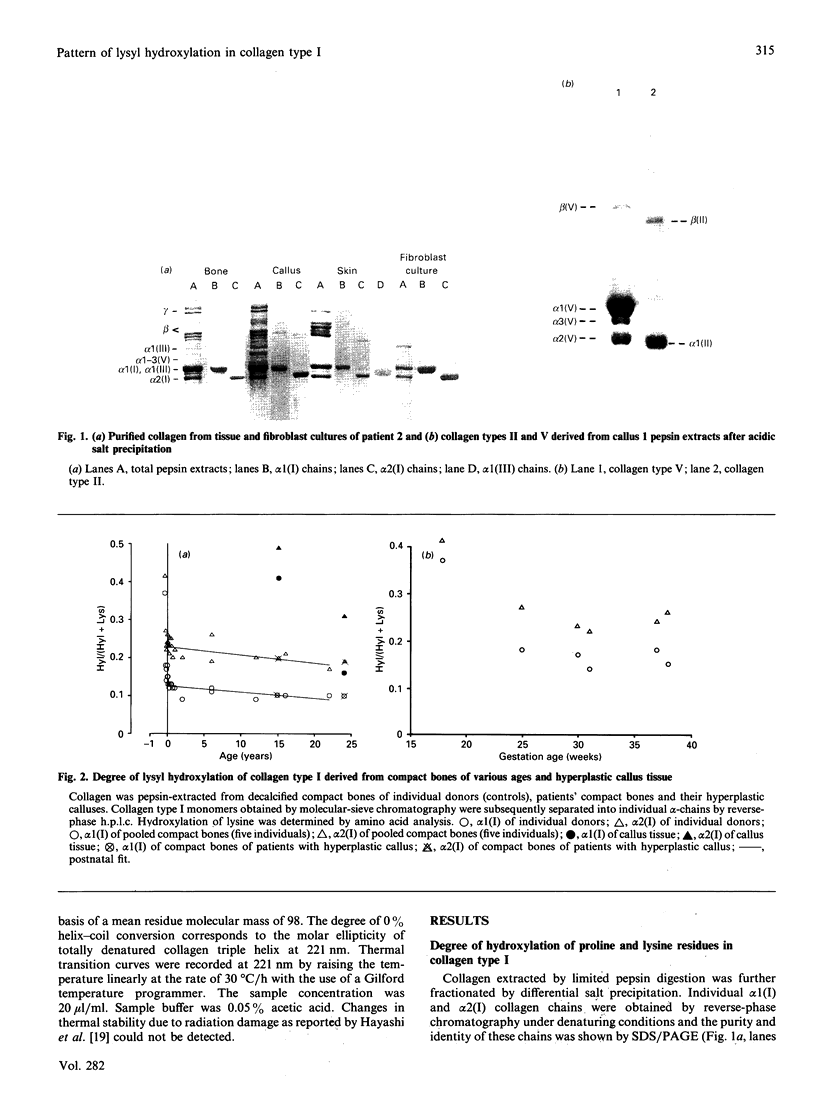
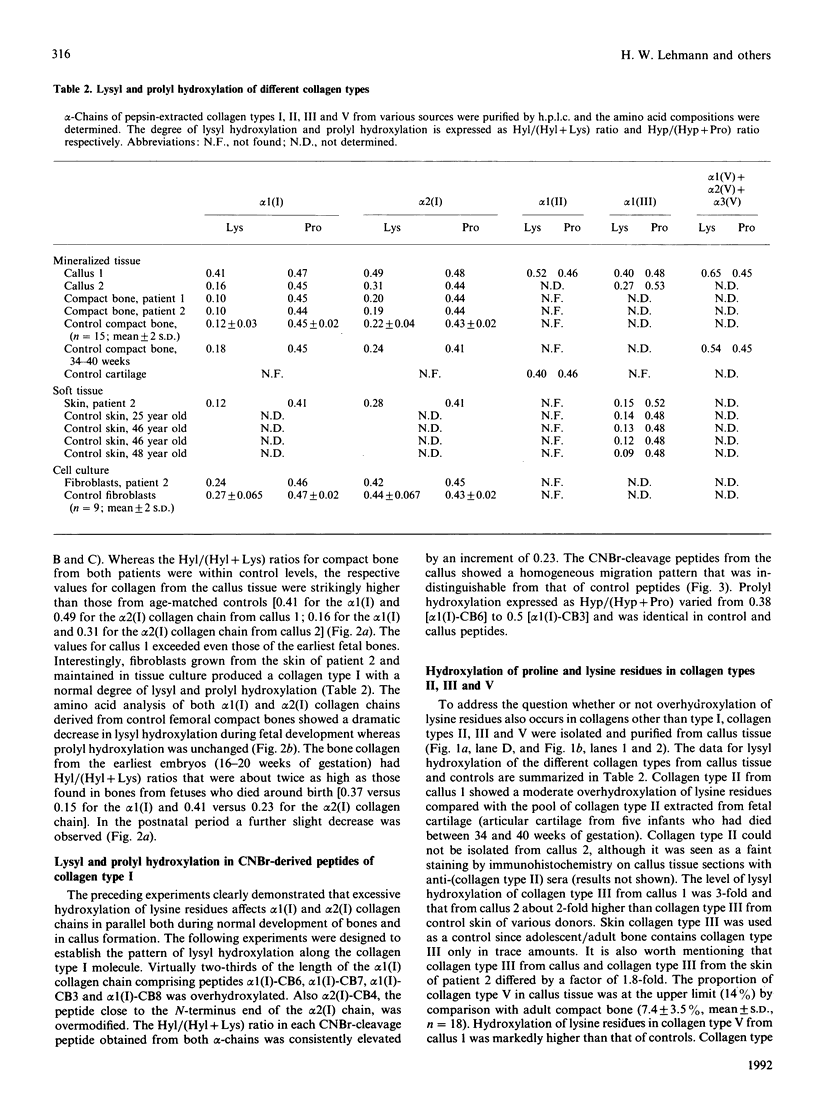
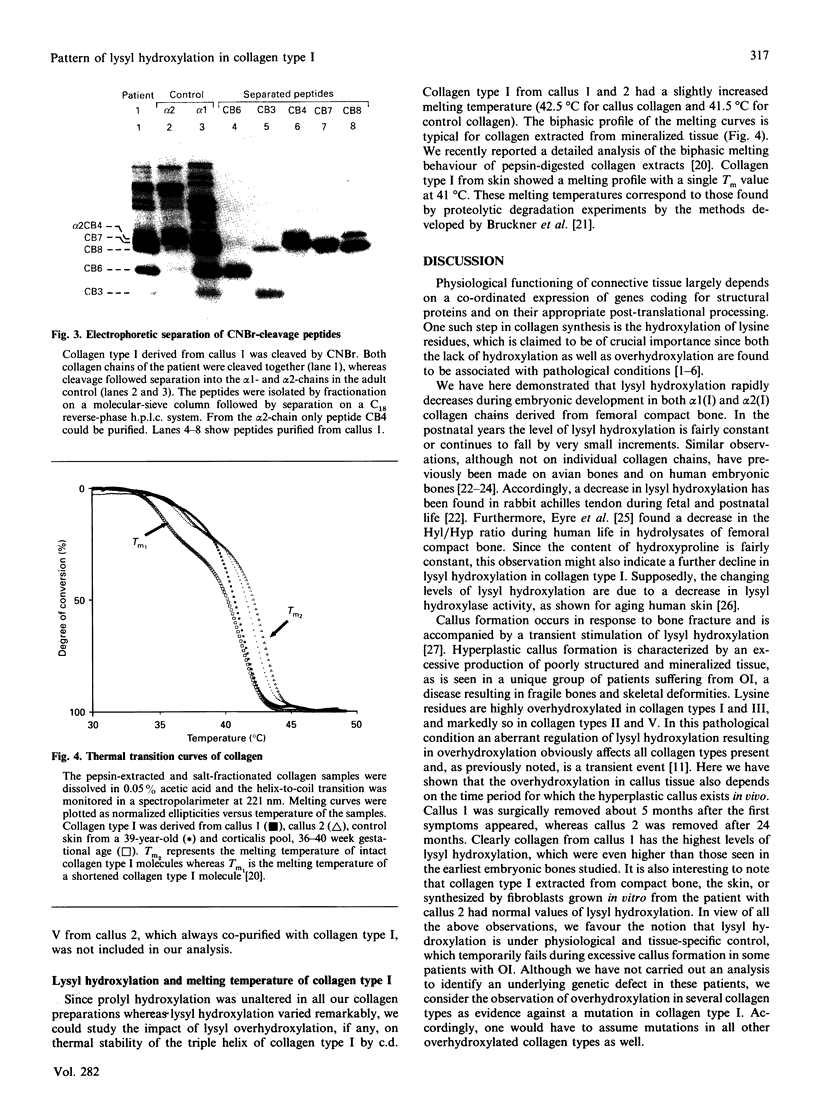
Abstract
Tissue from two patients with osteogenesis imperfecta suffering from a hyperplastic callus was studied. Although collagen type I from the compact bone and the skin and fibroblast cultures of these patients showed normal lysyl hydroxylation, collagen types I, II, III and V from the callus tissue were markedly overhydroxylated. Furthermore, the overhydroxylation of lysine residues covered almost equally the entire alpha 1 (I) collagen chain, as demonstrated by the analysis of individual CNBr-derived peptides. In addition, collagen type I was isolated from femoral compact bone of 33 individuals who died between the 16th week of gestational age and 22 years. Lysyl hydroxylation rapidly decreased in both collagen alpha 1 (I) and alpha 2 (I) chains during fetal development, and only little in the postnatal period. The transient increase in lysyl hydroxylation and the involvement of various collagen types in callus tissue argue for a regulatory mechanism that may operate in bone repair and during fetal development.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APLEY A. G. Hyperplastic callus in osteogenesis imperfecta; report of a case. J Bone Joint Surg Br. 1951 Nov;33-B(4):591–593. doi: 10.1302/0301-620X.33B4.591. [DOI] [PubMed] [Google Scholar]
- Anttinen H., Orava S., Ryhänen L., Kivirikko K. I. Assay of protocollagen lysyl hydroxylase activity in the skin of human subjects and changes in the activity with age. Clin Chim Acta. 1973 Aug 30;47(2):289–294. doi: 10.1016/0009-8981(73)90326-4. [DOI] [PubMed] [Google Scholar]
- Barnes M. J., Constable B. J., Morton L. F., Royce P. M. Age-related variations in hydroxylation of lysine and proline in collagen. Biochem J. 1974 May;139(2):461–468. doi: 10.1042/bj1390461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman J. F., Mascara T., Chan D., Cole W. G. Rapid fractionation of collagen chains and peptides by high-performance liquid chromatography. Anal Biochem. 1986 Apr;154(1):338–344. doi: 10.1016/0003-2697(86)90534-8. [DOI] [PubMed] [Google Scholar]
- Brenner R. E., Vetter U., Nerlich A., Wörsdorfer O., Teller W. M., Müller P. K. Biochemical analysis of callus tissue in osteogenesis imperfecta type IV. Evidence for transient overmodification in collagen types I and III. J Clin Invest. 1989 Sep;84(3):915–921. doi: 10.1172/JCI114253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckner P., Prockop D. J. Proteolytic enzymes as probes for the triple-helical conformation of procollagen. Anal Biochem. 1981 Jan 15;110(2):360–368. doi: 10.1016/0003-2697(81)90204-9. [DOI] [PubMed] [Google Scholar]
- Byers P. H. Inherited disorders of collagen gene structure and expression. Am J Med Genet. 1989 Sep;34(1):72–80. doi: 10.1002/ajmg.1320340114. [DOI] [PubMed] [Google Scholar]
- Bätge B., Notbohm H., Diebold J., Lehmann H., Bodo M., Deutzmann R., Müller P. K. A critical crosslink region in human-bone-derived collagen type I. Specific cleavage site at residue Leu95. Eur J Biochem. 1990 Aug 28;192(1):153–159. doi: 10.1111/j.1432-1033.1990.tb19208.x. [DOI] [PubMed] [Google Scholar]
- Chung E., Miller E. J. Collagen polymorphism: characterization of molecules with the chain composition (alpha 1 (3)03 in human tissues. Science. 1974 Mar;183(130):1200–1201. doi: 10.1126/science.183.4130.1200. [DOI] [PubMed] [Google Scholar]
- Cole W. G. Osteogenesis imperfecta. Baillieres Clin Endocrinol Metab. 1988 Feb;2(1):243–265. doi: 10.1016/s0950-351x(88)80014-4. [DOI] [PubMed] [Google Scholar]
- Eyre D. R., Dickson I. R., Van Ness K. Collagen cross-linking in human bone and articular cartilage. Age-related changes in the content of mature hydroxypyridinium residues. Biochem J. 1988 Jun 1;252(2):495–500. doi: 10.1042/bj2520495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher M. J., Shapiro F., Ellis R. D., Eyre D. R. Changes in tissue morphology and collagen composition during the repair of cortical bone in the adult chicken. J Bone Joint Surg Am. 1980 Sep;62(6):964–973. [PubMed] [Google Scholar]
- Hyashi T., Curran-Patel S., Prockop D. J. Thermal stability of the triple helix of type I procollagen and collagen. Precautions for minimizing ultraviolet damage to proteins during circular dichroism studies. Biochemistry. 1979 Sep 18;18(19):4182–4187. doi: 10.1021/bi00586a022. [DOI] [PubMed] [Google Scholar]
- Ibrahim J., Harding J. J. Pinpointing the sites of hydroxylysine glycosides in peptide alpha 1-CB7 of bovine corneal collagen, and their possible role in determining fibril diameter and thus transparency. Biochim Biophys Acta. 1989 Jul 21;992(1):9–22. doi: 10.1016/0304-4165(89)90044-5. [DOI] [PubMed] [Google Scholar]
- Kirsch E., Krieg T., Remberger K., Fendel H., Bruckner P., Müller P. K. Disorder of collagen metabolism in a patient with osteogenesis imperfecta (lethal type): increased degree of hydroxylation of lysine in collagen types I and III. Eur J Clin Invest. 1981 Feb;11(1):39–47. doi: 10.1111/j.1365-2362.1981.tb01763.x. [DOI] [PubMed] [Google Scholar]
- Krieg T., Feldmann U., Kessler W., Müller P. K. Biochemical characteristics of Ehlers-Danlos syndrome type VI in a family with one affected infant. Hum Genet. 1979 Jan 19;46(1):41–49. doi: 10.1007/BF00278900. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Martin G. R., Piez K. A., Powers M. J. Characterization of chick bone collagen and compositional changes associated with maturation. J Biol Chem. 1967 Dec 10;242(23):5481–5489. [PubMed] [Google Scholar]
- Pinnell S. R., Krane S. M., Kenzora J. E., Glimcher M. J. A heritable disorder of connective tissue. Hydroxylysine-deficient collagen disease. N Engl J Med. 1972 May 11;286(19):1013–1020. doi: 10.1056/NEJM197205112861901. [DOI] [PubMed] [Google Scholar]
- Pope F. M. Genetics of inherited defects of connective tissue. Baillieres Clin Rheumatol. 1988 Dec;2(3):673–702. doi: 10.1016/s0950-3579(88)80034-7. [DOI] [PubMed] [Google Scholar]
- Rao V. H., Steinmann B., de Wet W., Hollister D. W. Decreased thermal denaturation temperature of osteogenesis imperfecta mutant collagen is independent of post-translational overmodifications of lysine and hydroxylysine. J Biol Chem. 1989 Jan 25;264(3):1793–1798. [PubMed] [Google Scholar]
- STRACH E. H. Hyperplastic callus formation in osteogenesis imperfecta; report of a case and review of the literature. J Bone Joint Surg Br. 1953 Aug;35-B(3):417–422. doi: 10.1302/0301-620X.35B3.417. [DOI] [PubMed] [Google Scholar]
- Scott P. G., Veis A. The cyanogen bromide peptides of bovine soluble and insoluble collagens. I. Characterization of peptides from soluble type I collagen by sodium dodecylsulphate polyacrylamide gel electrophoresis. Connect Tissue Res. 1976;4(2):107–116. doi: 10.3109/03008207609152206. [DOI] [PubMed] [Google Scholar]
- Sillence D. O., Senn A., Danks D. M. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979 Apr;16(2):101–116. doi: 10.1136/jmg.16.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltz M. R., Dietrich S. L., Marshall G. J. Osteogenesis imperfecta. Perspectives. Clin Orthop Relat Res. 1989 May;(242):120–136. [PubMed] [Google Scholar]
- Sykes B., Puddle B., Francis M., Smith R. The estimation of two collagens from human dermis by interrupted gel electrophoresis. Biochem Biophys Res Commun. 1976 Oct 18;72(4):1472–1480. doi: 10.1016/s0006-291x(76)80180-5. [DOI] [PubMed] [Google Scholar]