Abstract
Background
Coronary artery calcification increases the procedural complexity of percutaneous coronary intervention and is associated with worse outcomes, especially in women. Intravascular lithotripsy (IVL) has been demonstrated to be safe and effective for vessel preparation in severely calcified stenotic lesions before stent implantation. Sex-based outcomes of IVL-facilitated stenting have not been defined.
Methods
We performed a patient-level pooled analysis of the 4 prospective, single-arm Disrupt CAD studies that evaluated the safety and efficacy of IVL-facilitated stenting. Patient baseline and procedural characteristics and clinical outcomes were examined based on sex. The primary safety end point was 30-day major adverse cardiovascular events, defined as the composite of cardiac death, myocardial infarction, or target vessel revascularization. The primary efficacy end point was procedural success, defined as stent delivery with residual in-stent stenosis ≤30% without in-hospital major adverse cardiovascular events.
Results
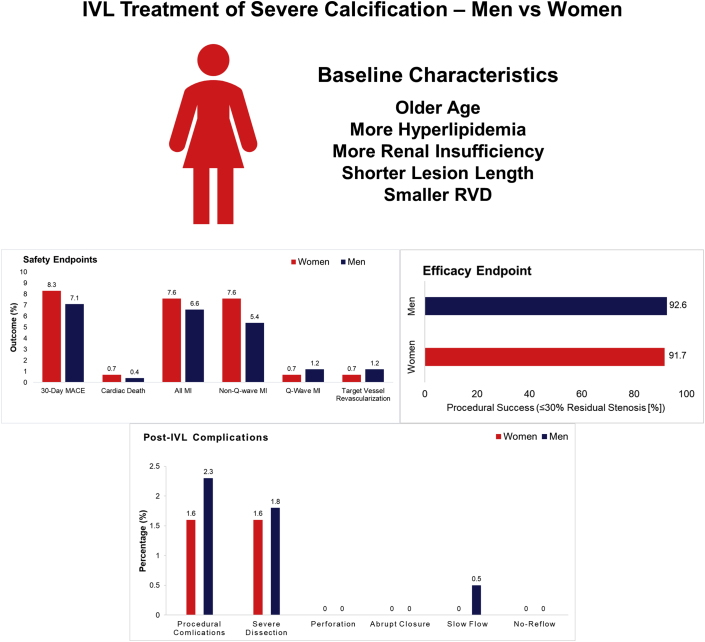
A total of 628 patients were included, of which 144 (22.9%) were women. Women were older (P < .001) and more likely to have hyperlipidemia (P = .03), renal insufficiency (P = .05), and prior myocardial infarction (P = .05). Women had smaller mean reference vessel diameter (2.7 ± 0.4 mm vs 3.0 ± 0.5 mm, P < .001), shorter lesion length (22.4 ± 10.3 mm vs 25.0 ± 11.7 mm, P = .01), and less side branch involvement (22.9% vs 32.4%, P = .03). Severe coronary calcification defined by angiography, stent delivery success, lesion predilatation, post-IVL dilatation, and poststent dilatation was similar between groups. There were no significant differences between women and men in the primary safety end point (8.3% vs 7.1%, P = .61; adjusted odds ratio 1.66; 95% confidence interval 0.78, 3.34; P = .17) or the primary efficacy end point (91.7% vs 92.6%, P = .72; adjusted odds ratio 0.58; 95% confidence interval 0.29, 1.24; P = .15). Post-IVL serious angiographic complications (flow-limiting dissection, perforation, abrupt closure, slow flow, no reflow) were similar for women and men (1.6% vs 2.3%, P = .75).
Conclusions
Despite more comorbidities and smaller vessel size, IVL-facilitated stenting of severely calcified lesions achieves similar safety and efficacy in women and men.
Keywords: Sex, Coronary artery disease, Intravascular lithotripsy, Calcium, Percutaneous coronary intervention.
Central Illustration

Highlights
-
•
In this sex-based analysis of 629 patients (23% women) with severe coronary calcium undergoing intravascular lithotripsy, there was no difference in 30-day major adverse cardiovascular events between women and men (8.3% vs 7.1%, P = .61).
-
•
Successful stent delivery with residual in-stent stenosis ≤30% without in-hospital major adverse cardiovascular events was equally high in women and men (91.7% vs 92.6%, P = .72).
-
•
Unlike prior experience with atherectomy devices, serious angiographic complications (flow-limiting dissection, perforation, abrupt closure, slow flow, no reflow) were low for both women and men (1.6% vs 2.3%, P = .75).
-
•
Intravascular lithotripsy is safe and effective in treating severely calcified lesions in women as well as men, where other plaque modification atherectomy devices have resulted in more acute complications.
Percutaneous coronary intervention (PCI) with second-generation drug-eluting stents (DES) is the standard of care for selected patients with coronary artery disease.1,2 Despite DES, treatment of calcified lesions remains challenging and is associated with significantly worse acute and long-term outcomes compared with noncalcified lesions.3, 4, 5 Calcified lesions now account for approximately a third of lesions treated in contemporary interventional practice as we face an aging population with a higher prevalence of diabetes, hypertension, and renal insufficiency.6 Women with moderate-to-severe lesion calcium are particularly vulnerable to poor outcomes. In a dedicated DES registry of 6371 female patients, of which 1622 (25.5%) had moderate/severe calcium, outcomes at 3 years were significantly worse with a reported 38% higher mortality, a 48% higher rate of death or myocardial infarction (MI), and a 56% higher rate of death, MI, or target lesion revascularization (TLR) compared with treatment of mildly or noncalcified lesions.7 Plaque modification with atherectomy improves lesion compliance, allowing optimal stent expansion, but is associated with increased periprocedural complications including coronary dissections, perforation, and higher rates of periprocedural MI.8, 9, 10 The procedural risks of atherectomy are accentuated in women who have rates of serious flow-limiting coronary dissections and cardiac tamponade that are 4- to 5-fold higher than men treated with rotational atherectomy, leading to 2-fold higher rates of in-hospital major adverse cardiac events (MACE).11 Similar results have been reported with orbital atherctomy.12 Acute procedural complications in women may limit the use of atherectomy to optimize DES expansion (one of the strongest predictors of subsequent stent thrombosis and restenosis13,14) and likely contribute to the poor outcomes reported in the longer term.
Intravascular lithotripsy (IVL) (Shockwave Medical Inc) is a novel approach that uses acoustic pressure waves to fracture both superficial and deep calcium deposits in situ.15 The safety and efficacy of lesion preparation with coronary IVL before DES implantation for patients undergoing PCI of severely calcified coronary lesions were evaluated in the observational Disrupt CAD I through IV studies.16, 17, 18, 19 We aimed to evaluate 30-day safety and procedural efficacy of IVL-facilitated stent implantation in women compared with men.
Methods
Study population and objectives
This is a sex-based comparison of a patient-level pooled analysis of the Disrupt CAD I-IV studies evaluating coronary IVL-facilitated stenting for de novo severely calcified coronary artery lesions in patients with stable or unstable angina or silent ischemia. Study designs, detailed inclusion criteria, and outcomes of the Disrupt CAD I-IV studies have been described previously.16,17,19, 20, 21 The Disrupt CAD I-IV studies had similar eligibility criteria (Supplemental Table S1). Severe calcification was defined by the angiographic appearance (before contrast injection) of radiopacities involving both sides of the arterial wall of at least 15 mm in length or based on intravascular imaging demonstrating a calcium angle ≥270° in at least 1 cross section. Lesion predilation was permitted, and IVL followed by DES implantation was consistently performed in each trial.16, 17, 18, 19, 20 The studies had similar end point definitions adjudicated by an independent clinical events committee, and angiograms were reviewed by the same independent core laboratory (Supplemental Table S2). Clinical follow-up was performed in all studies at 30 days. The Disrupt CAD studies were performed in accordance with the Declaration of Helsinki, institutional review board or ethics committee approval was obtained for each study at the participating centers (Supplemental Table S3), and all patients provided written informed consent.
Study end points
The primary safety end point was 30-day MACE, defined as the composite of cardiac death, MI, or target vessel revascularization (TVR). Periprocedural MI was defined in all studies as the peak post-PCI creatine kinase–myocardial band level >3× the upper limit of normal with or without new pathologic Q-waves, consistent with prior atherectomy studies.10,17,22 Spontaneous MI after discharge was defined using a creatine kinase–myocardial band threshold of >3× upper limit of normal for Disrupt CAD I and II and the Fourth Universal Definition of MI23 in Disrupt CAD III and IV. The primary efficacy end point was procedure success, defined as a residual in-stent stenosis ≤30% (angiographic core laboratory assessment) without in-hospital MACE. The secondary efficacy end points included procedural success with a residual diameter stenosis threshold of <50%, final postprocedural percent diameter stenosis, post-IVL and final serious angiographic complications (defined as dissection grade ≥D, perforation, abrupt closure, slow flow/no reflow), as well as target lesion failure, defined as the composite of cardiac death, target vessel MI, or ischemia-driven TLR at 30 days, and definite or probable stent thrombosis at 30 days as defined by the Academic Research Consortium.24
Statistical analysis
The primary analysis population was by intention-to-treat, consisting of all enrolled patients. Adjudicated patient-level data were pooled and compared based on sex. Continuous data are presented as mean ± standard deviation, and categorical variables are presented as percentages and frequencies. Multivariable logistic regression was performed to determine the independent relationship of sex to the primary 30-day safety and efficacy outcomes. The following variables were entered into each model: sex, age, diabetes, smoking, chronic kidney disease, reference vessel diameter, minimal luminal diameter, and lesion length. Primary end point heterogeneity was analyzed using a logistic regression model including an intercept and fixed effect for study. Point estimates and Clopper–Pearson 95% confidence intervals (CIs) were constructed for primary end points. All statistical analyses were performed with SAS, version 9.4 (SAS Institute).
Results
From December 21, 2015, to April 6, 2020, a total of 628 patients including 144 (22.9%) women and 484 (77.1%) men were enrolled at 72 centers from 12 countries in the United States and Europe. Follow-up at 30 days was available in 626 of 628 (99.7%) patients. Women were older and more frequently had hyperlipidemia and renal insufficiency than men (Table 1). Women had a smaller reference vessel diameter and less side branch involvement, but severe calcification and calcium length were not different compared with men (Table 1).
Table 1.
Baseline clinical and angiographic characteristics.
| Characteristic | Women, n = 144 | Men, n = 484 | P value |
|---|---|---|---|
| Age, y | 74.2 ± 9.0 | 71.1 ± 8.8 | <.001 |
| Diabetes mellitus | 59 (41.0) | 182 (37.6) | .47 |
| Hypertension | 124 (86.1) | 415 (85.7) | .91 |
| Hyperlipidemia | 130 (90.3) | 401 (83.0) | .03 |
| Prior myocardial infarction | 23 (16.0) | 114 (23.6) | .053 |
| Prior coronary artery bypass grafting | 9 (6.3) | 51 (10.5) | .12 |
| Prior stroke or transient ischemic attack | 13 (9.0) | 41 (8.5) | .83 |
| Current or former smoker | 62/143 (43.4) | 295/480 (61.5) | <.001 |
| Renal insufficiencya | 45/143 (31.5) | 112/482 (23.2) | .046 |
| Pacemaker or ICD/CRT-D | 7 (4.9) | 32/483 (6.6) | .44 |
| Canadian Cardiovascular Society angina classification | .03 | ||
| Class 0 | 17/139 (12.2) | 72/476 (15.1) | |
| Class I | 21/139 (15.1) | 121/476 (25.4) | |
| Class II | 57/139 (41.0) | 171/476 (35.9) | |
| Class III | 42/139 (30.2) | 101/476 (21.2) | |
| Class IV | 2/139 (1.4) | 11/476 (2.3) | |
| Angiographic characteristic (core laboratory) | |||
| Target vessel | .43 | ||
| Protected left main | 0 (0.0) | 9 (1.9) | |
| Left anterior descending | 87 (60.4) | 281 (58.1) | |
| Left circumflex | 17 (11.8) | 58 (12.0) | |
| Right | 40 (27.8) | 136 (28.1) | |
| Reference vessel diameter, mm | 2.74 ± 0.43 | 3.02 ± 0.51 (481) | <.001 |
| Minimum lumen diameter, mm | 1.00 ± 0.43 | 1.08 ± 0.38 (481) | .04 |
| Diameter stenosis, % | 63.2 ± 12.2 | 63.9 ± 11.7 (481) | .52 |
| Lesion length, mm | 22.4 ± 10.3 | 25.0 ± 11.7 (480) | .01 |
| Calcium length, mm | 38.7 ± 18.1 | 42.4 ± 20.4 (479) | .052 |
| Severe calcificationb | 138 (95.8) | 471 (97.3) | .08 |
| Bifurcation lesion with side branch involvement | 33 (22.9) | 157 (32.4) | .03 |
Values are n (%), n/N (%), mean ± standard deviation, or mean ± standard deviation (n).
ICD/CRT-D, implantable cardiac defibrillator with or without bi-ventricular pacing capability
Estimated glomerular filtration rate (using the Modification of Diet in Renal Disease [MDRD] formula) < 60 mL/min/1.73 m2.
Defined as radiopaque densities noted without cardiac motion generally involving both sides of the arterial wall.
Femoral access tended to be more common in women than in men, but the rate of successful IVL delivery, target lesion predilatation, and post-IVL and poststent dilatation was similar in women and men. Procedure duration was shorter in women, and fewer IVL catheters (1.2 ± 0.4 vs 1.4 ± 0.7, P < .001) and IVL pulses (63.0 ± 35.2 vs 78.1 ± 44.1, P < .001) were used than in men. Women had a significantly shorter lesion length (22.4 ± 10.3 vs 25.0 ± 11.7, P = .01). Stent implantation was similar in both sexes (99.3% vs 99.6%, P = 1.00), as was the median length of stay of 1 day (Table 2).
Table 2.
Procedural characteristics.
| Characteristic | Women, n = 144 | Men, n = 484 | P value |
|---|---|---|---|
| Total procedure time, min | 58.3 ± 26.5 | 66.2 ± 33.6 | .004 |
| Contrast volume, mL | 170.3 ± 77.8 | 182.6 ± 77.0 | .09 |
| Vascular accessa | .10 | ||
| Radial | 59/106 (55.7) | 222/342 (64.9) | |
| Femoral | 45/106 (42.5) | 118/342 (34.5) | |
| Brachial | 1/106 (0.9) | 2/342 (0.6) | |
| Ulnar | 1/106 (0.9) | 0/342 (0.0) | |
| Predilatation | 65 (45.1) | 234 (48.3) | .50 |
| Patients undergoing IVL | 140 (97.2) | 480 (99.2) | .09 |
| Maximum IVL inflation pressure, atm | 5.9 ± 0.4 | 6.0 ± 0.5 | .26 |
| Number of lithotripsy catheters | 1.2 ± 0.4 | 1.4 ± 0.7 | <.001 |
| IVL balloon-to-RVD ratio | 1.3 ± 0.2 | 1.2 ± 0.2 | .049 |
| Number of pulses | 63.0 ± 35.2 | 78.1 ± 44.1 | <.001 |
| Post-IVL dilatation | 15/114 (13.2) | 69/386 (17.9) | .24 |
| Stent delivery | 143 (99.3) | 482 (99.6) | 1.00 |
| Number of stents implanted | 1.2 ± 0.5 | 1.3 ± 0.5 | .24 |
| Poststent dilatation | 134 (93.1) | 454 (93.8) | .75 |
| Total stent length, mm | 31.3 ± 13.6 | 33.8 ± 14.6 | .07 |
| Duration of hospitalization, days | 1.0 (1.0, 1.0) | 1.0 (1.0, 1.0) | .95 |
Values are mean ± standard deviation, n/N (%), n (%), or median (first quartile, third quartile).
IVL, intravascular lithotripsy; RVD, reference vessel diameter.
Vascular access data collected in Disrupt CAD III and Disrupt CAD IV only.
Primary end points
The primary safety end point of 30-day MACE was similar in women and men (8.3% vs 7.1%, P = .61; adjusted odds ratio [OR] 1.66; 95% CI 0.78, 3.34; P = .17). The primary efficacy end point of procedural success with ≤30% residual stenosis was achieved at high rates in both women and men with no significant difference (91.7% vs 92.6%, P = .72; adjusted OR 0.58; 95% CI 0.29, 1.24; P = .15) (Table 3).
Table 3.
Primary and secondary endpoints.
| Endpoint | Women, n = 144 | Men, n = 484 | P value |
|---|---|---|---|
| In-hospital MACE | 11 (7.6) | 30 (6.2) | .54 |
| Cardiac death | 0 (0.0) | 1 (0.2) | 1.00 |
| All myocardial infarction | 11 (7.6) | 29 (6.0) | .48 |
| Non–Q-wave | 11 (7.6) | 25 (5.2) | .26 |
| Q-wave | 0 (0.0) | 4 (0.8) | .58 |
| Target vessel revascularization | 0 (0.0) | 2 (0.4) | 1.00 |
| 30-Day MACE | 12/144 (8.3) | 34/482 (7.1) | .61 |
| Cardiac death | 1/144 (0.7) | 2/482 (0.4) | 1.00 |
| All myocardial infarction | 11/144 (7.6) | 32/482 (6.6) | .67 |
| Non–Q-wave | 11/144 (7.6) | 26/482 (5.4) | .32 |
| Q-wave | 1/144 (0.7) | 6/482 (1.2) | .70 |
| Target vessel revascularization | 1/144 (0.7) | 6/482 (1.2) | .70 |
| Procedural success | |||
| Residual stenosis <50% | 132 (91.7) | 453 (93.6) | .42 |
| Residual stenosis ≤30% | 132 (91.7) | 448 (92.6) | .72 |
| Secondary end points at 30 d | |||
| Target lesion failure | 12/144 (8.3) | 33/482 (6.8) | .54 |
| Ischemia-driven target lesion revascularization | 1/144 (0.7) | 5/482 (1.0) | 1.00 |
| Stent thrombosis (definite or probable) | 0/144 (0.0) | 5/482 (1.0) | .35 |
Values are n (%) or n/N (%).
MACE, major adverse cardiovascular event.
Secondary end points
In-hospital outcomes including the MACE (7.6% vs 6.2%, P = .54) and its components (cardiac death, MI, and TVR) occurred with similar frequency in women and men (Table 3). At 30 days, the rates of cardiac death, MI, and TVR were also similar between groups. Ischemia-driven TLR and stent thrombosis were similar and infrequent between groups (Table 3).
Immediately after IVL, acute gain (0.78 ± 0.40 mm vs 0.84 ± 0.50 mm, P = .21) and residual diameter stenosis (34.1% ± 11.6% vs 35.8% ± 13.4%, P = .18) were similar in women and men (Table 4). The final residual stenosis after stenting and final balloon after dilatation were similar in women and men (11.4% ± 6.6% vs 12.3% ± 6.9%, P = .18). Severe angiographic complications immediately after IVL treatment were infrequent in women and in men (1.6% vs 2.3%, P = .75) and included low rates of flow-limiting dissection (1.6% vs 1.8%, P = .53) and slow flow (0.0% vs 0.5%, P = 1.00), with no perforations, abrupt closure, or no reflow after IVL. Final poststent serious angiographic complications occurred in 0.0% of women and 0.4% of men.
Table 4.
Core laboratory-assessed angiographic outcomes.
| Outcome | Women, n = 144 | Men, n = 484 | P value |
|---|---|---|---|
| Post-IVL angiographic outcomesa | n = 126 | n = 429 | |
| Acute gain, mm | 0.78 ± 0.40 | 0.84 ± 0.50 | .21 |
| Minimum lumen diameter, mm | 1.80 ± 0.39 | 1.92 ± 0.50 | .004 |
| Residual diameter stenosis, % | 34.1 ± 11.6 | 35.8 ± 13.4 | .18 |
| Post-IVL serious angiographic complications | 2/128 (1.6) | 10/435 (2.3) | .75 |
| Severe dissection (type D-F) | 2/126 (1.6) | 8/434 (1.8) | .53 |
| Perforation | 0/126 (0.0) | 0/435 (0.0) | — |
| Abrupt closure | 0/126 (0.0) | 0/435 (0.0) | — |
| Slow flow | 0/126 (0.0) | 2/435 (0.5) | 1.00 |
| No reflow | 0/126 (0.0) | 0/435 (0.0) | — |
| Final in-segment angiographic outcomes | |||
| Acute gain, mm | 1.37 ± 0.45 | 1.51 ± 0.49 | .004 |
| Minimum lumen diameter, mm | 2.39 ± 0.40 | 2.59 ± 0.48 | <.001 |
| Residual diameter stenosis, % | 16.3 ± 8.0 | 16.5 ± 8.4 | .85 |
| <50% | 143 (100.0) | 481 (99.4) | .59 |
| ≤30% | 136 (95.1) | 465 (96.1) | .61 |
| Final in-stent angiographic outcomes | |||
| Acute gain, mm | 1.59 ± 0.40 | 1.71 ± 0.48 | .002 |
| Minimum lumen diameter, mm | 2.60 ± 0.37 | 2.79 ± 0.45 | <.001 |
| Residual diameter stenosis, % | 11.4 ± 6.6 | 12.3 ± 6.9 | .18 |
| <50% | 143/143 (100.0) | 482/482 (100.0) | 1.00 |
| ≤30% | 143/143 (100.0) | 475/482 (98.5) | .21 |
| Final serious angiographic complications | 0 (0.0) | 2 (0.4) | 1.00 |
| Severe dissection (type D-F) | 0 (0.0) | 1 (0.2) | .51 |
| Perforation | 0 (0.0) | 1 (0.2) | 1.00 |
| Abrupt closure | 0 (0.0) | 1 (0.2) | 1.00 |
| Slow flow | 0 (0.0) | 0 (0.0) | — |
| No reflow | 0 (0.0) | 0 (0.0) | — |
Values are mean ± standard deviation or n/N (%).
IVL, intravascular lithotripsy.
Post-IVL angiographic data capture was not required per protocol in the Disrupt CAD studies.
Discussion
This patient-level pooled analysis from the Disrupt CAD I-IV studies is the largest series to evaluate sex-based outcomes of severely calcified coronary lesions treated with IVL lesion preparation before stent implantation. IVL-facilitated DES implantation was safe and effective independent of patient sex and was associated with infrequent angiographic complications, without evidence of excess acute angiographic or clinical complications in women (Central Illustration).
PCI of calcified lesions remains a significant predictor of procedural failure in the DES era.25 Lesions with severe calcification have higher rates of no reflow and abrupt closure after stent implantation,5 and coronary calcification is associated with stent under-expansion, deformation, and fracture and is a powerful independent predictor of restenosis and thrombosis.26, 27, 28 A recent patient-level meta-analysis of 18 randomized DES trials (19,833 patients) with 5-year follow-up reported that moderate and severe target lesion calcification, present in 31.3% of patients, was associated with higher rates of target lesion failure (hazard ratio [HR] 1.21; 95% CI 1.09-1.34, P < .001), cardiac death (HR 1.44; 95% CI 1.20-1.72, P < .001), MI (HR 1.15; 95% CI 1.00-1.33, P = .05), and repeat revascularization (HR 1.11; 95% CI 1.02-1.20, P = .02).29 In women, treatment of moderate/severe lesion calcium is particularly challenging. The Women in Innovation and Drug-Eluting Stents Collaboration showed that DES implantation of moderate and severely calcified lesions was associated with increased cardiac death (HR 1.44; 95% CI 1.00-2.07, P = .046), MI (HR 1.67; 95% CI 1.28-2.18, P = .0001), and TLR (HR 1.63; 95% CI 1.27-2.10, P = .0001) compared with lesions with no or mild calcification.7
Lesion preparation improves compliance of calcified lesions, allowing better stent expansion and improved long-term outcomes.22,30 Although atheroablative strategies have been the standard approach for calcified lesion preparation, they are not universally available and are associated particularly in women with an increased risk of procedural complications, including vessel perforation, abrupt vessel closure, and no reflow due to platelet activation and particulate embolization.9,10,31 In a retrospective analysis of 765 patients who underwent rotational atherectomy at a large referral center, periprocedural complications were significantly higher in women than those in men, including higher rates of coronary dissection (OR 3.78; 95% CI 1.42-10.05, P = .004), cardiac tamponade (OR 5.14; 95% CI 1.03-25.64, P = .026), and BARC 2 or greater bleeding (OR 2.37; 95% CI 1.07-5.23, P = .028) and a higher incidence of MACE at a median of 4.7 years of follow-up (HR 1.92; 95% CI 1.34-2.77, P < .001).11 Similarly, in the prospective, multicenter ORBIT II study, women undergoing orbital atherectomy had a higher risk of coronary dissection than men (OR 4.2; 95% CI 1.5-11.4, P = .005).12
Mechanisms of vascular injury in women may be due to direct mechanical injury related to smaller coronary arteries and higher burr:artery and balloon:artery ratios, increasing the risk of procedural complications.32,33 Inherent vascular fragility has been reported in postmenopausal women, who represent most women undergoing PCI of calcied lesions. Postmenopausal status has been associated with increased arterial stiffness and lack of vessel compliance and may increase the risk of vessel damage during atheroablation.34
In the current patient-level analysis of IVL-facilitated stenting, serious angiographic and procedural complications were infrequent in both women and men with no excess complication in women. This favorable safety profile is likely related to the mechanism of action of IVL which delivers acoustic energy circumferentially, resulting in multiplane calcium fracture without atheroembolic debris or platelet activation from frictional heat generation.35 In contrast to atheroablative technologies, IVL is not affected by wire bias or device size and has a rapid learning curve given its low-pressure balloon catheter delivery.18 By improving transmural vessel compliance, IVL removes the need for high-pressure balloon dilatation before stent delivery, thus reducing vessel trauma and arterial dissection.36 Significant improvements are observed in minimal lumen diameter and percent diameter stenosis after IVL alone despite a low IVL balloon pressure of 6 atm and the reduced need for post-IVL dilatation before stent delivery in both men and women. Despite smaller vessel size, IVL delivery was equally successful in women and men, and IVL allowed for >99% success in stent delivery in both sexes. Although women had fewer IVL catheters (1.2 ± 0.4 vs 1.4 ± 0.7, P < .001) and IVL pulses (63.0 ± 35.2 vs 78.1 ± 44.1, P < .001) used than men possibly due to less extensive lesion length (22.4 ± 10.3 vs 25.0 ± 11.7, P = .01), the rates of successful stent delivery after IVL were high for both women and men and appear similar from that reported with orbital atherectomy (99.4%) and rotational atherectomy (98.0%).12,31 With IVL, immediate procedural complications were rare (1.6% women and 2.3% men), with no perforations or abrupt closures, and final poststent complications were infrequent (0% women and 0.4% men); these rates appear to be significantly lower than have been reported with rotational or orbital atherectomy in women.11,12 These findings thus support the use of IVL for plaque modification before stenting of severely calcified lesions in women.
Limitations
Although all 4 Disrupt CAD studies were carefully conducted with independent core laboratory and clinical event committee adjudication, they were all single-arm studies lacking a concurrent control population. Women represented only 23% (n = 144) of the cohort, and although this is the largest series evaluating sex-based outcomes with IVL, it may be underpowered to detect differences in outcomes. Lack of randomization precludes comparisons with balloon-based or alternative atheroablative techniques for treatment of severely calcified lesions. The studies excluded patients with acute coronary syndromes and lesions that were ostial, unprotected left main, in-stent restenosis, length >40 mm, nondilatable, and bypass grafts, and the results are not generalizable to all comers. In addition, all target lesions were severely calcified; the outcomes of different approaches to moderately calcified target lesions deserve further study. While we report ORs for the primary end points, ORs are inherently limited when the assessed outcome is common, as is the case for the primary efficacy end point.37 Finally, the present report focused on 30-day outcomes only; ongoing follow-up will assess the long-term safety and efficacy of IVL use in women.
Conclusions
This patient-level pooled analysis demonstrates that IVL for lesion preparation of severely calcified lesions is safe and effective in both women and men. Women had more comorbidities and smaller vessel size but shorter target lesions than men. After adjustment for these and other baseline differences, women had similarly high rates of procedural success and low rates of 30-day MACE after IVL-facilitated DES implantation as men in severely calcified target lesions.
Acknowledgments
Declaration of competing interest
J. Dawn Abbott reported serving as a consultant for Philips, Medtronic, Abbott, and Recor and research grants from Boston Scientific and Microport. Dean J. Kereiakes reported serving as a consultant for SINO Medical Sciences Technologies, Elixir Medical, Svelte Medical Systems, Caliber Therapeutics/Orchestra BioMed, and Shockwave Medical and is a stockholder in Ablative Solutions. Carlo Di Mario reported research grants from Amgen, Behring, Chiesi, Daiichi-Sanyo, Edwards Lifesciences, Medtronic, Shockwave, and Volcano Philips. Shigeru Saito reported serving as a consultant for Terumo and Japan Lifeline. Robert F. Riley reported honoraria from Boston Scientific, Asahi Intecc, and Medtronic. Richard A. Shlofmitz reported serving as a speaker for Shockwave Medical. Ziad A. Ali reported grants from the National Heart, Lung, and Blood Institute, Abbott Vascular, Philips, Boston Scientific, Acist Medical, Opsens Medical, Medtronic, Abiomed and Cardiovascular Systems, has received personal fees from Amgen, AstraZeneca, and Boston Scientific, and holds equity in Shockwave Medical. Matthew J. Price reported consulting and speaker honoraria from Abbott Vascular, Boston Scientific, Biosense Webster, Medtronic, Shockwave Medical, and W.L. Gore. Jonathan M. Hill reported fees and grant support from Abbott Vascular, Boston Scientific, Abiomed, and Shockwave Medical and is a stockholder in Shockwave Medical. Gregg W. Stone has received speaker honoraria from Cook and Infraredx, has served as a consultant to Valfix, TherOx, Robocath, HeartFlow, Ablative Solutions, Vectorious, Miracor, Neovasc, Abiomed, Ancora, Elucid Bio, Occlutech, CorFlow, Apollo Therapeutics, Impulse Dynamics, Reva, Vascular Dynamics, Shockwave, V-Wave, Cardiomech, Gore, and has equity/options from Ancora, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, Valfix, MedFocus family of funds. The other authors have nothing to report.
Funding sources
Shockwave Medical funded the CAD I-IV studies and analysis.
Peer review statement
Given their roles as Deputy Editor and Editor in Chief, Dean J. Kereiakes and Alexandra Lansky had no involvement in the peer review of this article and have no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Andrew M. Goldsweig.
Footnotes
To access the supplementary material accompanying this article, visit the online version of the Journal of the Society for Cardiovascular Angiography & Interventions at https://doi.org/10.1016/j.jscai.2021.100011.
Supplementary material
References
- 1.Lawton J.S., Tamis-Holland J.E., Bangalore S., et al. ACC/AHA/SCAI guideline for coronary artery revascularization: executive summary: a report of the American College of Cardiology/American Heart Association Joint committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;79:197–215. doi: 10.1016/j.jacc.2021.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Windecker S., Kolh P., Alfonso F., et al. 2014 ESC/EACTS guidelines on myocardial revascularization: the task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur Heart J. 2014;35:2541–2619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 3.Madhavan M.V., Tarigopula M., Mintz G.S., Maehara A., Stone G.W., Généreux P. Coronary artery calcification: pathogenesis and prognostic implications. J Am Coll Cardiol. 2014;63:1703–1714. doi: 10.1016/j.jacc.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Huisman J., van der Heijden L.C., Kok M.M., et al. Impact of severe lesion calcification on clinical outcome of patients with stable angina, treated with newer generation permanent polymer-coated drug-eluting stents: a patient-level pooled analysis from TWENTE and Dutch PEERS (TWENTE II) Am Heart J. 2016;175:121–129. doi: 10.1016/j.ahj.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Généreux P., Madhavan M.V., Mintz G.S., et al. Ischemic outcomes after coronary intervention of calcified vessels in acute coronary syndromes. Pooled analysis from the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) and ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trials. J Am Coll Cardiol. 2014;63:1845–1854. doi: 10.1016/j.jacc.2014.01.034. [DOI] [PubMed] [Google Scholar]
- 6.Allison M.A., Criqui M.H., Wright C.M. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:331–336. doi: 10.1161/01.ATV.0000110786.02097.0c. [DOI] [PubMed] [Google Scholar]
- 7.Giustino G., Mastoris I., Baber U., et al. Correlates and Impact of coronary artery calcifications in women undergoing percutaneous coronary intervention with drug-eluting stents: from the Women in Innovation and Drug-Eluting Stents (WIN-DES) Collaboration. JACC Cardiovasc Interv. 2016;9:1890–1901. doi: 10.1016/j.jcin.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 8.Kini A.S., Vengrenyuk Y., Pena J., et al. Optical coherence tomography assessment of the mechanistic effects of rotational and orbital atherectomy in severely calcified coronary lesions. Catheter Cardiovasc Interv. 2015;86:1024–1032. doi: 10.1002/ccd.26000. [DOI] [PubMed] [Google Scholar]
- 9.Abdel-Wahab M., Richardt G., Joachim Büttner H., et al. High-speed rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: the randomized ROTAXUS (Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease) trial. JACC Cardiovasc Interv. 2013;6:10–19. doi: 10.1016/j.jcin.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 10.Chambers J.W., Feldman R.L., Himmelstein S.I., et al. Pivotal trial to evaluate the safety and efficacy of the orbital atherectomy system in treating de novo, severely calcified coronary lesions (ORBIT II) JACC Cardiovasc Interv. 2014;7:510–518. doi: 10.1016/j.jcin.2014.01.158. [DOI] [PubMed] [Google Scholar]
- 11.Ford T.J., Khan A., Docherty K.F., et al. Sex differences in procedural and clinical outcomes following rotational atherectomy. Catheter Cardiovasc Interv. 2020;95:232–241. doi: 10.1002/ccd.28373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim C.Y., Lee A.C., Wiedenbeck T.L., Lee M.S., Chambers J.W. Gender differences in acute and 30-day outcomes after orbital atherectomy treatment of de novo, severely calcified coronary lesions. Catheter Cardiovasc Interv. 2016;87:671–677. doi: 10.1002/ccd.26163. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi Y., Okura H., Kume T., et al. Impact of target lesion coronary calcification on stent expansion. Circ J. 2014;78:2209–2214. doi: 10.1253/circj.cj-14-0108. [DOI] [PubMed] [Google Scholar]
- 14.Choi K.H., Song Y.B., Lee J.M., et al. Impact of intravascular ultrasound-Guided percutaneous coronary intervention on long-term clinical outcomes in patients undergoing complex procedures. JACC Cardiovasc Interv. 2019;12:607–620. doi: 10.1016/j.jcin.2019.01.227. [DOI] [PubMed] [Google Scholar]
- 15.Yeoh J., Hill J. Intracoronary lithotripsy for the treatment of calcified plaque. Interv Cardiol Clin. 2019;8:411–424. doi: 10.1016/j.iccl.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Brinton T.J., Ali Z.A., Hill J.M., et al. Feasibility of Shockwave coronary intravascular lithotripsy for the treatment of calcified coronary stenoses. Circulation. 2019;139:834–836. doi: 10.1161/CIRCULATIONAHA.118.036531. [DOI] [PubMed] [Google Scholar]
- 17.Ali Z.A., Nef H., Escaned J., et al. Safety and Effectiveness of coronary intravascular lithotripsy for treatment of severely calcified coronary stenoses: the disrupt CAD II study. Circ Cardiovasc Interv. 2019;12:e008434. doi: 10.1161/CIRCINTERVENTIONS.119.008434. [DOI] [PubMed] [Google Scholar]
- 18.Hill J.M., Kereiakes D.J., Shlofmitz R.A., et al. Intravascular lithotripsy for treatment of severely calcified coronary artery disease: the disrupt CAD III study. J Am Coll Cardiol. 2020;76:2635–2646. doi: 10.1016/j.jacc.2020.09.603. [DOI] [PubMed] [Google Scholar]
- 19.Saito S., Yamazaki S., Takahashi A., et al. Disrupt CAD IV Investigators Intravascular lithotripsy for vessel preparation in severely calcified coronary arteries prior to stent placement- primary outcomes from the Japanese disrupt CAD IV study. Circ J. 2021;85:826–833. doi: 10.1253/circj.CJ-20-1174. [DOI] [PubMed] [Google Scholar]
- 20.Kereiakes D.J., Hill J.M., Ben-Yehuda O., Maehara A., Alexander B., Stone G.W. Evaluation of safety and efficacy of coronary intravascular lithotripsy for treatment of severely calcified coronary stenoses: design and rationale for the disrupt CAD III trial. Am Heart J. 2020;225:10–18. doi: 10.1016/j.ahj.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Kereiakes D.J., Di Mario C., Riley R.F., et al. Intravascular lithotripsy for treatment of calcified coronary lesions: patient-level pooled analysis of the disrupt CAD studies. JACC Cardiovasc Interv. 2021;14:1337–1348. doi: 10.1016/j.jcin.2021.04.015. [DOI] [PubMed] [Google Scholar]
- 22.Généreux P., Lee A.C., Kim C.Y., et al. Orbital atherectomy for treating de novo severely calcified coronary narrowing (1-year results from the pivotal ORBIT II trial) Am J Cardiol. 2015;115:1685–1690. doi: 10.1016/j.amjcard.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Thygesen K., Alpert J.S., Jaffe A.S., et al. Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 24.Cutlip D.E., Windecker S., Mehran R., et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 25.Levi A., Kornowski R., Vaduganathan M., et al. Incidence, predictors, and outcomes of failed primary percutaneous coronary intervention: a 10-year contemporary experience. Coron Artery Dis. 2014;25:145–151. doi: 10.1097/MCA.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 26.Mori S., Yasuda S., Kataoka Y., Morii I., Kawamura A., Miyazaki S. Significant association of coronary artery calcification in stent delivery route with restenosis after sirolimus-eluting stent implantation. Circ J. 2009;73:1856–1863. doi: 10.1253/circj.cj-09-0080. [DOI] [PubMed] [Google Scholar]
- 27.Fujii K., Carlier S.G., Mintz G.S., et al. Stent underexpansion and residual reference segment stenosis are related to stent thrombosis after sirolimus-eluting stent implantation: an intravascular ultrasound study. J Am Coll Cardiol. 2005;45:995–998. doi: 10.1016/j.jacc.2004.12.066. [DOI] [PubMed] [Google Scholar]
- 28.Sonoda S., Morino Y., Ako J., et al. Impact of final stent dimensions on long-term results following sirolimus-eluting stent implantation: serial intravascular ultrasound analysis from the sirius trial. J Am Coll Cardiol. 2004;43:1959–1963. doi: 10.1016/j.jacc.2004.01.044. [DOI] [PubMed] [Google Scholar]
- 29.Guedeney P., Claessen B.E., Mehran R., et al. Coronary calcification and long-term outcomes according to drug-eluting stent generation. JACC Cardiovasc Interv. 2020;13:1417–1428. doi: 10.1016/j.jcin.2020.03.053. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto M.H., Maehara A., Kim S.S., et al. Effect of orbital atherectomy in calcified coronary artery lesions as assessed by optical coherence tomography. Catheter Cardiovasc Interv. 2019;93:1211–1218. doi: 10.1002/ccd.27902. [DOI] [PubMed] [Google Scholar]
- 31.Abdel-Wahab M., Toelg R., Byrne R.A., et al. High-speed rotational atherectomy versus modified balloons prior to drug-eluting stent implantation in severely calcified coronary lesions. Circ Cardiovasc Interv. 2018;11:e007415. doi: 10.1161/CIRCINTERVENTIONS.118.007415. [DOI] [PubMed] [Google Scholar]
- 32.Mikhail G.W. Coronary revascularisation in women. Heart. 2006;92(suppl 3):iii19–iii23. doi: 10.1136/hrt.2005.070359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma S.K., Tomey M.I., Teirstein P.S., et al. North American expert review of rotational atherectomy. Circ Cardiovasc Interv. 2019;12:e007448. doi: 10.1161/CIRCINTERVENTIONS.118.007448. [DOI] [PubMed] [Google Scholar]
- 34.Samargandy S., Matthews K.A., Brooks M.M., et al. Arterial stiffness accelerates within 1 year of the final menstrual period: the SWAN Heart Study. Arterioscler Thromb Vasc Biol. 2020;40:1001–1008. doi: 10.1161/ATVBAHA.119.313622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kereiakes D.J., Virmani R., Hokama J.Y., et al. Principles of intravascular lithotripsy for calcific plaque modification. JACC Cardiovasc Interv. 2021;14:1275–1292. doi: 10.1016/j.jcin.2021.03.036. [DOI] [PubMed] [Google Scholar]
- 36.Biondi-Zoccai G.G., Agostoni P., Sangiorgi G.M., et al. Incidence, predictors, and outcomes of coronary dissections left untreated after drug-eluting stent implantation. Eur Heart J. 2006;27:540–546. doi: 10.1093/eurheartj/ehi618. [DOI] [PubMed] [Google Scholar]
- 37.Cummings P. The relative merits of risk ratios and odds ratios. Arch Pediatr Adolesc Med. 2009;163:438–445. doi: 10.1001/archpediatrics.2009.31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.