Abstract
Transcatheter pulmonary valve replacement was first performed by Dr Philip Bonhoeffer, who implanted a Medtronic Melody valve in a human in 2000. Over the past 2 decades, there have been many advances in transcatheter pulmonary valve technology. This includes the use of the SAPIEN transcatheter heart valve in the pulmonary position, modifications and refinements to valve implantation procedures, and development of self-expanding valves and prestents to treat large diameter native or patched right ventricular outflow tracts. This article reviews the current transcatheter pulmonary valve technologies with a focus on valve design, screening process, implant procedure, and clinical outcomes.
Keywords: tetralogy of Fallot, pulmonary valve replacement, transcatheter
Central Illustration
Highlights
-
•
Transcatheter pulmonary valve replacement was first performed over 20 years ago.
-
•
Valve technologies and implant techniques have evolved over time.
-
•
There are a wide variety of self-expanding valves and prestents available around the world.
-
•
These new technologies are safe and effective in the short-term.
Introduction
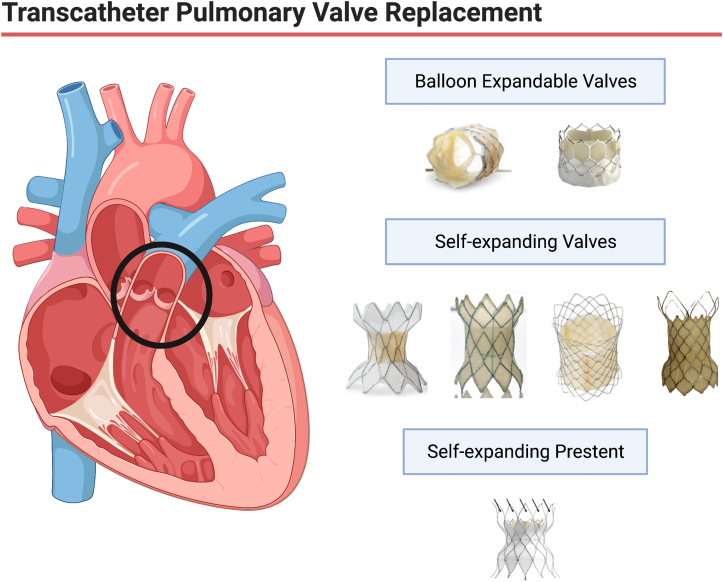
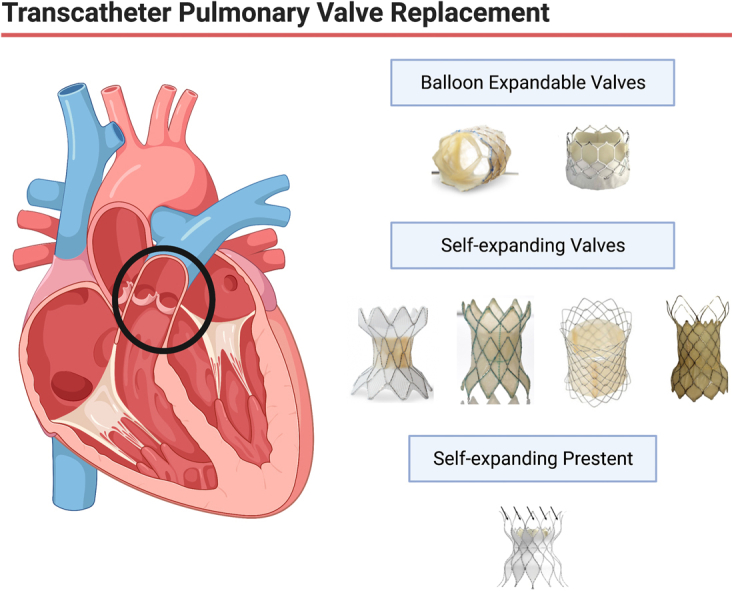
Over the past 22 years, transcatheter pulmonary valve replacement (TCPVR) has become the standard of care at most large centers for congenital heart disease. Although the initial balloon-expandable valves, the SAPIEN transcatheter heart valve (THV) from Edwards Lifesciences and the Melody valve from Medtronic, were initially approved and used in conduits and bioprosthetic valve TCPVR, many companies have now provided us with larger self-expanding valves and stent constructs, which have allowed us to extend TCPVR routinely to patients with native and “patched” right ventricular outflow tracts (RVOTs; Central Illustration).
Central Illustration.
Transcatheter pulmonary valve replacement has evolved significantly over the last 22 years. Current technologies include balloon expandable valves, self-expanding valves, and self-expanding prestents.
Although the balloon-expandable SAPIEN and Melody valves are available worldwide, the newer self-expanding valves and “prestents” are only available regionally. The Harmony valve (Medtronic) and Alterra Adaptive Prestent (Edwards Lifesciences) have been approved by the US Food and Drug Administration (FDA), but none of the other valves discussed have even begun trials in the United States. This review describes all the valves currently available around the world and details the selection process, implantation procedure, and data available for each valve/prestent. The intent is to provide an overview of all available TCPVR technologies (Figure 1).
Figure 1.
Comparison of valve and prestent types. TTE, transthoracic echo; RVOT, right ventricular outflow tract; NA, not applicable; S3 THV, Sapien 3 Transcatheter Heart Valve
Medtronic Melody valve
The Medtronic Melody valve was the first transcatheter valve implanted in a human in 2000 by Dr Philip Bonhoeffer.1 It is also the first valve to be commercially available in the world and the first to receive FDA approval in the United States, initially with a humanitarian device exemption, which was later upgraded to an investigational device exemption type FDA approval.2 Most importantly, Medtronic’s training program for the Melody valve implanters provided all congenital interventionalists with standardized training for conduit management and TCPVR. Both the device and its training and proctoring program worked to make TCPVR the standard of care in congenital heart disease and provided standardized worldwide training. The Melody valve is now indicated for pulmonary valve replacement in the dysfunctional right ventricle to pulmonary artery (RV-PA) conduits or surgical bioprosthetic valves in pediatric and adult patients. Since FDA approval, off-label Melody implantation into native/patched RVOTs with prestenting has also been adopted in many centers.3
Valve and delivery system
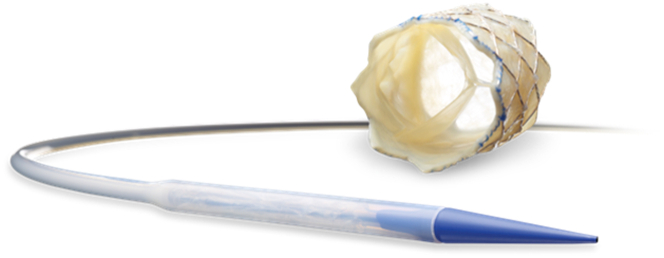
The Melody valve is made from a glutaraldehyde-treated valved bovine jugular vein sewn into a platinum-iridium stent (the Cheatham Platinum stent). The vein is sewn to the stent circumferentially at both ends. It is also sewn to the stent at each stent node with an interrupted suture. The bovine jugular veins are either 16 or 18 mm in diameter and are designated as TPV 20 and TPV 22, respectively. The intended maximum diameter for TPV 20 is 20 mm, and the maximum diameter for TPV 22 is 22 mm. The unexpanded valve height is 28 mm for TPV 20 and 30 mm for TPV 22 (Figure 2). It has been shown that the TPV 22 can be deployed or dilated to 24 mm without sacrificing valve function.4
Figure 2.
Melody Valve and Ensemble delivery system.
The valve is implanted on the Medtronic Ensemble II delivery system, which is available in 3 sizes: 18, 20, and 22 mm. It utilizes a balloon-in-balloon catheter mounted within a Teflon sheath. The distal portion of the sheath, which covers the valve, is 22F, and the remainder of the delivery system is 16F. The tip of the delivery system has a carrot, which tapers and is used as an introducer to allow for a smooth transition into the vein. The valve is hand-crimped onto the balloon-in-balloon catheter. The Ensemble delivery system has proven to be incredibly effective in TCPVR, even in patients weighing slightly less than 20 kg.5
Preprocedural evaluation
The preprocedural evaluation may include cardiac magnetic resonance imaging (MRI) or computed tomography to assess the RVOT anatomy and proximity of the coronary arteries, but it is not absolutely necessary. Although neither imaging modality can predict coronary compression with absolute certainty, they can help assess the risk of coronary artery compression.6,7
Implant procedure
The implant procedure depends on the type of pulmonary valve present: conduit versus bioprosthetic valve versus a native or “patched” RVOT. In general, most cases require the interventionalist to build and test the landing zone with balloons and stents before Melody valve insertion. In cases of RV-PA conduit stenosis, a stiff guide wire is positioned across the conduit. Serial balloon dilation of the conduit is performed, followed by coronary artery compression testing with either selective coronary or aortic root angiography with simultaneous angioplasty of the conduit intended to mimic the valve implantation. Patients with a prior Ross procedure or an anomalous origin of the coronaries in the tetralogy of Fallot are at the highest risk of coronary compression.8 Compression of the aorta has also been observed and, in its most severe form, can be a contraindication for transcatheter intervention.9 This approach is used for all balloon-expandable pulmonary valves.
Covered stent implantation may be required for the treatment of conduit tears and is sometimes placed prophylactically in conduits that are believed to be at higher risk for a tear or rupture. However, usually, coronary compression testing should precede any stent placement. Following dilation of the conduit, bare metal stents are implanted to provide radial strength and minimize the risk of valve-stent fracture, which was shown to be an issue in early studies. The valve is then implanted within the landing zone created by the previously placed stents. Mounting up to 3 prestents over the Melody valve on the Ensemble delivery system has also been shown to be an effective method of deploying the prestents and valve in 1 step.10,11 In smaller patients, the Melody valve has been delivered with a hybrid approach in which the surgeons access the RVOT, and a sheath is placed directly into the right ventricle.12 In some cases, surgeons have also created a landing zone for the Melody valve by reducing the RVOT surgically before valve delivery.13,14
In cases of bioprosthetic valves, a prestent is usually not necessary before Melody valve insertion, but for conduits and native RVOTs, prestenting has become almost universal.
When a bioprosthetic valve is present, the valve may be intentionally fractured in certain cases to allow for a larger valve diameter.15 In general, for older patients, pulmonary valves of >20 mm in internal diameter are preferred; thus, most of the smaller bioprosthetic valves will require fracture.
Clinical experience
Since the investigational device exemption study of the Melody valve began in 2007, there have been >18,000 implants and a vast amount of data and publications. In general, the Melody valves, which remain implanted and free of endocarditis, continue to have excellent valve function. On follow-up of the initial 149 patients followed long-term from the investigational device exemption cohort, there was 10-year freedom from mortality of 90% and freedom from reintervention of 60%.16 The rate of frame fracture has greatly diminished with improved prestenting techniques from 33.5% to 16.7%.17 The Munich Comparative Study showed equivalent results between 241 Melody valve implants and surgically implanted bioprosthetic valves. Both groups had 87% survival at 10 years. The annualized incidence of infective endocarditis (IE) was 1.6% in the Melody valve group and 0.5% in the surgical group; however, there was no difference in survival free of endocarditis.18
In early follow-up, multiple studies documented high freedom from reintervention and improved quality of life.19, 20, 21 However, IE has emerged as a threat to valve longevity, with most data sets reporting annualized incidence rates of IE of approximately 2% after Melody implantation, but some studies have documented annualized rates as high as >4%.22,23 The largest studies of balloon-expandable valves (which included both the Melody valve and SAPIEN THV) by McElhinney et al24,25 showed comparable rates of survival and reintervention to surgically placed valves with an incidence of endocarditis of 16.9% at 8 years. It has been very difficult to compare the Melody valve with the SAPIEN THV, as these comparisons are confounded by the differing landing zones and patient populations: the Melody valve is much more commonly used in conduits and bioprosthetic valves versus native landing zones for the SAPIEN THV. Although initial publications have shown comparable rates of endocarditis between the Melody valve and surgically placed valves, the latest data have trended toward a significant difference/increase in the rate of Melody valve IE versus surgical IE.19 Relationships between IE pathogenesis and TCPVR have been found in postimplant hemodynamics, prosthetic material, and immune function.22,25 It is hoped that identification of these risk factors can help minimize infection risk in all future TCPVR patients. Additionally, it is important to note that if significant valve dysfunction is not present, an explant of the valve may not be necessary.26
Edwards SAPIEN THV
The Edwards SAPIEN THV is a balloon-expandable valve made from the bovine pericardium. The SAPIEN THV was originally designed for placement in the aortic position but was found to have high success rates when placed in the pulmonary position, with the first successful pulmonary implantation in 2006.27 Although the SAPIEN XT and SAPIEN 3 Ultra versions can be used in the pulmonary position, currently, the SAPIEN 3 is the version being actively marketed and most commonly used for pulmonic implantation.
Valve and delivery system
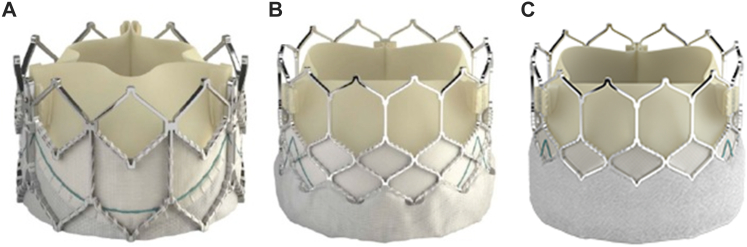
The bovine pericardium is shaped into a 3-leaflet valve and is mounted on a cobalt-chromium frame using polyethylene terephthalate (PET) fabric. The biomedical-grade chromium-cobalt frame consists of 4 rows and 4 columns, providing excellent radial strength to prevent fractures and promote uniform expansion. The valve tissue is treated with a patented Edwards ThermaFix process that uses a heat process to remove glutaraldehyde molecules and a patented chemical process that removes 98% phospholipids to prevent calcification. Compared with the Melody valve, the SAPIEN XT THV is short, with valve heights varying between 14.3 mm for the 23-mm valve and 19.1 mm for the 29-mm valve. The SAPIEN 3 THV has an additional PET skirt on the outer aspect of the frame that is intended to decrease the incidence of perivalvar leaks. The SAPIEN 3 THV has a slightly taller valve height than the XT, varying between 15.5 mm for the 20-mm valve and 22.5 mm for the 29-mm valve (Figure 3A, B). The most important difference between the SAPIEN 3 Ultra THV, the latest iteration, and SAPIEN 3 THV pertains to the textured PET outer skirt, which has an approximately 40% increased height designed to allow up to 50% more surface contact area aimed at improved annular sealing (Figure 3C).
Figure 3.
Edwards SAPIEN THV iterations. (A) SAPIEN XT valve, (B) SAPIEN 3 valve, (C) SAPIEN 3 Ultra valve.
The SAPIEN XT and SAPIEN 3 are mounted onto the Novaflex or Commander delivery systems (Edwards life sciences) and crimped using a specialized crimper developed by the company to provide uniform placement on the delivery system due to the strength of the frame. The delivery systems minimize profile by allowing the valve to be crimped onto the shaft of the balloon and then pushed onto the balloon in vivo once advanced through low-profile 14F and 16F expandable sheaths. The SAPIEN THV is available in diameters of 19, 23, 26, and 29 mm. These larger valve sizes allow for percutaneous intervention in patients with large native RVOTs, transannular patches, and large RV-PA conduits. Currently, the SAPIEN XT and SAPIEN 3 have received FDA approval for implantation in the pulmonary position within dysfunctional and bioprosthetic valves. There is published literature on using the SAPIEN THV within native or patched RVOT with significant success.28, 29, 30
Preprocedural evaluation
The details regarding the baseline anatomy and surgical correction are likely to impact candidacy and outcomes. The presence of a nonexpendable conduit or bioprosthetic valve might lead to under expansion of the deployed SAPIEN THV with significant residual stenosis. A baseline transthoracic echocardiogram should be performed to evaluate the biventricular function, right ventricular pressures, RVOT dysfunction (stenosis and degree of pulmonary regurgitation [PR]) and degree of tricuspid regurgitation. Advanced imaging with MRI or electrocardiogram (ECG)–gated computed tomography angiography (CTA) for volumetric assessment of the right ventricle, especially in patients with PR, is recommended. The evaluation of coronary artery proximity to the site of implantation can be performed, although compression testing is routinely performed.6,7
Implant procedure
The RVOT is evaluated using biplane or 3-dimensional rotational angiography to define dimensions in a stenotic conduit. Conduit rehabilitation in a stenotic RVOT involves serial angioplasty along with coronary compression testing, as described above. Prestenting might be performed before SAPIEN THV implantation if the area of stenosis is longer than the length of the implanted SAPIEN THV. Fracture of the bioprosthetic valve to increase the effective orifice is often performed before valve-in-valve implantation.15 Implantation of the SAPIEN THV within a native or patched RVOT is usually preceded by balloon sizing, especially in patulous and pulsatile outflow tracts, to better define the landing zone. Primary SAPIEN THV implantation without prestenting has been performed with good outcomes.28,29 Initially, the use of the Novaflex and Commander delivery systems required the valve to be advanced through the right heart uncovered, which resulted in tricuspid valve injury with an incidence of 6% to 8%.28,31 Thus, a Gore Dryseal (Gore and Associates) sheath is typically used to deliver the valve to the RVOT, which has been shown to significantly reduce the risk of tricuspid valve injury.31, 32, 30
Clinical experience
The COMPASSION trial (COngenital Multicenter trial of Pulmonic vAlve regurgitation Studying the SAPIEN interventional THV) was a prospective multicenter study to assess the safety and efficacy of the SAPIEN XT for the treatment of dysfunctional RV-PA conduits.33 A total of 69 patients were implanted from 2008 to 2010. Fifty-seven patients were accounted for at the 3-year follow-up visit from a total of 69 implantations in 81 enrolled patients. Functional improvement in New York Heart Association functional class was observed in 93.5% of patients. The mean peak conduit gradient decreased from 37.5 ± 25.4 to 17.8 ± 12.4 mm Hg (P < .001), and the mean right ventricular systolic pressure decreased from 59.6 ± 17.7 to 42.9 ± 13.4 mm Hg (P < .001). PR was mild or less in 91.1% of patients. Freedom from all-cause mortality at 3 years was 98.4%. Freedom from reintervention was 93.7% and that from endocarditis was 97.1% at 3 years. There were no observed stent fractures.
A multicenter retrospective study included 774 patients enrolled from 23 centers who had been implanted with a SAPIEN THV.30 The baseline demographic characteristics revealed 397 (51%) patients with a native/patched RVOT, 183 (24%) patients with a conduit, and 194 (25%) patients with a bioprosthetic valve. Most patients were implanted with the SAPIEN 3 (78%) and SAPIEN XT (22%), with most patients receiving a 29-mm (39%) or 26-mm (34%) valve. The implant was technically successful in 754 (97.4%) patients. Serious adverse events were reported in 67 (10%) patients, with no difference between the RVOT anatomy groups. Valve function at discharge was excellent in most patients, but moderate or severe PR or a maximum Doppler gradient of >40 mm Hg was documented in 58 (8.5%) patients. During the limited follow-up (n = 347; median, 12 months), 9 patients were diagnosed with endocarditis, and 18 additional patients underwent surgical valve replacement or valve-in-valve TCPVR (stenosis in 8, PR in 3, endocarditis in 4, unspecified in 3). One of the mechanisms of early valve failure is subclinical leaflet thrombosis, which can result in reduced leaflet motion. The ideal anticoagulation strategy postimplant remains unknown, with significant practice variation present among interventional cardiologists.34, 35, 36
Medtronic Harmony valve
The Medtronic Harmony valve is the first FDA-approved self-expanding valve designed specifically for the treatment of regurgitation in native or patched RVOTs. The initial concept for this self-expanding valve was championed by Dr Philipp Bonhoeffer, who realized that most patients with congenital heart disease in need of pulmonary valve replacement would not be able to be treated by smaller balloon-expandable valves. The Harmony valve design utilizes a wire frame knitted into a polymer cloth, which was originally a technology utilized in self-expanding stent grafts. Although the frame is designed to adapt to large-diameter RVOTs, the valve housings contain a 22- or 25-mm pulmonary valve. If needed in the future, a balloon-expandable valve can be placed within the Harmony valve frame.
Valve and delivery system
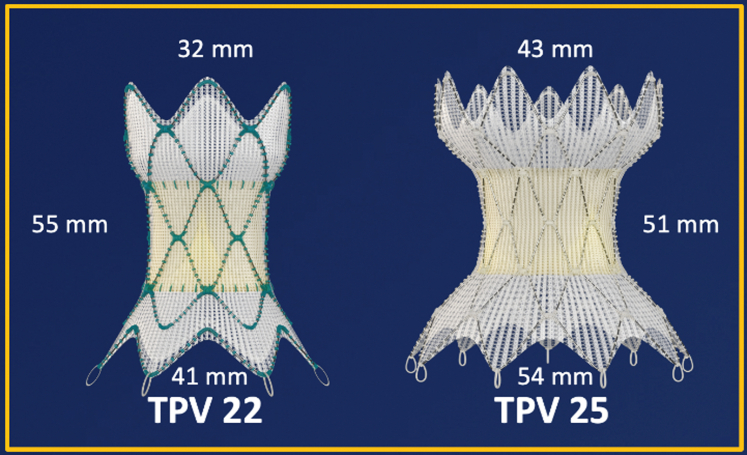
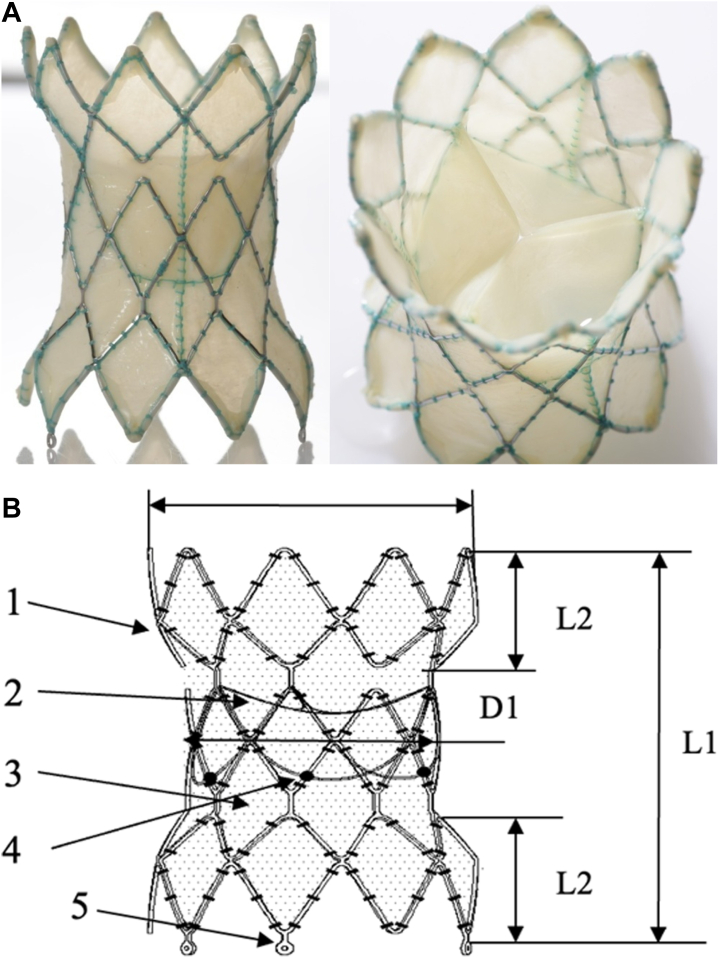
The Harmony valve is made from a knitted PET cloth covering with a nitinol wire frame sewn into it. The leaflets are α-amino oleic acid–treated porcine pericardial tissues. As shown in Figure 4, the original Harmony valve, now referred to as TPV 22, was longer and smaller in diameter than the second version, which is now referred to as TPV 25. The TPV 22 has a valve that is 22 mm in diameter, whereas the TPV 25 has a valve that is 25 mm in diameter. The TPV 22 version is 55 mm in length, whereas the TPV 25 is 51 mm in length. The TPV 25 is designed to treat larger RVOTs. The proximal skirt on the TPV 25 is 54 mm compared with 41 mm on the original TPV 22. In the clinical trial, there was an initial version of the TPV 25 originally called “clinical TPV 25,” which was less predictable in terms of deployment of the frame. The clinical TPV 25was modified to produce the current version of the TPV 25, which is now FDA approved.
Figure 4.
Harmony TPV 22 and TPV25. TPV, transcatheter pulmonary valve.
The delivery catheter system (DCS) was designed for both TPV 22 and TPV 25 based on the Ensemble delivery system. It is a 25F catheter that enables the Harmony valve to be compressed into a capsule. It has a nosecone that articulates with the capsule but can also be extended beyond the capsule. The delivery system allows the valve to be easily advanced into the RVOT and slowly uncovered from its distal and proximal end. When in position, the valve can be released from the delivery system by rotating the handle of the delivery system in a counter-clockwise fashion to release the bottom of the Harmony valve frame. After deployment from the capsule, the valve frame cannot be recaptured into the DCS but can be recaptured if a 26F DrySeal sheath is utilized.
Preprocedural evaluation
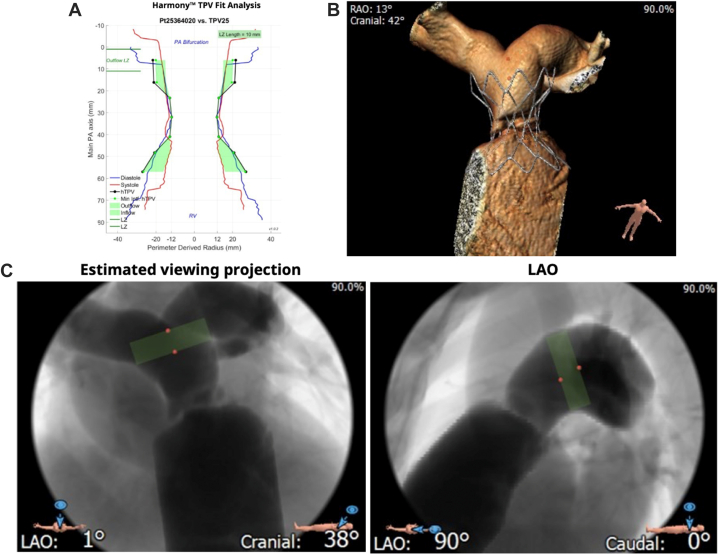
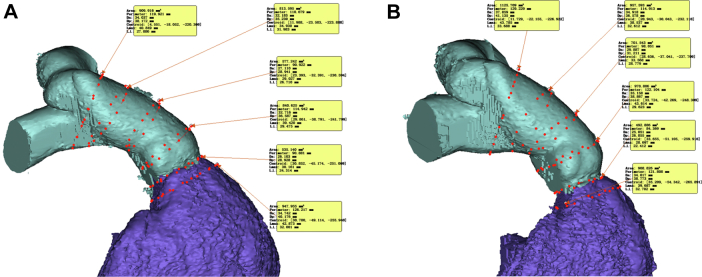
The screening for candidacy for a Harmony valve is performed completely through data from an ECG-gated CTA with reconstructions performed in both the systolic and diastolic frames. To simplify the screening process, Medtronic devised what is now known as the “perimeter plot” to graphically represent the anatomy of the RVOT. This plot, shown in Figure 5A, allows for a graphical comparison of the perimeter average diameter of the valve frame compared with the RVOT anatomy in both phases of the cardiac cycle. Good candidates for Harmony valve implantation have adequate interference of the anatomy of the RVOT with the valve frame both distally and proximally. Originally, Medtronic focused only on the diastolic anatomy, but more recently, there has been a focus on both the systolic and diastolic anatomy when evaluating valve fit. Essentially, the valve needs to be oversized vis a vis the patient’s RVOT anatomy both distally and proximally to be considered a good fit.37
Figure 5.
Harmony screening report. The report contains a (A) perimeter plot in systole and diastole, (B) virtual implantation, and (C) estimated fluoroscopic viewing projections with landing zone.
Implant procedure
Once the valve has been confirmed to be a good fit for patient anatomy, no further screening, balloon sizing, or coronary compression testing is performed. Typically, the RVOT is imaged after stiff guide wire placement in the distal pulmonary artery, and the valve is loaded and implanted with serial angiography in the anatomical position predicted to be favorable by the computed tomography scan. The biggest challenge to implantation is being able to correlate the position of the valve during delivery by angiography to the favorable position predicted by the computed tomography scan analysis. In addition to the perimeter plot, Medtronic provides operators with graphical predictions of the fluoroscopic appearance of the RVOT in several camera angles and 3-dimensional reconstructions of virtual implantations of the Harmony valve (Figure 5B, C).
Clinical experience
Before the FDA approval, 3 different clinical trials were conducted for both versions of the Harmony valve. The initial early feasibility study included only patients who received TPV 22.38 The pivotal study enrolled 50 patients, of which 10 were the modified, now commercially available TPV 25. The continued access study enrolled 37 patients, which included only 2 patients who received a TPV 22. Excluding the prototype clinical TPV, there were 87 patients in the pivotal and continued access studies who received the now commercially available TPV 22 and TPV 25.39
Four of 87 patients who received TPV 22 and TPV 25 required placement of the Harmony valve through a jugular vein approach because of lack of access from the femoral vein. In this patient cohort, there were no significant perivalvar leaks in any of the patients at the last follow-up, and there was nearly complete freedom from more than mild PR (1 patient had moderate PRs and none had severe PR at the 1-year follow-up). There were no patients in this 82-patient cohort with mortalities, sustained ventricular tachycardia (VT), or endocarditis in the 1-year follow-up data. Two patients who received TPV 22 in the early feasibility study required explant, 1 for valve fracture and 1 for migration because of inadequate interference. Several patients have required stenting and redo TCPVR because of intimal growth into the Harmony frames with resultant obstruction.40
There is concern that both balloon-expandable and self-expanding pulmonary valves can trigger VT and hinder VT ablations. Especially self-expanding valves, which interact with the muscular RVOT, can cover up the critical isthmus for VT ablations.41 Therefore, as clinical experience with Harmony increases, we will continue to need to monitor for sustained VT cases and explore the benefits of electrophysiology studies before implantation.
Edwards SAPIEN 3 TPV with the Alterra Adaptive Prestent
The Alterra Adaptive Prestent (Edwards Lifesciences) was designed to internally remodel a wide variety and sizes of RVOT morphologies, thereby creating a suitable “landing zone” for the implantation of a 29-mm SAPIEN 3 THV. The FDA has approved the use of the Edwards SAPIEN 3 TPV system with the Alterra Adaptive Prestent (SAPIEN 3 with Alterra) for patients with severe PR in 2021.42
Valve and delivery system
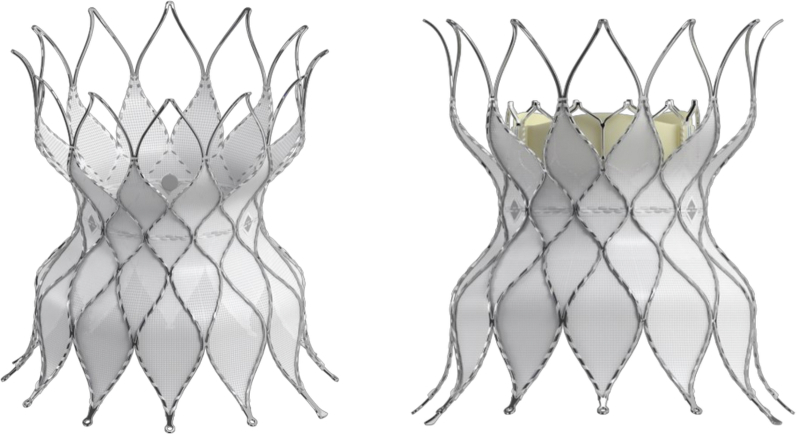
The Alterra Adaptive Prestent is designed to be used as a docking adaptor for the 29-mm SAPIEN 3 THV within the RVOT. It comprises a self-expanding, radiopaque, nitinol frame assembly and PET covering and has designated inflow and outflow ends (Figure 6). Three radiopaque markers are positioned at the prestent waist to facilitate the placement of the SAPIEN 3 THV. The inflow section is identifiable by the presence of 2 triangular tabs that are attached to the delivery system and circumferential covering of all cells.
Figure 6.
Alterra Adaptiveprestent. The Alterra Adaptive prestent is shown with the SAPIEN 3 THV. The proximal inflow portion of the pre-stent is covered, while the distal outflow portion has an open-cell design.
The outflow section is distinguished by open cells designed to facilitate blood flow into the branch pulmonary arteries should the device need to extend into the orifice of 1 or both of these structures. The PET fabric is attached by sutures to the inner surface of the frame to create a seal at the inflow section. The device has a symmetrical frame design, with the inflow and outflow diameters equal to 40 mm, and the central section measures 27 mm to provide a rigid landing zone for a SAPIEN 3 THV (29 mm). Although the total unconstrained device length is 49 mm, the nonfabric covered row of cells at the outflow results in a completely covered length of approximately 30 mm, allowing for the treatment of shorter RVOT by placing the distal apices across the orifice of the branch pulmonary arteries if needed. Currently, suitable anatomy is deemed to have a landing zone diameter between 27 and 38 mm with a minimum length ≥35 mm. Importantly, the Alterra Adaptive Prestent can be recaptured within the delivery system after up to 50% deployment, allowing for 2 attempts at placement, if required.42
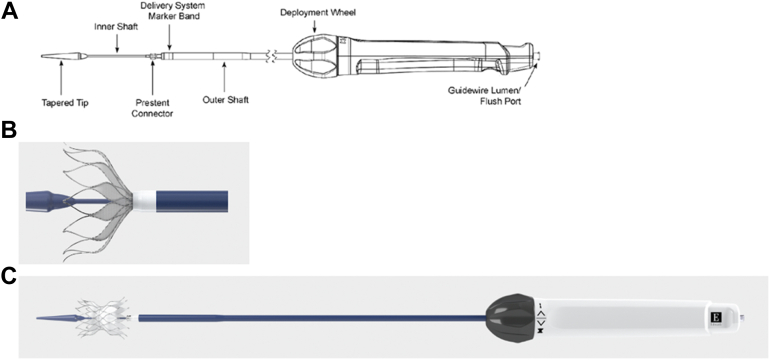
The Alterra Adaptive Prestent comes fully loaded in a custom delivery system consisting of a handle, retractable outer shaft, inner delivery shaft (upon which the stent sits), prestent connector, and a tapered tip meant to facilitate tracking through the vasculature (Figure 7). The delivery handle consists of a single knob that ergonomically allows for a 1-handed, slow, controlled deployment and potential recapture. The entire system fits through a 16F eSheath (Edwards Lifesciences).42
Figure 7.
Alterra Prestent delivery system. (A) Schematic diagram showing the Alterra Prestent delivery system components. (B) Enlarged image of the distal end of the delivery system with approximately 50% of the Alterra Prestent deployed. At this point, recovering, recapture, and subsequent redeployment are possible. (C) With the outer shaft completely withdrawn, the Alterra Prestent is completely deployed.
Screening process
The screening process for the Alterra Adaptive Prestent is similar to that of the Harmony valve. A perimeter plot in systole is created from an ECG-gated CTA to assess for adequate interference between the prestent and the patient’s RVOT.43 The main difference in screening for Alterra is that the screening process currently focuses on the systolic anatomy, whereas the Harmony reports are graded primarily on the diastolic fit.
Clinical experience
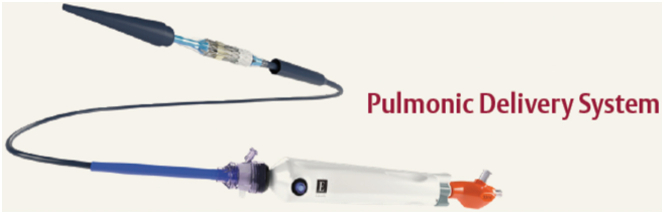
Patients with greater than moderate PR were prospectively evaluated for entry into a single arm, multicenter trial for the Edwards SAPIEN 3 TPV system with the Alterra Adaptive Prestent. Recently, 1-year data were evaluated from that study. Over 100 patients were evaluated, and 60 patients were enrolled following the screening process, resulting in a screen pass rate of 56%. The Alterra Adaptive Prestent and SAPIEN 3 THV were implanted in the intended location in 100% of patients during a single procedure in all but 1 patient in which the procedure was staged. There were no instances of device embolization or death at implant or in follow-up. Transient arrhythmias were reported in 33% of the patients within the first month after implant, but no arrhythmias were noted after 30 days out to 1 year. New or worsening tricuspid regurgitation was noted in 16.7% of patients, which is most likely secondary to chordal damage sustained with the use of the Commander delivery system in the right heart. The newly designed covered Edwards Pulmonic Delivery System, designed specifically to navigate a 29-mm SAPIEN 3 THV through the right heart, should greatly simplify this procedure and minimize the risk of tricuspid valve damage (Figure 8). In patients with available paired 1 year MRI data, significant improvements in pulmonary regurgitant fraction and right ventricular end-diastolic volume were noted. The mean echocardiographic Doppler RVOT gradient remained stable for the group through 1 year. Minor type 1 frame fractures of the Alterra Adaptive Prestent were noted in 12 patients at 1 year with no apparent clinical impact at this time. There were no reported cases of IE, and all patients remained with the device in place at 1 year.44
Figure 8.
Edwards Pulmonicdeliverysystem. The system features a covered balloon assembly that protects the SAPIEN 3 THV and cardiac structures from one another during navigation through the right side of the heart. THV, transcatheter heart valve.
Med-Zenith PT-Valve
The Med-Zenith PT-Valve (Beijing Med-Zenith) is a self-expandable frame housing with a tissue pulmonary valve designed to treat PR in native or patched RVOTs. It is currently in clinical trials only in China.
Valve and delivery system
Beijing Med-Zenith Medical Scientific manufactured a symmetric-shaped, laser-cut, nitinol wire frame covered with porcine pericardium. Inside, a 3-leaflet porcine pericardial valve with sealed membranes is sewn in the middle of the frame. The proximal and distal ends of the frame are softer, wider, and equal in diameter. The valve housing is smaller and stiffer in an attempt to resist valve leaflet deformation. There are radiopaque markers at the bottom of the leaflets, with 3 anchors at the distal frame for attachment and release. There are 3 valve sizes: 20, 23, and 26 mm, and there are 5 frame sizes (Figure 9).45 The delivery system has a crossing profile of 21F. The loading accessories consist of 3 hooks to attach to the anchors with a loading funnel and a unique attached crimper to facilitate loading the nitinol frame in ice-cold sterile saline in a bowl.
Figure 9.
Med-Zenith PT valve. (A) Med-Zenith PT-valve with a (B) schematic diagram. 1, nitinol stent; 2, leaflets; 3, seal membrane; 4, markers; 5, anchors.
Preprocedural evaluation
Like Harmony and Alterra, screening for appropriate patients for the Med-Zenith PT-Valve is solely performed using an ECG-gated CTA of the RVOT and pulmonary arteries. Perimeter plots in systole and diastole are created to assess for adequate interference between the valve and the patient’s RVOT (Figure 10).
Figure 10.
Med-Zenith PT-valvescreening report. Perimeter plots in (A) diastole and (B) systole are generated from an ECG-gated CTA. CTA, computed tomography angiography; ECG, electrocardiogram.
Implant procedure
The PT-Valve is washed in ice-cold normal saline and loaded onto the 21F delivery catheter. The valve is then delivered to the intended distal landing zone using a siting angiographic catheter delivered from the contralateral vein. The valve is then expanded using the clockwise rotation of the delivery handle until the 3 loading hooks release the anchors.
Clinical experience
Thirty-seven patients were screened, and 22 patients with significant PR and right ventricle dilation were enrolled in the Chinese clinical trial. Valve implantation was successful in all 22 patients. Acutely, there were no cases of device embolization/malposition, coronary compression, ventricular arrhythmias, tricuspid valve injury, or >2+ PR or parivalvar leak. During a follow-up period of 13 to 35 months, 5 patients developed grade 1 PR or perivalvar leak.46
Pulsta valve
The Pulsta valve (TaeWoong Medical Co) is a self-expanding valve with flared ends that was developed in South Korea to accommodate TCPVR within larger RVOTs that preclude the placement of balloon-expandable valves. It is a unique self-expanding valve that does not have the hourglass shape of the longer Harmony, Alterra, and Med-Zenith PT constructs.
Valve and delivery system
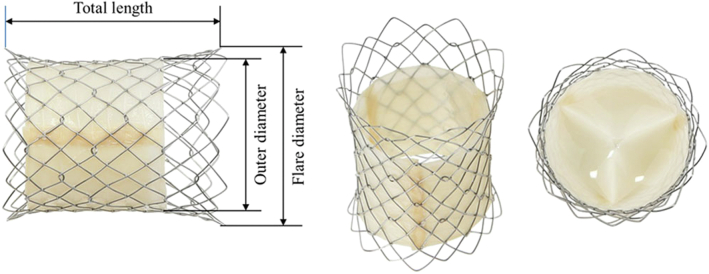
The Pulsta valve comprises a nitinol stent frame, which is made using 0.0115-inch thick knitted double-strand wire and is covered by the treated porcine pericardium. Both ends of the valve are flared to 4 mm wider than the outer diameter for stable valve adaptation to various RVOT geometries (Figure 11). The proximal and distal ends of the stent frame are uncovered and can be positioned without concern of flow obstruction if they protrude into the branch pulmonary arteries or RVOT. The trileaflet valve is made using treated porcine pericardium for maximal tissue preservation. This porcine pericardium is tightly hand sewn to the stent wall with 5-0 braided polyester to allow for good valve coaptation. The valve diameter ranges from 18 to 28 mm with 2-mm increments. The total length of the valve is 28 to 38 mm.47
Figure 11.
Pulsta valve. The valve diameter ranges from 18 to 32 mm with 2-mm increments. Both ends of the valve are flared 4 mm wider than the outer diameter and are uncovered.
The delivery system has a tapered tip, which is 17 mm in length and conical in shape to aid in insertion and a usable catheter length of 110 cm. The diameter of the outer sheath varies from 18F to 20F in the region of the valve loading zone based on the valve size. The catheter shaft is 12F (Figure 3C). The proximal aspect of the stent frame is inserted onto a hook block positioned at the proximal part of the valve loading zone, thus securing the frame and allowing for controlled deployment and positioning of the valve. The valve is then crimped onto the delivery cable using the Heart Valve Crimper (Model RVS, Blockwise Engineering LLC).47
Preprocedural evaluation
Anatomic suitability for the Pulsta valve requires the RVOT diameter to be <28 mm as measured on transthoracic echocardiography or CTA.47,48
Implant procedure
Valve sizing determination is based on preprocedural imaging and confirmed by angiography and balloon sizing of the RVOT. The valve chosen is 2 to 4 mm larger than the narrowest area of the main pulmonary artery and equal to or slightly larger than the mean main pulmonary artery size. Serial v angiography is performed during valve deployment. The valve deployment is performed by rotating the knob on the delivery catheter clockwise, which retracts the outer sheath and partially uncovers the valve. After confirming the device position, complete deployment of the valve is performed by pulling the slider.47,48
Clinical experience
Twenty-five patients with significant PR and right ventricle dilation were screened and enrolled in the Korean multicenter clinical trial. The mean age of the patients was 21.6 ± 6.6 years (range, 11.2-38.5 years), and the mean body weight was 55.7 ± 12.7 kg (range, 36.8-81.3 kg). All patients at baseline had severe PR without significant stenosis with right ventricle dilation on cardiac MRI and mild-to-moderate exercise intolerance. The Pulsta valve was successfully implanted in all of the enrolled patients without any major adverse events.48 Follow-up evaluation after Pulsta valve placement performed at a mean of 33.1 ± 14.3 months (range, 12.0-50.6 months) showed excellent outcomes. There was no significant stenosis, with a mean gradient of 6.5 ± 3.0 mm Hg (range, 2.0-16.0 mm Hg) and no significant PR in all patients, but mild perivalvar leak was seen in 4 patients. The New York Heart Association functional class of patients improved to class I in all patients, and the peak oxygen consumption also improved significantly.48 The Pulsta valve has also been implanted in the branch pulmonary arteries in a patient with severely dilated RVOT that was >30 mm in diameter. Given the shorter stent frame and uncovered proximal and distal regions of the stent, there was no jailing of the upper lobe branches or overhang into the main pulmonary artery.49
Venus P-valve
The Venus P-valve (Venus MedTech) is a self-expanding transcatheter valve developed by Venus Medtech in Shanghai, China. Similar to many of the other valves described above, it was designed to treat patients with patched RVOTs and secondary PR and right ventricle dilation.
Valve and delivery system
The Venus P-valve consists of a self-expanding nitinol stent with a trileaflet porcine pericardial valve sutured inside the stent (Figure 12). The valve is available in diameters of 18 to 36 mm, and lengths of the straight section of the stent of 25 to 30 mm. There are flares at both ends, the proximal one at the RVOT being covered and the distal one at the pulmonary artery end being uncovered. The latter allows maintaining patency of the pulmonary artery branches when the flare is placed in the distal main pulmonary artery. Both the flares are 10 mm larger than the straight section of the stent. There are radiopaque markers at each end of the straight section of the stent at the junction with the flares.
Figure 12.

Venus P-valve. Self-expanding nitinol stent with a trileaflet porcine pericardial valve.
The delivery catheter system has a radiopaque tip at its distal end and a radiopaque marker on the distal part of the protective sheath covering, which maintains the valve when it has been crimped and compressed (note that the Harmony delivery system is also referred to as the DCS). There are 2 ears for attaching the valve at the proximal flare to aid with crimping. At the proximal end of the handle, there is a precise release handle, which is used to load and deploy the valve in a controlled manner and eventually release it. The DCS can be passed over a 0.035-inch stiff guide wire in the pulmonary artery branches. The DCS can be delivered through 22F or 24F sheaths. The 28- to 30-mm diameter valves can be accommodated in a 22F sheath and 32- to 36-mm diameter valves in a 24F sheath.
Preprocedural evaluation
Unlike many of the self-expanding valve technologies described above, only MRI is needed to screen patients for the Venus P-valve. The Venus P-valve is intended for a population of patients who require a valve of >29 mm. Because it is being implanted in an expansile RVOT and because the valve is mounted in a self-expanding stent, some oversizing is required for satisfactory placement. Therefore, from the initial studies, it became clear that the valve could be implanted in RVOTs with the narrowest diameters (usually at the pulmonary valve annulus and evaluated by balloon interrogation) of 32 to 34 mm.
Clinical experience
Since 2013, various publications have reported on the early results of the Venus P-valve implantation on a compassionate use basis in patients fulfilling the criteria for percutaneous pulmonary valve implantation.50, 51, 52, 53 The valve implantations were reported from China, Thailand, and South America.50,51,53 The results have described successful valve implantation and adequate remodeling of the right ventricle with reduction of the indexed right ventricular end-diastolic volume and abolition of PR. Subsequently, Venus P-valves have been implanted in many countries, including the United Kingdom. A recent publication has reported on the international multicenter medium-term results of Venus P-valve implantation.52 Data were collected from 38 patients in whom valve implantation was attempted. Failure to implant a valve occurred in only 1 patient. During a mean follow-up of 25 months, there were no valve failures or deterioration of valve performance. Frame fractures, which were actively and systematically screened for, occurred in 27% of patients and were minor, involving single struts of the stents. On MRI follow-up assessment, there was a reduction in the index right ventricular end-diastolic volume from a mean of 151 mL/m2 before to 112 mL/m2 after valve implantation. The pulmonary regurgitant fraction reduced from a mean of 48% before to 4% after valve implantation. There were no episodes of IE, although further long-term data are needed.
The advantages of the Venus P-valve include the ability to implant the valve precisely, to deal with larger RVOTs, good durability, and low incidence of IE. Although some RVOTs were challenging for valve implantation, the recent use of DrySeal sheaths has made the procedure much easier. When larger sizes of the valve are implanted, it allows for future valve-in-valve procedures if needed.
Conclusion
Transcatheter pulmonary valve replacement has come a long way since the first procedure in 2000.1 After the initial development and use of balloon-expandable valves for TCPVR, it became evident that larger self-expanding valve technologies were needed to treat most of our patients needing pulmonary valve replacement. Over the last decade, several new self-expanding valves and prestents have been developed worldwide, which have been shown to be quite effective in the short- and medium-term and have proven to be an effective treatment modality for this patient population. Long-term data for balloon-expandable valves in a multicenter study of 2476 patients have shown that survival and freedom from reintervention are comparable with surgically placed pulmonary valves, but the incidence of IE was 16.9% at 8 years.24,25 Given the large size of this experience and identification of risk factors such as residual gradients, younger age, and history of immunosuppression and prior endocarditis, it is possible that we can use this significant experience to guide future procedures and the development and design of the next generation of TPV to improve further on outcomes in this patient population.
Acknowledgments
Declaration of competing interest
Dr Levi is a consultant and proctor for Edwards Lifesciences and Medtronic Inc. Dr Cheatham is a consultant, principal investigator, proctor, advisory board member for Medtronic Inc; consultant for NuMED; consultant, research support, and proctor for Bejing Med-Zenith Scientific Medical Co; data and safety monitoring board member for Xeltis; and data monitoring committee member for Autus Valve Technologies. Dr Qureshi is a consultant and proctor for NuMed, Medtronic Inc, Occlutech, Lifetech, and Venus Medtech and principal investigator for Venus Medtech. Dr Shahanavaz is a consultant and proctor for Medtronic Inc and Edwards Lifesciences. Dr Zahn is a consultant and proctor for Medtronic Inc and Abbott; consultant, proctor, and national principal investigator for Edwards Lifesciences; data and safety monitoring board member for valve trial and consultant for WL Gore; and chief medical officer for Renata Medical. Dr Patel reported no financial interests.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Bonhoeffer P., Boudjemline Y., Saliba Z., et al. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet. 2000;356(9239):1403–1405. doi: 10.1016/S0140-6736(00)02844-0. [DOI] [PubMed] [Google Scholar]
- 2.Zahn E.M., Hellenbrand W.E., Lock J.E., McElhinney D.B. Implantation of the melody transcatheter pulmonary valve in patients with a dysfunctional right ventricular outflow tract conduit early results from the U.S. clinical trial. J Am Coll Cardiol. 2009;54(18):1722–1729. doi: 10.1016/j.jacc.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 3.Martin M.H., Meadows J., McElhinney D.B., et al. Safety and feasibility of melody transcatheter pulmonary valve replacement in the native right ventricular outflow tract: a multicenter pediatric heart network scholar study. JACC Cardiovasc Interv. 2018;11(16):1642–1650. doi: 10.1016/j.jcin.2018.05.051. [DOI] [PubMed] [Google Scholar]
- 4.Cheatham S.L., Holzer R.J., Chisolm J.L., Cheatham J.P. The Medtronic Melody® transcatheter pulmonary valve implanted at 24-mm diameter – it works. Catheter Cardiovasc Interv. 2013;82(5):816–823. doi: 10.1002/ccd.24821. [DOI] [PubMed] [Google Scholar]
- 5.Berman D.P., McElhinney D.B., Vincent J.A., Hellenbrand W.E., Zahn E.M. Feasibility and short-term outcomes of percutaneous transcatheter pulmonary valve replacement in small (<30 kg) children with dysfunctional right ventricular outflow tract conduits. Circ Cardiovasc Interv. 2014;7(2):142–148. doi: 10.1161/CIRCINTERVENTIONS.113.000881. [DOI] [PubMed] [Google Scholar]
- 6.Malone L., Fonseca B., Fagan T., et al. Preprocedural risk assessment prior to PPVI with CMR and cardiac CT. Pediatr Cardiol. 2017;38(4):746–753. doi: 10.1007/s00246-017-1574-0. [DOI] [PubMed] [Google Scholar]
- 7.Romans R.A., Lu J.C., Balasubramanian S., et al. Cardiac magnetic resonance to predict coronary artery compression in transcatheter pulmonary valve implantation into conduits. JACC Cardiovasc Interv. 2022;15(9):979–988. doi: 10.1016/j.jcin.2022.02.047. [DOI] [PubMed] [Google Scholar]
- 8.Morray B.H., McElhinney D.B., Cheatham J.P., et al. Risk of coronary artery compression among patients referred for transcatheter pulmonary valve implantation: a multicenter experience. Circ Cardiovasc Interv. 2013;6(5):535–542. doi: 10.1161/CIRCINTERVENTIONS.113.000202. [DOI] [PubMed] [Google Scholar]
- 9.Torres A.J., McElhinney D.B., Anderson B.R., et al. Aortic root distortion and aortic insufficiency during balloon angioplasty of the right ventricular outflow tract prior to transcatheter pulmonary valve replacement. J Interv Cardiol. 2016;29(2):197–207. doi: 10.1111/joic.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guyon P.W., Mohammad Nijres B., Justino H., et al. Expanded use of the one-step technique for simultaneous landing zone stenting and placement of the melody transcatheter pulmonary valve. J Invasive Cardiol. 2021;33(12):E954–E959. doi: 10.25270/jic/20.00739. [DOI] [PubMed] [Google Scholar]
- 11.Boudjemline Y. A new one-step procedure for pulmonary valve implantation of the melody valve: simultaneous prestenting and valve implantation. Catheter Cardiovasc Interv. 2018;91(1):64–70. doi: 10.1002/ccd.27332. [DOI] [PubMed] [Google Scholar]
- 12.Breatnach C.R., Mcguinness J., Ng L.Y., et al. Procedural technique for hybrid pulmonary valve replacement in infants and small children. Eur J Cardiothorac Surg. 2021;59(4):823–830. doi: 10.1093/ejcts/ezaa410. [DOI] [PubMed] [Google Scholar]
- 13.Porras D., Gurvitz M., Marshall A.C., Emani S.M. Hybrid approach for off-pump pulmonary valve replacement in patients with a dilated right ventricular outflow tract. Ann Thorac Surg. 2015;100(5):e99–e101. doi: 10.1016/j.athoracsur.2015.02.124. [DOI] [PubMed] [Google Scholar]
- 14.Travelli F.C., Herrington C.S., Ing F.F. A novel hybrid technique for transcatheter pulmonary valve implantation within a dilated native right ventricular outflow tract. J Thorac Cardiovasc Surg. 2014;148(2):e145–e146. doi: 10.1016/j.jtcvs.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 15.Shahanavaz S., Asnes J.D., Grohmann J., et al. Intentional fracture of bioprosthetic valve frames in patients undergoing valve-in-valve transcatheter pulmonary valve replacement. Circ Cardiovasc Interv. 2018;11(8) doi: 10.1161/CIRCINTERVENTIONS.118.006453. [DOI] [PubMed] [Google Scholar]
- 16.Jones T.K., McElhinney D.B., Vincent J.A., et al. Long-term outcomes after melody transcatheter pulmonary valve replacement in the US investigational device exemption trial. Circ Cardiovasc Interv. 2022;15(1) doi: 10.1161/CIRCINTERVENTIONS.121.010852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cardoso R., Ansari M., Garcia D., Sandhu S., Brinster D., Piazza N. Prestenting for prevention of melody valve stent fractures: a systematic review and meta-analysis. Catheter Cardiovasc Interv. 2016;87(3):534–539. doi: 10.1002/ccd.26235. [DOI] [PubMed] [Google Scholar]
- 18.Georgiev S., Ewert P., Eicken A., et al. Munich comparative study: prospective long-term outcome of the transcatheter melody valve versus surgical pulmonary bioprosthesis with up to 12 years of follow-up. Circ Cardiovasc Interv. 2020;13(7) doi: 10.1161/CIRCINTERVENTIONS.119.008963. [DOI] [PubMed] [Google Scholar]
- 19.Abdelghani M., Nassif M., Blom N.A., et al. Infective endocarditis after melody valve implantation in the pulmonary position: a systematic review. J Am Heart Assoc. 2018;7(13) doi: 10.1161/JAHA.117.008163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Dijck I., Budts W., Cools B., et al. Infective endocarditis of a transcatheter pulmonary valve in comparison with surgical implants. Heart. 2015;101(10):788–793. doi: 10.1136/heartjnl-2014-306761. [DOI] [PubMed] [Google Scholar]
- 21.Nordmeyer J., Ewert P., Gewillig M., et al. Acute and midterm outcomes of the post-approval MELODY registry: a multicentre registry of transcatheter pulmonary valve implantation. Eur Heart J. 2019;40(27):2255–2264. doi: 10.1093/eurheartj/ehz201. [DOI] [PubMed] [Google Scholar]
- 22.Sadeghi S., Wadia S., Lluri G., et al. Risk factors for infective endocarditis following transcatheter pulmonary valve replacement in patients with congenital heart disease. Catheter Cardiovasc Interv. 2019;94(4):625–635. doi: 10.1002/ccd.28474. [DOI] [PubMed] [Google Scholar]
- 23.Georgiev S., Ewert P., Tanase D., et al. A low residual pressure gradient yields excellent long-term outcome after percutaneous pulmonary valve implantation. JACC Cardiovasc Interv. 2019;12(16):1594–1603. doi: 10.1016/j.jcin.2019.03.037. [DOI] [PubMed] [Google Scholar]
- 24.McElhinney D.B., Zhang Y., Levi D.S., et al. Reintervention and survival after transcatheter pulmonary valve replacement. J Am Coll Cardiol. 2022;79(1):18–32. doi: 10.1016/j.jacc.2021.10.031. [DOI] [PubMed] [Google Scholar]
- 25.McElhinney D.B., Zhang Y., Aboulhosn J.A., et al. Multicenter study of endocarditis after transcatheter pulmonary valve replacement. J Am Coll Cardiol. 2021;78(6):575–589. doi: 10.1016/j.jacc.2021.05.044. [DOI] [PubMed] [Google Scholar]
- 26.Davtyan A., Guyon P.W., El-Sabrout H.R., et al. Selective valve removal for melody valve endocarditis: practice variations in a multicenter experience. Pediatr Cardiol. 2022;43(4):894–902. doi: 10.1007/s00246-021-02801-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garay F., Webb J., Hijazi Z.M. Percutaneous replacement of pulmonary valve using the Edwards-Cribier percutaneous heart valve: first report in a human patient. Catheter Cardiovasc Interv. 2006;67(5):659–662. doi: 10.1002/ccd.20753. [DOI] [PubMed] [Google Scholar]
- 28.Sinha S., Aboulhosn J., Asnes J., et al. Initial results from the off-label use of the Sapien S3 valve for percutaneous transcatheter pulmonary valve replacement: a multi-institutional experience. Catheter Cardiovasc Interv. 2019;93(3):455–463. doi: 10.1002/ccd.27973. [DOI] [PubMed] [Google Scholar]
- 29.Morgan G.J., Sadeghi S., Salem M.M., et al. SAPIEN valve for percutaneous transcatheter pulmonary valve replacement without “pre-stenting”: a multi-institutional experience. Catheter Cardiovasc Interv. 2019;93(2):324–329. doi: 10.1002/ccd.27932. [DOI] [PubMed] [Google Scholar]
- 30.Shahanavaz S., Zahn E.M., Levi D.S., et al. Transcatheter pulmonary valve replacement with the Sapien prosthesis. J Am Coll Cardiol. 2020;76(24):2847–2858. doi: 10.1016/j.jacc.2020.10.041. [DOI] [PubMed] [Google Scholar]
- 31.Stapleton G.E., Gowda S.T., Bansal M., Khan A., Qureshi A.M., Justino H. Sapien S3 valve deployment in the pulmonary position using the gore DrySeal sheath to protect the tricuspid valve. Catheter Cardiovasc Interv. 2020;96(6):1287–1293. doi: 10.1002/ccd.29120. [DOI] [PubMed] [Google Scholar]
- 32.Kenny D., Morgan G.J., Murphy M., et al. Use of 65 cm large caliber Dryseal sheaths to facilitate delivery of the Edwards SAPIEN valve to dysfunctional right ventricular outflow tracts. Catheter Cardiovasc Interv. 2019;94(3):409–413. doi: 10.1002/ccd.28409. [DOI] [PubMed] [Google Scholar]
- 33.Kenny D., Rhodes J.F., Fleming G.A., et al. 3-year outcomes of the Edwards SAPIEN transcatheter heart valve for conduit failure in the pulmonary position from the COMPASSION multicenter clinical trial. JACC Cardiovasc Interv. 2018;11(19):1920–1929. doi: 10.1016/j.jcin.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Riahi M., Blanke P., Webb J., Carere R.G. Early leaflet thrombosis complicating transcatheter implantation of a Sapien 3 valve in a native right ventricular outflow tract. Catheter Cardiovasc Interv. 2018;92(5):925–929. doi: 10.1002/ccd.27183. [DOI] [PubMed] [Google Scholar]
- 35.Shibbani K., Garg R., Zahn E.M., Mclennan D. Aspirin use and transcatheter pulmonary valve replacement, the need for consistency. Pediatr Cardiol. 2021;42(7):1640–1646. doi: 10.1007/s00246-021-02652-8. [DOI] [PubMed] [Google Scholar]
- 36.Hammadah M., Han B.K., de Oliveira Nunes M., et al. Hypoattenuated leaflet thickening after transcatheter pulmonary valve replacement with the SAPIEN 3 valve. JACC Cardiovasc Imaging. 2021;14(10):2047–2048. doi: 10.1016/j.jcmg.2021.04.023. [DOI] [PubMed] [Google Scholar]
- 37.Gillespie M.J., Benson L.N., Bergersen L., et al. Patient selection process for the harmony transcatheter pulmonary valve early feasibility study. Am J Cardiol. 2017;120(8):1387–1392. doi: 10.1016/j.amjcard.2017.07.034. [DOI] [PubMed] [Google Scholar]
- 38.Gillespie M.J., Bergersen L., Benson L.N., Weng S., Cheatham J.P. 5-year outcomes from the harmony native outflow tract early feasibility study. JACC Cardiovasc Interv. 2021;14(7):816–817. doi: 10.1016/j.jcin.2021.01.046. [DOI] [PubMed] [Google Scholar]
- 39.Gillespie M., McElhinney D., Jones T., Levi D., Weng S., Cheatham J. Midterm outcomes from the harmony transcatheter pulmonary valve pivotal trial and continued access study. Pediatr Cardiol. 2021;42(8):1903–1904. [Google Scholar]
- 40.Levi D.S., Gillespie M.J., McElhinney D.B., et al. O-6 | One-year outcomes in an expanded cohort of harmony transcatheter pulmonary valve recipients. J Soc Cardiovasc Angiogr Interv. 2022;1(3):100326–100327. doi: 10.1016/j.jscai.2022.100326. [DOI] [Google Scholar]
- 41.Combes N., Bartoletti S., Heitz F., Waldmann V. Critical isthmus of ventricular tachycardia covered by transcatheter pulmonary valve in a patient with tetralogy of Fallot. Eur Heart J. 2020;41(5):723. doi: 10.1093/eurheartj/ehz610. [DOI] [PubMed] [Google Scholar]
- 42.Zahn E.M., Chang J.C., Armer D., Garg R. First human implant of the Alterra Adaptive Prestent TM: a new self-expanding device designed to remodel the right ventricular outflow tract. Catheter Cardiovasc Interv. 2018;91(6):1125–1129. doi: 10.1002/ccd.27581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shahanavaz S., Balzer D., Babaliaros V., et al. Alterra adaptive Prestent and SAPIEN 3 THV for congenital pulmonic valve dysfunction: an early feasibility study. JACC Cardiovasc Interv. 2020;13(21):2510–2524. doi: 10.1016/j.jcin.2020.06.039. [DOI] [PubMed] [Google Scholar]
- 44.Zahn E., Dimas V., Babaliaros V., Kim D., Lim S., Morgan G. Transcatheter pulmonary valve implantation with the Alterra adaptive Prestent and SAPIEN 3 transcatheter heart valve: one-year outcomes of the ALTERRA pivotal trial. Pediatr Cardiol. 2021;42(8):1910–1911. [Google Scholar]
- 45.Shang X., Chen S., Zhang C., et al. First-in-man implantation of Med-zenith PT-Valve in right ventricular outflow tract for pulmonary regurgitation. JACC Cardiovasc Interv. 2019;12(19):1989–1990. doi: 10.1016/j.jcin.2019.07.036. [DOI] [PubMed] [Google Scholar]
- 46.Shang X., Dong N., Zhang C., Wang Y. The clinical trial outcomes of Med-zenith PT-Valve in the treatment of patients with severe pulmonary regurgitation. Front Cardiovasc Med. 2022;9:887886. doi: 10.3389/fcvm.2022.887886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim G.B., Song M.K., Bae E.J., et al. Successful feasibility human trial of a new self-expandable percutaneous pulmonary valve (Pulsta valve) implantation using knitted nitinol wire backbone and trileaflet α-Gal-free porcine pericardial valve in the native right ventricular outflow tract. Circ Cardiovasc Interv. 2018;11(6) doi: 10.1161/CIRCINTERVENTIONS.118.006494. [DOI] [PubMed] [Google Scholar]
- 48.Lee S.Y., Kim G.B., Kim S.H., et al. Mid-term outcomes of the Pulsta transcatheter pulmonary valve for the native right ventricular outflow tract. Catheter Cardiovasc Interv. 2021;98(5):E724–E732. doi: 10.1002/ccd.29865. [DOI] [PubMed] [Google Scholar]
- 49.Kim J.Y., Kim S.H., Jang S.I. Bilateral branch pulmonary artery Pulsta valve implantation for treatment of large right ventricular outflow tract in a high-risk patient. Catheter Cardiovasc Interv. 2021;98(5):923–927. doi: 10.1002/ccd.29857. [DOI] [PubMed] [Google Scholar]
- 50.Garay F., Pan X., Zhang Y.J., Wang C., Springmuller D. Early experience with the Venus p-valve for percutaneous pulmonary valve implantation in native outflow tract. Neth Heart J. 2017;25(2):76–81. doi: 10.1007/s12471-016-0932-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cao Q.L., Kenny D., Zhou D., et al. Early clinical experience with a novel self-expanding percutaneous stent-valve in the native right ventricular outflow tract. Catheter Cardiovasc Interv. 2014;84(7):1131–1137. doi: 10.1002/ccd.25544. [DOI] [PubMed] [Google Scholar]
- 52.Morgan G., Prachasilchai P., Promphan W., et al. Medium-term results of percutaneous pulmonary valve implantation using the Venus P-valve: international experience. EuroIntervention. 2019;14(13):1363–1370. doi: 10.4244/EIJ-D-18-00299. [DOI] [PubMed] [Google Scholar]
- 53.Promphan W., Prachasilchai P., Siripornpitak S., Qureshi S.A., Layangool T. Percutaneous pulmonary valve implantation with the Venus P-valve: clinical experience and early results. Cardiol Young. 2016;26(4):698–710. doi: 10.1017/S1047951115001067. [DOI] [PubMed] [Google Scholar]