Abstract
Background and Aims
There is clinical interest in the sustainability or otherwise of prebiotic, microbial, and antibiotic treatments to both prevent and treat inflammatory bowel diseases. This study examined the role of antibiotic manipulation of the gut microbiome to treat spontaneous and induced murine models of colitis.
Methods
Symptomatic, histological, molecular, and microbial ecology and bioinformatic readouts were used to study the effect of a 10-day antibiotic cocktail and then follow-up over 2 months in the spontaneous Winnie colitis mouse preclinical model of ulcerative colitis and also the indirect antibiotic and Winnie microbiotic gavage effects in an acute dextran sodium sulfate–induced colitis model in wild-type mice.
Results
The antibiotics elicited a striking reduction in both colitis symptoms and blinded histological colitis scores, together with a convergence of the microbial taxonomy of the spontaneous colitis and wild-type control mice, toward a taxonomic phenotype usually considered to be dysbiotic. The improvement in colitis was sustained over the following 8 weeks although the microbial taxonomy changed. In vitro, fecal waters from the antibiotic-treated colitis and wild-type mice suppressed the inflammatory tenor of colonic epithelial cells, and gavaged cecal slurries from these mice moderated the acute induced colitis.
Conclusion
The results clearly show the possibility of a sustained remission of colitis by microbial manipulation, which is relevant to clinical management of inflammatory bowel diseases. The beneficial effects appeared to depend on the microbial metabolome rather than its taxonomy.
Keywords: Metabolome, Antibiotic, IBD, Dysbiosis
Introduction
Inflammatory bowel disease (IBD) etiology is becoming better understood. Etiological factors for these conditions involve an interplay of host genetic predispositions and external environmental and host microbial factors resulting in inappropriate inflammation in the gut as well as extraintestinal manifestations.1, 2, 3 Treatment paradigms in IBDs have evolved toward inducing and maintaining remission, achieving mucosal healing, avoiding surgical resection, and reducing the risk of cancer as a result of chronic inflammation.4, 5, 6 Modulation of the host’s innate inflammatory and adaptive immune responses by glucocorticosteroids, immunomodulators, biologics, and newer small molecules remains the cornerstone of treatment for IBDs.7
The interactions between the host immune system, the target of most IBD treatments, and the microbiome are complex. In Crohn’s disease (CD), diversion of the fecal stream improves disease activity, whereas reinfusion of the fecal stream causes recurrence of inflammation.8 On the other hand, with ulcerative colitis (UC), diversion of the fecal stream can result in inflammation, so called diversion colitis.9 These clinical observations underscore that the gut microbiome or its metabolome can positively and negatively regulate gut inflammation.
Understanding of the gut microbiome has improved with developments in bioinformatic tools and high-throughput molecular sequencing techniques. Numerous studies have demonstrated reduced bacterial diversity of the microbiome in IBDs,10 which may predispose to reduced stability of the microbiome over time. Many studies also report overrepresentation and underrepresentation of particular bacterial species, suggesting possible roles for these species in the pathogenesis of IBDs.10,11,12 This has spawned the term ‘dysbiosis’, which is generally used to describe an imbalance in the microbial community, which in some manner is associated with disease.
Therefore, there has been considerable interest in modulation of the microbiome by prebiotic, probiotic, or antibiotic interventions. However, by and large, these treatments individually have not proven to be reliable. Exceptions include antibiotic treatment of perianal CD, antibiotic treatment of inflammation of a postoperative ileal pouch anal anastomosis (pouchitis),13 postsurgical ileitis,14 sepsis complicating CD where some efficacy has been established,15,16 and antimycobacterial regimes for uncomplicated inflammatory CD, but the clinical response is not reliably sustained after their cessation.17
Although evidence to support the use of antibiotics in UC is even less persuasive, there being a lack of properly powered randomized controlled trials,18 there continues to be great interest in treatment with fecal microbial transplant (FMT) to re-establish a more healthy microbial community.19 FMT combines microbial and prebiotic manipulation. It is a well-proven established treatment for Clostridium difficile infection.20 Furthermore, randomised control trial data are accumulating to support FMT for UC.21
However, questions around the mechanism and durability of a clinical response to microbial manipulation in UC remain unanswered. In this article, we show that manipulation of the gut microbiome with antibiotics ameliorated the chronic spontaneous colitis in the Winnie mouse model. Winnie mice harbor a single-nucleotide polymorphism mutation in the D3 domain of the Muc2 gene, which leads to misfolding of the Muc2 protein. This nonimmune defect leads to spontaneous colitis starting at 4 weeks of age, which include signs of inflammation including diarrhea and rectal bleeding, as well as molecular and histologic changes including increased endoplasmic reticulum stress, depleted goblet cells and depleted secreted mucus layer, and a proximal-distal gradient Th17-dominant intestinal inflammation resembling human UC.22,23 Surprisingly, the improvement seen with antibiotic treatment was sustained. In addition, surprisingly, the successful antibiotic treatment resulted in a striking shift in the microbiome with a loss of diversity consistent with ‘dysbiosis’. Fecal waters from the antibiotic-treated mice reduced NF-kB expression by epithelial cells in an in vitro assay, consistent with microbial function, not microbial taxonomy, being a determinant of the mucosal immune tone.
Materials and Methods
Animal Experiments
Mice
All animal experiments were approved by the University of Queensland Animal Ethics Committee (MED/TRI/MRI-UQ/104/16/NHMRC/Manipulating the microbiome and mucosal biology to treat colitis). Experiments conformed to the Animal Research: Reporting of in Vivo Experiments guidelines.
Antibiotic Experiments
C57Bl/6 wild-type (WT) controls and Winnie male or female Winnie mice were in-bred over >7 generations and housed in a specific-pathogen-free facility at the Translational Research Institute (Brisbane, Australia). WT or Winnie mice (4-week-old) were gavaged for a duration of 10 days an antibiotic cocktail similar to that popularized by Rakoff-Nahoum et al 200424 but with the addition of gentamicin. Specifically, mice were gavaged 20 μL/g mouse weight daily with either water control or a cocktail of 5 antibiotics called AB5 consisting of 1 g/L ampicillin (Sigma), 0.2 g/L metronidazole (Mater Pharmacy, Brisbane), 1 g/L neomycin sulfate (Mater Pharmacy, Brisbane), 0.5 g/L vancomycin (Hospira), and 0.1 g/L gentamicin (Pfizer) suspended in water. Winnie mice and controls treated with +/− AB5 were observed before sacrifice at the end of the AB5 treatment. In addition, 2 batches of Winnie mice treated +/− AB5 were housed in separate single cages (one mouse per cage) after AB5 treatment for observation before sacrifice at 4 and 8 weeks.
DSS Colitis
Four-week-old WT mice purchased from University of Queensland Biological Resources were accommodated in the TRI animal facility for 2 weeks and then provided with either water or 1% dextran sodium sulfate (DSS) in drinking water bottles to induce colitis. The WT mice on DSS were treated for 7 days with 10% w/v anaerobic slurries, prepared from cecal contents collected at sacrifice on day 10 from WT and Winnie mice treated +/− AB5. Specifically, the anaerobic cecal contents were prepared by suspending in anaerobic glycerol solution and stored at −70 °C before use; 20 μL/g body weight was administered intragastrically daily from day 0 to day 6 to the DSS mice. A control group of WT DSS mice were gavaged daily with anaerobic Ringer’s solution (vehicle).
Disease Activity Scores
The disease activity index for both spontaneous and DSS-induced colitis was scored daily by one of 3 unblinded scorers for the duration of the experiments.22
Histology and Histological Colitis Scoring
Colons were sectioned and stained for haematoxylin and eosin, and whole slide scanning was performed using the Olympus VS120 slide scanner. Histological images were analyzed using OlyVIA 2.6, and scoring for spontaneous and DSS-induced colitis was performed blinded to the mouse genotype and treatment as previously described.22
Bacterial 16S Ribosomal Ribonucleic Acid (16S rRNA) Gene Sequencing
The 16S rRNA amplicons produced from the genomic DNA extracted from the cecal mucosal tissues were sequenced using the Illumina MiSeq platform available at the University of Queensland’s Australian Centre for Ecogenomics (ACE). The V5–8 regions of the bacterial/archaeal 16S rRNA gene from the individual DNA samples were amplified by polymerase chain reaction (PCR) using primers 803F and 1392R.25 Bar-coded amplicon libraries were constructed and sequenced by the ACE facility.
Sequence Analysis and Bioinformatics Workflows
The raw sequences were trimmed by ACE using the ACE mitag pipeline. The first 20 bases of all fastq files were trimmed to remove the primer sequence. The raw sequences were then quality trimmed using Trimmomatic software with an average base quality above 15. All reads were then hard trimmed to 250 bases, and any reads with fewer than 250 bases were excluded. Fastq files were then converted to fasta files. The sequence data were then analyzed using the Quantitative Insights Into Microbial Ecology software package26 on a Ubuntu Linux virtual machine (v5.0.12). Briefly, Quantitative Insights Into Microbial Ecology was used to demultiplex fasta files and perform quality control checking and filtering of the sequence data, and only high-quality full-length sequences were further analyzed. Sequences were run through USEARCH 6.1 to perform reference-based chimera detection, and any candidate chimeric sequences were removed before performing significant analysis on data sets. The sequences were then clustered into Operational Taxonomic Units (OTUs) using a threshold setting of 97% sequence identity, and using the open-reference OTU picking method, individual sequences were assigned to their respective OTUs as per the Greengenes database 75.27 Tables of the OTUs containing taxonomic and abundance data were generated for each individual sample, and any OTUs that were not identified as bacteria or archaea, and/or OTUs that comprised ≤0.01% of the total sample sequence count, were removed. The median coverage of the biodiversity present in the samples (7526 [4677–10,814]) was then assessed by rarefaction analysis, and a normalized subsampled OTU table was subsequently generated by random sampling to the minimum (4677) reads for cecal mucosal microbiome samples. The subsampled OTU table was used to generate taxonomy plots from phylum to genus levels and to calculate alpha (within sample) and beta (between samples) diversity metrics. Alpha diversity considers both the species richness and evenness of the species present. Alpha diversity was assessed using the Shannon index (a key alpha diversity metric). To determine how diverse the microbial communities were affected with AB5 treatment, beta diversity (difference in taxonomic abundance profiles from different samples) was assessed by weighted UniFrac shown by a principal coordinate analysis (PCoA) plot in 3-dimensional space. The statistical clustering of the observed clustering was determined using canonical correspondence analysis.
Cecal mucosal samples were collected from 6-week-old WT and Winnie mice +/− 10 days of AB5 treatment, and 16S rRNA sequencing analysis was performed to examine changes in the cecal mucosa–associated microbiota. A total of 90,087 16S rRNA reads were generated from cecal mucosal samples of WT and Winnie mice +/− AB5 treatment with an average of 7526 (±1835 standard deviation) per sample. Rarefaction curves for WT and Winnie mice +/− AB5 treatment at 97% similarity levels were established by sampling to the minimum reads (4677) for cecal mucosal microbiome samples.
Quantitative PCR and Primer Sets
See Figure A6.
Functional Experiments
Fecal waters were prepared by dispersing the fecal pellets collected from WT and Winnie mice +/− day-10 AB5 treatment in sterile 37 °C phosphate buffered saline containing 20% fetal bovine serum (FBS) and 2 mM ethylenediaminetetraacetic acid to a final concentration of 1 g/mL. Fecal samples were then vortexed for 1 minute and incubated at 37 °C for 10 minutes. After incubation, samples were centrifuged at 21,000g for 10 minutes at room temperature to remove insoluble materials. The centrifugation step was repeated if necessary. Clear supernatants were collected into fresh 1.5-mL Eppendorf tubes and filtered through 0.2-μm nylon filters to remove microbiota. The filtered supernatants were stored at −20 °C until required.28
NF-κB Reporter Cell Line
LS174T cells (REF) were treated with transduction medium (Dulbecco's Modified Eagle Medium supplemented with 10% v/v FBS and 1% v/v Glutamax [Gibco] supplemented with 6 μg/mL polybrene) and 2 × 104 colony-forming units of NF-kB firefly luciferase reporter lentivirus (Cellomics Technology) and centrifuged at 1200g for 90 minutes at 32 °C. The transduction medium was replaced after 24 hours with a complete growth medium, and transduced cells were recovered after puromycin selection (2.5 μg/mL) for 48 hours.29
Measurement of Fecal Waters’ NF-κB Immunomodulatory Activities
Ninety-six–well microtiter plates were seeded with 20,000 LS174T NF-κB reporter cells per well in complete media (Dulbecco's Modified Eagle Medium supplemented with 5% FBS and 1% Glutamax [Gibco]) and cultured for 24 hours. Fecal waters, culture vehicle, or AB5 were added to the cultured cells (10% v/v in complete media) and further incubated for 4 hours at 37 °C. The activation of NF-κB was assessed using the Pierce Firefly Luc One-Step Glow Assay Kit (Thermo Fisher Scientific) as per the manufacturer’s instructions and read out on a PHERAstar FS micro plate reader.29
Viable fecal bacteria were determined by culture on blood agar plates (Blood Agar Base #2 supplemented with 7% defibrinated horse blood [Oxoid, UK]) either aerobically or anaerobically in anaerobic jars containing Oxoid AnaeroGen sachet at 37 °C. Anaerobic condition was confirmed using the Oxoid anaerobic indicator. The latter culture method will only allow facultative anaerobes to survive and be cultured as the preparation procedures before incubation in the anaerobic gas jar did not prevent exposure to some atmospheric oxygen.
Statistical Analyses
All statistical analyses were performed using Prism v7.01 (GraphPad Software). Experimental data where normally distributed were graphically represented as mean ± standard deviation. All comparisons were performed with nonparametric statistical tests. For the time- and dose-related experiments, significance was assessed by 2-way analysis of variance with Tukey’s multiple comparison tests. For comparison of groups, the 1-way analysis of variance nonparametric Kruskal-Wallis test with Dunn’s multiple comparison tests was used. P = .1234 (ns), 0.0332 (∗ or # or &), 0.0021 (∗∗ or ## or &&), 0.0002 (∗∗∗ or ### or &&&), <0.0001 (∗∗∗∗ or #### or &&&&).
Results
A 10-day Antibiotic Intervention With AB5 Ameliorated Spontaneous Colitis in Winnie Mice
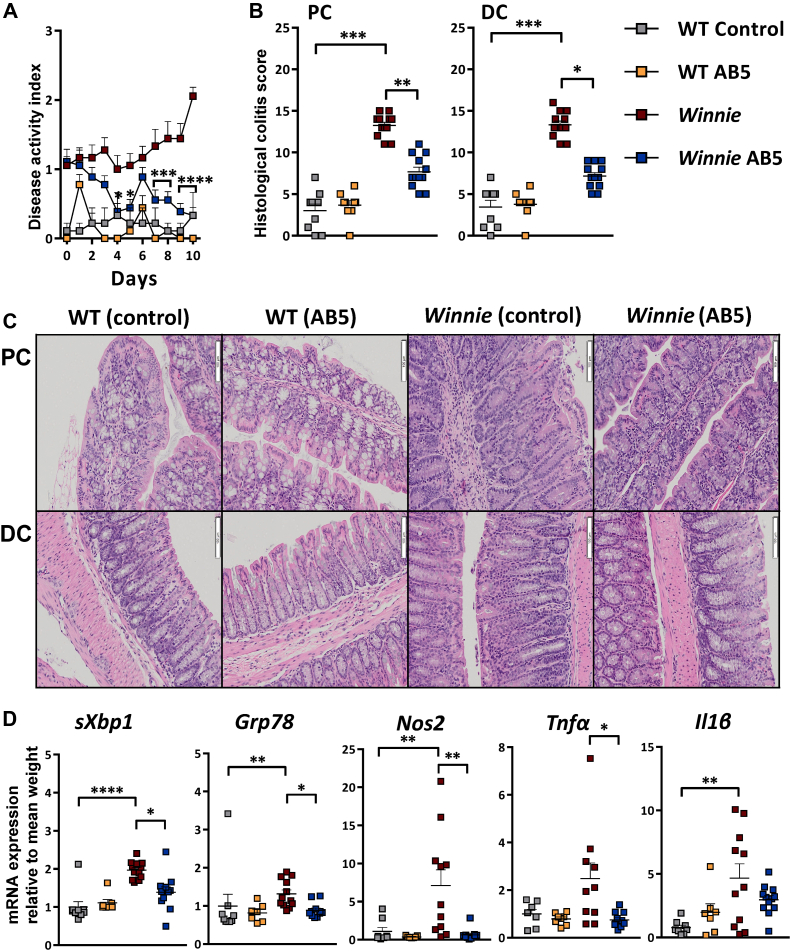
The gut microbiota shapes the inflammatory response in Winnie mice because germ-free (GF) Winnie mice do not develop significant inflammation,30 but it is not known whether microbiota-directed treatments can modulate disease. To assess this, Winnie mice were gavaged daily with either the AB5 cocktail (20 μL/g body weight) or water (as controls) for 10 days. The AB5 treatment resulted in striking improvements of both disease activity index (Figure 1A) and histologic colonic inflammation scores, compared with Winnie control mice (Figure 1B and C). Notably, blinded histological colitis scores in the AB5-treated Winnie mice were significantly reduced relative to Winnie control mice. Conventional haematoxylin and eosin histology and subscoring showed that the AB5-treated Winnie mice were more similar to WT mice, having more mucus-filled goblet cells, shorter crypt lengths, and reduced inflammatory cell infiltrates (Figure A1). The Muc2 missense mutation in Winnie results in endoplasmic reticulum (ER) stress, and as expected, expression of ER stress (sXbp1 and Grp78) and oxidative stress (Nos2) genes was elevated in the control Winnie mice compared with the WT control mice. AB5 treatment of Winnie mice decreased both ER and oxidative stress (Figure 1D) and also reduced expression of the inflammatory cytokines tumour necrosis factor-alpha (Tnf-α) and interleukin-1β to levels similar to those in the WT groups (Figure 1D).
Figure 1.
Ten-day AB5 treatment ameliorated spontaneous colitis in Winnie mice. (A) Daily disease activity index (DAI); (B) blinded histological colitis proximal colon (PC) and distal colon (DC) scoring on day 10, for WT and Winnie AB5-treated and control animals; (C) representative H & E histology of colonic Swiss rolls; (D) fold change mRNA relative to mean WT sXbp1, Grp78, Nos2, Tnfα, Il6. Statistical analyses and significance (see Materials and Methods).
Both Winnie and WT mice treated with AB5 for 10 days had similarly enlarged ceca (Figure A2A), increased cecal cross-sectional area and weight (Figure A2B and C, respectively), and decreased spleen weight as well as a reduced number of visible Peyer’s patches (Figure A2D and E, respectively). These macroscopic findings after antibiotic treatment are similar to those first reported in comparative studies of GF and conventionally raised mice,31 and although reported previously,32 they are not well known. However, and in contrast to GF mice, the AB5 treatment did not eliminate the microbiota within the gastrointestinal tract of either WT or Winnie mice during the course of the experiment. In that context, after 9 days of AB5 intervention, the median total bacterial count as measured by bacteria-domain qPCR/g of stool was reduced 280-fold in the WT mice (Mann Whitney P = .002) but interestingly was not decreased significantly in the Winnie mice (MW P = .7, N = 9). Culture-based counts of the viable aerobic and facultative anaerobic fecal bacteria corroborated the real-time PCR results. Aerobic bacteria were reduced 18.6-fold in WT mice on day 9 (P = .04) compared with day 0 but was statistically unchanged for Winnie. Facultative anaerobe counts were reduced 7.2-fold in WT (P = .001) and only 1.8-fold in Winnie (P = .02). In summary, AB5 treatment for 10 days reduced colitis symptoms in Winnie mice and was associated with improvements in histology, ER and oxidative stress, and reduced expression of proinflammatory cytokines. Although these host responses are consistent with those reported in GF mice, the AB5 treatment did not result in the reduction of gastrointestinal bacteria in Winnie mice to the same magnitude measured for the WT mice. These results suggested that the reduction of colitis symptoms and inflammatory tenor in Winnie mice after AB5 treatment must be linked with the community profile rather than bacterial density in the large intestine.
Antibiotic Treatment, Not the Host Genotype, Was the Major Driver for an Altered Microbiome
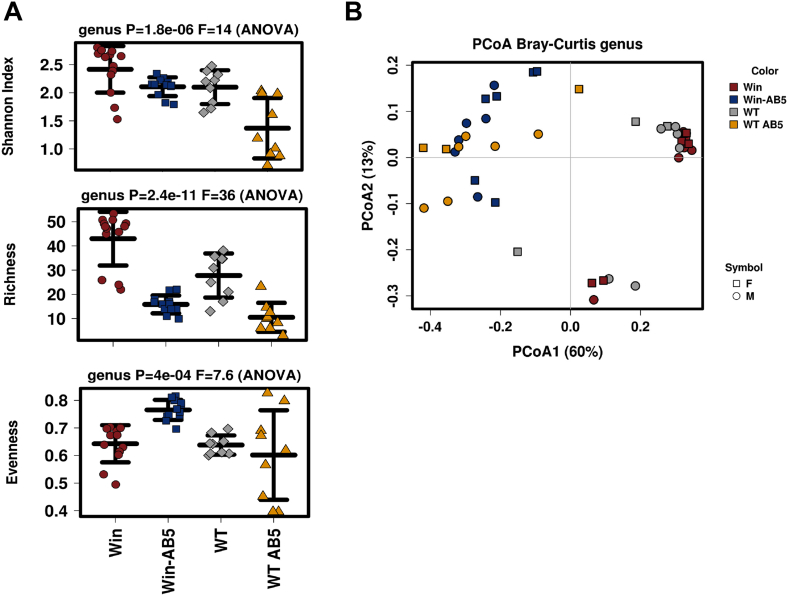
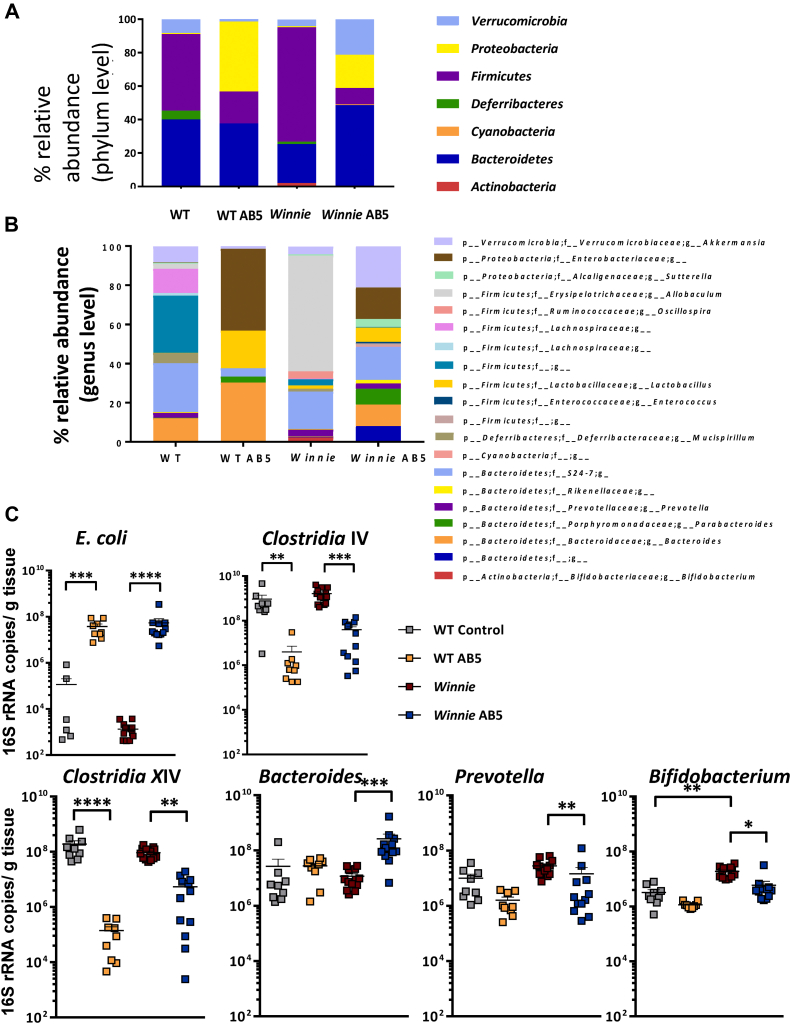
We next assessed the impact of the AB5 treatment on the composition of the microbiome using 16S rRNA gene amplicon profiling. The AB5 treatment for 10 days decreased microbial richness indices in both WT and Winnie feces (Figure 2A), and the PCoA plots of the weighted UniFrac distance metrics revealed a distinct clustering of mice relative to AB5 treatment more so than the animal genotype (Figure 2B; P = .0002, canonical correspondence analysis statistical analysis). The AB5 treatment increased the relative abundance of Proteobacteria and decreased the relative abundance of Firmicutes in both WT and Winnie feces (Figure 3A). Interestingly, the relative abundance of Verrucomicrobia was increased in the feces of AB5-treated Winnie mice but not WT mice, and the reduction in the relative abundance of Firmicutes in the AB5-treated mice did not involve taxa affiliated with the genus Lactobacillus (Figure 3B).
Figure 2.
AB5 was the major driver of an altered microbiome. (A) Alpha diversity (diversity within a sample) Shannon, richness, and evenness indices at 10 days; (B) beta diversity (diversity between samples) PCoA of the Bray Curtis distance for WT and Winnie AB5-treated and control animals at 10 days.
Figure 3.
AB5 drove a ‘dysbiotic’ microbiome. UniFrac relative abundance analysis of (A) microbial phyla and (B) genus; (C) log-scale: rtPCR quantification of E.coli, Clostridium IV, Clostridium XIV, Bacteroides, Prevotella, and Bifidobacterium associated with cecal mucosa.
The community profiles evident in mouse feces were largely recapitulated in cecal mucosa as assessed by the qPCR assays targeting key bacterial groups (Figure 3C). Both the WT and Winnie AB5-treated mice showed a 30- to 50-fold increase in Escherichia coli (P = .0002 and P < .0001, respectively, Figure 3C). In contrast, there was a 10- and 25-fold decrease in the absolute abundance of taxa affiliated with Clostridium cluster IV in the AB5-treated Winnie and WT mice (P = .002 and P = .01, respectively) as well as a 15- and 40-fold decrease in the absolute abundance of taxa affiliated with Clostridium cluster XIVa in the AB5-treated Winnie and WT mice (P = .002 and P < .0001, respectively). Although the Bacteroides-specific qPCR assays showed no significant differences between the WT control and AB5-treated groups vs WT control mice (P > .3), there was a 2-log increase in the Winnie AB5 group vs Winnie control (P = .0002) (Figure 3C).
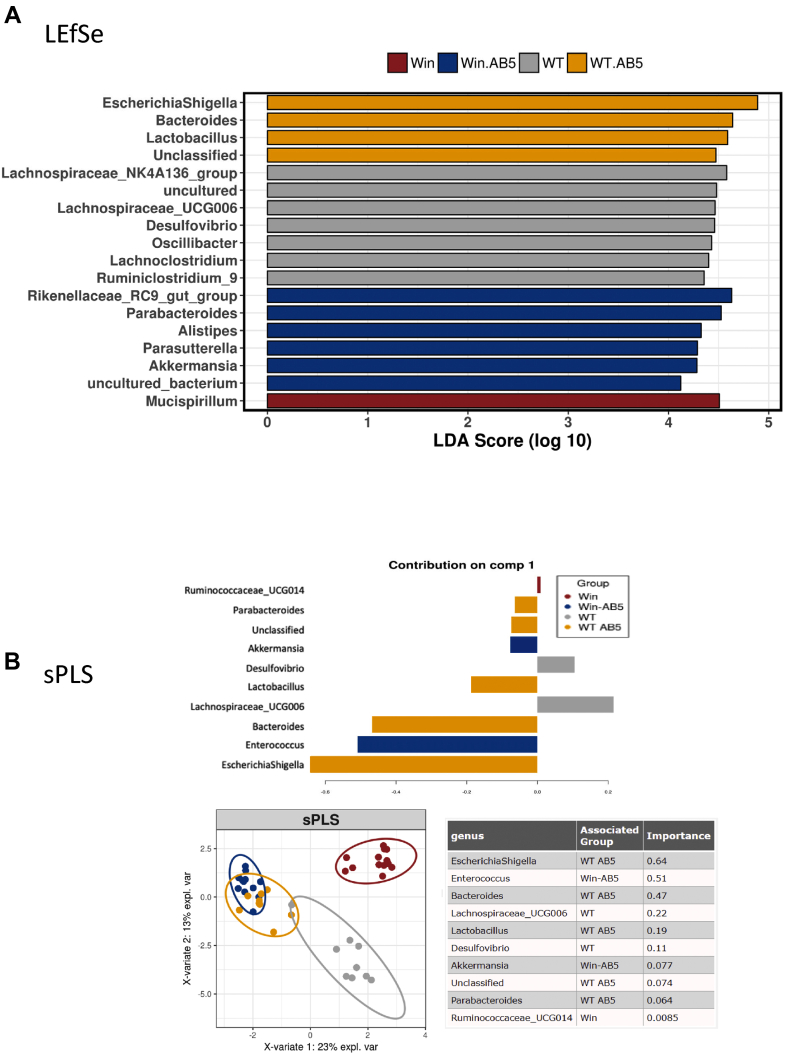
These alterations in response to AB5 treatment were substantiated and further resolved using linear discriminant analysis effect size (LEfSe) and sparse Partial Least Squares regression discriminatory analysis (sPLS-DA) discriminatory analyses of the top 20 bacterial taxa (Figure 4A and B, respectively). The LEfSe analysis showed Escherichia/Shigella was the most discriminatory in AB5-treated WT mice and that Parasutterella and Akkermansia were discriminatory of the microbial communities of AB5-treated Winnie mice, whereas sPLS-DA showed Escherichia/Shigella taxa were most discriminatory of the AB5-treated WT mice but Enterococcus and Akkermansia spp. were most discriminatory of the AB5-treated Winnie mice. The Akkermansia spp. bloom in Winnie AB5 feces is consistent with restoration of mucin production, which is the main substrate for Akkermansia spp.25 In summary, the 3 different analytical methodologies show that AB5 treatment drove alterations to the microbiome characterized by decreases in Clostridia IV and XIV and an increase in Enterobacteriaceae. These changes, which were largely independent of the mouse genotype, resulted in macroscopic changes in the host gut and immune structures.
Figure 4.
Analysis of top 20 discriminating taxa between each group at day 10. (A) LEfSe unconstrained analysis to determine microbial differences most likely to explain differences between groups; (B) sparse partial least squares regression constrained principal component analysis of the WT and Winnie AB5-treated and control animals at 10 days.
Improvement in Colitis Was Sustained in Quarantined AB5-treated Winnie Mice
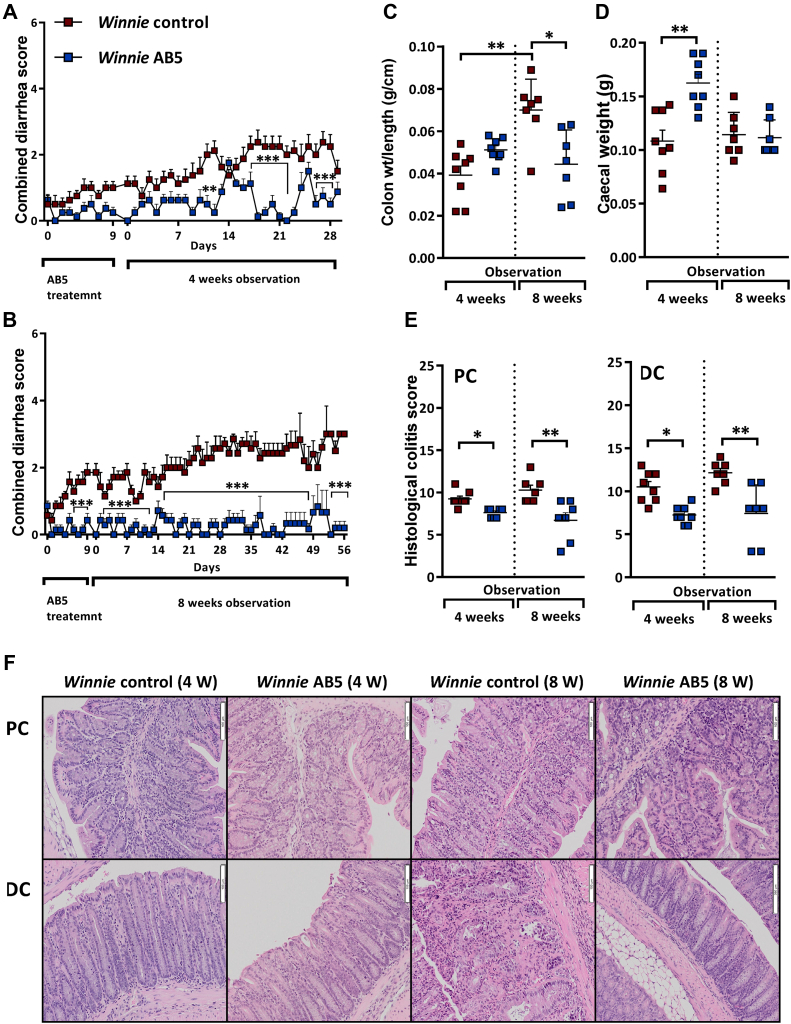
In uncomplicated CD or UC, successful antibiotic treatment is not usually associated with a cure after cessation of antibiotic treatment. To explore the possibility that the ameliorative effect of antibiotics in the model could be sustained, AB5-treated Winnie and the respective matched control Winnie mice were observed for up to 8 weeks after 10 days of AB5 treatment. We have observed on other occasions but not published that antibiotic-treated Winnie relapsed quickly when they were free living with untreated Winnie, likely due to coprophagy, and so the animals in this set of experiments were kept in separate cages, one animal per cage. Although the diarrhea progressed in matched Winnie controls to become increasingly bloody and was associated with rectal prolapse, the Winnie AB5-treated group remained largely disease-free. Figure 5A and B show the results, being for 2 batches of animals sacrificed, respectively, at 4 and 8 weeks after AB5.
Figure 5.
Sustained improvement in AB5-treated Winnie over 8 weeks. (A, B) Serial DAI over 4 and 8 weeks in treated and untreated Winnie controls; (C) colon weight/length and (D) cecal weights at 4 and 8 weeks; (E) blinded histological colitis scores from PC and DC at 4 and 8 weeks; (F) representative H & E histology from PC and DC at 4 and 8 weeks in treated and untreated Winnie controls. DAI, disease activity index; DC, distal colon; PC, proximal colon.
At 8 weeks, the AB5-treated mice, which were not prolapsing, as well as the control Winnie mice showing rectal prolapse, were culled to comply with the approved ethics protocol. The colon weight/length ratio—a reliable indicator of colitis severity—was significantly greater in the control Winnie mice sacrificed at 8 weeks than that in those control mice sacrificed at 4 weeks (Figure 5C). This was not unexpected as the severity of the spontaneous colitis, which commences around 4 weeks, increases with age.22,23
At 4 weeks after the 10-day AB5 treatment, the cecal weight remained increased, but interestingly, by the end of 8 weeks, the GF-like cecal phenotype was lost (Figure 5D). Remarkably, the symptom reduction observed in AB5-treated Winnie mice persisted throughout the 8-week period, which was corroborated by the lower blinded histological colitis scores. Histology subscores were also generally lower in proximal and distal sections of the colon in the Winnie AB5 group vs the Winnie controls (Figure 5E and F and Figure A3).
Taken together, these results showed that AB5 treatment moderated disease risk in colitis-susceptible Winnie mice. The AB5 cocktail resulted in sustained colitis remission in those mice housed in isolation after the AB5 treatment, as evidenced by colon weight/length ratios and blinded histology. However, the GF-like changes in cecal weight were not sustained at 8 weeks.
Antibiotic Treatment Suppresses the Inflammatory Phenotype of the Microbiome
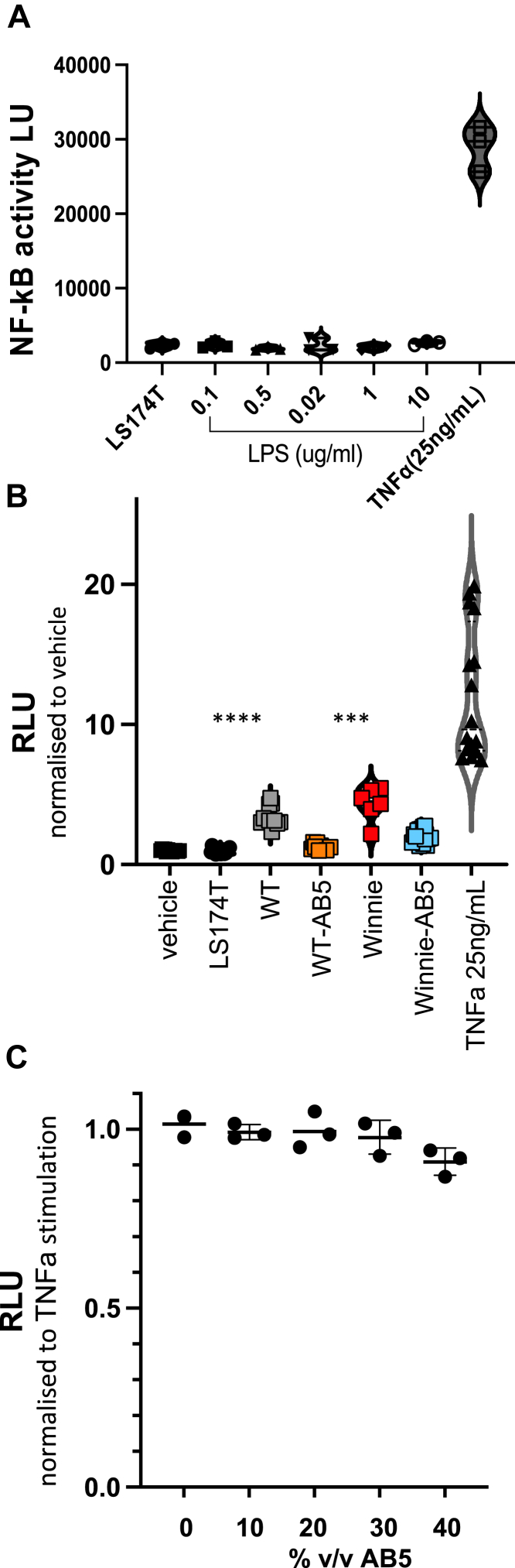
NF-κB is a major regulator of inflammation, and gut epithelial integrity and tonic low-level stimulation by proinflammatory host and bacterial signals are required to maintain the integrity of the colonic epithelium.33,34 However, elevated NF-κB signaling is associated with maladaptive inflammation. We reasoned on the strength of the successful AB5 treatment that either the perturbation(s) of the microbiota was somehow structurally or functionally beneficial in the setting of the Winnie predisposition to colitis or that there had been a direct effect from the AB5 cocktail on the large intestinal mucosa. To test this possibility, we explored the effect of bacteria-free fecal waters from the various experimental conditions using an in vitro LS174T gut epithelial reporter cell line for NF-κB (see Materials and Methods).
TNFα (25 μg/mL), but not lipopolysaccharide (0.1–10 μg/mL), variably increased LS174T NF-κB activity 8- to 15-fold (Figure 6A). Bacterial-free fecal waters prepared from 10% w/v slurries of the 6-week-old WT and Winnie mice were also proinflammatory (∼4-fold) in this assay, and proinflammatory activity was statistically greater with untreated Winnie fecal supernatant extracts than that with untreated WT fecal water (P = .014) (Figure 6B). On the other hand, bacterial-free fecal waters prepared from 10% w/v slurries of both WT-AB5- and Winnie-AB5-treated mice reduced NF-κB luciferase activity in these cells to near baseline levels (Figure 6B). However, the antibiotics themselves within the AB5 cocktail did not affect NF-κB luciferase activity in these cells: a 10% v/v AB5 cocktail had no effect on TNFα-stimulated NF-κB activity (Figure 6C).
Figure 6.
AB5 fecal waters from 10-day mice suppressed NF-kB activity in colonic epithelial cells. Activity of the NF-kB reporter in LS174T cell cultures expressed as light units (LUs) or relative light units normalized to vehicle controls (RLUs). (A) LUs comparing vehicle, LPS, TNFα; (B) RLUs with AB5- or vehicle-treated WT and Winnie 10% v/v fecal supernatants; (C) RLU activity in TNFα-stimulated cultures with addition of AB5 10%–40% v/v.
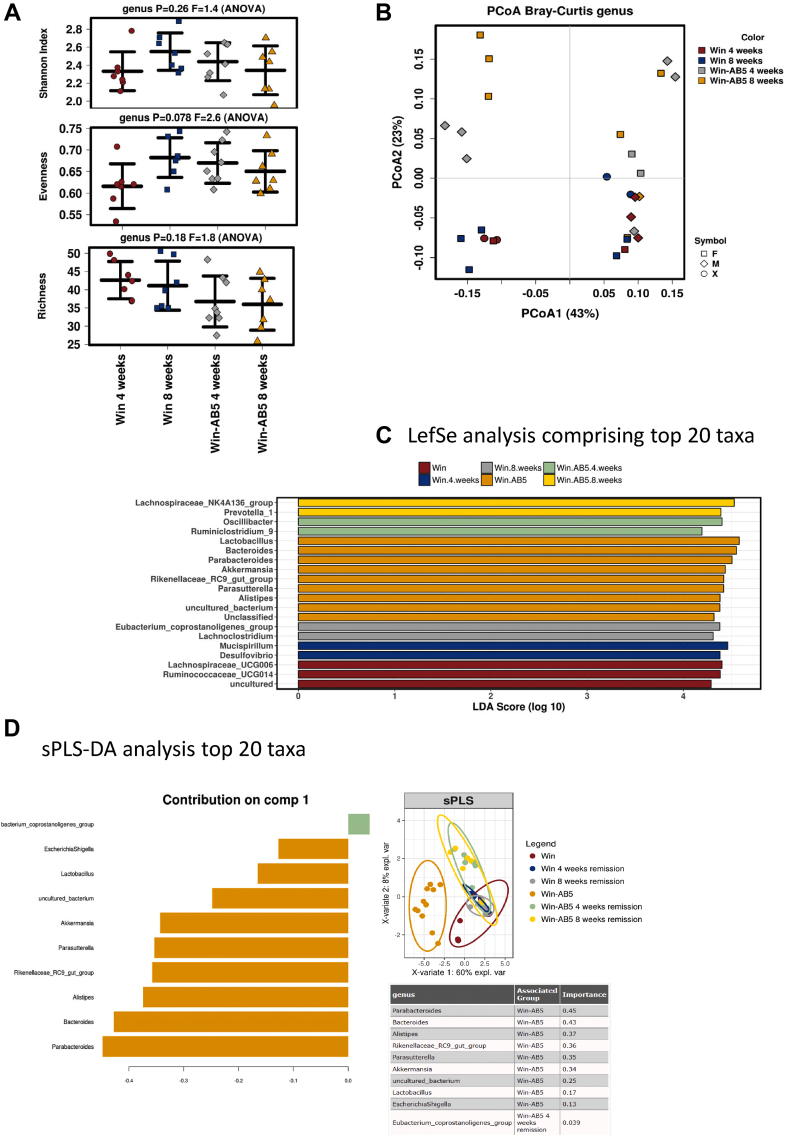
The Fecal Microbiota Profile of Winnie AB5-treated Mice Was Unstable Over Time
We hypothesized that the clinical durability of the AB5 treatment should be paralleled by a stable microbiome over the period of observation, and consistent with this hypothesis, within-group fecal sample diversity measures were stable between 10 days and after 4 weeks and 8 weeks for dummy-treated Winnie. However, the Shannon and richness diversity measures were increased from 4 weeks in AB5-exposed Winnie fecal samples (Figure A4). Thus, although the Winnie fecal microbiome was stable in its alpha diversity despite the worsening colitis in the host animal, a significant increase in alpha diversity had evolved in those Winnie previously exposed to AB5, but whose colitis remained in abeyance. However, within-sample variation (Shannon index, richness, and evenness) was not significantly different at 4 and 8 weeks in AB5-treated and untreated Winnie 4- and 8-week feces (Figure 7A). In contrast to fecal sample diversity at 10 days, diversity between groups at 4 and 8 weeks was not adequately explained on the basis of any of AB5 treatment, sex, or time period (Figure 7B), there being no clear groupings when using PCoA Bray-Curtis.
Figure 7.
Winnie fecal microbiome structures were unstable over time. (A) Alpha diversity Shannon, richness, and evenness indices at 4 and 8 weeks; (B) beta diversity PCoA of the Bray Curtis distance for Winnie AB5-treated and control animals at 4 and 8 weeks. (Color as per groups and symbols as per gender); (C) LEfSe analysis and (D) sPLS comparing (10 days) AB5-treated and control Winnie animals with AB5-treated and control Winnie at 4 and 8 weeks.
LefSe analysis comparing the evolution of the fecal microbiome at post-4- and post-8-week AB5 with day-10 AB5 Winnie indicated many differences at a genus classification level, although the bacteria after 4 and 8 weeks were all common to Firmicutes and Bacteroidetes phyla (Figure 7C). This contrasted with AB5-exposed Winnie fecal samples, where Proteobacteria (Parasutterella) and Verrucomicrobia (Akkermansia) were represented frequently at 10 days but not after 4 or 8 weeks, at which times members of the Firmicutes phylum were almost exclusively the dominant species, apart from Prevotella in the Bacteroidetes phylum. Winnie day-10 AB5 remained well separated from the other groups (Winnie control day-10 and Winnie 4 and 8 weeks +/− AB5) (sPLS-DA analysis, Figure 7D).
In conclusion, the stable colonic mucosal response after AB5 treatment was not paralleled by a stable AB5-microbiome taxonomy, whereas the Winnie control microbiome appeared more stable. Winnie control microbiomes after 4 and 8 weeks could not be distinguished at a phylum level of taxonomy from post-4- and post-8-week AB5 fecal microbiomes. Thus, the documented AB5 dysbiosis and the larger diversity of the microbiome taxonomy in the absence of the AB5 driver suggested that shifts in taxonomy (OTUs, phyla, species) were not immediately critical in moderating the colitis in the Winnie model.
Winnie AB5 Cecal Gavages Trended to Improve DSS-induced Colitis in C57Bl/6 WT Mice
To further test the hypothesis that the improvement in gut inflammation related to the microbiome or its metabolome, rather than a direct effect of the AB5 treatment on the colon or immune system, we examined in vivo the effect of gavaging the cecal contents from Winnie-AB5 and control mice on the phenotype of acute DSS-induced colitis in WT mice.
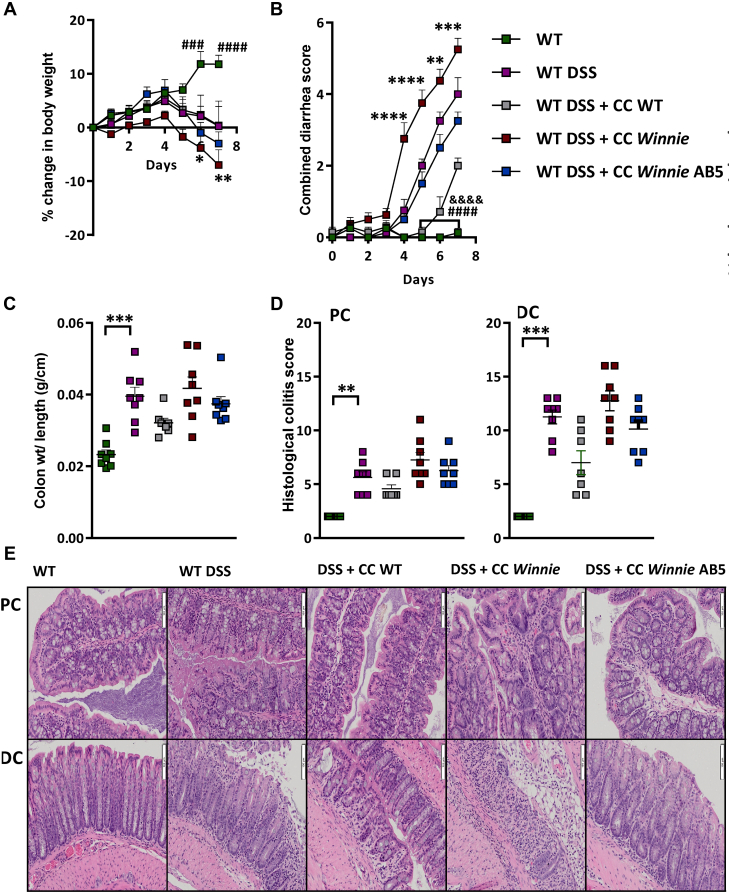
We reasoned that the success of the AB5 treatment was mediated via alteration of the microbiome and/or its metabolome, having shown in the previous section that there was NF-κB-suppressor activity in the fecal microbial supernatant of AB5-treated mice, which was not due to the AB5 cocktail. We therefore tested in vivo the effect of gavaging daily for 6 days the microbiome from the different experimental conditions into WT mice exposed to a chemically induced colitis. Briefly, acute colitis was induced in 4 groups of C57Bl/6 mice (N = 8 per group) using 1% DSS in drinking water for 7 days. During the induction of colitis, 3 of the groups of mice were cotreated with anaerobic cecal microbial gavages (CMGs) prepared from cecal contents collected from Winnie +/− AB5 treatment and WT mice at their sacrifice on day 10 of the acute AB5 intervention experiment (refer to the Materials and Methods section).
WT mice treated with 1% DSS lost significant weight from day 6 compared with a group of untreated WT mice, as expected. The degree of weight loss trended greater in WT DSS mice receiving CMG from untreated Winnie (Figure 8A), which suggested that untreated Winnie CMG was aggravating the DSS-induced colitis. The combined diarrhea-rectal bleeding scores started to increase with DSS treatment from day 4 and were significantly increased in the WT DSS vs WT group from day 5 as expected. Interestingly, attenuated diarrhea-rectal bleeding scores were observed in animals gavaged with WT CMG vs those DSS-treated control animals gavaged with the vehicle only (Figure 8B). Conversely, the diarrhea-rectal bleeding scores appeared to worsen in the animals receiving Winnie CMG but improve in animals receiving AB5 Winnie CMG compared with DSS-treated controls. Colon weight/length ratio followed similar trends (Figure 8C).
Figure 8.
Winnie AB5 cecal gavages trended to improve acute DSS-induced colitis. WT and WT DSS controls vs WT DSS + cecal content (CC) gavages from untreated Winnie, AB5-treated Winnie, or untreated WT. (A) Daily weight. Statistical analyses: 2-way ANOVA (Tukey’s multiple comparison test). # indicates comparison between WT DSS vs WT; ∗ indicates comparison between WT DSS + Winnie CC vs WT DSS; (B) combined diarrhea score (frequency + rectal bleeding). # indicates comparison between WT DSS vs WT; & indicates comparison between WT DSS + WT CC vs WT DSS; ∗ indicates comparison between WT DSS + Winnie CC vs WT DSS; (C) colonic weight length ratios; (D) blinded histological colitis scores of PC and DC; and (E) representative H & E histology. ANOVA, analysis of variance.
Consistent with the disease activity scores, histological colitis scores were lower in animals receiving Winnie AB5 CMG vs animals receiving untreated Winnie CMG. Remarkably, among the DSS-treated groups, the group receiving WT CMG had the lowest colitis score (Figure 8D and E). The subscores are reported in Figure A5. The most parsimonious interpretation of this experiment is that CMG from the less colitic animals was associated with less severe inflammation from DSS.
Discussion
Our experiments examined the effects of antibiotic administration on the microbiome and inflammatory phenotype of Winnie mice, a preclinical animal model of UC. Although the 5 antibiotics (AB5) did not eliminate gut bacteria, the antibiotic treatment had profound effects on the microbiome and host gut physiology. Typically, spontaneous colitis is apparent in Winnie mice by ∼4 weeks of age and progresses to maximum severity by 12 weeks. Here, we found that a 10-day regimen of the AB5 cocktail, given early in disease development, significantly improved disease activity and histological inflammation in Winnie mice. Furthermore, the improvements induced by this short course of antibiotic treatment were sustained over 8 weeks in Winnie mice housed individually under specific-pathogen-free conditions. At 12 weeks of age, most Winnie have diarrhea, rectal bleeding, and rectal prolapse, but the AB5-treated mice remained largely asymptomatic and histologically free of disease when housed in separate cages.
We also found that when cecal microbial slurries from Winnie mice were gavaged into WT mice, this appeared to worsen inflammation in the model of acute DSS colitis compared with mice gavaged with either cecal microbial slurries prepared from dummy-treated WT mice or prepared from AB5-treated Winnie mice. (However, this experiment had limitations. It lacked an AB5-treated WT mice control, and changes although internally consistent were not statistically significant. Even an antibiotic effect was not completely excluded because nonabsorbable antibiotic may still have been present in the cecal contents.)
Taken together despite limitations, the results suggest that the AB5 treatment shifted the microbiome to a less inflammatory state, which counteracted the genetically driven spontaneous colitis in Winnie mice, and the treatment was also able to ameliorate the induced colitis in the acute DSS model. At 6 weeks of age, both the WT and Winnie stool microbiomes after the 10-day AB5 cocktail were taxonomically distinct from the untreated controls, with profound alterations of the stool and mucosa-associated microbiota in both WT and Winnie mouse genotypes. Strikingly, the AB5 treatment produced dramatic reductions in microbial richness, and with the notable exception of the increased relative abundance of Akkermansia muciniphila in AB5-treated Winnie mice, there was a taxonomic convergence of the microbial communities across both host genotypes, as revealed by the PCoA analysis of the weighted UniFrac distance metrics from both mouse genotypes (Figures 2 and 3).
A muciniphila is well recognized for its mucin degradation and its utilization as a growth substrate.25 Indeed, an increase in the relative abundance of A muciniphila has been reported in a case series of Crohn’s children who had sustained remission with exclusive enteral nutrition35 which should favor the growth of mucin-associated bacteria. With limited exceptions,36 our group25 and others have reported the relative abundance of A muciniphila to be reduced manyfold in active CD37 and even more so in UC where it has been touted as a marker for disease severity.38
In contrast, the ‘dysbiotic’ community in AB5-Winnie mice observed at 10 days (Figure 3) and its anti-inflammatory tenor (Figure 6) included a mucosal bloom (6-log increase) of taxa affiliated with E coli. The Proteobacteria are widely believed to be pathobionts in some colitis models and IBD39,40 although, alternatively, some strains are recognized as commensal or beneficial, for example, E coli Nissle 1917.41 Furthermore, there was also a 2-log reduction in Clostridium spp, affiliated with cluster IV in the AB5-treated mice. In that context, Faecalibacterium prausnitzii is affiliated with Clostridium cluster IV and may represent more than 5% of the bacteria in the colon, making it one of the most common gut bacteria in ‘healthy’ individuals.42 It is generally considered to boost the immune system via a variety of metabolites and other secreted products.43 Reductions in the relative abundances of both F prausnitzii and other members of Clostridium cluster IV (and XIVa) have been associated with CD. However, in this study, colitis was abrogated despite the substantial (2-log) reductions in the relative and total abundance of taxa affiliated with Clostridium clusters IV and XIVa in both the AB5-treated WT and Winnie mice. Interestingly, similar effects with respect to reduced microbial richness and reduced abundance of F prausnitzii have been reported in patients with CD treated with exclusive enteral nutrition therapy.44,45
Rather than microbial taxonomy, our data, given the effects seen with fecal waters in our reporter assays, suggest that microbial-derived secreted factors, likely secondary metabolites, determine the efficacy of the AB5 intervention. In gross terms, the AB5 treatment over 10 days produced alterations in taxonomy not unlike those commonly described as the “dysbiosis” that has been called a hallmark of IBD. However, this “dysbiotic” community from the AB5-treated Winnie mice was functionally silent from an inflammatory context or immunologically suppressive. This functional quiescence was not directly attributable to the AB5 cocktail because the cocktail per se did not directly influence the NF-κB tone in the in vitro NF-κB reporter system. Fecal cell-free supernatants from Winnie mice increased NF-κB activation, whereas those from the AB5-treated Winnie mice reduced NF-κB activation (Figure 6). These functional in vitro findings are consistent with the in vivo amelioration of symptoms and histological measures of colitis severity in the AB5-treated Winnie mice.
Gut homeostasis is underpinned by an intact mucosal barrier and regulated production of antimicrobial molecules.46 Although we did not assess the microbial metabolomes in this study, the results of the in vitro experiment suggest that uncharacterized non-lipopolysaccharide components of the microbial fecal water directly modulate the inflammatory tone of the colonic epithelium. Given the normalization of cecal weight in Winnie by 8 weeks after AB5 treatment and the changing taxonomy, it is deduced that the microbial-mucosal interaction evolved in the absence of AB5 pressure. It is possible that the evolving microbiome (and its metabolome) would have eventually resulted in a relapse in colitis in the AB5-Winnie animals if they had been permitted to live longer, or alternatively, there was inertia for functional change in the microbial metabolome given the reliable supply of the endogenous mucus substrate in the bacterial mucosal niche and the absence of an inflamed mucosa in the Winnie mice previously treated with the AB5 cocktail.
Although the once daily AB5 gavage cocktail for 10 days shifted the structure of the microbiome and reduced its diversity, similarly in both WT and Winnie mice, it was remarkable that while AB5 appreciably reduced total bacteria as measured by bacteria-domain 16S rRNA and culture methods in WT, AB5 did not reduce total bacteria in Winnie. (There was a small 1.8-fold reduction in facultative aerobes in Winnie at day 10). The reason for this is not known, although Winnie mice are different especially with regard to mucus. Interestingly, it was recently reported in a careful study comparing antibiotic protocols in murine models that once daily vs twice daily antibiotic gavage predisposed to reduced bacterial depletion efficiency. The limited decrease in fecal bacterial densities correlated with the hyperproliferation of specific Gammaproteobacteria,47 as we found to occur in both our WT and Winnie AB5-treated mice.
There remains clinical interest in antibiotic treatment for acute severe UC requiring hospitalization, which is a particularly difficult medical problem because of the acute morbidity and the frequent necessity for colectomy in the longer term. It was therefore disappointing that although the day-5 outcome was reported to be improved in hospitalized pediatric patients in the treatment arm of a randomized single blinded quadruple intravenous antibiotic trial in Canada, 3 antibiotic-treated patients had colectomies at 12 months vs 2 in the control arm.48
FMTs, which are believed to act through a direct effect on colonic inflammation by the FMT’s microbiota and prebiotic material, are more promising clinically than antibiotic interventions. In addition to the well-established benefit of FMT for C difficile colitis, there are randomized controlled trials that support the use of FMT for UC.21,49
A major clinical goal is to ensure a reliable and durable response to FMT, but determining the durability of a clinical response after FMT and any associated factors that influence reliability and sustainability has proved challenging. This is especially so given the generally relapsing-remitting natural history of UC, in contrast to Winnie mice, which uniformly develop a progressive proximal-to-distal colitis. The gavage results of our DSS-induced colitis experiment, where we showed improvement more likely with WT and AB5 fecal gavages, are consistent with the concept of a “super” donor for FMT,50 and so add some experimental support for FMT donor selection in the clinic. It has been suggested that dietary therapy after FMT could reduce the likelihood of relapse of UC.51 In the context of the current SARS-2 pandemic, it seems reasonable to extrapolate from our experimental results that clinical relapse in UC after FMT should be less likely if patients pay careful attention to social distancing and hand hygiene to prevent feco-oral spread. Alternatively, a sustained clinical response could be ensured by regular prescribing of FMT or, preferably, regular prescribing of a consortium of defined commensal bacteria52 in a capsule. This could be a more palatable microbial treatment and management to prevent relapse or to induce and maintain remission.
Acknowledgments
Authors' Contributions:
Mark Morrison, Jakob Begun, and Timothy H. Florin conceived the overall project and experimental plan, which was performed in the laboratory headed by Jakob Begun; Ramya Movva, Nida Murtaza, Rabina Giri, Chin Wen Png, and Saleh Alabbas contributed to experimental work; Julie Davies, Iulia Oancea, and Páraic Ó'Cuív contributed with analysis; Ramya Movva and Nida Murtaza wrote the sections of the first draft; Timothy H. Florin wrote the final drafts and is overall responsible with input from Mark Morrison and Jakob Begun.
Footnotes
Conflicts of Interest: The authors disclose no conflicts.
Funding: The authors report no funding.
Ethical Statement: The corresponding author, on behalf of all authors, jointly and severally, certifies that their institution has approved the protocol for any investigation involving humans or animals and that all experimentation was conducted in conformity with ethical and humane principles of research.
Data Transparency Statement: Data, analytic methods, and study materials will be available on request to interested researchers.
Material associated with this article can be found in the online version at https://doi.org/10.1016/j.gastha.2021.12.008.
Supplementary Materials
References
- 1.Xavier R.J., Podolsky D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 2.Ananthakrishnan A.N., Bernstein C.N., Iliopoulos D., et al. Environmental triggers in IBD: a review of progress and evidence. Review. Nat Rev Gastroenterol Hepatol. 2018;15:39–49. doi: 10.1038/nrgastro.2017.136. [DOI] [PubMed] [Google Scholar]
- 3.Batura V., Muise A.M. Very early onset IBD: novel genetic aetiologies. Review. Curr Opin Allergy Clin Immunol. 2018;18:470–480. doi: 10.1097/ACI.0000000000000486. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein C. Treatment of IBD: where we are and where we are going. Am J Gastroenterol. 2015;110:114–126. doi: 10.1038/ajg.2014.357. [DOI] [PubMed] [Google Scholar]
- 5.Egan C., Doherty G.A. Why do we need to improve monitoring of patients with inflammatory bowel disease (IBD) on biologic treatment? Expert Opin Biol Ther. 2019;19:907–918. doi: 10.1080/14712598.2019.1615050. [DOI] [PubMed] [Google Scholar]
- 6.Sheng Y.H., Giri R., Davies J., et al. A nucleotide analog prevents colitis-associated cancer via beta-catenin independently of inflammation and autophagy. Cell Mol Gastroenterol Hepatol. 2021;11:33–53. doi: 10.1016/j.jcmgh.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colombel J.F., D'Haens G., Lee W.J., et al. Outcomes and strategies to support a treat-to-target approach in inflammatory bowel disease: a systematic review. J Crohns Colitis. 2020;14:254–266. doi: 10.1093/ecco-jcc/jjz131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutgeerts P., Goboes K., Peeters M., et al. Effect of faecal stream diversion on recurrence of Crohn's disease in the neoterminal ileum. Lancet. 1991;338:771–774. doi: 10.1016/0140-6736(91)90663-a. [DOI] [PubMed] [Google Scholar]
- 9.Tominaga K., Kamimura K., Takahashi K., et al. Diversion colitis and pouchitis: a mini-review. World J Gastroenterol. 2018;24:1734–1747. doi: 10.3748/wjg.v24.i16.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wohlgemuth S., Haller D., Blaut M., et al. Reduced microbial diversity and high numbers of one single Escherichia coli strain in the intestine of colitic mice. Environ Microbiol. 2009;11:1562–1571. doi: 10.1111/j.1462-2920.2009.01883.x. [DOI] [PubMed] [Google Scholar]
- 11.Dalal S.R., Chang E.B. The microbial basis of inflammatory bowel diseases. J Clin Invest. 2014;124:4190–4196. doi: 10.1172/JCI72330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pascal V., Pozuelo M., Borruel N., et al. A microbial signature for Crohn's disease. Gut. 2017;66:813–822. doi: 10.1136/gutjnl-2016-313235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gionchetti P., Califiore A., Riso D., et al. The role of antibiotics and probiotics in pouchitis. Ann Gastroenterol. 2012;25:100–105. [PMC free article] [PubMed] [Google Scholar]
- 14.Rutgeerts P. Review article: recurrence of Crohn's disease after surgery - the need for treatment of new lesions. Aliment Pharmacol Ther. 2006;24 Suppl 3:29–32. doi: 10.1111/j.1365-2036.2006.03056.x. [DOI] [PubMed] [Google Scholar]
- 15.Townsend C.M., Parker C.E., MacDonald J.K., et al. Antibiotics for induction and maintenance of remission in Crohn's disease. Cochrane Database Syst Rev. 2019;2:CD012730. doi: 10.1002/14651858.CD012730.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abraham B., Quigley E.M.M. Antibiotics and probiotics in inflammatory bowel disease: when to use them? Frontline Gastroenterol. 2020;11:62–69. doi: 10.1136/flgastro-2018-101057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selby W., Pavli P., Crotty B., et al. Two-year combination antibiotic therapy with clarithromycin, rifabutin and clofazimine for Crohn's disease. Gastroenterology. 2007;132:2313–2319. doi: 10.1053/j.gastro.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 18.Present D.H. Ciprofloxacin as a treatment for ulcerative colitis—not yet. Gastroenterology. 1998;115:1289–1291. doi: 10.1016/s0016-5085(98)70104-0. [DOI] [PubMed] [Google Scholar]
- 19.Costello S.P., Conlon M.A., Vuaran M.S., et al. Faecal microbiota transplant for recurrent Clostridium difficile infection using long-term frozen stool is effective: clinical efficacy and bacterial viability data. Aliment Pharmacol Ther. 2015;42:1011–1018. doi: 10.1111/apt.13366. [DOI] [PubMed] [Google Scholar]
- 20.Cammarota G., Ianiro G., Tilg H., et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569–580. doi: 10.1136/gutjnl-2016-313017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costello S.P., Hughes P.A., Waters O., et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA. 2019;321:156–164. doi: 10.1001/jama.2018.20046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heazlewood C.K., Cook M.C., Eri R., et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008;5:e54. doi: 10.1371/journal.pmed.0050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eri R.D., Adams R.J., Tran T.V., et al. An intestinal epithelial defect conferring ER stress results in inflammation involving both innate and adaptive immunity. Mucosal Immunol. 2011;4:354–364. doi: 10.1038/mi.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rakoff-Nahoum S., Paglino J., Eslami-Varzaneh F., et al. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Png C.W., Simms L.A., Gilshenan K.S., et al. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am J Gastroenterol. 2010;105:2420–2428. doi: 10.1038/ajg.2010.281. [DOI] [PubMed] [Google Scholar]
- 26.Pelzer E.S., Willner D., Buttini M., et al. A role for the endometrial microbiome in dysfunctional menstrual bleeding. Antonie Van Leeuwenhoek. 2018;111:933–943. doi: 10.1007/s10482-017-0992-6. [DOI] [PubMed] [Google Scholar]
- 27.DeSantis T.Z., Hugenholtz P., Larsen N., et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mar J.S., LaMere B.J., Lin D.L., et al. Disease severity and immune activity relate to distinct interkingdom gut microbiome states in ethnically distinct ulcerative colitis patients. mBio. 2016;7 doi: 10.1128/mBio.01072-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O Cuiv P., de Wouters T., Giri R., et al. The gut bacterium and pathobiont Bacteroides vulgatus activates NF-kappaB in a human gut epithelial cell line in a strain and growth phase dependent manner. Anaerobe. 2017;47:209–217. doi: 10.1016/j.anaerobe.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Wang R., Moniruzzaman M., Wong K., et al. Gut microbiota shape the inflammatory response in mice with an epithelial defect. Gut Microbes. 2021;13:1–18. doi: 10.1080/19490976.2021.1887720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson G.R., Trexler P.C. Gastrointestinal structure and function in germ-free or gnotobiotic animals. Gut. 1971;12:230–235. doi: 10.1136/gut.12.3.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kennedy E.A., King K.Y., Baldridge M.T. Mouse microbiota models: comparing germ-free mice and antibiotics treatment as tools for modifying gut bacteria. Front Physiol. 2018;9:1534. doi: 10.3389/fphys.2018.01534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rogler G., Brand K., Vogl D., et al. Nuclear factor κB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 1998;115:357–369. doi: 10.1016/s0016-5085(98)70202-1. [DOI] [PubMed] [Google Scholar]
- 34.Wullaert A., Bonnet M.C., Pasparakis M. NF-kappaB in the regulation of epithelial homeostasis and inflammation. Cell Res. 2011;21:146–158. doi: 10.1038/cr.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunn K.A., Moore-Connors J.M., MacIntyre B., et al. Early changes in microbial community structure are associated with sustained remission following nutritional treatment of pediatric Crohn's disease. Inflamm Bowel Dis. 2016;22:2853–2862. doi: 10.1097/MIB.0000000000000956. [DOI] [PubMed] [Google Scholar]
- 36.Lopez-Siles M., Enrich-Capo N., Aldeguer X., et al. Alterations in the abundance and co-occurrence of Akkermansia muciniphila and Faecalibacterium prausnitzii in the colonic mucosa of inflammatory bowel disease subjects. Front Cell Infect Microbiol. 2018;8:281. doi: 10.3389/fcimb.2018.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajilic-Stojanovic M., Shanahan F., Guarner F., et al. Phylogenetic analysis of dysbiosis in ulcerative colitis during remission. Inflamm Bowel Dis. 2013;19:481–488. doi: 10.1097/MIB.0b013e31827fec6d. [DOI] [PubMed] [Google Scholar]
- 38.Pittayanon R., Lau J.T., Leontiadis G.I., et al. Differences in gut microbiota in patients with vs without inflammatory bowel diseases: a systematic review. Gastroenterology. 2020;158:930–946.e1. doi: 10.1053/j.gastro.2019.11.294. [DOI] [PubMed] [Google Scholar]
- 39.Mukhopadhya I., Hansen R., El-Omar E.M., et al. IBD-what role do Proteobacteria play? Nat Rev Gastroenterol Hepatol. 2012;9:219–230. doi: 10.1038/nrgastro.2012.14. [DOI] [PubMed] [Google Scholar]
- 40.Palmela C., Chevarin C., Xu Z., et al. Adherent-invasive Escherichia coli in inflammatory bowel disease. Gut. 2018;67:574–587. doi: 10.1136/gutjnl-2017-314903. [DOI] [PubMed] [Google Scholar]
- 41.Kruis W., Fric P., Pokrotnieks J., et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53:1617–1623. doi: 10.1136/gut.2003.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miquel S., Martin R., Rossi O., et al. Faecalibacterium prausnitzii and human intestinal health. Curr Opin Microbiol. 2013;16:255–261. doi: 10.1016/j.mib.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 43.Miquel S., Leclerc M., Martin R., et al. Identification of metabolic signatures linked to anti-inflammatory effects of Faecalibacterium prausnitzii. mBio. 2015;6:e00300–e00315. doi: 10.1128/mBio.00300-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jia W., Whitehead R.N., Griffiths L., et al. Is the abundance of Faecalibacterium prausnitzii relevant to Crohn's disease? FEMS Microbiol Lett. 2010;310:138–144. doi: 10.1111/j.1574-6968.2010.02057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerasimidis K., Bertz M., Hanske L., et al. Decline in presumptively protective gut bacterial species and metabolites are paradoxically associated with disease improvement in pediatric Crohn's disease during enteral nutrition. Inflamm Bowel Dis. 2014;20:861–871. doi: 10.1097/MIB.0000000000000023. [DOI] [PubMed] [Google Scholar]
- 46.Elshaer D.B., Begun J. The role of barrier function, autophagy, and cytokines in maintaining intestinal homeostasis. J Semin Cell Dev Biol. 2017;61:51–59. doi: 10.1016/j.semcdb.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 47.Tirelle P., Breton J., Riou G., et al. Comparison of different modes of antibiotic delivery on gut microbiota depletion efficiency and body composition in mouse. BMC Microbiol. 2020;20:340. doi: 10.1186/s12866-020-02018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turner D., Bishai J., Reshef L., et al. Antibiotic cocktail for pediatric acute severe colitis and the microbiome: the PRASCO randomized controlled trial. Inflamm Bowel Dis. 2020;26:1733–1742. doi: 10.1093/ibd/izz298. [DOI] [PubMed] [Google Scholar]
- 49.Moayyedi P., Surette M.G., Kim P.T., et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. 2015;149:102–109.e6. doi: 10.1053/j.gastro.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 50.Wilson B.C., Vatanen T., Jayasinghe T.N., et al. Strain engraftment competition and functional augmentation in a multi-donor fecal microbiota transplantation trial for obesity. Microbiome. 2021;9:107. doi: 10.1186/s40168-021-01060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Costello S.P., Day A., Yao C.K., et al. Faecal microbiota transplantation (FMT) with dietary therapy for acute severe ulcerative colitis. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2019-233135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petrof E.O., Khoruts A. From stool transplants to next-generation microbiota therapeutics. Gastroenterology. 2014;146:1573–1582. doi: 10.1053/j.gastro.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.