Abstract
Background
Supersaturated oxygen (SSO2) delivered into the left anterior descending coronary artery after percutaneous coronary intervention (PCI) for anterior ST-segment elevation myocardial infarction (STEMI) has been shown to reduce infarct size, but its effects on microvascular obstruction (MVO) are unknown. The aim of this study was to compare MVO in patients with anterior STEMI treated with SSO2 after successful primary PCI from 2 studies (the optimized SSO2 pilot and IC-HOT) with similar patients from 7 randomized trials who underwent primary PCI without SSO2 treatment.
Methods
A total of 874 patients with anterior STEMI who underwent MVO assessment using cardiac magnetic resonance imaging within 10 days after primary PCI were included, of whom 90 patients (10.3%) were treated with SSO2. The primary end point was the extent of MVO as a continuous measure in a weighted multivariable model. The secondary end point was the presence of MVO.
Results
SSO2 therapy was independently associated with a lower extent of MVO compared with no SSO2 therapy (coefficient, −1.35; 95% CI, −2.58 to −0.11; P = .03). SSO2 therapy was also associated with a borderline lower risk of any MVO (adjusted odds ratio, 0.56; 95% CI, 0.31-1.00; P = .051).
Conclusions
In the present individual patient data pooled analysis from 9 studies, SSO2 therapy was associated with less MVO after successful primary PCI for anterior STEMI.
Keywords: cardiac magnetic resonance imaging, microvascular obstruction, pooled analysis ST-elevation myocardial infarction, supersaturated oxygen therapy
Introduction
Although early reperfusion by primary percutaneous coronary intervention (PCI) has improved the prognosis of patients with ST-segment elevation myocardial infarction (STEMI),1, 2, 3, 4, 5, 6 approximately 20% of patients with STEMI still develop heart failure (HF).7 Infarct size and microvascular obstruction (MVO) are both strong predictors of HF and death after STEMI.8,9
The intracoronary infusion of supersaturated oxygen (SSO2) in the left anterior descending (LAD) coronary artery after successful primary PCI for anterior STEMI significantly reduced infarct size in the randomized Acute Myocardial Infarction With Hyperoxemic Therapy II (AMIHOT II) trial (NCT00175058) but was associated with a numerical increase in-stent thrombosis events, possibly related to the 90-minute dwell time of the infusion catheter in the LAD.10 The delivery system was modified so that SSO2 was delivered to the origin of the left main coronary artery for 60 minutes via a diagnostic catheter after successful primary PCI (optimized SSO2 delivery). Following the optimized SSO2 pilot study,11 the safety of optimized SSO2 delivery was demonstrated in the prospective single-arm Evaluation of Intracoronary Hyperoxemic Oxygen Therapy in Anterior Acute Myocardial Infarction Patients (IC-HOT) study (NCT02603835), leading to U.S. Food and Drug Administration approval of SSO2 therapy for patients with anterior STEMI undergoing primary PCI within 6 hours of symptom onset. In this study, infarct size was consistent with prior studies in which SSO2 was delivered by an intracoronary infusion catheter,10,11 and the 1-year clinical outcomes were improved compared with those in other studies of similar patients in which SSO2 was not administered.12 However, although SSO2 therapy has been shown to reduce endothelial cell swelling and induce capillary vasodilation in experimental animal models,13, 14, 15 it has not been assessed whether MVO after primary PCI in patients with STEMI treated with SSO2 is reduced.
We therefore sought to examine the presence and extent of MVO in patients treated with optimized SSO2 therapy compared with those in patients not treated with SSO2 after successful primary PCI for anterior STEMI.
Methods
Study design, study population, and definitions
The SSO2 treatment group consisted of patients from the optimized SSO2 and IC-HOT studies.11,16 The study designs of the optimized SSO2 pilot study11 and the IC-HOT trial (NCT02603835)16 have been previously published. In brief, 20 patients with anterior STEMI in the optimized SSO2 pilot study and 100 patients with anterior STEMI in the IC-HOT (single arm) study who underwent successful primary PCI (postprocedural Thrombolysis in Myocardial Infarction [TIMI] 2 or 3 flow) of the proximal or mid LAD within 6 hours of symptom onset) and who did not have cardiogenic shock were treated with 60 minutes of SSO2 delivered through a 5F diagnostic catheter seated in the origin of the left main coronary artery. Cardiac magnetic resonance (CMR) imaging was performed between 2 and 7 days after PCI to assess infarct size. MVO was also assessed by analyzing late gadolinium enhancement imaging at a core laboratory (Cardiovascular Research Foundation [CRF]) as previously described.9 MVO was denoted by the lack of gadolinium enhancement within the hyperenhanced infarct zone and was expressed as a percentage of the total left ventricular (LV) myocardial mass.
MVO results from these 2 studies were compared to the MVO findings similarly determined from patients with anterior STEMI who underwent LAD PCI without SSO2 from 7 trials that have been previously described.17, 18, 19, 20, 21, 22, 23 To ensure that the comparator population was similar to the SSO2 treatment group, patients were included in this analysis if they fulfilled the following criteria: (1) age 80 years or younger; (2) time from symptom onset to device <6 hours; (3) CMR assessment within 10 days with available data on MVO; (4) Killip class <4 (ie, absence of cardiogenic shock) at presentation; and (5) post-PCI TIMI flow grade 2 or 3.
All patients signed informed written consent for each study. Data from these 9 studies (2 SSO2 treatment studies, and 7 SSO2 untreated control studies) were pooled into a common database at CRF. ZOLL Circulation, Inc, provided funding to CRF for data analysis. The outcomes were interpreted by the authors and the manuscript prepared independent of the study sponsor.
End points
The primary end point of interest for the present analysis was the extent of MVO assessed by CMR imaging, measured as a continuous variable, read by a core laboratory. The secondary end point was the presence of any MVO.
Statistical analysis
Categorical variables are presented as percentages and were compared with the χ2 test. Continuous variables are presented as mean ± SD and median with IQR and were compared with the Wilcoxon rank-sum test. The association of SSO2 and MVO was calculated using linear regression for the end point of the extent of MVO and logistic regression for the end point of the presence of MVO, each adjusted for a prespecified covariate set known to affect MVO, infarct size, and prognosis after anterior STEMI consisting of age, sex, diabetes, hypertension, current smoking, time from symptom onset to device, and baseline TIMI flow grade ≤1 vs ≥2. All statistical analyses were performed with SAS v9.4 (SAS Institute). A 2-sided P value of <.05 was considered statistically significant.
Results
Baseline characteristics
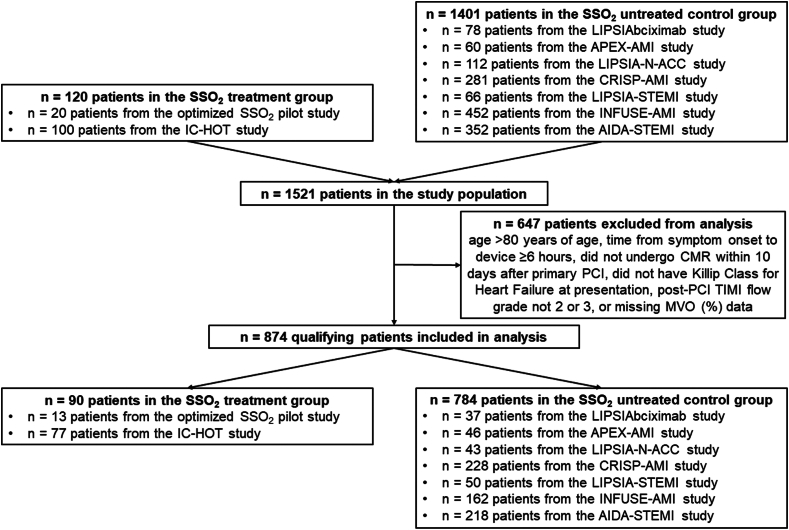
A total of 874 qualifying patients with anterior STEMI who underwent CMR within 10 days after primary PCI and in whom data on MVO were available were included; 90 received SSO2 and 784 did not (Figure 1). Features of the individual studies are shown in Supplemental Tables S1 and S2. As shown in Table 1, patients in the SSO2-treated group were more likely to be smokers and have diabetes and had shorter duration from the onset of chest pain to device time compared with the control group. There were no differences between the groups in sex, age, hypertension, baseline TIMI flow grade, or final TIMI flow grade.
Figure 1.
CONSORT diagram. CMR, cardiac magnetic resonance; PCI, percutaneous coronary intervention; SSO2, supersaturated oxygen; TIMI, Thrombolysis in Myocardial Infarction.
Table 1.
Baseline clinical, angiographic, and procedural characteristics in patients treated with and without supersaturated oxygen.
| Characteristic | SSO2-treated group (n = 90) | Control group (n = 784) | Unadjusted P |
|---|---|---|---|
| Male sex | 76/90 (84.4) | 631/784 (80.5) | .37 |
| Age, y | 58.0 [50.0, 65.0] | 58.8 [50.0, 67.1] | .83 |
| Current smoker | 34/58 (58.6) | 332/767 (43.3) | .02 |
| Hypertension | 47/90 (52.2) | 373/783 (47.6) | .41 |
| Diabetes | 22/90 (24.4) | 120/783 (15.3) | .03 |
| Symptom onset to device time, h | 2.6 [1.8, 3.1] | 2.8 [2.0, 3.9] | .01 |
| Baseline TIMI flow grade 0/1 | 53/90 (58.9) | 535/778 (68.8) | .058 |
| Final TIMI flow grade 3a | 88/90 (97.8) | 740/784 (94.4) | .17 |
Data presented as n/N (%) or median [Q1, Q3], where applicable.
TIMI, Thrombolysis in Myocardial Infarction; SSO2, supersaturated oxygen.
The remainder of patients had TIMI flow grade 2.
Unadjusted MVO results
As shown in Table 2, the median extent of MVO was 0.2% [0.0%-2.1%] of the LV mass in SSO2-treated patients (mean, 1.5% ± 2.3%) and median 0.8% [0.0%-3.8%] of the LV mass in the control patients (mean, 2.9% ± 4.9%; P = .052). Any MVO was present in 48 of 90 patients (53.3%) treated with SSO2 and in 459 of 784 (58.5%) patients not treated with SSO2 (P = .35). Infarct size was also lower in SSO2 treatment arm (Table 2). Further details of the MVO results in the individual studies are presented in Supplemental Tables S3 and S4.
Table 2.
Microvascular obstruction in patients treated with and without supersaturated oxygen.
| Characteristic | SSO2-treated group (n = 90) | Control group (n = 784) | Unadjusted P |
|---|---|---|---|
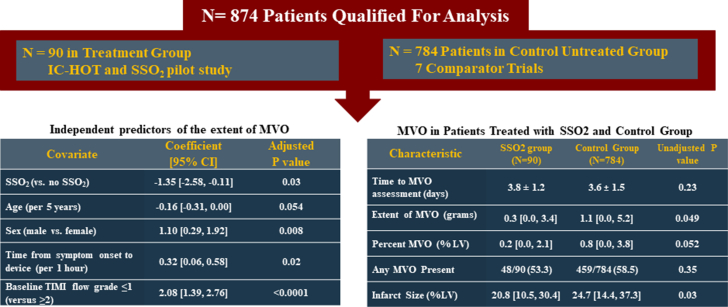
| Time to MVO assessment, d | 3.8 ± 1.2 | 3.6 ± 1.5 | .23 |
| Extent of MVO, g | 0.3 [0.0, 3.4] | 1.1 [0.0, 5.2] | .049 |
| Percent MVO, % LV | 0.2 [0.0, 2.1] | 0.8 [0.0, 3.8] | .052 |
| Any MVO present | 48/90 (53.3) | 459/784 (58.5) | .35 |
| Infarct size, % LV | 20.8 [10.5, 30.4] | 24.7 [14.4, 37.3] | .03 |
Data presented as n/N (%), mean ± SD, or median [Q1, Q3], where applicable.
LV, left ventricular; MVO, microvascular obstruction; SSO2, supersaturated oxygen.
Adjusted MVO results
After multivariable adjustment, SSO2 therapy was an independent predictor of the extent of MVO (coefficient, −1.35; 95% CI, −2.58 to −0.11; P = .03) (Table 3 and Central Illustration). SSO2 was a borderline predictor of freedom from any MVO, although the difference did not reach statistical significance (adjusted odds ratio, 0.56; 95% CI, 0.31-1.00; P = .051) (Table 4). Other predictors of the extent and presence of MVO are summarized in Tables 3 and 4. Propensity-adjusted multivariable analysis resulted in comparable findings (Supplemental Tables S5 and S6).
Table 3.
Independent predictors of the extent of microvascular obstruction.
| Covariate | Coefficient (95% CI) | Adjusted P |
|---|---|---|
| SSO2 (vs no SSO2) | −1.35 (−2.58 to −0.11) | .03 |
| Age (per 5 y) | −0.16 (−0.31 to 0.00) | .054 |
| Sex (male vs female) | 1.10 (0.29-1.92) | .008 |
| Diabetes | 1.36 (0.47-2.25) | .003 |
| Hypertension | −0.36 (−1.02 to 0.31) | .29 |
| Current smoking | −0.62 (−1.29 to 0.06) | .07 |
| Time from symptom onset to device (per 1 h) | 0.32 (0.06-0.58) | .02 |
| Baseline TIMI flow grade ≤1 (vs ≥2) | 2.08 (1.39-2.76) | <.0001 |
Estimates and 95% CI were estimated using multiple linear models.
SSO2, supersaturated oxygen; TIMI, Thrombolysis in Myocardial Infarction.
Central Illustration.
Microvascular obstruction in patients with anterior STEMI treated with supersaturated oxygen pooled analysis. Data are presented as mean ± SD or median [Q1, Q3], where applicable. Estimates and 95% CI are estimated by multiple linear models. LV, left ventricular; MVO, microvascular obstruction; SSO2, supersaturated oxygen; STEMI, ST-elevation myocardial infarction; TIMI, Thrombolysis in Myocardial Infarction.
Table 4.
Independent predictors of the presence of microvascular obstruction.
| Covariate | Adjusted OR (95% CI) | Adjusted P |
|---|---|---|
| SSO2 (vs no SSO2) | 0.56 (0.31-1.00) | .051 |
| Age (per 5 y) | 1.09 (1.01-1.18) | .02 |
| Sex (male vs female) | 1.35 (0.92-1.97) | .12 |
| Diabetes | 1.59 (1.03-2.45) | .03 |
| Hypertension | 0.98 (0.72-1.33) | .88 |
| Current smoking | 1.07 (0.78-1.47) | .66 |
| Time from symptom onset to device (per 1 h) | 1.03 (0.91-1.16) | .63 |
| Baseline TIMI flow grade ≤1 (vs ≥2) | 3.45 (2.51-4.74) | <.0001 |
Odds ratios and 95% CI were estimated using multiple logistic models.
OR, odds ratio; SSO2, supersaturated oxygen; TIMI, Thrombolysis in Myocardial Infarction.
Discussion
To our knowledge, this is the first study to examine whether post-PCI SSO2 infusion, a therapy known to reduce infarct size after anterior STEMI as shown in the AMIHOT II trial,10 is also associated with reduced MVO. From this nonrandomized multivariable-adjusted comparison of the outcomes of SSO2 therapy in 2 studies vs control patients from 7 studies in which all patients had anterior STEMI treated with primary PCI with MVO assessed by late gadolinium enhancement of an early CMR scan postreperfusion, SSO2 therapy was associated with a significant reduction in the extent of MVO and a borderline reduction in the presence of any MVO. These findings likely contribute to the benefit of SSO2 therapy in reducing infarct size.9
Several pharmacologic and mechanical strategies have been utilized to improve microcirculatory function in patients with STEMI to prevent and reduce the extent of MVO, including mechanical circulatory support, intracoronary adenosine, nitroprusside, and intracoronary abciximab.17, 18, 19, 20, 21, 22, 23, 24 However, none of these treatments, except possibly for intracoronary abciximab, have been shown to reduce MVO or improve clinical outcomes. The randomized AMIHOT II trial established the ability of SSO2 to reduce infarct size. However, technitium-99m sestamibi single-photon emission computed tomography and not CMR was used to assess the degree of myonecrosis in that study,10 precluding assessment of MVO. In the nonrandomized IC-HOT study, patients with anterior STEMI treated with SSO2 had a low incidence of MVO after primary PCI as assessed with CMR, although the absence of a comparator group challenged the interpretation of this finding.16 This study demonstrates that the extent of MVO may be reduced with SSO2 after successful primary PCI of the LAD compared with a matched control population, contributing to the reduction in infarct size with this therapy.9 Moreover, patients treated with SSO2 have been shown to have lower rates of mortality and HF hospitalization at 1-year follow-up compared with a matched control population from the INFUSE-AMI trial treated without SSO2.12 These data are consistent with those in prior studies that have shown a strong association among MVO, infarct size, and all-cause mortality and HF hospitalization within 1 year.9,25 Thus, this study extends these prior findings; the lesser extent of MVO with SSO2 therapy after primary PCI in anterior STEMI likely underlies its mechanism in decreasing infarct size, potentially improving the prognosis of high-risk patients with anterior STEMI.
MVO is associated with infarct size,9 a major determinant of freedom from death and HF hospitalization after STEMI.25 However, MVO may also adversely affect prognosis through mechanisms that are independent of infarct size.9,26 Specifically, anterior STEMI patients with large extent of MVO (defined as above the median value of 0.47%) were 3.2 times (hazard ratio, 3.21; 95% CI, 1.60-6.46) more likely to die or have a HF hospitalization within 1 year compared patients with small extent of MVO.9 The pathophysiology behind these adverse events is multifactorial where MVO limits the delivery of endogenous factors involved in postinfarction remodeling and clearing of cellular debris.27,28 MVO has been associated with increased myocardial stiffness and reduced elasticity, impaired remodeling predisposing the heart to HF and lethal ventricular arrhythmias, as well as with more severe myocardial wall thinning over the first several months after myocardial infarction.26,29,30 In patients treated with SSO2 therapy, the observed extent of MVO of 0.2% is low compared with 0.8% seen in the control group in which SSO2 was not administered. Hence, a treatment such as SSO2 that appears to reduce MVO may favorably affect prognosis and clinical long-term outcomes through multiple pathways.
Limitations
This study comprised a nonrandomized comparison of patients from different trials and should be considered hypothesis generating. Despite multivariable adjustments for factors known to affect MVO, infarct size, and prognosis after STEMI, we cannot exclude the presence of unmeasured confounders. For example, data on specific lesion location (eg, proximal LAD vs. mid LAD) were not available from all studies. The present study was limited to STEMI patients with anterior STEMI who presented without cardiogenic shock in whom primary PCI was successful and who survived until CMR. The findings of this study may thus not apply to patients with nonanterior STEMI, those with cardiogenic shock, or in whom PCI was unsuccessful in restoring at least TIMI 2 flow.
Conclusions
The principal finding from this nonrandomized individual patient data pooled analysis from 9 studies is that treatment with SSO2 after successful primary PCI in anterior STEMI was an independent predictor of less MVO as assessed by CMR. As SSO2 has also been demonstrated to reduce infarct size after primary PCI in anterior STEMI, an appropriately powered randomized trial is warranted to definitively determine whether SSO2 treatment improves long-term clinical outcomes in patients with anterior STEMI who undergo primary PCI.
Acknowledgments
Declaration of competing interests
Björn Redfors has received consultant fees from Pfizer and Boehringer Ingelheim. Gregg W. Stone has received speaker honoraria from Medtronic, Pulnovo, Infraredx, Abiomed, Amgen, and Boehringer Ingelheim; has served as a consultant to Abbott, Daiichi Sankyo, Ablative Solutions, CorFlow, Cardiomech, Robocath, Miracor, Vectorious, Apollo Therapeutics, Valfix, TherOx, HeartFlow, Neovasc, Ancora, Elucid Bio, Occlutech, Impulse Dynamics, Adona Medical, Millennia Biopharma, Oxitope, Cardiac Success, and HighLife; and has equity/options from Ancora, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, Valfix, and Xenter. Gregg Stone’s employer, Mount Sinai Hospital, receives research grants from Abbott, Abiomed, BioVentrix, Cardiovascular Systems Inc, Philips, Biosense-Webster, Shockwave Medical, Vascular Dynamics, Pulnovo, and V-wave. Suzanne de Waha has received speaker honoraria from AstraZeneca, Boehringer Ingelheim, and TherOx and has served as a consultant to Pfizer-BMS and TherOx/Zoll Medical. All other coauthors have no relevant disclosures.
Funding sources
ZOLL Circulation sponsored the IC-HOT trial and provided funding to Cardiovascular Research Foundation for statistical support.
Ethics statement and patient consent
The studies in the SSO2-treated group and the control group were conducted according to the relevant ethical guidelines, and all patients provided written informed consent.
Footnotes
To access the supplementary material accompanying this article, visit the online version of the Journal of the Society for Cardiovascular Angiography & Interventions at 10.1016/j.jscai.2024.101356.
Supplementary material
References
- 1.Nabel E.G., Braunwald E. A tale of coronary artery disease and myocardial infarction. N Engl J Med. 2012;366(1):54–63. doi: 10.1056/NEJMra1112570. [DOI] [PubMed] [Google Scholar]
- 2.Harold J.G., Bass T.A., Bashore T.M., et al. ACCF/AHA/SCAI 2013 update of the clinical competence statement on coronary artery interventional procedures: a report of the American College of Cardiology Foundation/American Heart Association/American College of Physicians Task Force on Clinical Competence and Training (writing committee to revise the 2007 clinical competence statement on cardiac interventional procedures) J Am Coll Cardiol. 2013;62(4):357–396. doi: 10.1016/j.jacc.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Ford E.S., Ajani U.A., Croft J.B., et al. Explaining the decrease in U.S. deaths from coronary disease, 1980-2000. N Engl J Med. 2007;356(23):2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 4.Mensah G.A., Wei G.S., Sorlie P.D., et al. Decline in cardiovascular mortality: possible causes and implications. Circ Res. 2017;120(2):366–380. doi: 10.1161/CIRCRESAHA.116.309115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krumholz H.M., Normand S.T., Wang Y. Twenty-year trends in outcomes for older adults with acute myocardial infarction in the United States. JAMA Netw Open. 2019;2(3):e191938. doi: 10.1001/jamanetworkopen.2019.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Granger C.B., Bates E.R., Jollis J.G., et al. Improving care of STEMI in the United States 2008 to 2012. J Am Heart Assoc. 2019;8(1):e008096. doi: 10.1161/JAHA.118.008096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faridi K.F., et al. New heart failure after myocardial infarction (from the National Cardiovascular Data Registries [NCDR] linked with all-payer claims) Am J Cardiol. 2021;151:70–77. doi: 10.1016/j.amjcard.2021.04.019. [DOI] [PubMed] [Google Scholar]
- 8.Wu K.C., Zerhouni E.A., Judd R.M., et al. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 1998;97(8):765–772. doi: 10.1161/01.cir.97.8.765. [DOI] [PubMed] [Google Scholar]
- 9.de Waha S., Patel mR., Granger C.B., et al. Relationship between microvascular obstruction and adverse events following primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: an individual patient data pooled analysis from seven randomized trials. Eur Heart J. 2017;38(47):3502–3510. doi: 10.1093/eurheartj/ehx414. [DOI] [PubMed] [Google Scholar]
- 10.Stone G.W., Martin J.L., de Boer M.J., et al. Effect of supersaturated oxygen delivery on infarct size after percutaneous coronary intervention in acute myocardial infarction. Circ Cardiovasc Interv. 2009;2(5):366–375. doi: 10.1161/CIRCINTERVENTIONS.108.840066. [DOI] [PubMed] [Google Scholar]
- 11.Hanson I.D., David S.W., Dixon S.R., et al. "Optimized" delivery of intracoronary supersaturated oxygen in acute anterior myocardial infarction: a feasibility and safety study. Catheter Cardiovasc Interv. 2015;86(Suppl 1):S51–S57. doi: 10.1002/ccd.25773. [DOI] [PubMed] [Google Scholar]
- 12.Chen S., David S.W., Khan Z.A., et al. One-year outcomes of supersaturated oxygen therapy in acute anterior myocardial infarction: the IC-HOT study. Catheter Cardiovasc Interv. 2021;97(6):1120–1126. doi: 10.1002/ccd.29090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartorelli A.L. Hyperoxemic perfusion for treatment of reperfusion microvascular ischemia in patients with myocardial infarction. Am J Cardiovasc Drugs. 2003;3(4):253–263. doi: 10.2165/00129784-200303040-00004. [DOI] [PubMed] [Google Scholar]
- 14.Spears J.R., Prcevski P., Xu R., et al. Aqueous oxygen attenuation of reperfusion microvascular ischemia in a canine model of myocardial infarction. ASAIO J. 2003;49(6):716–720. doi: 10.1097/01.mat.0000094665.72503.3c. [DOI] [PubMed] [Google Scholar]
- 15.Spears J.R., Prcevski P., Jiang A., Brereton G.J., Vander Heide R. Intracoronary aqueous oxygen perfusion, performed 24 h after the onset of postinfarction reperfusion, experimentally reduces infarct size and improves left ventricular function. Int J Cardiol. 2006;113(3):371–375. doi: 10.1016/j.ijcard.2005.11.099. [DOI] [PubMed] [Google Scholar]
- 16.David S.W., Khan Z.A., Patel N.C., et al. Evaluation of intracoronary hyperoxemic oxygen therapy in acute anterior myocardial infarction: the IC-HOT study. Catheter Cardiovasc Interv. 2019;93(5):882–890. doi: 10.1002/ccd.27905. [DOI] [PubMed] [Google Scholar]
- 17.Thiele H., Schindler K., Friedenberger J., et al. Intracoronary compared with intravenous bolus abciximab application in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention: the randomized Leipzig immediate percutaneous coronary intervention abciximab IV versus IC in ST-elevation myocardial infarction trial. Circulation. 2008;118(1):49–57. doi: 10.1161/CIRCULATIONAHA.107.747642. [DOI] [PubMed] [Google Scholar]
- 18.Patel M.R., Worthley S.G., Stebbins A., et al. Pexelizumab and infarct size in patients with acute myocardial infarction undergoing primary percutaneous coronary Intervention: a delayed enhancement cardiac magnetic resonance substudy from the APEX-AMI trial. J Am Coll Cardiol Img. 2010;3(1):52–60. doi: 10.1016/j.jcmg.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 19.Thiele H., Hildebrand L., Schirdewahn C., et al. Impact of high-dose N-acetylcysteine versus placebo on contrast-induced nephropathy and myocardial reperfusion injury in unselected patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. The LIPSIA-N-ACC (Prospective, Single-Blind, Placebo-Controlled, Randomized Leipzig Immediate PercutaneouS Coronary Intervention Acute Myocardial Infarction N-ACC) Trial. J Am Coll Cardiol. 2010;55(20):2201–2209. doi: 10.1016/j.jacc.2009.08.091. [DOI] [PubMed] [Google Scholar]
- 20.Patel M.R., Smalling R.W., Thiele H., et al. Intra-aortic balloon counterpulsation and infarct size in patients with acute anterior myocardial infarction without shock: the CRISP AMI randomized trial. JAMA. 2011;306(12):1329–1337. doi: 10.1001/jama.2011.1280. [DOI] [PubMed] [Google Scholar]
- 21.Thiele H., Eitel I., Meinberg C., et al. Randomized comparison of pre-hospital-initiated facilitated percutaneous coronary intervention versus primary percutaneous coronary intervention in acute myocardial infarction very early after symptom onset: the LIPSIA-STEMI trial (Leipzig immediate prehospital facilitated angioplasty in ST-segment myocardial infarction) J Am Coll Cardiol Intv. 2011;4(6):605–614. doi: 10.1016/j.jcin.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 22.Stone G.W., Maehara A., Witzenbichler B., et al. Intracoronary abciximab and aspiration thrombectomy in patients with large anterior myocardial infarction: the INFUSE-AMI randomized trial. JAMA. 2012;307(17):1817–1826. doi: 10.1001/jama.2012.421. [DOI] [PubMed] [Google Scholar]
- 23.Eitel I., Wöhrle J., Suenkel H., et al. Intracoronary compared with intravenous bolus abciximab application during primary percutaneous coronary intervention in ST-segment elevation myocardial infarction: cardiac magnetic resonance substudy of the AIDA STEMI trial. J Am Coll Cardiol. 2013;61(13):1447–1454. doi: 10.1016/j.jacc.2013.01.048. [DOI] [PubMed] [Google Scholar]
- 24.Nazir S.A., McCann G.P., Greenwood J.P., et al. Strategies to attenuate micro-vascular obstruction during P-PCI: the randomized reperfusion facilitated by local adjunctive therapy in ST-elevation myocardial infarction trial. Eur Heart J. 2016;37(24):1910–1919. doi: 10.1093/eurheartj/ehw136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stone G.W., et al. Relationship between infarct size and outcomes following primary PCI: patient-level analysis from 10 randomized trials. J Am Coll Cardiol. 2016;67(14):1674–1683. doi: 10.1016/j.jacc.2016.01.069. [DOI] [PubMed] [Google Scholar]
- 26.Baks T., van Geuns R.-J., Biagini E., et al. Effects of primary angioplasty for acute myocardial infarction on early and late infarct size and left ventricular wall characteristics. J Am Coll Cardiol. 2006;47(1):40–44. doi: 10.1016/j.jacc.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Wu K.C. CMR of microvascular obstruction and hemorrhage in myocardial infarction. J Cardiovasc Magn Reson. 2012;14(1):68. doi: 10.1186/1532-429X-14-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sutton M.G., Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101(25):2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 29.Gerber B.L., Rochitte C.E., Melin J.A., et al. Microvascular obstruction and left ventricular remodeling early after acute myocardial infarction. Circulation. 2000;101(23):2734–2741. doi: 10.1161/01.cir.101.23.2734. [DOI] [PubMed] [Google Scholar]
- 30.Bodi V., Gavara J., Lopez-Lereu M.P., et al. Impact of persistent microvascular obstruction late after STEMI on adverse LV remodeling: a CMR study. J Am Coll Cardiol Img. 2023;16(7):919–930. doi: 10.1016/j.jcmg.2023.01.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.