Abstract
Peripheral artery disease (PAD) commonly refers to atherosclerotic narrowing of noncoronary arteries, primarily those supplying the lower extremities. The risk factors for PAD include smoking, hyperlipidemia, hypertension, and diabetes mellitus. Patients with PAD are at a heightened risk of major adverse cardiovascular events (including myocardial infarction, stroke, and cardiovascular death) and major adverse limb events (including progressive symptoms or limb ischemia requiring peripheral revascularization, amputation, and acute limb ischemia), highlighting the need for guideline-directed therapies. Lifestyle modifications and medical therapies are utilized to improve function and outcomes in this patient population. Adherence to a healthy diet and smoking cessation are both associated with better outcomes in patients with PAD. Medical therapies targeting axes of risk, including lipid-modifying therapies, antithrombotic therapies, and targeted diabetes therapies, are available to reduce this risk in patients with PAD; however, significant residual risk remains. Unfortunately, despite guideline recommendations and efforts at education, even available medical therapies remain underutilized in patients with PAD. Continued development of novel therapies and efforts to improve the provision of care in patients with PAD are needed.
Keywords: lifestyle modification, medical therapy, peripheral arterial disease, pharmacotherapy
Central Illustration
Highlights
-
•
Patients with peripheral artery disease are at an increased risk of major adverse cardiovascular events and major adverse limb events.
-
•
Lifestyle modification, including smoking cessation and healthy diet, are important first steps.
-
•
Medical therapies can reduce the risk of MACE and/or MALE.
-
•
Despite guideline recommendations, these patients remain undertreated.
Introduction
Peripheral artery disease (PAD) commonly refers to atherosclerotic narrowing of noncoronary arteries; this review focuses on atherosclerosis of the lower extremities. The prevalence of PAD in patients aged ≥50 years is 12% to 20% and increases to ∼50% at the age of ≥85 years.1 The risk factors for PAD include smoking, hyperlipidemia, hypertension, and diabetes mellitus (DM).2 Patients with PAD are at a heightened risk of major adverse cardiovascular events (MACE), including myocardial infarction (MI), stroke, and cardiovascular (CV) death. Importantly, PAD is a manifestation of systemic atherosclerosis: in the Study Comparing Cardiovascular Effects of Ticagrelor and Clopidogrel with Patients with Peripheral Artery Disease (EUCLID) trial, 44% of patients with symptomatic PAD had concomitant coronary artery and/or cerebrovascular disease.3 These patients with polyvascular disease are at the highest risk of MACE.4
In addition to MACE, patients with PAD experience limb-related disabilities and adverse events, which represent dominant morbidity. Impaired lower-extremity perfusion can cause exertional leg discomfort and impaired walking performance.5 Severe impairment can lead to critical limb ischemia (CLI) and increases the risk of major adverse limb events (MALE) such as peripheral revascularization, amputation, and acute limb ischemia (ALI). Prior peripheral revascularization has been shown to be associated with a ∼4-fold increased risk of ALI.6 Importantly, the pathophysiology of PAD is complex and involves inflammation, atherogenesis, and thrombosis.7 In addition to obstructive PAD, microvascular disease (MVD) may contribute to an increased risk of amputation in high-risk subgroups, for example, patients with chronic renal insufficiency and diabetes.8
Medical therapies have been shown to reduce the risk of MACE and/or MALE in patients with PAD; yet, the undertreatment of PAD, especially relative to coronary artery disease (CAD), remains well recognized.9,10 This undertreatment may be related to multiple factors, including lack of awareness, fragmentation of care, and racial and ethnic disparities regarding delayed diagnosis and treatment.11,12 Raising awareness about PAD and understanding treatment options are key for optimizing care for these high-risk patients. The following review discusses available medical management strategies to improve outcomes in patients with PAD.
Risk factor modification
The treatment of patients with PAD using proven therapies can lower the incidences of MACE and MALE, highlighting the importance of guideline-directed therapies. In addition, a major component of the treatment strategy includes lifestyle modifications such as dietary changes, exercise, and smoking cessation. The American Heart Association has created “Life’s Essential 8,” a resource for patients and providers that outlines 8 lifestyle choices to improve CV health.13
Dietary modifications
Adherence to a healthy diet is associated with a lower incidence of clinical PAD. This was demonstrated in the Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts (PREDIMED) trial, in which a Mediterranean diet was associated with a reduced risk of PAD.14 Among patients with PAD, poor nutrition has been shown to be associated with worse outcomes in terms of CLI, and there is evidence supporting chronic inflammation in patients with PAD.15 Diets enriched with foods with anti-inflammatory and antioxidant properties have not been proven beneficial in patients with PAD16; however, dietary modifications and access to high-quality and healthy nutrition are important both for prevention of PAD and improved outcomes in patients with established PAD.
Smoking cessation
Tobacco smoking promotes arterial damage through adverse effects on platelets, endothelial tissue, and coagulation pathways through oxidative stress and inhibition of nitric oxide,17,18 and cigarette smoking is associated with reduced patency in patients who have undergone bypass grafting.19 Cigarette smoke not only contains nicotine, which has been shown to accelerate plaque production,20 but also has other volatile organic compounds, such as acrolein, that have been shown to induce arterial endothelial damage.21 Beyond tobacco, both marijuana and “vaping” have also been shown to promote arterial damage in a similar manner.22,23
Smoking cessation has been shown to be associated with improvement of the arterial vasodilatory ischemic response, suggesting that some of the drivers of PAD can be reversed through smoking cessation.24 Smoking cessation is associated with a decreased risk of progression of PAD to CLI, amputation, and all-cause mortality.25 Further, patients who stop smoking have improved walking ability and decreased claudication symptoms.26
In addition to counseling, pharmacologic approaches increase the rates of smoking cessation. In the Study Evaluating the Safety and Efficacy of Varenicline and Bupropion for Smoking Cessation in Subjects With and Without a History of Psychiatric Disorders (EAGLES) trial, the effect of varenicline or bupropion versus that of single nicotine replacement therapy was studied, and it was found that that varenicline was superior to both nicotine replacement and bupropion27; varenicline is now considered first-line therapy.
Lipid-lowering therapy
Lipid-lowering therapies have been shown to slow the natural history of the progression of atherosclerosis in patients with PAD.28 Associations between higher levels of low-density lipoprotein-cholesterol (LDL-C) and increased risks of MACE and MALE have been consistently demonstrated.29,30 The Heart Protection Study (HPS) demonstrated that statins reduce MACE compared with a placebo, with consistent benefits in a subgroup of 6748 patients with PAD, wherein simvastatin reduced MACE by 22% and reduced the risk of the first acute peripheral vascular event (characterized by noncoronary revascularization, major amputation, aneurysm repair, or death due to PAD) by 16%.31 Confirmatory findings for the limb benefits of statins have largely been derived from observational data.32, 33, 34 In the Reduction of Atherothrombosis for Continued Health (REACH) registry, the use of statins was associated with lower rates of adverse limb events, characterized by worsening claudication or new CLI, limb revascularization, or amputation.34 A subsequent analysis from Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events—Thrombolysis in Myocardial Infarction 50 (TRA 2P-TIMI 50) demonstrated that statin therapy was associated with a 23% lower risk of MACE and 27% lower risk of an ischemic limb event,35 with greater benefit according to the intensity of the statin used.
Ezetimibe lowers the LDL level by inhibiting the gastrointestinal absorption of cholesterol. In the Ezetimibe Added to Statin Therapy After Acute Coronary Syndromes (IMPROVE-IT) trial, the effect of ezetimibe plus statin versus that of statin alone was studied in patients with prior acute coronary syndrome. Ezetimibe was associated with an 8% relative risk reduction in CV death, major coronary events, and stroke.36 The benefit was consistent among patients with polyvascular disease, including patients with PAD and cerebrovascular disease.
Proprotein convertase subtilisin/kexin type 9 inhibitors are effective therapies for reducing LDL-C levels by inhibiting the hepatic protease that internalizes LDL receptors into lysosomes for destruction, thus preventing the destruction of LDL receptors. The effect of evolocumab was studied in patients with stable atherosclerotic cardiovascular disease (ASCVD) in Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER).37 Among 3642 patients with PAD, evolocumab reduced the primary composite end point of CV death, MI, stroke, and hospitalization for unstable angina or coronary revascularization by 15% and reduced the risk of MALE by 37%. A linear relationship between risk reduction and LDL-C lowering down to an LDL-C level of <10 mg/dL was observed. In the ODYSSEY Outcomes: Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment with Alirocumab trial, the effect of alirocumab was studied in patients with recent acute coronary syndrome, which showed a 15% risk reduction in death related to CAD, nonfatal MI, ischemic stroke, or hospitalization for unstable angina while also reducing the risk of PAD events (CLI, limb revascularization, and amputation for ischemia) by 31%.38 The limb-related benefit of alirocumab appeared to be related to reductions in the levels of both lipoprotein(a) and LDL-C, supporting an important role of lipoprotein(a). Although inclisiran has been shown to reduce the LDL-C level by ∼50% in addition to statin therapy in patients with ASCVD or CV risk factors, data on its outcomes are not yet available.39
Icosapent ethyl is an eicosapentaenoic acid ethyl ester that reduces the levels of triglycerides. In the Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia (REDUCE-IT) trial, the effect of icosapent ethyl was studied in patients with established CV disease or diabetes and other risk factors as well as those with elevated fasting levels of triglycerides while on statin therapy.40 Icosapent ethyl significantly reduced the risk of CV events by 25%, with no benefit described for limb outcomes. The efficacy was consistent in a subgroup of 688 patients with PAD.41 Questions regarding the mechanism and magnitude of the benefit of icosapent ethyl have been raised in a post hoc analysis that demonstrated minimal differences in lipid and inflammatory biomarkers between treatment groups.42
Additional classes of lipid-lowering therapies have not been shown to be beneficial or have not yet been studied in patients with PAD. In Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglyceride: Impact on Global Health Outcomes (AIM-HIGH), the effect of niacin was examined in statin-treated patients with polyvascular disease, including PAD. Although niacin treatment did increase the level of high-density lipoprotein and lower the levels of LDL or triglycerides, it did not reduce MACE, and MALE was not reported.43 A combination of niacin plus lovastatin versus a dietary intervention also showed no effect on walking function or claudication onset time in patients with PAD.44 The effects of fibrates, which lower the levels of LDL-C and triglycerides and increase that of high-density lipoprotein-cholesterol via activation of lipoprotein lipase, on CV and limb events in patients with PAD have not been well studied. However, a 36% reduction in the risk of the first amputation with fenofibrate versus that with a placebo in patients with diabetes was observed in the Effect of Fenofibrate on Amputation Events in People With Type 2 Diabetes Mellitus (FIELD) study.45 Further studies may provide an insight into the mechanism of this benefit.
Glucose-lowering therapy
Diabetes promotes atherosclerosis and endothelial dysfunction and is a major risk factor for the development of PAD. Patients with diabetes have a heightened risk of MACE,46 and relative to PAD alone, the addition of DM to PAD is associated with a high risk of amputation.11 DM-related MVD involves impairment of vascular tone and autoregulation of blood flow. MVD and its role in limb complications are often discussed in the context of impaired wound healing and neuropathy in patients with diabetes. However, a recent analysis demonstrated that MVD not only is an independent risk factor for amputation but also potentiates the risk of amputation in patients with PAD.47
The data to support glycemic control to improve outcomes in patients with PAD and DM are mixed. In the United Kingdom Prospective Diabetes Study (UKPDS), glucose lowering with medical therapy versus with dietary restrictions showed benefits for the treatment of MVD and long-term benefits for reduction in the incidence of MI at 10 years.48 Other studies of intensive glucose lowering demonstrated no benefit for MACE reduction up to 5 years,49,50 whereas 1 trial reported lower amputation rates but excess mortality with intensive glucose control.49 Newer diabetes therapies have demonstrated large benefits for patients with DM that cannot be explained by glycemic control alone. These novel therapies are discussed below.
Glucagon-like peptide 1 receptor agonists
Glucagon-like peptide 1 receptor agonists (GLP1-RAs) exert pharmacologic effects by increasing glucose-dependent insulin secretion from pancreatic beta cells, decreasing glucagon secretion, and delaying gastric emptying. The beneficial CV effects of these drugs are driven by weight loss, improved blood pressure, decreased levels of glycosylated hemoglobin, and anti-inflammatory effects, in addition to inhibition of platelet aggregation and endothelial signaling.51 A subgroup analysis of the placebo-controlled Exenatide Study of Cardiovascular Event Lowering (EXSCEL) trial demonstrated no benefit for reduction in MACE or amputations in patients with PAD and diabetes treated with exenatide.52 However, in Semaglutide and Cardiovascular Outcomes in Patients With Type 2 Diabetes (SUSTAIN-6), it was found that patients with diabetes and PAD treated with semaglutide versus those treated with a placebo had a 39% reduction in the risk of MACE.53 Beyond CV benefits, a post hoc analysis of Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) demonstrated a significant reduction of 35% in the risk of amputation in patients with diabetes treated with liraglutide versus that in patients treated with a placebo.54
Observational data have reported decreased risks of MACE and MALE associated with GLP1-RAs when compared to dipeptidyl peptidase-4 (DPP-4) inhibitors.54 The trials of GLP1-RAs specifically in patients with DM and PAD include the Liraglutide and Peripheral Artery Disease Trial (STARDUST; NCT04881110), which examined the effects of liraglutide versus those of aggressive risk factor treatment on transcutaneous oxygen pressure, and Effects of Semaglutide on Functional Capacity in Patients with Type 2 Diabetes and Peripheral Artery Disease Trial (STRIDE; NCT04560998), which will assess for improvement of walking function with semaglutide.
Sodium-glucose cotransporter 2 inhibitors
Sodium-glucose cotransporter 2 (SGLT2) inhibitors impair the renal tubular reabsorption of glucose, leading to reduction in the concentration of blood glucose. Treatment with SGLT2 inhibitors reduces MACE, hospitalizations for heart failure (HF), and progression of kidney disease.55 However, a signal for an increased risk of amputation was observed with canagliflozin in patients with DM at a high CV risk (hazard ratio [HR], 1.97; 95% CI, 1.41-2.75).56 This finding was not confirmed in the subsequent Evaluation of the Effects of Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy (CREDENCE) trial, which examined the effect of canagliflozin versus that of a placebo in patients with DM and chronic kidney disease.57 The Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58 (DECLARE-TIMI 58) trial demonstrated a broad benefit of dapagliflozin in patients with DM with or at the risk of ASCVD for reduction in hospitalizations for HF as well as renal complications; a benefit for MACE reduction was noted predominantly in patients with established vascular disease.58 Patients with PAD versus those without PAD enrolled in DECLARE-TIMI 58 had an increased risk of limb events.59 The benefit of dapagliflozin for reduction in CV deaths or hospitalizations for HF was consistent among patients with PAD (HR, 0.86; 95% CI, 0.60-1.24), although the absolute benefits of dapagliflozin were greater given the increased ischemic risk at baseline in the population with PAD. No increased risk of amputation with dapagliflozin was observed.
More supporting data on SLGT2i in patients with PAD were obtained from Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) trial, which assessed the effect of empagliflozin versus that of a placebo in patients with diabetes and CV risk factors.60 In patients with PAD, compared with the placebo, empagliflozin reduced CV deaths (HR, 0.57; 95% CI, 0.37-0.88) as well as hospitalizations for HF (HR, 0.56; 95% CI, 0.35-0.92) and worsening kidney disease (HR, 0.54; 95% CI, 0.41-0.71). Empagliflozin was also associated with a 16% reduced risk of limb amputations in the patients with PAD.60 Similar results were seen in Cardiovascular Outcomes Following Ertugliflozin Treatment in Type 2 Diabetes Mellitus Participants With Vascular Disease (VERTIS-CV), which examined the effect of ertugliflozin versus that of a placebo in patients with DM and ASCVD.61 Ertugliflozin was not inferior to the placebo for MACE reduction, with a consistent reduction in hospitalizations for HF (HR, 0.70; 95% CI, 0.54-0.90) without any increase in the amputation rate (2.0% and 1.6% in the ertugliflozin and placebo groups, respectively). A meta-analysis of 15 randomized SGLT2 inhibitor trials and >63,000 patients confirmed no significant difference in amputation events.62 More recently, DM, polyvascular disease, and chronic kidney disease were found to be independent predictors of hospitalization for HF in a population with stable atherosclerotic disease.63 Taken together, these data suggest clear benefits of the use of SGLT2 inhibitors in patients with PAD and DM, especially in high-risk patients with HF or chronic kidney disease.
Antithrombotic therapy
In patients with PAD, dysfunction and disruption of platelets in coagulation pathways contribute to CV risk,64 and MALE is driven by thrombus formation, even in the absence of a large atherosclerotic burden.65 Few trials have studied the effect of antithrombotic therapy in a primary population with PAD (Table 1), and most of the early data regarding antithrombotic therapy have been derived from a subgroup analysis of larger trials of patients with ASCVD, including PAD, in which therapies that target MACE reduction were studied. MALE is a more common end point in contemporary antithrombotic trials, allowing for a better understanding of therapeutic benefits in the population with PAD.
Table 1.
Key antithrombotic trials in a primary population with peripheral artery disease.
| Study | Population | Treatment | Outcomes | Bleeding |
|---|---|---|---|---|
| POPADAD67 | Patients with diabetes with an ABI of <0.99 | Aspirin versus placebo | No difference in the composite of death due to CAD or stroke, nonfatal MI or stroke, or amputation (HR, 0.98; 95% CI, 0.76-1.26) | No difference in gastrointestinal bleeding (HR, 0.90; 95% CI, 0.53-1.52) |
| AAA68 | Asymptomatic PAD | Aspirin versus placebo | No difference in the composite of fatal or nonfatal coronary event, stroke, or revascularization (HR, 1.03; 95% CI, 0.84-1.27) | Increase in major bleeding (HR, 1.71; 95% CI, 0.99-2.97) |
| EUCLID72 | Patients with symptomatic PAD | Ticagrelor versus clopidogrel | Ticagrelor not superior to clopidogrel for MACE reduction (HR, 1.02; 95% CI, 0.92-1.13) | No increase in bleeding (HR, 1.1; 95% CI, 0.84-1.43) |
| WAVE82 | Patients with PAD | Warfarin + antiplatelet versus antiplatelet | No MACE reduction (RR, 0.92; 95% CI, 0.73-1.16) | Increased life-threatening or moderate bleeding (RR, 3.21; 95% CI, 2.02-5.08) |
| Dutch BOA87 | Patients with PAD after infrainguinal arterial grafting | Oral anticoagulant (phenprocoumon or acenocoumarol; coumarin derivatives) versus aspirin equivalent | No difference in graft occlusion (HR, 0.95; 95% CI, 0.82-1.11); no difference in the composite of vascular mortality, MI, stroke, or amputation (HR, 0.89; 95% CI, 0.75-1.06) | Increase in severe bleeding (HR, 1.96; 95% CI, 1.42-2.71) |
| CASPAR88 | Patients with PAD undergoing below-knee bypass grafting | Clopidogrel + aspirin versus aspirin + placebo | No reduction in the composite of graft occlusion, revascularization, major amputation, or death (HR, 0.98; 95% CI, 0.78-1.23) | No difference in severe bleeding (2.1% vs 1.2%) |
| MIRROR89 | Patients with PAD undergoing endovascular LER | Clopidogrel + aspirin versus placebo + aspirin | Decreased risk of target lesion revascularization (5% vs 8%, P = .04) at 6 mo but no difference at 1 y (25% vs 32%, P = .35) | No increase in bleeding (2.5% vs 5%, P = .56) |
| ePAD90 | Patients with PAD after endovascular LER | Edoxaban + aspirin versus aspirin + clopidogrel | No difference in restenosis or reocclusion of femoropopliteal targets (HR, 0.89; 95% CI, 0.59-1.34) | No difference in bleeding (RR, 0.56; 95% CI, 0.19-1.62) |
| VOYAGER PAD91 | Patients with PAD after LER | Rivaroxaban + aspirin versus placebo + aspirin | Composite of reduction of MACE and MALE (HR, 0.85; 95% CI, 0.76-0.96) | No difference in TIMI major bleeding (HR, 1.43; 95% CI, 0.97-2.10); increase in ISTH major bleeding (HR, 1.42; 95% CI, 1.10-1.84) |
AAA, Aspirin for the Prevention of Cardiovascular Events in a General Population Screened for a Low Ankle Brachial Index; ABI, ankle brachial index; CAD, coronary artery disease; CASPAR, Clopidogrel and Acetylsalicylic Acid in Bypass Surgery for Peripheral Arterial Disease; Dutch BOA, Dutch Bypass Oral Anticoagulants or Aspirin Study; ePAD, Oral Anticoagulation With Edoxaban Plus Aspirin vs Dual Antiplatelet Therapy in Endovascular Treatment of Patients with Peripheral Artery Disease; EUCLID, Study Comparing Cardiovascular Effects of Ticagrelor and Clopidogrel with Patients with Peripheral Artery Disease Trial; HR, hazard ratio; ISTH, International Society on Thrombosis and Haemostasis; LER, lower-extremity revascularization; MACE, major adverse cardiovascular events; MALE, major adverse limb events; MI, myocardial infarction; MIRROR, Management of Peripheral Arterial Interventions with Mono or Dual Antiplatelet Therapy; PAD, Peripheral artery disease; POPADAD, Prevention of Progression of Arterial Disease and Diabetes; RR, risk reduction; TIMI, Thrombolysis in Myocardial Infarction; VOYAGER PAD, Vascular Outcomes Study of Aspirin Along with Rivaroxaban in Endovascular or Surgical Limb Revascularizations for Peripheral Artery Disease; WAVE, Warfarin Antiplatelet Vascular Evaluation.
Aspirin
The use of aspirin in the treatment of PAD is based on the AntiThrombotic Trialists meta-analysis, which involved 22 trials.66 Antiplatelet therapy was associated with a 22% reduction in MACE but a 60% relative excess in major extracranial bleeding. Although generally taken as evidence for the benefit of low-dose aspirin, this trial included multiple doses of aspirin as well as nonaspirin antiplatelet agents such as picotamide. Two trials have studied the effect of aspirin in patients with an abnormal ankle brachial index (ABI). In the Prevention of Progression of Arterial Disease and Diabetes (POPADAD) trial, aspirin had no effect compared with a placebo in terms of MACE or major amputation in diabetic patients with an ABI of <0.99.67 In the Aspirin for the Prevention of Cardiovascular Events in a General Population Screened for a Low Ankle Brachial Index (AAA) trial, the CV outcomes with aspirin were examined in patients with abnormal ABI but no evidence or symptoms of atherosclerosis. There was no benefit in terms of fatal coronary events, stroke, or revascularization, whereas major bleeding was increased with aspirin (HR, 1.71; 95% CI, 0.99-2.97).68 No randomized trial has demonstrated the benefit of aspirin monotherapy for reduction in MALE. Based on these data, aspirin monotherapy is recommended for MACE reduction.
P2Y12 receptor inhibition
In 1990, in The Swedish Ticlopidine Multicenter Study (STIMS), a 29.1% lower mortality was observed (64 vs 89, P = .015), driven mainly by reduced mortality from ischemic heart disease, with first-generation thienopyridine ticlopidine as monotherapy versus a placebo (no antiplatelet therapy) in 687 patients with intermittent claudication leading to a 2.5% increase in bleeding.69 In the Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) trial, the effect of second-generation thienopyridne clopidogrel versus that of aspirin on MACE was evaluated in a broad population of 19,185 patients with atherosclerosis, including 6452 patients with symptomatic PAD.70 Overall, the trial showed that clopidogrel was superior to aspirin as monotherapy (5.32% vs 5.83%, respectively; HR, 8.7%; 95% CI, 0.3-16.5; P = .043), although the magnitude of benefit was considered modest (8.7% relative risk reduction), and there was no benefit in terms of amputation (52 events with clopidogrel vs 47 events with aspirin). In a subgroup analysis, an effect modification was suggested, with greater benefit in those with PAD versus that in others (Pinteraction = .0028); however, this was driven by apparent worse outcomes with clopidogrel versus those with aspirin in patients with MI (risk reduction increase, 3.7%). In the context of subsequent studies demonstrating favorable outcomes in patients with coronary disease with P2Y12 monotherapy and the lack of validation of this observation,71 the robustness of the potential interaction is uncertain.
The use of ticagrelor, a third-generation P2Y12 receptor inhibitor, in patients with PAD was examined in EUCLID,72 the first large CV outcomes antithrombotic therapy trial conducted in a primary population with PAD. In EUCLID, ticagrelor monotherapy was not superior to clopidogrel for reduction in MACE (HR, 1.02; 95% CI, 0.92-1.13) but did not increase bleeding (HR, 1.10; 95% CI, 0.84-1.43). Similar results were observed in patients with prior lower-extremity revascularization (LER).73 However, an interaction favoring ticagrelor for the primary efficacy end point was observed in a subgroup of patients with previous coronary or carotid revascularization or previous coronary stenting.3 It should be noted that although the results of the overall EUCLID trial have been interpreted by some as demonstrating equivalence between ticagrelor and clopidogrel in patients with symptomatic PAD, EUCLID was not designed or powered to show noninferiority, and this claim has not been made by the primary authors of the article or the sponsors. Furthermore, the Food and Drug Administration has not given ticagrelor an indication for use in patients with PAD.
Dual-antiplatelet therapy
The use of dual antiplatelet therapy (DAPT) with aspirin and clopidogrel in patients with stable atherosclerosis was assessed in the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial.74 The study found no benefit of DAPT over aspirin for MACE reduction; however, subgroup analyses have raised the hypothesis that there may be a benefit of DAPT in high-risk patients with prior MI (HR, 0.78; 95% CI, 0.61-0.98), prior ischemic stroke (HR, 0.78; 95% CI, 0.62-0.97), and a history of PAD (HR, 0.83; 95% CI, 0.72-0.95).75 The effect of treatment with more intensive DAPT using aspirin and ticagrelor in patients with PAD was studied in a subgroup analysis of Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin—Thrombolysis in Myocardial Infarction (PEGASUS-TIMI 54).76 In this trial, the efficacy of different ticagrelor doses or a placebo plus aspirin was evaluated for secondary prevention after MI. In patients with concomitant CAD and PAD, ticagrelor reduced CV mortality by 53% and reduced MALE, characterized by ALI or peripheral revascularization for limb ischemia, by ∼40%. However, the combined ticagrelor dose increased Thrombolysis in Myocardial Infarction (TIMI) major bleeding (HR, 1.32; 95% CI, 0.41-4.29).77 The effect of aspirin plus ticagrelor versus that of aspirin alone in patients with diabetes and stable coronary disease was studied in The Effect of Ticagrelor on Health Outcomes in Diabetes Mellitus Patients Intervention Study (THEMIS),78 which demonstrated a lower risk of MACE, as well as a 55% reduction in MALE, with DAPT, although there was an increase in TIMI major bleeding, especially in intracranial hemorrhage (HR, 2.32; 95% CI, 1.82-2.94).
In the TRA 2P-TIMI 50 trial, the effect of aspirin and/or clopidogrel plus vorapaxar, a competitive and selective antagonist of the thrombin receptor protease-activated receptor-1 (PAR-1), was examined in patients with a history of MI, stroke, or PAD.79 Overall, the vorapaxar cohort had a 12% reduction in CV death, MI, stroke, or recurrent ischemia leading to revascularization but a higher rate of Global Use of Strategies to Open Occluded Arteries (GUSTO) moderate-severe bleeding versus that with a placebo (HR, 1.66; 95% CI, 1.43-1.93) in patients with prior stroke. A subsequent investigation of a subgroup with PAD revealed a 42% reduction in hospitalizations for ALI in individuals in the vorapaxar arm, with no evidence of increased GUSTO moderate-severe bleeding or intracranial hemorrhage in patients with CAD or PAD.80 Additional analyses have demonstrated greater absolute risk reduction for MACE in those with concomitant PAD and CAD compared with that in patients with PAD alone (−2.2% vs 0.1%, respectively), and the benefit for MALE reduction was most favorable in those with prior LER (2.5 vs 0.2%).81 Currently, DAPT with ticagrelor is approved for use in the treatment of CAD, and vorapaxar is approved for use in the treatment of PAD or CAD.
Dual-pathway inhibition
Dual-pathway inhibition is characterized by the concomitant use of antiplatelet therapy plus an anticoagulant. In the Warfarin Antiplatelet Vascular Evaluation (WAVE) trial, the effect of the combination of therapeutic warfarin plus aspirin versus that of aspirin alone was evaluated in a population with PAD.82 There were no differences in MACE, and there was a significant increase in life-threatening bleeding in the combination therapy arm (risk reduction, 3.41; 95% CI, 1.84-6.35). Dual-pathway inhibition with the factor Xa inhibitor rivaroxaban plus aspirin was studied in patients with stable atherosclerosis, including PAD, in the Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS) trial.83 Relative to aspirin alone, 2.5 mg of rivaroxaban plus aspirin decreased MACE (HR, 0.76; 95% CI, 0.66-0.86), with a greater risk of severe bleeding in the rivaroxaban arm (HR, 1.70; 95% CI, 1.40-2.05) but no significant difference in intracranial or fatal bleeding between the groups. This benefit was consistent in the subgroup with PAD.84 In patients with PAD, 2.5 mg of rivaroxaban twice daily plus aspirin versus aspirin also reduced MALE by 43%. Although there was a significant reduction in major amputations with low-dose rivaroxaban plus aspirin in COMPASS, the event rates were low, with 13 major amputation events in the pooled treatment arm and 15 in the placebo arm.85
Antithrombotic therapy after LER
Patients with PAD undergoing LER are at an increased risk of MALE, particularly ALI.86 Until recently, antithrombotic therapies had not been proven to be beneficial in this acute setting. In the Dutch Bypass Oral Anticoagulants or Aspirin (Dutch BOA) study, warfarin versus aspirin in patients undergoing infrainguinal grafting did not reduce the composite outcome of vascular mortality, MI, stroke, or amputation but did increase severe bleeding (HR, 1.96; 95% CI, 1.42-2.71).87 Similarly, aspirin and clopidogrel versus aspirin alone in patients undergoing lower-extremity bypass did not reduce MACE or MALE but did increase bleeding in the Clopidogrel and Acetylsalicylic Acid in Bypass Surgery for Peripheral Arterial Disease (CASPAR) trial.88 Antithrombotic therapy with aspirin plus clopidogrel versus that with aspirin after endovascular LER was assessed in Management of Peripheral Arterial Interventions with Mono or Dual Antiplatelet Therapy (MIRROR).89 The primary end points were markers of platelet activation and clopidogrel resistance. Target lesion revascularization, a secondary end point, was reduced with DAPT at 6 months (5% vs 8%, P = .04); however, this effect did not persist for 1 year. In the Oral anticoagulation with Edoxaban Plus Aspirin vs Dual Antiplatelet Therapy in Endovascular Treatment of Patients With Peripheral Artery Disease (ePAD) trial, the effect of edoxaban plus aspirin versus that of DAPT with aspirin and clopidogrel after endovascular revascularization was assessed.90 No differences in restenosis or reocclusion of femoropopliteal target lesions or serious bleeding were noted between the groups. However, this study was focused on safety and not powered for efficacy.
In the Vascular Outcomes Study of Aspirin Along with Rivaroxaban in Endovascular or Surgical Limb Revascularizations for Peripheral Artery Disease (VOYAGER PAD) trial, participants undergoing surgical or endovascular revascularization for symptomatic PAD were randomized to receive either 2.5 mg of rivaroxaban twice daily or a placebo on a background of low-dose aspirin.91 Rivaroxaban plus aspirin reduced the risk of the primary composite end point of ALI, major amputation for vascular etiology, MI, ischemic stroke, and CV death by 15%. There was more TIMI major bleeding with rivaroxaban, although this was not statistically significant (HR, 1.43; 95% CI, 0.97-2.10). The overall benefit-risk ratio was 6:1 in favor of the use of rivaroxaban plus aspirin after LER to reduce severe CV and limb events. In VOYAGER PAD, rivaroxaban also reduced the total primary end point events (HR, 0.86; 95% CI, 0.75-0.98) and total vascular events (HR, 0.86; 95% CI, 0.79-0.95).92
Secondary analyses from VOYAGER PAD have confirmed the consistent benefit and safety of rivaroxaban plus aspirin after LER. The benefit of rivaroxaban was particularly robust in preventing ALI (absolute risk reduction of 2.6% at 3 years; HR, 0.67; 95% CI, 0.55-0.82), irrespective of a surgical or endovascular approach and the use of clopidogrel.6 Consistent efficacy and safety of rivaroxaban have also been demonstrated in older patients aged >75 years.93 Among patients undergoing surgical LER, rivaroxaban reduced the risk of the primary composite end point by 19% without significantly increasing major bleeding events.94
Although DAPT with clopidogrel and aspirin is often used after peripheral revascularization,95 robust randomized data related to PAD to support this practice are lacking. In VOYAGER PAD, the benefit of rivaroxaban was consistent irrespective of the concomitant use of clopidogrel (Pinteraction = .92), and the increase in TIMI major bleeding observed with rivaroxaban relative to that observed with aspirin was similar regardless of the use of clopidogrel (Pinteraction = .71). However, rivaroxaban was associated with more International Society on Thrombosis and Haemostasis (ISTH) major bleeding in patients treated with clopidogrel for >30 days than in those treated for shorter durations (HR, 3.2; 95% CI, 1.44-7.13; P = .06).96 Taken together, these data suggest that DAPT is insufficient and should be used for limited durations, and the addition of low-dose rivaroxaban should be considered after peripheral revascularization.
Therapies to improve function
Patients with PAD often have functional impairments, including diminished walking distance, speed, and balance. Cilostazol has been shown to increase the maximal and pain-free walking distances by ∼40 and 30 m, respectively.46 However, cilostazol is contraindicated in patients with HF because of its similarity to other phosphodiesterase 3 inhibitors that have previously been shown to increase mortality in this patient population. Pentoxifylline, a derivative of theophylline, has also been approved for use in patients with PAD and claudication. Unfortunately, small studies have shown that the benefit of pentoxifylline in this population is small or nonexistent.97,98
Exercise therapy
Supervised exercise therapy (SET) is an effective strategy to reduce claudication symptoms and improve functional outcomes and is recommended for the treatment of symptomatic PAD.99 The potential mechanisms of benefit include vasodilation and greater microvascular flow, improved skeletal muscle metabolism and oxidative capacity, and reduction of inflammation. SET using a structured regimen is recommended and has been proven to improve walking performance.100 In contrast, the data for home-based training are mixed: an older meta-analysis did not demonstrate an increase in walking distance between home-based structured exercise and walking advice101; however, the Low-Intensity Exercise Intervention in PAD (LITE) trial reported a benefit of high-intensity versus low-intensity home-based exercise therapy for increasing walking distance.102 More recent comparisons have included SET combined with LER and found that the combination is superior to either treatment alone.103 The Claudication: Exercise Versus Endoluminal Revascularization (CLEVER) trial demonstrated that SET and revascularization improved peak walking time (4.7 and 3.0 minutes, respectively) compared with optimal medical therapy alone.104 The Endovascular Revascularization and Supervised Exercise for Peripheral Artery Disease and Intermittent Claudication (ERASE) trial demonstrated increased maximum walking distance, pain-free walking distance, and better quality of life with SET plus revascularization in patients with aortoiliac or femoropopliteal PAD compared with those with SET alone.105 These data highlight the importance of SET in patients with PAD even after revascularization.
Selection of therapy
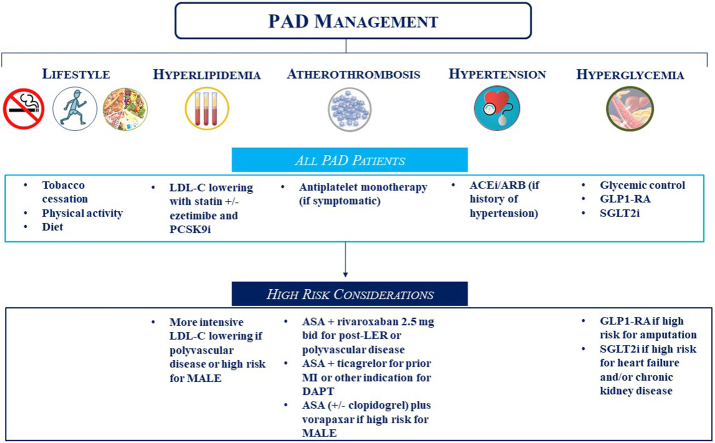
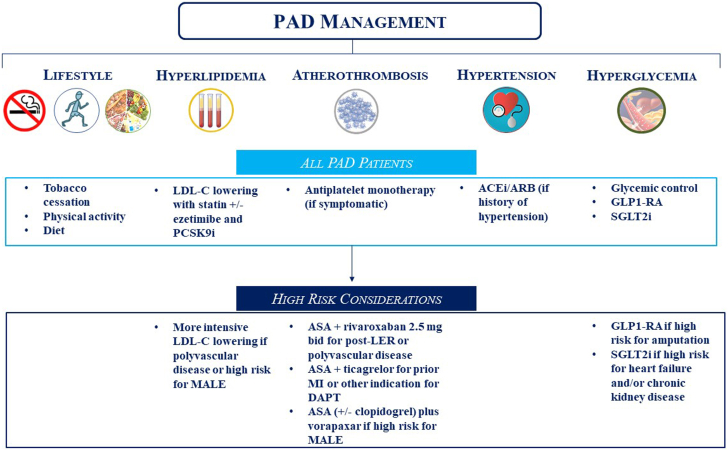
Although patients with PAD are at overall heightened risks of MACE and MALE, heterogeneity of risk exists and should be accounted for while selecting therapies for individualized care (Central Illustration). All patients should undergo lifestyle modifications with adequate exercise along with smoking cessation and blood pressure control, if applicable. Targeted LDL-C-lowering therapies should be utilized to reduce the risks of MACE and MALE, with more intensive LDL-C-lowering strategies planned for patients at a higher risk of MACE, such as those with polyvascular disease, or a higher risk of MALE, such as those with a history of amputation or LER. Similarly, although antiplatelet monotherapy should be prescribed for patients with symptomatic PAD for MACE prevention, more intensive antithrombotic therapy should be considered for higher-risk patients. Robust data support the use of a combination of aspirin plus low-dose rivaroxaban in patients with chronic PAD and polyvascular disease as well as in the acute setting of LER. Data also support the use of DAPT with aspirin and ticagrelor in patients with prior MI or another indication for DAPT (eg, percutaneous coronary intervention), and vorapaxar can be considered in patients at a high risk of MALE. In patients with diabetes, GLP1-RAs may be prioritized in those at a high risk of amputation, whereas SGLT2 inhibitors may be favored for those with HF or chronic kidney disease.
Central Illustration.
Medical therapy for peripheral artery disease. ACEi/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; ASA, acetylsalicylic acid; DAPT, dual-antiplatelet therapy; GLP-1 RA, glucagon-like peptide-1 receptor agonist; LDL-C, low-density lipoprotein-cholesterol; LER, lower-extremity revascularization; MALE, major adverse limb events; MI, myocardial infarction; PAD, peripheral artery disease; PCSK9i, proprotein convertase subtilisin/kexin type 9 inhibitor; SGLT2i, sodium-glucose cotransporter 2 inhibitor.
Unfortunately, the medical treatment of PAD in most real-world observational studies remains suboptimal despite evidence supporting the efficacy of recommended therapies. For example, >33% of smoking patients with symptomatic PAD presenting to a health care provider are not offered evidence-based smoking cessation counseling or therapy.106 Although data support the use of SET, the utilization of this therapy has also been reported to be as low as 1.3%.107 Lipid-lowering therapies are consistently underutilized in patients with PAD, particularly compared with their utilization in patients with coronary disease.10 Additional efforts and studies based on implementation science are needed to help providers deliver optimal care to patients with PAD. An example is the Implementation of Vascular Care Team to Improve Medical Management of PAD Patients (OPTIMIZE PAD-1) study, which is examining the use of a multidisciplinary care team approach and algorithm-based management for lipid lowering in patients with PAD (NCT04400409). Beyond pivotal trials proving the efficacy of medical therapies, these types of studies can help translate the evidence into practice to improve outcomes in patients with PAD.
Conclusion
Although patients with PAD are at a very high risk of ischemic events, many therapeutic options exist to reduce this risk. Initial treatment should include lifestyle alterations such as smoking cessation, dietary modifications, and exercise training. Additional medical therapies have been shown to reduce the risks of MACE and MALE while also showing benefits in functional outcomes, and treatment strategies should be multifaceted and personalized to the risk profiles of individual patients. Despite the availability of proven therapies for PAD, more work is needed to translate the evidence into practice to optimize care for this high-risk population and improve outcomes.
Declaration of competing interest
Drs King, Canonico, Bonaca, and Hess receive salary support from CPC, a nonprofit academic research organization affiliated with the University of Colorado that receives research grant/consulting funding from Abbott, Agios, Alexion Pharma, Amgen, AstraZeneca, Bayer, Better Therapeutics, Brigham and Women’s Hospital, Bristol-Myers Squibb, Cambrian Biopharma, Cook Medical, Cook Regentec, Eidos Therapeutics, EPG Communications Holdings, Epizon Pharma, Exicon Consulting, Faraday, Insmed, Janssen, Lexicon, Medpace, Merck, NovoNordisk, PPD Development, Prothena Biosciences, Regeneron, Regio Biosciences, Sanifit Therapeutics, Regents of the University of Colorado, Stealth BioTherapeutics, VarmX, and Wraser. Dr Bonaca receives support from the AHA SFRN under award numbers 18SFRN3390085 (BWH-DH SFRN Center) and 18SFRN33960262 (BWH-DH Clinical Project). Dr Bonaca also reports stock in Medtronic and Pfizer.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics statement
This research has adhered to the relevant ethical guidelines.
References
- 1.Benjamin E.J., Virani S.S., Callaway C.W., et al. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 2.Song P., Rudan D., Zhu Y., et al. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Health. 2019;7(8):e1020–e1030. doi: 10.1016/S2214-109X(19)30255-4. [DOI] [PubMed] [Google Scholar]
- 3.Hiatt W.R., Fowkes F.G., Heizer G., et al. Ticagrelor versus clopidogrel in symptomatic peripheral artery disease. N Engl J Med. 2017;376(1):32–40. doi: 10.1056/NEJMoa1611688. [DOI] [PubMed] [Google Scholar]
- 4.Gutierrez J.A., Mulder H., Jones W.S., et al. Polyvascular disease and risk of major adverse cardiovascular events in peripheral artery disease: a secondary analysis of the Euclid trial. JAMA Netw Open. 2018;1(7):e185239–e185240. doi: 10.1001/jamanetworkopen.2018.5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDermott M.M., Dayanidhi S., Kosmac K., et al. Walking exercise therapy effects on lower extremity skeletal muscle in peripheral artery disease. Circ Res. 2021;128(12):1851–1867. doi: 10.1161/CIRCRESAHA.121.318242. [DOI] [PubMed] [Google Scholar]
- 6.Hess C.N., Debus E.S., Nehler M.R., et al. Reduction in acute limb ischemia with Rivaroxaban versus placebo in peripheral artery disease after lower extremity revascularization: insights from VOYAGER PAD. Circulation. 2021;144(23):1831–1841. doi: 10.1161/CIRCULATIONAHA.121.055146. [DOI] [PubMed] [Google Scholar]
- 7.Hamburg N.M., Creager M.A. Pathophysiology of intermittent claudication in peripheral artery disease. Circ J. 2017;81(3):281–289. doi: 10.1253/circj.CJ-16-1286. [DOI] [PubMed] [Google Scholar]
- 8.Beckman J.A., Duncan M.S., Damrauer S.M., et al. Microvascular disease, peripheral artery disease, and amputation. Circulation. 2019;140(6):449–458. doi: 10.1161/CIRCULATIONAHA.119.040672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hua S., Isasi C.R., Kizer J.R., et al. Underuse of cardiovascular medications in individuals with known lower extremity peripheral artery disease: HCHS. SOL. J Am Heart Assoc. 2020;9(16):e015451–e015452. doi: 10.1161/JAHA.119.015451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reynolds K., Mues K.E., Harrison T.N., et al. Trends in statin utilization among adults with severe peripheral artery disease including critical limb ischemia in an integrated healthcare delivery system. Vasc Med. 2020;25(1):3–12. doi: 10.1177/1358863X19871100. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch A.T., Criqui M.H., Treat-Jacobson D., et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286(11):1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 12.Hackler E.L., III, Hamburg N.M., White Solaru K.T. Racial and ethnic disparities in peripheral artery disease. Circ Res. 2021;128(12):1913–1926. doi: 10.1161/CIRCRESAHA.121.318243. [DOI] [PubMed] [Google Scholar]
- 13.Lloyd-Jones D.M., Allen N.B., Anderson C.A., et al. Life’s Essential 8: updating and enhancing the American Heart Association’s construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. 2022;146(5):e18–e43. doi: 10.1161/CIR.0000000000001078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.López-Laguna N., Martínez-González M.A., Toledo E., et al. Risk of peripheral artery disease according to a healthy lifestyle score: the PREDIMED study. Atherosclerosis. 2018;275:133–140. doi: 10.1016/j.atherosclerosis.2018.05.049. [DOI] [PubMed] [Google Scholar]
- 15.du Mont L.S., Leclerc B., Morgant M.C., et al. Impact of nutritional state on critical limb ischemia early outcomes (DENUCRITICC study) Ann Vasc Surg. 2017;45:10–15. doi: 10.1016/j.avsg.2017.04.030. [DOI] [PubMed] [Google Scholar]
- 16.Nosova E.V., Conte M.S., Grenon S.M. Advancing beyond the ‘heart-healthy diet’ for peripheral arterial disease. J Vasc Surg. 2015;61(1):265–274. doi: 10.1016/j.jvs.2014.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tibuakuu M., Kamimura D., Kianoush S., et al. The association between cigarette smoking and inflammation: The Genetic Epidemiology Network of Arteriopathy (GENOA) study. PLoS One. 2017;12(9):e0184914–e0184915. doi: 10.1371/journal.pone.0184914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark D., III, Cain L.R., Blaha M.J., et al. Cigarette smoking and subclinical peripheral arterial disease in blacks of the Jackson Heart Study. J Am Heart Assoc. 2019;8(3):e010674–e010675. doi: 10.1161/JAHA.118.010674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willigendael E.M., Teijink J.A., Bartelink M.L., Peters R.J., Büller H.R., Prins M.H. Smoking and the patency of lower extremity bypass grafts: a meta-analysis. J Vasc Surg. 2005;42(1):67–74. doi: 10.1016/j.jvs.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 20.Heeschen C., Jang J.J., Weis M., et al. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat Med. 2001;7(7):833–839. doi: 10.1038/89961. [DOI] [PubMed] [Google Scholar]
- 21.Wheat L.A., Haberzettl P., Hellmann J., et al. Acrolein inhalation prevents vascular endothelial growth factor-induced mobilization of Flk-1+/Sca-1+ cells in mice. Arterioscler Thromb Vasc Biol. 2011;31(7):1598–1606. doi: 10.1161/ATVBAHA.111.227124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Subramaniam V.N., Menezes A.R., DeSchutter A., Lavie C.J. The cardiovascular effects of marijuana: are the potential adverse effects worth the high? Mo Med. 2019;116(2):146–153. [PMC free article] [PubMed] [Google Scholar]
- 23.Fetterman J.L., Keith R.J., Palmisano J.N., et al. Alterations in vascular function associated with the use of combustible and electronic cigarettes. J Am Heart Assoc. 2020;9(9):e014570–e014571. doi: 10.1161/JAHA.119.014570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moreno H., Jr., Chalon S., Urae A., et al. Endothelial dysfunction in human hand veins is rapidly reversible after smoking cessation. Am J Physiol. 1998;275(3):H1040–H1045. doi: 10.1152/ajpheart.1998.275.3.H1040. [DOI] [PubMed] [Google Scholar]
- 25.Armstrong E.J., Wu J., Singh G.D., et al. Smoking cessation is associated with decreased mortality and improved amputation-free survival among patients with symptomatic peripheral artery disease. J Vasc Surg. 2014;60(6):1565–1571. doi: 10.1016/j.jvs.2014.08.064. [DOI] [PubMed] [Google Scholar]
- 26.Quick C.R., Cotton L.T. The measured effect of stopping smoking on intermittent claudication. Br J Surg. 1982;69(suppl 6):S24–S26. doi: 10.1002/bjs.1800691309. [DOI] [PubMed] [Google Scholar]
- 27.Tønnesen P., Lawrence D., Tonstad S. Medication-assisted quit rates in participants with smoking-related diseases in EAGLES: post hoc analyses of a double-blind, randomized, placebo-controlled clinical trial. Tob Induc Dis. 2022;20:46–47. doi: 10.18332/tid/146567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Youssef F., Seifalian A.M., Jagroop I.A., et al. The early effect of lipid-lowering treatment on carotid and femoral intima media thickness (IMT) Eur J Vasc Endovasc Surg. 2002;23(4):358–364. doi: 10.1053/ejvs.2002.1611. [DOI] [PubMed] [Google Scholar]
- 29.Grundy S.M., Cleeman J.I., Merz C.N., et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 30.Reiner Z., Catapano A.L., De Becker G., et al. ESC/EAS guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Eur Heart J. 2011;32(14):1769–1818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 31.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 32.Steg P.G., Bhatt D.L., Wilson P.W., et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297(11):1197–1206. doi: 10.1001/jama.297.11.1197. [DOI] [PubMed] [Google Scholar]
- 33.Arya S., Khakharia A., Binney Z.O., et al. Association of statin dose with amputation and survival in patients with peripheral artery disease. Circulation. 2018;137(14):1435–1446. doi: 10.1161/CIRCULATIONAHA.117.032361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumbhani D.J., Steg P.G., Cannon C.P., et al. Statin therapy and long-term adverse limb outcomes in patients with peripheral artery disease: insights from the REACH registry. Eur Heart J. 2014;35(41):2864–2872. doi: 10.1093/eurheartj/ehu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilchrist I.C., Jr., Morrow D.A., Creager M.A., et al. Efficacy and safety of vorapaxar by intensity of background lipid-lowering therapy in patients with peripheral artery disease: insights from the TRA2P-TIMI 50 trial. J Am Heart Assoc. 2021;10(20):e021412–e021413. doi: 10.1161/JAHA.121.021412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cannon C.P., Blazing M.A., Giugliano R.P., et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 37.Bonaca M.P., Nault P., Giugliano R.P., et al. Low-density lipoprotein cholesterol lowering with evolocumab and outcomes in patients with peripheral artery disease: insights from the FOURIER trial (further cardiovascular outcomes research with PCSK9 inhibition in subjects with elevated risk) Circulation. 2018;137(4):338–350. doi: 10.1161/CIRCULATIONAHA.117.032235. [DOI] [PubMed] [Google Scholar]
- 38.Jukema J.W., Szarek M., Zijlstra L.E., et al. Alirocumab in patients with polyvascular disease and recent acute coronary syndrome: Odyssey OUTCOMES trial. J Am Coll Cardiol. 2019;74(9):1167–1176. doi: 10.1016/j.jacc.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 39.Ray K.K., Wright R.S., Kallend D., et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020;382(16):1507–1519. doi: 10.1056/NEJMoa1912387. [DOI] [PubMed] [Google Scholar]
- 40.Bhatt D.L., Steg P.G., Miller M., et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380(1):11–22. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 41.Bhatt D.L., Steg P.G., Miller M., et al. Benefits of icosapent ethyl in patients with prior peripheral artery disease: REDUCE-IT PAD. Circulation. 2021;144(suppl 1) [Google Scholar]
- 42.Ridker P.M., Rifai N., MacFadyen J., et al. Effects of randomized treatment with icosapent ethyl and a mineral oil comparator on interleukin-1β, interleukin-6, C-reactive protein, oxidized low-density lipoprotein cholesterol, homocysteine, lipoprotein (a), and lipoprotein-associated phospholipase A2: a REDUCE-IT biomarker substudy. Circulation. 2022;146(5):372–379. doi: 10.1161/CIRCULATIONAHA.122.059410. [DOI] [PubMed] [Google Scholar]
- 43.Aim-High Investigators Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365(24):2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 44.Hiatt W.R., Hirsch A.T., Creager M.A., et al. Effect of niacin ER/lovastatin on claudication symptoms in patients with peripheral artery disease. Vasc Med. 2010;15(3):171–179. doi: 10.1177/1358863X09360579. [DOI] [PubMed] [Google Scholar]
- 45.Rajamani K., Colman P.G., Li L.P., et al. Effect of fenofibrate on amputation events in people with type 2 diabetes mellitus (FIELD study): a prespecified analysis of a randomised controlled trial. Lancet. 2009;373(9677):1780–1788. doi: 10.1016/S0140-6736(09)60698-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonaca M.P., Hamburg N.M., Creager M.A. Contemporary medical management of peripheral artery disease. Circ Res. 2021;128(12):1868–1884. doi: 10.1161/CIRCRESAHA.121.318258. [DOI] [PubMed] [Google Scholar]
- 47.Behroozian A., Beckman J.A. Microvascular disease increases amputation in patients with peripheral artery disease. Arterioscler Thromb Vasc Biol. 2020;40(3):534–540. doi: 10.1161/ATVBAHA.119.312859. [DOI] [PubMed] [Google Scholar]
- 48.Holman R.R., Paul S.K., Bethel M.A., Matthews D.R., Neil H.A. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 49.Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.ADVANCE Collaborative Group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 51.Das S.R., Everett B.M., Birtcher K.K., et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76(9):1117–1145. doi: 10.1016/j.jacc.2020.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Badjatiya A., Merrill P., Buse J.B., et al. Clinical outcomes in patients with type 2 diabetes mellitus and peripheral artery disease: results from the EXSCEL trial. Circ Cardiovasc Interv. 2019;12(12):e008018–e008019. doi: 10.1161/CIRCINTERVENTIONS.119.008018. [DOI] [PubMed] [Google Scholar]
- 53.Verma S., Al-Omran M., Leiter L.A., et al. Cardiovascular efficacy of liraglutide and semaglutide in individuals with diabetes and peripheral artery disease. Diabetes Obes Metab. 2022;24(7):1288–1299. doi: 10.1111/dom.14700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin D.S., Lee J.K., Chen W.J. Major adverse cardiovascular and limb events in patients with diabetes treated with GLP-1 receptor agonists vs DPP-4 inhibitors. Diabetologia. 2021;64(9):1949–1962. doi: 10.1007/s00125-021-05497-1. [DOI] [PubMed] [Google Scholar]
- 55.Paul S.K., Bhatt D.L., Montvida O. The association of amputations and peripheral artery disease in patients with type 2 diabetes mellitus receiving sodium-glucose cotransporter type-2 inhibitors: real-world study. Eur Heart J. 2021;42(18):1728–1738. doi: 10.1093/eurheartj/ehaa956. [DOI] [PubMed] [Google Scholar]
- 56.Matthews D.R., Li Q., Perkovic V., et al. Effects of canagliflozin on amputation risk in type 2 diabetes: the CANVAS Program. Diabetologia. 2019;62(6):926–938. doi: 10.1007/s00125-019-4839-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perkovic V., Jardine M.J., Neal B., et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 58.Wiviott S.D., Raz I., Bonaca M.P., et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 59.Bonaca M.P., Wiviott S.D., Zelniker T.A., et al. Dapagliflozin and cardiac, kidney, and limb outcomes in patients with and without peripheral artery disease in DECLARE-TIMI 58. Circulation. 2020;142(8):734–747. doi: 10.1161/CIRCULATIONAHA.119.044775. [DOI] [PubMed] [Google Scholar]
- 60.Verma S., Mazer C.D., Al-Omran M., et al. Cardiovascular outcomes and safety of empagliflozin in patients with type 2 diabetes mellitus and peripheral artery disease: a subanalysis of EMPA-REG OUTCOME. Circulation. 2018;137(4):405–407. doi: 10.1161/CIRCULATIONAHA.117.032031. [DOI] [PubMed] [Google Scholar]
- 61.Cannon C.P., Pratley R., Dagogo-Jack S., et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383(15):1425–1435. doi: 10.1056/NEJMoa2004967. [DOI] [PubMed] [Google Scholar]
- 62.See R.M., Teo Y.N., Teo Y.H., et al. Effects of sodium-glucose cotransporter 2 on amputation events: a systematic review and meta-analysis of randomized-controlled trials. Pharmacology. 2022;107(3-4):123–130. doi: 10.1159/000520903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Freedman B.L., Berg D.D., Scirica B.M., et al. Epidemiology of heart failure hospitalization in patients with stable atherothrombotic disease: insights from the TRA 2° P-TIMI 50 trial. Clin Cardiol. 2022;45(8):831–838. doi: 10.1002/clc.23843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grant P.J., Cosentino F. The 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: new features and the ‘Ten Commandments’ of the 2019 guidelines are discussed by Professor Peter J. Grant and Professor Francesco Cosentino, the Task Force chairmen. Eur Heart J. 2019;40(39):3215–3217. doi: 10.1093/eurheartj/ehz687. [DOI] [PubMed] [Google Scholar]
- 65.Armstrong E.J., Chen D.C., Westin G.G., et al. Adherence to guideline-recommended therapy is associated with decreased major adverse cardiovascular events and major adverse limb events among patients with peripheral arterial disease. J Am Heart Assoc. 2014;3(2) doi: 10.1161/JAHA.113.000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baigent C., Blackwell L., Collins R., et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373(9678):1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Belch J., MacCuish A., Campbell I., et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ. 2008;337(7677):a1840. doi: 10.1136/bmj.a1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fowkes F.G., Price J.F., Stewart M.C., et al. Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. JAMA. 2010;303(9):841–848. doi: 10.1001/jama.2010.221. [DOI] [PubMed] [Google Scholar]
- 69.Janzon L., Bergqvist D., Boberg J., et al. Prevention of myocardial infarction and stroke in patients with intermittent claudication; effects of ticlopidine. Results from STIMS, the Swedish Ticlopidine Multicentre Study. J Intern Med. 1990;227(5):301–308. doi: 10.1111/j.1365-2796.1990.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 70.Dennis M., CAPRIE Steering Committee A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348(9038):1329–1339. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 71.Mehran R., Baber U., Sharma S.K., et al. Ticagrelor with or without aspirin in high-risk patients after PCI. N Engl J Med. 2019;381(21):2032–2042. doi: 10.1056/NEJMoa1908419. [DOI] [PubMed] [Google Scholar]
- 72.Berger J.S., Abramson B.L., Lopes R.D., et al. Ticagrelor versus clopidogrel in patients with symptomatic peripheral artery disease and prior coronary artery disease: insights from the Euclid trial. Vasc Med. 2018;23(6):523–530. doi: 10.1177/1358863X18775594. [DOI] [PubMed] [Google Scholar]
- 73.Jones W.S., Baumgartner I., Hiatt W.R., et al. Ticagrelor compared with clopidogrel in patients with prior lower extremity revascularization for peripheral artery disease. Circulation. 2017;135(3):241–250. doi: 10.1161/CIRCULATIONAHA.116.025880. [DOI] [PubMed] [Google Scholar]
- 74.Bhatt D.L., Fox K.A., Hacke W., et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354(16):1706–1717. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- 75.Bhatt D.L., Flather M.D., Hacke W., et al. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol. 2007;49(19):1982–1988. doi: 10.1016/j.jacc.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 76.Marciniak T.A. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;373(13):1272–1273. doi: 10.1056/NEJMc1508692. [DOI] [PubMed] [Google Scholar]
- 77.Bonaca M.P., Bhatt D.L., Storey R.F., et al. Ticagrelor for prevention of ischemic events after myocardial infarction in patients with peripheral artery disease. J Am Coll Cardiol. 2016;67(23):2719–2728. doi: 10.1016/j.jacc.2016.03.524. [DOI] [PubMed] [Google Scholar]
- 78.Steg P.G., Bhatt D.L., Simon T., et al. Ticagrelor in patients with stable coronary disease and diabetes. N Engl J Med. 2019;381(14):1309–1320. doi: 10.1056/NEJMoa1908077. [DOI] [PubMed] [Google Scholar]
- 79.Morrow D.A., Braunwald E., Bonaca M.P., et al. Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med. 2012;366(15):1404–1413. doi: 10.1056/NEJMoa1200933. [DOI] [PubMed] [Google Scholar]
- 80.Bonaca M.P., Scirica B.M., Creager M.A., et al. Vorapaxar in patients with peripheral artery disease: results from TRA2° P-TIMI 50. Circulation. 2013;127(14):1522–1529. doi: 10.1161/CIRCULATIONAHA.112.000679. [DOI] [PubMed] [Google Scholar]
- 81.Qamar A., Morrow D.A., Creager M.A., et al. Effect of vorapaxar on cardiovascular and limb outcomes in patients with peripheral artery disease with and without coronary artery disease: analysis from the TRA 2 P-TIMI 50 trial. Vasc Med. 2020;25(2):124–132. doi: 10.1177/1358863X19892690. [DOI] [PubMed] [Google Scholar]
- 82.Warfarin Antiplatelet Vascular Evaluation Trial Investigators Oral anticoagulant and antiplatelet therapy and peripheral arterial disease. N Engl J Med. 2007;357(3):217–227. doi: 10.1056/NEJMoa065959. [DOI] [PubMed] [Google Scholar]
- 83.Eikelboom J.W., Connolly S.J., Bosch J., et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377(14):1319–1330. doi: 10.1056/NEJMoa1709118. [DOI] [PubMed] [Google Scholar]
- 84.Kaplovitch E., Eikelboom J.W., Dyal L., et al. Rivaroxaban and aspirin in patients with symptomatic lower extremity peripheral artery disease: a subanalysis of the COMPASS randomized clinical trial. JAMA Cardiol. 2021;6(1):21–29. doi: 10.1001/jamacardio.2020.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Anand S.S., Caron F., Eikelboom J.W., et al. Major adverse limb events and mortality in patients with peripheral artery disease: the COMPASS trial. J Am Coll Cardiol. 2018;71(20):2306–2315. doi: 10.1016/j.jacc.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 86.Hess C.N., Wang T.Y., Weleski Fu J., et al. Long-term outcomes and associations with major adverse limb events after peripheral artery revascularization. J Am Coll Cardiol. 2020;75(5):498–508. doi: 10.1016/j.jacc.2019.11.050. [DOI] [PubMed] [Google Scholar]
- 87.Dutch Bypass Oral Anticoagulants or Aspirin (BOA) Study Group Efficacy of oral anticoagulants compared with aspirin after infrainguinal bypass surgery (The Dutch Bypass Oral anticoagulants or Aspirin study): a randomised trial. Lancet. 2000;355(9201):346–351. [PubMed] [Google Scholar]
- 88.Belch J.J., Dormandy J., CASPAR Writing Committee Results of the randomized, placebo-controlled clopidogrel and acetylsalicylic acid in bypass surgery for peripheral arterial disease (CASPAR) trial. J Vasc Surg. 2011;53(2) 564-564. [Google Scholar]
- 89.Tepe G., Bantleon R., Brechtel K., et al. Management of peripheral arterial interventions with mono or dual antiplatelet therapy—the MIRROR study: a randomised and double-blinded clinical trial. Eur Radiol. 2012;22(9):1998–2006. doi: 10.1007/s00330-012-2441-2. [DOI] [PubMed] [Google Scholar]
- 90.Moll F., Baumgartner I., Jaff M., et al. Edoxaban plus aspirin vs dual antiplatelet therapy in endovascular treatment of patients with peripheral artery disease: results of the ePAD trial. J Endovasc Ther. 2018;25(2):158–168. doi: 10.1177/1526602818760488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bonaca M.P., Bauersachs R.M., Anand S.S., et al. Rivaroxaban in peripheral artery disease after revascularization. N Engl J Med. 2020;382(21):1994–2004. doi: 10.1056/NEJMoa2000052. [DOI] [PubMed] [Google Scholar]
- 92.Bauersachs R.M., Szarek M., Brodmann M., et al. Total ischemic event reduction with Rivaroxaban after peripheral arterial revascularization in the VOYAGER PAD trial. J Am Coll Cardiol. 2021;78(4):317–326. doi: 10.1016/j.jacc.2021.05.003. [DOI] [PubMed] [Google Scholar]
- 93.Krantz M.J., Debus S.E., Hsia J., et al. Low-dose rivaroxaban plus aspirin in older patients with peripheral artery disease undergoing acute limb revascularization: insights from the VOYAGER PAD trial. Eur Heart J. 2021;42(39):4040–4048. doi: 10.1093/eurheartj/ehab408. [DOI] [PubMed] [Google Scholar]
- 94.Debus E.S., Nehler M.R., Govsyeyev N., et al. Effect of rivaroxaban and aspirin in patients with peripheral artery disease undergoing surgical revascularization: insights from the VOYAGER PAD trial. Circulation. 2021;144(14):1104–1116. doi: 10.1161/CIRCULATIONAHA.121.054835. [DOI] [PubMed] [Google Scholar]
- 95.Jones W.S., Mi X., Qualls L.G., et al. Significant variation in P2Y12 inhibitor use after peripheral vascular intervention in Medicare beneficiaries. Am Heart J. 2016;179:10–18. doi: 10.1016/j.ahj.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 96.Hiatt W.R., Bonaca M.P., Patel M.R., et al. Rivaroxaban and aspirin in peripheral artery disease lower extremity revascularization: impact of concomitant clopidogrel on efficacy and safety. Circulation. 2020;142(23):2219–2230. doi: 10.1161/CIRCULATIONAHA.120.050465. [DOI] [PubMed] [Google Scholar]
- 97.Broderick C., Forster R., Abdel-Hadi M., Salhiyyah K. Pentoxifylline for intermittent claudication. Cochrane Database Syst Rev. 2020;10(10):CD005262–CD005263. doi: 10.1002/14651858.CD005262.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Creager M.A., Pande R.L., Hiatt W.R. A randomized trial of iloprost in patients with intermittent claudication. Vasc Med. 2008;13(1):5–13. doi: 10.1177/1358863X07084910. [DOI] [PubMed] [Google Scholar]
- 99.Gerhard-Herman M.D., Gornik H.L., Barrett C., et al. AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;69(11):e71–e126. doi: 10.1016/j.jacc.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 100.Treat-Jacobson D., McDermott M.M., Beckman J.A., et al. Implementation of supervised exercise therapy for patients with symptomatic peripheral artery disease: a science advisory from the American Heart Association. Circulation. 2019;140(13):e700–e710. doi: 10.1161/CIR.0000000000000727. [DOI] [PubMed] [Google Scholar]
- 101.Hageman D., Fokkenrood H.J., Gommans L.N., van den Houten M.M., Teijink J.A. Supervised exercise therapy versus home-based exercise therapy versus walking advice for intermittent claudication. Cochrane Database Syst Rev. 2018;4(4):CD005263–CD005264. doi: 10.1002/14651858.CD005263.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McDermott M.M., Spring B., Tian L., et al. Effect of low-intensity vs high-intensity home-based walking exercise on walk distance in patients with peripheral artery disease: the LITE randomized clinical trial. JAMA. 2021;325(13):1266–1276. doi: 10.1001/jama.2021.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Biswas M.P., Capell W.H., McDermott M.M., et al. Exercise training and revascularization in the management of symptomatic peripheral artery disease. JACC Basic Transl Sci. 2021;6(2):174–188. doi: 10.1016/j.jacbts.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Murphy T.P., Cutlip D.E., Regensteiner J.G., et al. Supervised exercise, stent revascularization, or medical therapy for claudication due to aortoiliac peripheral artery disease: the CLEVER study. J Am Coll Cardiol. 2015;65(10):999–1009. doi: 10.1016/j.jacc.2014.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fakhry F., Spronk S., van der Laan L., et al. Endovascular revascularization and supervised exercise for peripheral artery disease and intermittent claudication: a randomized clinical trial. JAMA. 2015;314(18):1936–1944. doi: 10.1001/jama.2015.14851. [DOI] [PubMed] [Google Scholar]
- 106.Patel K.K., Jones P.G., Ellerbeck E.F., et al. Underutilization of evidence-based Smoking Cessation support strategies despite high smoking addiction burden in peripheral artery disease specialty care: insights from the international PORTRAIT registry. J Am Heart Assoc. 2018;7(20):e010076–e010077. doi: 10.1161/JAHA.118.010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Divakaran S., Carroll B.J., Chen S., Shen C., Bonaca M.P., Secemsky E.A. Supervised exercise therapy for symptomatic peripheral artery disease among Medicare beneficiaries between 2017 and 2018: participation rates and outcomes. Circ Cardiovasc Qual Outcomes. 2021;14(8):e007953–e007954. doi: 10.1161/CIRCOUTCOMES.121.007953. [DOI] [PMC free article] [PubMed] [Google Scholar]