell death and preceded substantial CD4+ T-cell losses in vivo. These observations suggest strongly that early immune dysfunction among CD4+ T cells is due to inappropriately high activation of lymphocytes during the first weeks of infection. Moreover, the kinetics of lymphocyte activation indicate the action of extracellular factors originating from the virus, the host, or both. Viral proteins, including the envelope protein and Tat, are known to induce apoptosis of uninfected CD4+ T cells (1, 8, 16, 34). It is also possible that specific cytokines induced early in infection, such as gamma interferon and tumor necrosis factor alpha (10, 14), promote apoptosis.
There are numerous examples of susceptibility to AICD during asymptomatic infection of HIV in humans (11, 19) or simian immunodeficiency virus in macaques (7, 9). Among HIV-1-infected persons, the extent of apoptosis in LN lymphocytes was associated directly with cell activation in these tissues (20). In a previous study, we observed loss of proliferative responses to p27 and PHA as early as 1 week after i.r. SHIV89.6PD infection in p27-immunized rhesus macaques; depressed mitogen responses were transient and returned to normal levels by 8 weeks after infection, while proliferative responses to Gag antigen did not recover (31). Similar observations of transient lymphocyte unresponsiveness were reported in HIV-1+ persons during acute retroviral syndrome (5, 22).
Our analysis of acute infection seeks to identify the earliest events in viral pathogenesis and to assess their impact on subsequent disease progression. The present studies show that lymphocyte activation, triggered as a nearly immediate consequence of virus infection, begins the process of CD4+ T-cell depletion and renders the remaining lymphocytes more permissive for virus replication. The extent of acute CD4+ T-cell depletion likely limits the remaining immune repertoire and establishes a host set point, that we define as a measure of immune competence for controlling virus replication and modulating subsequent disease progression. The foundation of immune competence is the breadth and strength of an intact, virus-specific, CD4+ T-cell repertoire. CD4+ T cells provide crucial cytokine help for antiviral cytotoxic T lymphocytes and for the generation of T-cell-dependent virus-neutralizing antibodies that are associated with long-term nonprogression (3, 24). Lesions in the CD4+ T-cell repertoire are not repaired rapidly by antiviral therapy, despite increased CD4+ T-cell numbers (4). Strategies for managing acute infection should include efforts to control virus replication but may also need to account for lymphocyte activation and AICD via apoptosis that also promote destruction of the helper repertoire.
The host set point describes the capacity for immune control of disease progression. One part of this control is the ability to modulate virus replication and to establish a viral set point that provides useful quantitative values for prognosis and evaluation of therapy (17). However, to better understand AIDS pathogenesis, we need to direct our investigations closer to the time of initial exposure to uncover the host mechanisms affecting and being affected by early events in virus infection. Studies with the macaque model show that the earliest events of acute infection remodel the CD4+ T-cell repertoire, establish the host set point, and dictate the subsequent levels of virus replication and rates of disease progression to AIDS.
ACKNOWLEDGMENTS
We are grateful to James Thomson for microscopic pathology of necropsy tissues and to Maria S. Salvato and Miroslav Malkovsky for helpful comments and discussions.
These studies were supported by PHS grants AI38491 and AI/RR42534 (C.D.P.) and grant RR00167 (Regional Primate Center Support). M.W. was supported by Virology Oncology Training Grant 5T32 CA09075.
Footnotes
Publication 39-014 from the Wisconsin Regional Primate Research Center.
REFERENCES
- 1.Banda N K, Bernier J, Kurahara D K, Kurrle R, Haigwood N, Sekaly R P, Finkel T H. Crosslinking CD4 by human immunodeficiency virus gp120 primes T cells for activation-induced apoptosis. J Exp Med. 1992;176:1099–1106. doi: 10.1084/jem.176.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark S J, Saag M S, Decker W D, Campbell-Hill S, Roberson J L, Veldkamp P J, Kappes J C, Hahn B H, Shaw G M. High titers of cytopathic virus in plasma of patients with symptomatic primary HIV-1 infection. N Engl J Med. 1991;324:954–960. doi: 10.1056/NEJM199104043241404. [DOI] [PubMed] [Google Scholar]
- 3.Cohen O J, Kinter A, Fauci A S. Host factors in the pathogenesis of HIV disease. Immunol Rev. 1997;159:31–48. doi: 10.1111/j.1600-065x.1997.tb01005.x. [DOI] [PubMed] [Google Scholar]
- 4.Connors M, Kovacs J A, Krevat S, Gea-Banacloche J C, Sneller M C, Flanigan M, Metcalf J A, Walker R E, Falloon J, Baseler M, Stevens R, Feuerstein I, Masur H, Lane H C. HIV infection induces changes in CD4+ T-cell phenotype and depletions within the CD4+ T-cell repertoire that are not immediately restored by antiviral or immune-based therapies. Nat Med. 1997;3:533–540. doi: 10.1038/nm0597-533. [DOI] [PubMed] [Google Scholar]
- 5.Cooper D A, Tindall B, Wilson E J, Imrie A A, Penny R. Characterization of T lymphocyte responses during primary infection with human immunodeficiency virus. J Infect Dis. 1988;157:889–896. doi: 10.1093/infdis/157.5.889. [DOI] [PubMed] [Google Scholar]
- 6.Daar E S, Moudgil T, Meyer R D, Ho D D. Transient high levels of viremia in patients with primary human immunodeficiency virus type 1 infection [see comments] N Engl J Med. 1991;324:961–964. doi: 10.1056/NEJM199104043241405. [DOI] [PubMed] [Google Scholar]
- 7.Estaquier J, Idziorek T, de Bels F, Barre-Sinoussi F, Hurtrel B, Aubertin A M, Venet A, Mehtali M, Muchmore E, Michel P, et al. Programmed cell death and AIDS: significance of T-cell apoptosis in pathogenic and nonpathogenic primate lentiviral infections. Proc Natl Acad Sci USA. 1994;91:9431–9435. doi: 10.1073/pnas.91.20.9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finkel T H, Tudor-Williams G, Banda N K, Cotton M F, Curiel T, Monks C, Baba T W, Ruprecht R M, Kupfer A. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med. 1995;1:129–134. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- 9.Gougeon M L, Garcia S, Heeney J, Tschopp R, Lecoeur H, Guetard D, Rame V, Dauguet C, Montagnier L. Programmed cell death in AIDS-related HIV and SIV infections. AIDS Res Hum Retroviruses. 1993;9:553–563. doi: 10.1089/aid.1993.9.553. [DOI] [PubMed] [Google Scholar]
- 10.Groux H, Monte D, Plouvier B, Capron A, Ameisen J C. CD3-mediated apoptosis of human medullary thymocytes and activated peripheral T cells: respective roles of interleukin-1, interleukin-2, interferon-gamma and accessory cells. Eur J Immunol. 1993;23:1623–1629. doi: 10.1002/eji.1830230734. [DOI] [PubMed] [Google Scholar]
- 11.Groux H, Torpier G, Monté D, Mouton Y, Capron A, Ameisen J C. Activation-induced death by apoptosis in CD4+ T cells from human immunodeficiency virus-infected asymptomatic individuals. J Exp Med. 1992;175:331–340. doi: 10.1084/jem.175.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henrard D R, Phillips J F, Muenz L R, Blattner W A, Wiesner D, Eyster M E, Goedert J J. Natural history of HIV-1 cell-free viremia. JAMA. 1995;274:554–558. [PubMed] [Google Scholar]
- 13.Hirsch V M, Dapolito G, Johnson P R, Elkins W R, London W T, Montali R J, Goldstein S, Brown C. Induction of AIDS by simian immunodeficiency virus from an African green monkey: species-specific variation in pathogenicity correlates with the extent of in vivo replication. J Virol. 1995;69:955–967. doi: 10.1128/jvi.69.2.955-967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaneko H, Hishikawa T, Sekigawa I, Hashimoto H, Okumura K, Kaneko Y. Role of tumour necrosis factor-alpha (TNF-alpha) in the induction of HIV-1 gp120-mediated CD4+ T cell anergy. Clin Exp Immunol. 1997;109:41–46. doi: 10.1046/j.1365-2249.1997.4231325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lecoeur H, Ledru E, Prevost M C, Gougeon M L. Strategies for phenotyping apoptotic peripheral human lymphocytes comparing ISNT, annexin-V and 7-AAD cytofluorometric staining methods. J Immunol Methods. 1997;209:111–123. doi: 10.1016/s0022-1759(97)00138-5. [DOI] [PubMed] [Google Scholar]
- 16.Li C J, Friedman D J, Wang C, Metelev V, Pardee A B. Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science. 1995;268:429–431. doi: 10.1126/science.7716549. [DOI] [PubMed] [Google Scholar]
- 17.Lifson J D, Nowak M A, Goldstein S, Rossio J L, Kinter A, Vasquez G, Wiltrout T, Brown C, Schneider D, Wahl L, Lloyd A L, Williams J, Elkins W R, Fauci A S, Hirsch V A. The extent of early viral replication is a critical determinant of the natural history of simian immunodeficiency virus infection. J Virol. 1997;71:9508–9514. doi: 10.1128/jvi.71.12.9508-9514.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu Y C, Pauza C D, Lu X S, Montefiori D C, Miller C J. Rhesus macaques that become systemically infected with pathogenic SHIV89.6-PD after intravenous, rectal, or vaginal inoculation and fail to make an antiviral antibody response rapidly develop AIDS. J Acquired Immune Defic Syndr Hum Retrovirol. 1998;19:6–18. doi: 10.1097/00042560-199809010-00002. [DOI] [PubMed] [Google Scholar]
- 19.Medina E, Borthwick N, Johnson M A, Miller S, Bofill M. Flow cytometric analysis of the stimulatory response of T cell subsets from normal and HIV-1+ individuals to various mitogenic stimuli in vitro. Clin Exp Immunol. 1994;97:266–272. doi: 10.1111/j.1365-2249.1994.tb06079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muro-Cacho C A, Pantaleo G, Fauci A S. Analysis of apoptosis in lymph nodes of HIV-infected persons: intensity of apoptosis correlates with the general state of activation of the lymphoid tissue and not with stage of disease or viral burden. J Immunol. 1995;154:5555–5566. [PubMed] [Google Scholar]
- 21.Niu M T, Stein D S, Schnittman S M. Primary human immunodeficiency virus type 1 infection: review of pathogenesis and early treatment intervention in humans and animal retrovirus infections. J Infect Dis. 1993;168:1490–1501. doi: 10.1093/infdis/168.6.1490. [DOI] [PubMed] [Google Scholar]
- 22.Pedersen C, Dickmeiss E, Gaub J, Ryder L P, Platz P, Lindhardt B O, Lundgren J D. T-cell subset alterations and lymphocyte responsiveness to mitogens and antigen during severe primary infection with HIV: a case series of seven consecutive HIV seroconverters. AIDS. 1990;4:523–526. doi: 10.1097/00002030-199006000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Pedersen C, Lindhardt B O, Jensen B L, Lauritzen E, Gerstoft J, Dickmeiss E, Gaub J, Scheibel E, Karlsmark T. Clinical course of primary HIV infection: consequences for subsequent course of infection. B Med J. 1989;299:154–157. doi: 10.1136/bmj.299.6692.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pilgrim A K, Pantaleo G, Cohen O J, Fink L M, Zhou J Y, Zhou J T, Bolognesi D P, Fauci A S, Montefiori D C. Neutralizing antibody responses to human immunodeficiency virus type 1 in primary infection and long-term-nonprogressive infection. J Infect Dis. 1997;176:924–932. doi: 10.1086/516508. [DOI] [PubMed] [Google Scholar]
- 25.Reimann K A, Li J T, Veazy R, Halloran M, Park I-W, Karlsson G B, Sodroski J, Letvin N L. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J Virol. 1996;70:6922–6928. doi: 10.1128/jvi.70.10.6922-6928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schacker T W, Hughes J P, Shea T, Coombs R W, Corey L. Biological and virologic characteristics of primary HIV infection. Ann Intern Med. 1998;128:613–620. doi: 10.7326/0003-4819-128-8-199804150-00001. [DOI] [PubMed] [Google Scholar]
- 27.Schenkel A R, Uno H, Pauza C D. Asymptomatic simian immunodeficiency virus infection decreases blood CD4+ T cells by accumulating recirculating lymphocytes in the lymphoid tissues. J Virol. 1999;73:601–607. doi: 10.1128/jvi.73.1.601-607.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmid I, Uittenbogaart C H, Keld B, Giorgi J V. A rapid method for measuring apoptosis and dual-color immunofluorescence by single laser flow cytometry. J Immunol Methods. 1994;170:145–157. doi: 10.1016/0022-1759(94)90390-5. [DOI] [PubMed] [Google Scholar]
- 29.Steger K K, Dykhuizen M, Mitchen J, Hinds P W, Preuninger B L, Wallace M, Thomson J, Lu Y, Pauza C D. CD4+ T cell and CD20+ B cell changes that predict rapid disease progression after SHIV89.6PD inoculation of rhesus macaques. J Virol. 1998;72:1600–1605. doi: 10.1128/jvi.72.2.1600-1605.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steger K K, Pauza C D. Immunization of Macaca mulatta with aroA-attenuated Salmonella typhimurium expressing SIV p27 antigen. J Med Primatol. 1997;26:44–50. doi: 10.1111/j.1600-0684.1997.tb00318.x. [DOI] [PubMed] [Google Scholar]
- 31.Steger K K, Waterman P M, Pauza C D. Acute effects of pathogenic simian-human immunodeficiency virus challenge on vaccine-induced cellular and humoral responses to Gag in rhesus macaques. J Virol. 1999;73:1853–1859. doi: 10.1128/jvi.73.3.1853-1859.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tindall B, Cooper D A. Primary HIV infection: host responses and intervention strategies. AIDS. 1991;5:1–14. [PubMed] [Google Scholar]
- 33.Trivedi P, Horejsh D, Hinds S B, Hinds II P W, Wu M S, Salvato M S, Pauza C D. Intrarectal transmission of simian immunodeficiency virus in rhesus macaques: selective amplification and host responses to transient or persistent viremia. J Virol. 1996;70:6876–6883. doi: 10.1128/jvi.70.10.6876-6883.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westendorp M O, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin K-M, Krammer P H. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature. 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]