Abstract
Background
While not available for clinical use in the United States, dedicated drug-coated balloons (DCB) are currently under investigation for the management of coronary in-stent restenosis (ISR). Peripheral drug-coated balloons (P-DCB) have been used off-label for coronary ISR. Further data regarding this practice are needed. We aimed to describe outcomes in patients who underwent off-label P-DCB angioplasty for coronary ISR.
Methods
We analyzed data on P-DCB angioplasty for coronary ISR at a single high-volume center between April 1, 2015, and December 30, 2017. Demographic and procedural details were collected, with systematic follow-up as clinically indicated.
Results
Data from 31 patients treated with P-DCB angioplasty (mean age 68.0 ± 10.7 years) with coronary ISR (17 recurrent and 14 first time) were analyzed. Most patients presented with high-grade angina (81%) or myocardial infarction (13%). Treated ISR lesions were in native coronary arteries (68%), saphenous vein grafts (SVG, 23%), and the left internal mammary artery (10%). Diffuse intrastent ISR was common (69%) with a mean lesion length of 21.7 ± 12.4 mm. No postprocedural myocardial infarction occurred and 1 nonprocedural mortality occurred during index admission. At follow-up (median: 283, interquartile range [IQR]: 354 days), repeat angiography was performed in 19 patients (median: 212, IQR: 188 days), and 11 patients had target lesion recurrent ISR (Kaplan-Meier event-free survival estimate: 44.7%, 95% CI, 26.1%-76.5%).
Conclusions
In the absence of availability of dedicated coronary DCB, treatment of coronary ISR using P-DCB angioplasty was feasible, although follow-up demonstrated continued risk for recurrent ISR in this high-risk population.
Keywords: angioplasty, coronary arteries, drug-coated balloon, in-stent restenosis, paclitaxel
Central Illustration
Introduction
Despite advances in-stent technology, patients remain at risk for in-stent restenosis (ISR),1 which continues to be clinically relevant and challenging issue.2 Even with contemporary drug-eluting stents (DES), the incidence of ISR is approximately 5%-15% in all-comers patients and lesions with rates approaching the lower end of that range with newer generation stents.3, 4, 5 Patients who develop ISR are at risk for the development of recurrent symptoms, repeat revascularization, acute coronary syndromes, and increased risk for mortality.6, 7, 8 The occurrence of ISR can be influenced by patient factors (ie, age and diabetes mellitus), lesion characteristics (ie, length, vessel caliber), and procedural technique (ie, minimal stent area, under expansion).9, 10, 11
When patients present with ISR, the optimal management strategy is individualized and guided by the clinical presentation, type of underlying stent (bare metal stent [BMS] vs DES), and mechanism and pattern of restenosis.12 While implantation of a second layer of stent, typically DES, is considered to be a Class I indication in both the American College of Cardiology (ACC)/American Heart Association (AHA) guidelines and the European Society of Cardiology (ESC)/European Association for Cardio-Thoracic Surgery (EACTS) guidelines,13,14 multiple stent layers can contribute to further narrowing of the luminal area, increasing risk for recurrent ISR, more difficulty achieving optimal stent expansion, and other stent-related complications such as stent thrombosis.10,15
Drug-coated balloons (DCB) have become an increasingly attractive treatment option for ISR in the coronary vasculature due to the ability to deliver antiproliferative drugs to sites of ISR without necessitating the placement of an additional layer stent,16,17 and previous studies have demonstrated similar rates of restenosis with minimal differences in clinical outcomes between DCB and DES in the treatment of ISR.18 This has led the ESC/EACTS to deem the use of DCB a Class I recommendation in this setting.14 While dedicated coronary DCB are currently under clinical investigation and are not commercially available in the United States, peripheral drug-coated balloons (P-DCB) are approved. These devices were initially designed with indications for use in de novo or restenotic lesions in native superficial femoral or popliteal arteries. In the absence of availability of dedicated coronary DCB, P-DCB has been used in selected cases off-label. We sought to study the clinical characteristics and outcomes of 31 patients undergoing angioplasty with P-DCB for ISR treatment at our high-volume, academic, cardiac catheterization laboratory.
Methods
Patients undergoing diagnostic coronary angiography as part of routine clinical care between April 1, 2015, and December 30, 2017 were screened. Data from patients with evidence of ISR, defined as >50% narrowing within a stented segment or within 5 mm of the stent edge by visual estimation, who were treated with commercially available P-DCB off-label at the discretion of the performing operator were analyzed. ISR was characterized as first time if this was the first instance of restenosis in the stented segment or recurrent if evidence of prior treatment for restenosis was documented. Clinical and procedural characteristics were collected through chart review. All patients were contacted after the index procedure to identify any subsequent clinically indicated coronary angiograms and interventions that were performed at outside institutions. Institutional Review Board approval was obtained for this study and informed consent from the patients was obtained when applicable.
Procedural technique
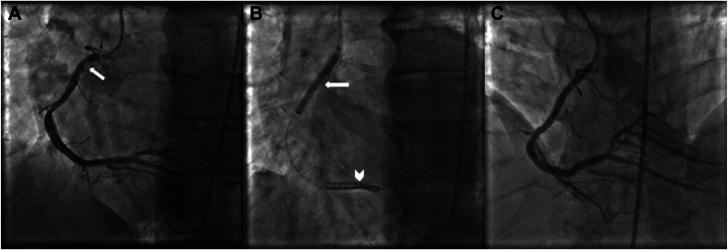
All patients underwent 7F or 8F access to accommodate the delivery of equipment. Diagnostic coronary angiograms were taken as per usual practice. After the vessel was adequately prepared according to the discretion of the operator (ie, via balloon angioplasty, atherectomy, cutting balloons, etc.), paclitaxel-coated P-DCB IN.PACT Admiral (Medtronic) or LUTONIX (Bard Peripheral Vascular) were delivered over 2 or three 0.14” supportive coronary wires or a single 0.18” wire. The distal portion of the balloon was advanced into the lesion. The shortest available peripheral P-DCB is 40 mm; therefore the proximal end of the P-DCB may have remained in the aorta in proximal cases (Figure 1). The P-DCB was inflated for ideally 180 seconds or as long as clinically tolerated. The use of intravascular imaging (Figure 2) or treatment of residual stenosis or complications of balloon inflation was left to the discretion of the operator, although imaging was used in the vast majority of cases.
Figure 1.
Angiogram of treatment of in-stent restenosis (ISR) with peripheral paclitaxel drug-coated balloon. (A) Proximal focal ISR demonstrated in the right coronary artery (arrow). (B) Inflation of peripheral drug-coated balloon (P-DCB; arrow) over two 0.014′′ support wires (arrowhead). (C) Excellent angiographic result and improvement in ISR after P-DCB treatment.
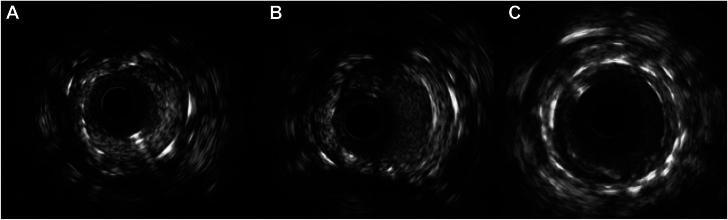
Figure 2.
Intravascular imaging of treatment of in-stent restenosis (ISR) with peripheral paclitaxel drug-coated balloon. (A) Proximal focal ISR of the right coronary artery with severe neointimal hyperplasia. Minimal luminal area measured to be 2.1 mm2. (B) Improvement in minimal luminal area of ISR lesion to 3.8 mm2 after lesion preparation with scoring balloon catheter. (C) Significant improvement in minimal luminal area (5.5 mm2) with evidence of mild neointimal hyperplasia after treatment with peripheral paclitaxel drug-coated balloon.
Statistical analysis
Continuous values are summarized using mean ± standard deviation. Time-to-event analysis was performed by the Kaplan-Meier method. Analyses were performed with SAS version 9.4 (SAS Institute) and RStudio version 4.2.0 (RStudio).
Results
Between April 1st, 2015, and December 30th, 2017, a total of 31 patients with ISR underwent treatment with P-DCB at our institution. Baseline clinical characteristics are summarized in Table 1. The mean age of patients included was 68.0 ± 10.5 years. The majority of patients were male (78%) with a high prevalence of diabetes mellitus (59%), hypertension (78%), and prior coronary artery bypass graft (CABG) surgery (69%).
Table 1.
Baseline clinical characteristics.
| Variable | N = 31 |
|---|---|
| Age, y | 68.0 ± 10.7 |
| Male sex | 24 (78%) |
| Race | |
| Black | 1 (3%) |
| Caucasian | 20 (65%) |
| Hispanic | 1 (3%) |
| Other | 9 (29%) |
| Diabetes mellitus | 18 (58%) |
| Hypertension | 24 (77%) |
| Cerebrovascular accident | 4 (13%) |
| Peripheral arterial disease | 10 (29%) |
| Prior coronary artery bypass graft | 21 (68%) |
| Smoking history | 5 (16%) |
| Thoracic radiation treatment history | 1 (3%) |
| Clinical presentation | |
| High-grade angina | 25 (81%) |
| Non-ST-elevation myocardial infarction | 4 (13%) |
| Cardiogenic shock | 2 (6%) |
| Ejection fraction, % | 46.0 ± 12.8 |
| Creatinine, mg/dL | 1.4 ± 1.3 |
Data presented as mean ± SD or n (%).
Table 2 summarizes procedural details. Femoral access (n = 29, 91%) was predominantly used with 7F (17, 55%) and 8F (13, 42%) sheaths. Coronary angiography demonstrated recurrent ISR in 53% of patients and first-time ISR in 47% of patients. The majority of patients had a diffuse intrastent (Mehran class II) pattern of ISR19 (68%) with a mean lesion length of 22.0 ± 12.6 mm by visual assessment. Target lesion locations were in native coronary arteries in 69% of lesions and grafted vessels in 32% of lesions (23% in SVG and 10% in left internal mammary arteries). The right coronary artery was the most frequently treated ISR lesion location (34%). Four (13%) patients were previously treated with brachytherapy.
Table 2.
Index procedure summary.
| Variable | N = 31 |
|---|---|
| Target vessel | |
| Left main | 3 (10%) |
| Left anterior descending | 2 (7%) |
| Left circumflex | 4 (13%) |
| Right coronary artery | 11 (35%) |
| Bypass graft | 10 (32%) |
| In-stent restenosis | |
| First time | 14 (45%) |
| Recurrent | 17 (55%) |
| Prior brachytherapy | 4 (13%) |
| Access | |
| Radial | 3 (10%) |
| Femoral | 28 (90%) |
| Contrast volume, mL | 145.0 ± 84.3 |
| Radiation time, min | 29.1 ± 12.2 |
| Radiation dose, Gy | 2094.8 ± 1990.0 |
| Length of stay, d | 4.3 ± 8.9 |
Data presented as mean ± SD or n (%).
P-DCB, peripheral drug-coated balloon.
For lesion preparation, a combination of cutting or specialty balloons was used in (21, 66%) patients, noncompliant balloons alone were used in (8, 25%) cases, and atherectomy in combination with noncompliant balloons and/or specialty balloons was used in (3, 9%) patients (rotational atherectomy in 1 patient and laser atherectomy in 2 patients). After lesion preparation, the lesion was treated with P-DCB. IN.PACT Admiral DCB were used in 19 (61%) and LUTONIX DCB were used in 10 (32%) cases; 2 patients underwent P-DCB treatment but the specific balloon device was not recorded. P-DCB crossed the target lesion in all but 1 lesion captured in this analysis. The average inflation pressure was 7.8 ± 3.6 atm. There were no perforations or dissections requiring further intervention, and no postprocedural myocardial infarctions (MI) were identified. One nonprocedural death occurred during the index admission (cardiac arrest in the setting of mesenteric ischemia).
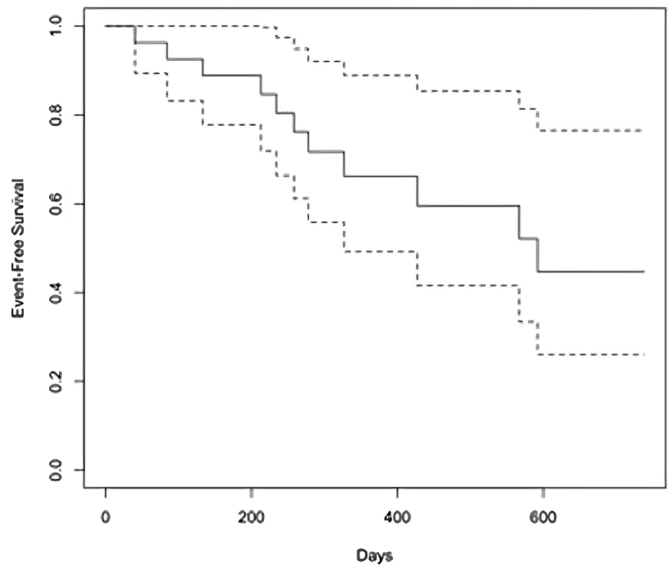
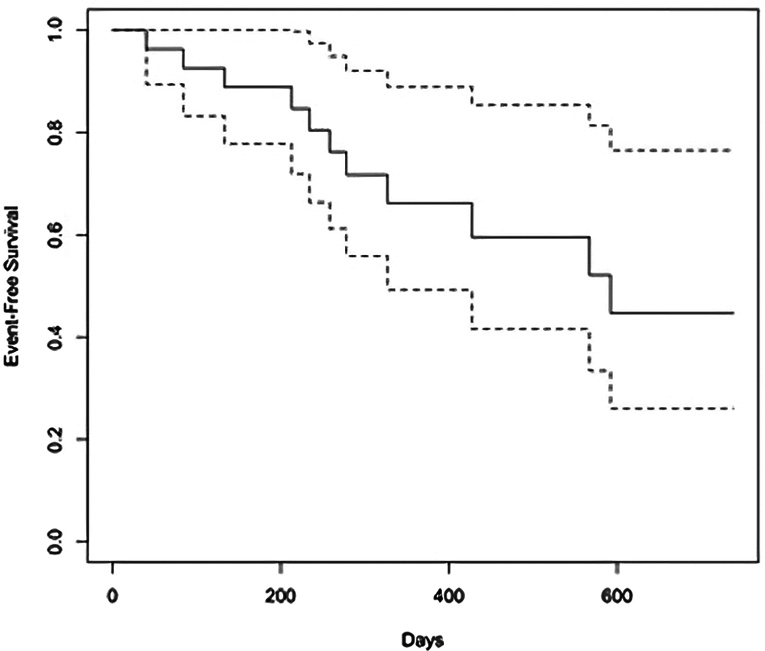
As summarized in Table 3, at a median follow-up of 283 (interquartile range [IQR]: 354) days, repeat elective angiography was performed in 19 patients for recurrent symptoms (median: 212, IQR: 188 days), and recurrent ISR of the target lesion was reported in 11 patients (42%). The Central Illustration depicts the Kaplan-Meier estimate for the occurrence of recurrent ISR. The Kaplan-Meier survival estimate for event-free survival at the longest follow-up available was 44.7% (95% CI, 26.1%-76.5%). The Kaplan-Meier survival estimate for event-free survival at 1 year was 66.2% (95% CI, 49.3-88.9). The pattern of recurrent ISR was in the same location as the index lesion in 8/11 (80%) patients and 2/11 (20%) had progression to a chronic total occlusion (Figure 3). One angiogram was not available for review. Recurrent ISR was treated by brachytherapy (4 patients), repeat P-DCB (1 patient), balloon angioplasty (2 patients), laser atherectomy (1 patient), medical therapy (2 patients), and 1 patient with graft vessel ISR had revascularization of the native chronically occluded artery (Table 3). There was no difference in outcome by the specific balloon used.
Table 3.
Repeat angiography and intervention summary.
| Clinically indicated repeat angiography | N = 19 |
|---|---|
| Days to repeat angiography | 212 (188) |
| Recurrent ISR | 11 (34%) |
| Treatment of recurrent ISR | |
| Balloon angioplasty | 2/11 (18%) |
| Brachytherapy | 4/11 (36%) |
| Laser atherectomy | 1/11 (9%) |
| Repeat P-DCB | 1/11 (9%) |
| Native vessel revascularization | 1/11 (9%) |
| Medical management only | 2/11 (18%) |
Data presented as median (IQR) or n (%).
ISR, in-stent restenosis; P-DCB, peripheral drug-coated balloon.
Central Illustration.
Survival from recurrent in-stent restenosis (ISR) after treatment with peripheral drug-coated balloon. Kaplan-Meier time-to-event survival curves demonstrate only 44.7% of patients were free of ISR at a median follow-up of 283 days, and interquartile range of 354 days. Repeat elective angiography was performed in 19 patients, 11 of whom exhibited evidence of recurrent ISR.
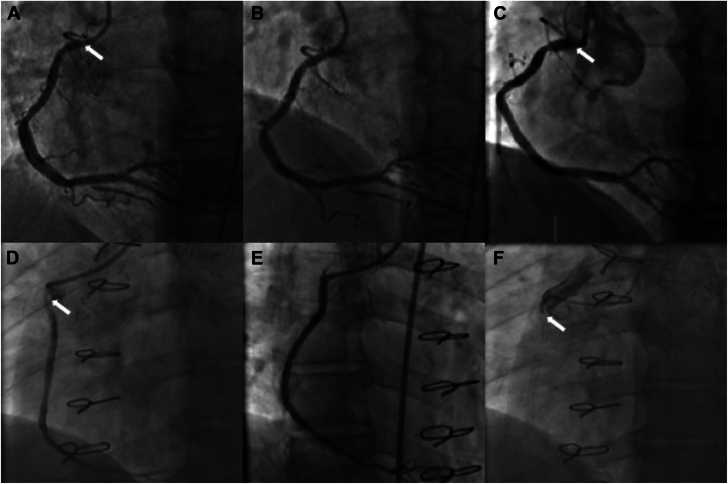
Figure 3.
Patterns of in-stent restenosis (ISR). Panels A-C are a representative example of recurrent ISR after paclitaxel peripheral drug-coated balloon (P-DCB) treatment. Panels D-F are a representative example of progression to complete total occlusion after treatment with P-DCB. (A) Ostial ISR of the right coronary artery (arrow). (B) Improvement of ISR post P-DCB. (C) Recurrence of ostial ISR. (D) Ostial and proximal ISR of saphenous vein graft to the right posterior descending artery. (E) Improvement of ISR post P-DCB; (F) Recurrence of ISR with progression to complete total occlusion.
Discussion
In this report, we describe our institutional experience with the use of paclitaxel-coated P-DCB in the treatment of ISR in coronary lesions in 31 patients. P-DCB was successfully used for ISR treatment in all but 1 patient, and no procedural adverse events were noted. In this high-risk population, there was a high rate of symptom-related repeat angiography during the follow-up period, with 19 patients undergoing coronary angiography and 11 patients having recurrent ISR necessitating treatment.
ISR remains a significant clinical problem even in the contemporary DES era. While the proportion of patients with ISR is decreasing due to increasing usage of DES and optimal image-guided stent implantation techniques, data from the Veterans Affairs Clinical Assessment and Tracking (VA-CART) registry demonstrate that ISR lesions comprise 10.5% of percutaneous coronary intervention (PCI) procedures.4 Similarly, data from the National Cardiovascular Data Registry showed that 10.6% of the 5,100,394 patients who underwent PCI between 2009 and 2017 underwent treatment for ISR lesions.5 Further, when ISR occurs, it is becoming increasingly more challenging to treat.20,21 ISR seen in DES (DES-ISR) is different in both pattern and histopathology when compared to bare metal stent ISR (BMS-ISR). When compared to BMS-ISR, DES-ISR is usually more focal in nature and is characterized by proteoglycan-rich neointimal hyperplasia with a smaller proportion of vascular smooth muscle cells.22 However, DES-ISR is less likely to be successfully treated with first-line approaches like placement of a DES compared with BMS-ISR.23 This may be secondary to a variety of reasons, including innate resistance to the antiproliferative drug on the DES, or due to deployment of the stent in small coronary arteries or bifurcation side branches that are at higher risk of restenosis.24
DCB is 1 potential therapy that may be employed for the treatment of ISR, and unlike the placement of a DES, this strategy leaves no scaffold behind. Therefore, the sustained effect of the drug is purely dependent on the properties of the drug and the excipient carrier itself. Paclitaxel is a highly lipophilic and potent microtubular inhibitor whose properties permit passive absorption through cell membranes, thus allowing a sustained effect on cell proliferation after the balloon is removed from the patient.25 Conversely, antiproliferative agents such as sirolimus and everolimus are less lipophilic and have demonstrated less reliable uptake and response without significant engineering of the excipient for drug delivery.26 At present, only P-DCB are available for commercial use within the United States, with several trials of dedicated coronary DCB either in the planning stages or currently underway.
Available data has demonstrated paclitaxel-based dedicated coronary DCB to have similar outcomes as DES for the treatment of both BMS-ISR and DES-ISR and superior outcomes to plain old balloon angioplasty (POBA). Scheller et al17 compared DCB to POBA in the treatment of BMS-ISR and demonstrated that at 6 months follow-up, angiographic restenosis was 43% in the POBA group vs 5% in the DCB group (P < .002). In the Intracoronary Stenting and Angiographic Results: Drug-Eluting Stent In-Stent Restenosis: 3 Treatment Approaches (ISAR-DESIRE 3) trial, there was no significant difference in the primary end point of percent diameter stenosis at 6 to 8 months of follow-up when comparing DCB to DES.16 In the Restenosis Intra-stent of Bare-Metal Stents: Paclitaxel-eluting Balloon vs. Everolimus-eluting Stent (RIBS V) trial, while the primary outcome of minimum lumen diameter was significantly greater in patients receiving a DES, the secondary outcomes including major adverse cardiovascular events and binary restenosis were not significantly different.27 Though notably, this study was underpowered for clinical outcomes. Several other meta-analyses and clinical trials have reported similar outcomes with DES showing larger acute luminal gain but typically no significant difference in clinical outcomes.18,28,29 Most recently, a meta-analysis of 10 randomized clinical trials suggested that while repeat stenting with DES may be associated with moderately lower rates of target lesion revascularization at 3 years compared with DCB, rates of the primary safety end point of all-cause death, MI, and target lesion thrombosis were similar after DES when compared with DCB.30 Most recently, Scheller and colleagues demonstrated noninferiority between sirolimus-coated and paclitaxel-coated balloons in 101 patients with ISR randomized to these treatment strategies with regards to late-lumen loss at 6 months follow-up.31
Dedicated coronary DCB are widely available and used in Europe for the treatment of both BMS-ISR and DES-ISR, recently gaining a Class I recommendation in the ESC/EACTS guidelines. However, these devices are not currently available in the United States. For physicians and patients wishing to avoid a second layer of stent, outside of clinical trials, the current options are limited to balloon angioplasty alone, atherectomy, brachytherapy, or off-label use of P-DCB in the coronaries. P-DCB is considerably different than dedicated coronary DCB, with different drug concentrations and excipients. In particular, the majority of patients in the present series were treated with IN.PACT Admiral DCB which relies upon a urea-based excipient with more crystalline paclitaxel than the LUTONIX device. Greater particulate embolization has been demonstrated by this device, and consequently, periprocedural complications would be more commonly expected and perhaps impact our outcomes.32,33 Most importantly, the sizes and deliverability are different with the smallest available balloons being 4.0 mm in diameter and 40 mm in length, and 0.035” or 0.038” wire lumens. In the latter half of the time period of our study, the LUTONIX balloon was introduced on a 0.018” guide wire compatible platform, and this resulted in a shift in our practice pattern toward the more deliverable LUTONIX device. In either case, these limitations typically allow for large proximal and ostial coronary lesions to be treated with P-DCB, although P-DCB was delivered to the left internal mammary artery (LIMA) graft in this cohort (including 1 case of distal graft intervention). In this analysis, we demonstrate that with appropriate lesion selection, P-DCB can be safely used to treat coronary ISR. There were no periprocedural MI, perforations, clinically relevant dissections, or deaths related to P-DCB use. As suggested in the peripheral vasculature literature, the use of DCB can result in the development of slow flow due to particulate embolization, and this phenomenon can be associated with adverse outcomes.34
While safe, the rate of recurrent ISR in our population is significantly higher than that reported in randomized clinical trials for dedicated coronary DCB. In Restenosis Intra-Stent of Drug-Eluting Stents: Drug-Eluting Balloons vs. Everolimus-Eluting Stents (RIBS IV), the target vessel revascularization (TVR) rate at 1 year was 16% in the DCB arm.28 In the drug-eluting balloon for in-stent restenosis (DARE) trial, TVR at 1 year was 8.8% in the DCB arm.18 In our cohort, the rate ISR at a mean follow-up of 307 days was 34%. This is likely due to the fact that we studied a very high-risk population. Most patients (97%) had DES-ISR and more than half the population had recurrent ISR. The fraction of patients with prior coronary artery bypass graft (CABG) surgery was 69%, and 30% of target lesions were in the LIMA or SVG. For comparison, in the DCB arm of the DARE trial, only 19% of patients had prior CABG and 0.7% of lesions were in SVG. In addition, given that P-DCB are not designed for the coronary space, the adequacy of drug delivery and amount of drug uptake in coronary lesions is unknown, especially as the pharmacokinetics of these devices are tailored for de novo peripheral arterial disease rather than coronary ISR. The recently presented AGENT IDE trial demonstrated significantly lower rates of target lesion failure (cardiac mortality, target-vessel myocardial infarction [MI], target lesion revascularization) with dedicated coronary DCB compared with POBA in the first 480 patients randomized.35 Results of the complete trial population (N = 600) and other ongoing dedicated coronary DCB trials will help provide further information regarding the safety and efficacy of DCB in contemporary PCI practice.
Our study has limitations which should be acknowledged when interpreting these findings. Inherent to retrospective analysis, our study is limited by small sample size and selection bias as we have studied a highly select population with angiographic and/or clinical factors limiting the ability to offer standard therapies. This was a higher-risk patient cohort with more than half presenting with recurrent ISR, and thus, long-term event rates may have reflected this challenging-to-treat patient population. Additionally, there was variability in the duration of inflations and reliability of documentation in the electronic medical record. While repeat angiography was largely clinically driven after P-DCB treatment, select higher-risk patients may have undergone planned surveillance angiography to assess for restenosis. Lastly, although procedural success in this cohort was high, there may have been additional selected cases in which P-DCB treatment was attempted and did not cross the lesion of interest which were not captured in the electronic medical record.
Conclusions
In conclusion, in the absence of commercially available dedicated coronary DCB, it is feasible to use P-DCB in appropriately selected patients and lesions for the treatment of ISR. However, the high rates of target-vessel failure following this treatment modality require further study.
Acknowledgments
Peer review statement
Associate Editor Sahil A. Parikh had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Associate Editor Andrew M. Goldsweig.
Declaration of competing interest
Mahesh V. Madhavan reports an institutional educational grant to Columbia University from Boston Scientific. Dimitri Karmpaliotis reports honoraria from Boston Scientific and Abbott Vascular and equity in Saranas, Soundbite, and Traverse Vascular. Ziad A. Ali reports grants from Abbott Vascular and Cardiovascular Systems Inc, personal fees from Amgen, AstraZeneca, Boston Scientific, and equity in Shockwave Medical. Torsten P. Vahl reports institutional funding to Columbia University Irving Medical Center from Boston Scientific, Edwards Lifesciences, JenaValve, Medtronic, and Siemens Healthineers, and he personally received consulting fees from Abbott Vascular, Boston Scientific, and Siemens Healthineers. Tamim M. Nazif reports consulting fees or honoraria from Medtronic, Boston Scientific, and Teleflex. Khady N. Fall reports consulting fees from Boston Scientific. Ajay J. Kirtane reports institutional funding to Columbia University and/or Cardiovascular Research Foundation from Medtronic, Boston Scientific, Abbott Vascular, Amgen, Cardiovascular Systems Inc, Philips, ReCor Medical, Neurotronic, Biotronik, Chiesi, Bolt Medical, Magenta Medical, Canon, SoniVie, Shockwave Medical, and Merck. In addition to research grants, institutional funding includes fees paid to Columbia University and/or Cardiovascular Research Foundation for consulting and/or speaking engagements in which Ajay Kirtane controlled the content. Personal: Consulting from IMDS; travel expenses/meals from Medtronic, Boston Scientific, Abbott Vascular, Cardiovascular Systems Inc, Siemens, Philips, ReCor Medical, Chiesi, OpSens, Zoll, and Regeneron. Jeffrey W. Moses reports equity in Orchestra Biomed. The other authors reported no financial interests.
Funding sources
This work was not supported by funding agencies in the public, commercial, or not-for-profit sectors.
Ethics statement and patient consent
All patients were contacted after the index procedure to identify any subsequent clinically indicated coronary angiograms and interventions that were performed at outside institutions. Institutional review board approval was obtained for this study, and informed consent from the patients was obtained when applicable.
References
- 1.Madhavan M.V., Kirtane A.J., Redfors B., et al. Stent-related adverse events >1 year after percutaneous coronary intervention. J Am Coll Cardiol. 2020;75(6):590–604. doi: 10.1016/j.jacc.2019.11.058. [DOI] [PubMed] [Google Scholar]
- 2.Dangas G.D., Claessen B.E., Caixeta A., Sanidas E.A., Mintz G.S., Mehran R. In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol. 2010;56(23):1897–1907. doi: 10.1016/j.jacc.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 3.Torrado J., Buckley L., Durán A., et al. Restenosis, stent thrombosis, and bleeding complications: navigating between Scylla and Charybdis. J Am Coll Cardiol. 2018;71(15):1676–1695. doi: 10.1016/j.jacc.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 4.Waldo S.W., O’Donnell C.I., Prouse A., et al. Incidence, procedural management, and clinical outcomes of coronary in-stent restenosis: insights from the National Va CART Program. Catheter Cardiovasc Interv. 2018;91(3):425–433. doi: 10.1002/ccd.27161. [DOI] [PubMed] [Google Scholar]
- 5.Moussa I.D., Mohananey D., Saucedo J., et al. Trends and outcomes of restenosis after coronary stent implantation in the United States. J Am Coll Cardiol. 2020;76(13):1521–1531. doi: 10.1016/j.jacc.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Piccolo R., Bonaa K.H., Efthimiou O., et al. Drug-eluting or bare-metal stents for percutaneous coronary intervention: a systematic review and individual patient data meta-analysis of randomised clinical trials. Lancet. 2019;393(10190):2503–2510. doi: 10.1016/S0140-6736(19)30474-X. [DOI] [PubMed] [Google Scholar]
- 7.Cassese S., Byrne R.A., Schulz S., et al. Prognostic role of restenosis in 10 004 patients undergoing routine control angiography after coronary stenting. Eur Heart J. 2015;36(2):94–99. doi: 10.1093/eurheartj/ehu383. [DOI] [PubMed] [Google Scholar]
- 8.Palmerini T., Della Riva D., Biondi-Zoccai G., et al. Mortality following nonemergent, uncomplicated target lesion revascularization after percutaneous coronary intervention: an individual patient data pooled analysis of 21 randomized trials and 32,524 patients. JACC Cardiovasc Interv. 2018;11(9):892–902. doi: 10.1016/j.jcin.2018.01.277. [DOI] [PubMed] [Google Scholar]
- 9.Alfonso F. Treatment of drug-eluting stent restenosis the new pilgrimage: quo vadis? J Am Coll Cardiol. 2010;55(24):2717–2720. doi: 10.1016/j.jacc.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 10.Fujii K., Mintz G.S., Kobayashi Y., et al. Contribution of stent underexpansion to recurrence after sirolimus-eluting stent implantation for in-stent restenosis. Circulation. 2004;109(9):1085–1088. doi: 10.1161/01.CIR.0000121327.67756.19. [DOI] [PubMed] [Google Scholar]
- 11.Alfonso F., Pérez-Vizcayno M.J., Hernández R., et al. Long-term outcome and determinants of event-free survival in patients treated with balloon angioplasty for in-stent restenosis. Am J Cardiol. 1999;83(8):1268–1270. doi: 10.1016/s0002-9149(99)00071-5. A9. [DOI] [PubMed] [Google Scholar]
- 12.Her A.Y., Shin E.S. Current management of in-stent restenosis. Korean Circ J. 2018;48(5):337–349. doi: 10.4070/kcj.2018.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith S.C., Jr., Feldman T.E., Hirshfeld J.W., Jr., et al. 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology. J Am Coll Cardiol. American Heart Association Task Force on Practice Guidelines. 2006;47(1):e1–e121. doi: 10.1016/j.jacc.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Neumann F.J., Sousa-Uva M., Ahlsson A., et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165. doi: 10.1093/eurheartj/ehy394. [DOI] [PubMed] [Google Scholar]
- 15.Fujii K., Carlier S.G., Mintz G.S., et al. Stent underexpansion and residual reference segment stenosis are related to stent thrombosis after sirolimus-eluting stent implantation an intravascular ultrasound study. J Am Coll Cardiol. 2005;45(7):995–998. doi: 10.1016/j.jacc.2004.12.066. [DOI] [PubMed] [Google Scholar]
- 16.Byrne R.A., Neumann F.J., Mehilli J., et al. Paclitaxel-eluting balloons, paclitaxel-eluting stents, and balloon angioplasty in patients with restenosis after implantation of a drug-eluting stent (ISAR-DESIRE 3): a randomised, open-label trial. Lancet. 2013;381(9865):461–467. doi: 10.1016/S0140-6736(12)61964-3. [DOI] [PubMed] [Google Scholar]
- 17.Scheller B., Hehrlein C., Bocksch W., et al. Treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. N Engl J Med. 2006;355(20):2113–2124. doi: 10.1056/NEJMoa061254. [DOI] [PubMed] [Google Scholar]
- 18.Baan J., Jr., Claessen B.E., Dijk K.B., et al. A randomized comparison of paclitaxel-eluting balloon versus everolimus-eluting stent for the treatment of any in-stent restenosis: the DARE Trial. JACC Cardiovasc Interv. 2018;11(3):275–283. doi: 10.1016/j.jcin.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 19.Mehran R., Dangas G., Abizaid A.S., et al. Angiographic patterns of in-stent restenosis: classification and implications for long-term outcome. Circulation. 1999;100(18):1872–1878. doi: 10.1161/01.cir.100.18.1872. [DOI] [PubMed] [Google Scholar]
- 20.Hong S.J., Kim B.K., Shin D.H., et al. Effect of intravascular ultrasound-guided vs angiography-guided everolimus-eluting stent implantation: the IVUS-XPL randomized clinical Trial. JAMA. 2015;314(20):2155–2163. doi: 10.1001/jama.2015.15454. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J., Gao X., Kan J., et al. Intravascular ultrasound versus angiography-guided drug-eluting stent implantation: the ULTIMATE Trial. J Am Coll Cardiol. 2018;72(24):3126–3137. doi: 10.1016/j.jacc.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 22.Byrne R.A., Joner M., Kastrati A. Stent thrombosis and restenosis: what have we learned and where are we going? The Andreas Gruntzig Lecture ESC 2014. Eur Heart J. 2015;36(47):3320–3331. doi: 10.1093/eurheartj/ehv511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alfonso F., Pérez-Vizcayno M.J., García Del Blanco B., et al. Everolimus-eluting stents in patients with bare-metal and drug-eluting in-stent restenosis: results from a patient-level pooled analysis of the RIBS IV and V trials. Circ Cardiovasc Interv. 2016;9(7) doi: 10.1161/CIRCINTERVENTIONS.115.003479. [DOI] [PubMed] [Google Scholar]
- 24.Farooq V., Gogas B.D., Serruys P.W. Restenosis: delineating the numerous causes of drug-eluting stent restenosis. Circ Cardiovasc Interv. 2011;4(2):195–205. doi: 10.1161/CIRCINTERVENTIONS.110.959882. [DOI] [PubMed] [Google Scholar]
- 25.Ng V.G., Mena C., Pietras C., Lansky A.J. Local delivery of paclitaxel in the treatment of peripheral arterial disease. Eur J Clin Investig. 2015;45(3):333–345. doi: 10.1111/eci.12407. [DOI] [PubMed] [Google Scholar]
- 26.Lemos P.A., Farooq V., Takimura C.K., et al. Emerging technologies: polymer-free phospholipid encapsulated sirolimus nanocarriers for the controlled release of drug from a stent-plus-balloon or a stand-alone balloon catheter. EuroIntervention. 2013;9(1):148–156. doi: 10.4244/EIJV9I1A21. [DOI] [PubMed] [Google Scholar]
- 27.Alfonso F., Pérez-Vizcayno M.J., Cárdenas A., et al. A randomized comparison of drug-eluting balloon versus everolimus-eluting stent in patients with bare-metal stent-in-stent restenosis: the RIBS V Clinical Trial (restenosis Intra-stent of Bare Metal Stents: paclitaxel-eluting balloon vs. everolimus-eluting stent) J Am Coll Cardiol. 2014;63(14):1378–1386. doi: 10.1016/j.jacc.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Alfonso F., Pérez-Vizcayno M.J., Cárdenas A., et al. A prospective randomized trial of drug-eluting balloons versus everolimus-eluting stents in patients with in-stent restenosis of drug-eluting stents: the RIBS IV randomized clinical Trial. J Am Coll Cardiol. 2015;66(1):23–33. doi: 10.1016/j.jacc.2015.04.063. [DOI] [PubMed] [Google Scholar]
- 29.Cai J.Z., Zhu Y.X., Wang X.Y., et al. Comparison of new-generation drug-eluting stents versus drug-coated balloon for in-stent restenosis: a meta-analysis of randomised controlled trials. BMJ Open. 2018;8(2) doi: 10.1136/bmjopen-2017-017231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giacoppo D., Alfonso F., Xu B., et al. Paclitaxel-coated balloon angioplasty vs. drug-eluting stenting for the treatment of coronary in-stent restenosis: a comprehensive, collaborative, individual patient data meta-analysis of 10 randomized clinical trials (Daedalus study) Eur Heart J. 2020;41(38):3715–3728. doi: 10.1093/eurheartj/ehz594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheller B, Mangner N, Kader MA, et al. One-year outcomes of two parallel randomized trials of sirolimus-coated and paclitaxel-coated balloons in coronary in-stent restenosis lesions. Presented at: Transcatheter Cardiovascular Therapeutics; November 6, 2021. [DOI] [PubMed]
- 32.Granada J.F., Ferrone M., Melnick G., et al. Downstream paclitaxel released following drug-coated balloon inflation and distal limb wound healing in swine. JACC Basic Transl Sci. 2021;6(5):416–427. doi: 10.1016/j.jacbts.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parikh S.A., Finn M.T. Can paclitaxel coated balloons have a deep impact on critical limb ischemia? JACC Basic Transl Sci. 2021;6(5):428–430. doi: 10.1016/j.jacbts.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang T.Y., Sulaiman M.S.B., Soon S.X.Y., Yap C.J.Q., Patel A., Chong T.T. Slow-flow phenomena following lower limb paclitaxel- and sirolimus-coated balloon angioplasty in the setting of chronic limb threatening ischaemia-a case series. Quant Imaging Med Surg. 2022;12(3):2058–2065. doi: 10.21037/qims-21-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeh RW. Primary outcomes of a pivotal multicenter randomized trial comparing the AGENT paclitaxel-coated balloon with conventional balloon angioplasty for in-stent restenosis. Presented at: TCT 2023; October 25, 2023; San Francisco, USA.