Abstract
As the average maternal age advances with increasing concurrent cardiovascular disease risk factors, more women are entering pregnancy with or at risk for various cardiovascular conditions. Although rare, pregnant patients may require various cardiac interventions in the catheterization laboratory. An understanding of indications for intervention in pregnant patients with conditions such as myocardial infarction, severe valvular disease, and cardiogenic shock is critical to optimizing both fetal and maternal outcomes. This document highlights the most common cardiovascular conditions that may be encountered during pregnancy that may require intervention and highlights indications for intervention and periprocedural considerations to facilitate favorable maternal and fetal outcomes.
Keywords: cardiogenic shock, maternal-fetal medicine, myocardial infarction, percutaneous coronary intervention, peripartum cardiomyopathy, pregnancy-associated spontaneous coronary artery dissection
Central Illustration
Highlights
-
•
Cardiovascular conditions during pregnancy are associated with significant morbidity and mortality.
-
•
Myocardial infarction, cardiogenic shock, and valvular disease may require intervention during pregnancy.
-
•
Interdisciplinary care is critical in optimizing maternal and fetal outcomes.
Introduction
Maternal mortality and morbidity continue to increase, with cardiovascular disease as the leading cause of adverse maternal outcomes.1 The reasons for these trends are multifaceted; however, advancing maternal age, socioeconomic disparities, and coexistent cardiovascular risk factors contribute to such adverse outcomes. During pregnancy, alterations in the hormonal state, coagulation, and maternal hemodynamics increase the risk for various maternal cardiovascular conditions (Figure 1). Acute conditions such as acute decompensated heart failure, cardiogenic shock (CS), acute coronary syndromes, and severe valvular disease contribute to these unfavorable outcomes and may require interventional cardiology expertise as part of cardio-obstetrics interdisciplinary care. With advancing technology, including hemodynamic support devices and percutaneous interventions for valvular conditions, a growing array of therapies may be available during pregnancy. However, consideration for intervention during pregnancy is complex in light of elevated maternal and fetal risk. This article discusses broad considerations for pregnant patients with acute cardiovascular conditions in whom percutaneous or surgical intervention is being considered. Although preconception counseling and delivery planning are of the utmost importance, these issues will not be addressed here, and readers should refer to the Journal of the American College of Cardiology Focus Seminar series on Cardio-Obstetrics.2,3
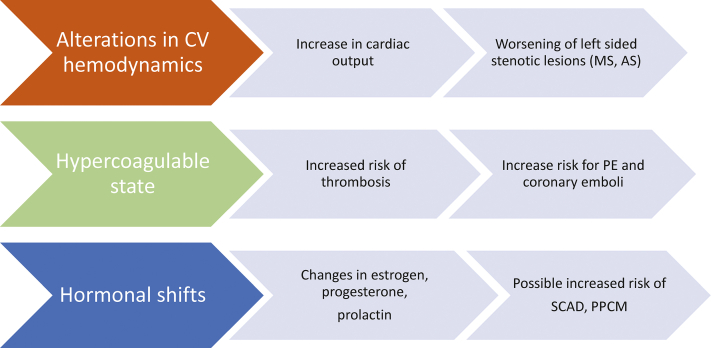
Figure 1.
Maternal changes in pregnancy and risk of CV conditions. Significant changes during pregnancy can increase the risk of various cardiovascular conditions. AS, aortic stenosis; CV, cardiovascular; MS, mitral stenosis; PE, pulmonary embolism; PPCM, peripartum cardiomyopathy; SCAD, spontaneous coronary artery dissection.
Material changes in pregnancy and associated cardiovascular disease risk
Pregnancy is associated with a significant change in cardiovascular hemodynamics, including increases in heart rate with associated increase in cardiac output up to 50% and a decline in systemic vascular resistance. Although these changes are intended to support a growing fetus, such changes can contribute to the development of new cardiovascular conditions or exacerbate existing conditions such as volume overload in patients with existing cardiomyopathy or decompensation in patients with left-sided valvular lesions. Such hemodynamic changes may unmask the previously undiagnosed cardiovascular conditions and lead to adverse outcomes for both the mother and fetus. Several risk classification schemes can be used to assess cardiovascular risk in pregnant patients, including the modified World Health Organization, ZAHARA (Zwangerschap bij Aangeboren HARtAfwijking [Pregnancy in Women With Congenital Heart Disease]), and CARPREG II (Cardiac Disease in Pregnancy Study) classifications that are the most commonly used in clinical practice.2 Such risk assessment methods should be combined with patient-specific clinical history and parameters to guide preconception planning, follow-up during pregnancy, and delivery management.
General considerations for pregnant patients when cardiac intervention is being considered
Interventional cardio-obstetrics team care
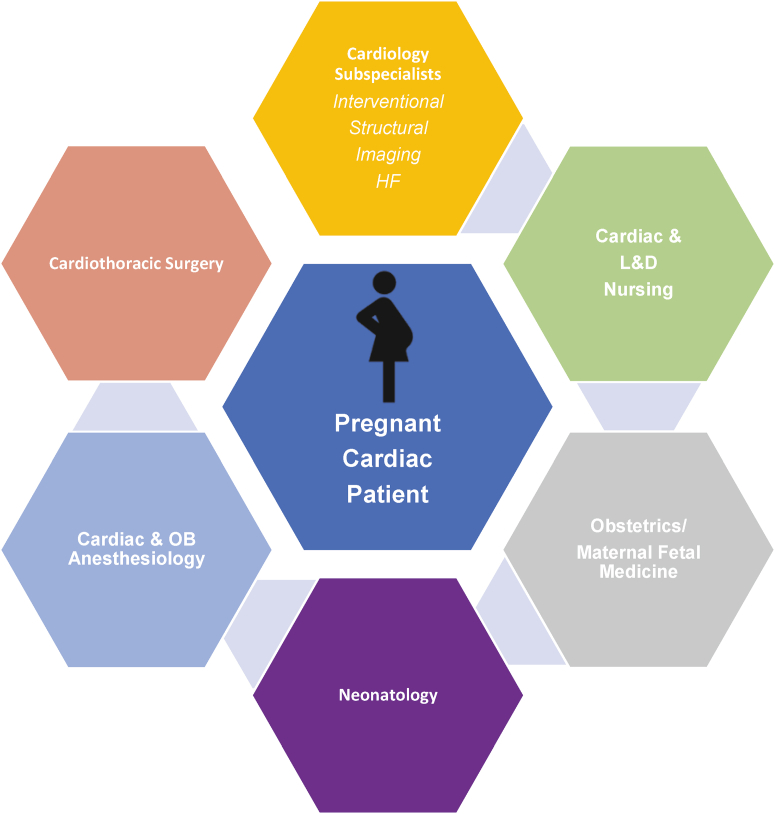
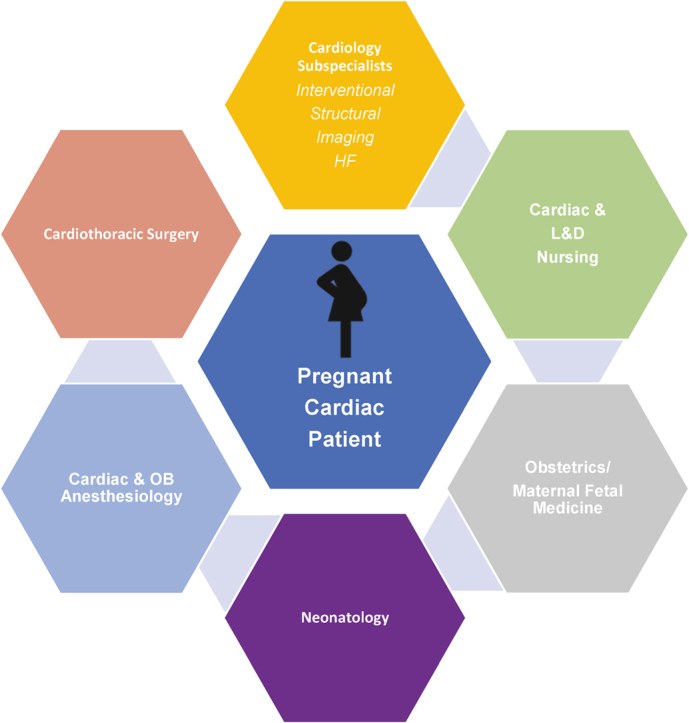
Core representation on the cardio-obstetrics team should be multidisciplinary across levels of care, including cardio-obstetrics specialists, interventional cardiology and cardiothoracic surgery, obstetrics/maternal-fetal medicine, nursing, neonatology and obstetric and cardiac anesthesiology (Central lIlustration). Team members should be easily identified and consulted for emergent cases. For elective cases, a plan should be formulated, broadly communicated, and shared in the electronic medical record with contingencies for emergent care.4 Membership of the team should be tailored to the specific requirements of each patient, with representation from subspecialties within the core fields. Within nursing, representation from labor and delivery nursing is critical, as is input from cardiac intensive care and cardiac catheterization staff. Representation from obstetric anesthesiology is often needed in combination with input from cardiothoracic anesthesiology. In cases where open cardiac surgery may be considered, careful assessment with the heart team/cardiothoracic surgery is critical as there is a significant risk of fetal loss associated with cardiac surgery in pregnancy.5,6
Central Illustration.
Components of an interventional cardio-obstetrics team. Interdisciplinary team care across various levels of clinical care and expertise is critical in treating pregnant patients with cardiovascular disease. HF, heart failure; L&D, labor and delivery; OB, obstetrics.
Radiation exposure
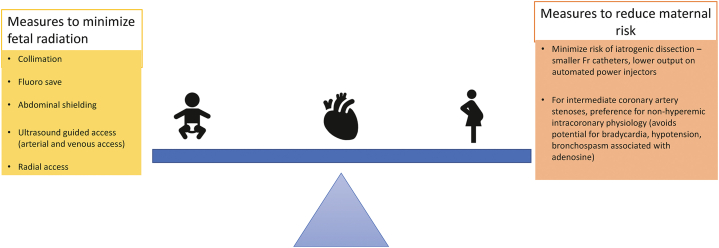
Fetal radiation exposure is a concern when considering interventional procedures. However, most interventions can be performed with exposure of <50 mGy; at this threshold, no reported fetal anomalies have been noted (Table 1).7,8 The potential for fetal malformation correlates with gestational age, such that exposure during the first trimester, the period of organogenesis, is associated with the highest risk, with declining risk as gestational age advances. If possible, consideration for intervention should take into account gestational age and be deferred until after the first trimester. However, the concern for fetal radiation exposure should not preclude maternal intervention, particularly as preserving maternal health is key to maintaining fetal health. Every effort should still be made to reduce radiation exposure with the recommendations outlined in Figure 2. Such measures include collimation, the use of fluorosave features, and ultrasound-guided access.
Table 1.
In utero fetal radiation effects and average radiation exposure during interventional procedures.
| Summary of suspected in utero-induced deterministic radiation effects | |||
|---|---|---|---|
| Gestational age (wks) | <50 mGy | 50-100 mGy | >100 mGy |
| 0-2 | None | None | None |
| 3-4 | None | Probably none | Possible spontaneous abortion |
| 5-10 | None | Scientifically uncertain and probably too subtle to be clinically detectable | Possible malformation risk increases with increasing dose |
| 11-17 | None | Scientifically uncertain and probably too subtle to be clinically detectable | Risk of diminished IQ increases with increasing dose |
| 18-27 | None | None | IQ deficits not detectable at diagnostic doses |
| >27 | None | None | None applicable to diagnostic medicine |
| Average radiation exposure for interventional procedures: 3-20 mGya | |||
| Diagnostic angiography, percutaneous coronary intervention, balloon aortic valvuloplasty, mitral valvuloplasty, hemodynamic support insertion, catheter-directed thrombolysis/thrombectomy | |||
Of note, these are average rates of exposure and do not account for complex interventions when indicated.
Figure 2.
Measures to reduce maternal and fetal risk during coronary angiography and percutaneous coronary intervention. Procedural considerations to reduce maternal and fetal risk during cardiac catheterization and intervention are noted here.
Medications and imaging agents
Medications commonly used in the cardiac catheterization laboratory setting are compatible with pregnancy, including heparin, metoprolol, verapamil, and nitroglycerin.3 Low-dose aspirin is safe and preferred over higher doses that may lead to hemorrhage and teratogenic effects.9 Although experience in human pregnancy is limited, clopidogrel has not been associated with adverse effects in animal models, and maternal use should be individualized based on risk assessment and benefit through shared decision-making.9 The safety of other antiplatelet agents such as prasugrel, ticagrelor, and cangrelor is unknown. Intravenous heparin is the preferred anticoagulant for pregnant patients in the catheterization laboratory with a standard assessment of ACTs per protocol to allow real-time procedural adjustment. Specific dose adjustment of intravenous heparin during pregnancy is not required, but careful attention should be paid to maintaining therapeutic ACTs in the setting of known hypercoagulable state during pregnancy. Intravenous or arterial iodinated contrast media may cross the placenta and enter the amniotic fluid and fetal circulation with potential for fetal thyroid function suppression.10 However, its use should not be withheld if indicated for the procedure. Excretion of iodinated contrast into breastmilk is exceedingly low, and the American College of Obstetricians and Gynecologists recommends no alteration in breastfeeding.8,11 Anticoagulation and dual antiplatelet therapy have implications for the choice of regional techniques (spinal or epidural) for labor analgesia or cesarean delivery. Neuraxial anesthesia is generally preferred over general anesthesia. However, this is associated with an increased risk of spinal epidural hematoma if the patient was recently on antiplatelet or anticoagulant therapy and may necessitate cesarean delivery under general anesthesia.2 A team approach with obstetric anesthesiology, obstetrics, or maternal-fetal medicine is needed for optimal delivery planning.12
Periprocedural considerations
There are unique aspects to planning cardiac interventions for a pregnant patient (Table 2). Sedatives and opioids can be administered if needed, with caution, as sleep-disordered breathing is more common in high-risk pregnant patients, and appropriate reversal agents and experienced anesthesiology teams should be readily available.13 The maternal upper airway is hyperemic and edematous, and highly skilled clinicians are needed for airway management, particularly in patients at risk of respiratory decompensation while recumbent. Fetal monitoring should occur before and after intervention by Doppler ultrasound and during the procedure where possible, although initiating monitoring should not necessarily delay the indicated procedure in the event of an emergent situation. In the event that a premature delivery would be considered, the administration of steroids for fetal lung maturation should be given in consultation with obstetrics or maternal-fetal medicine. Neonatology should be available in the event of urgent delivery for supportive care for the neonate as indicated.
Table 2.
Periprocedural maternal and fetal considerations in the catheterization laboratory.
| Maternal considerations |
| Avoid excessive sedation |
| Adequate anticoagulation |
| Left lateral recumbent positioning |
| BLS/ACLS - manual left uterine displacement |
| Anesthesia support for airway management |
| Fetal considerations |
| Measures to limit in utero radiation |
| MFM present at the time of the procedure |
| Neonatology consultation |
| Fetal monitoring |
| Consideration for preemptive steroids in discussion with MFM |
| C-section tray readily available |
ACLS, advanced cardiovascular life support; BLS, basic life support; MFM, maternal-fetal medicine.
Although infrequent, cardiac arrest during pregnancy has significant implications given coexistent maternal and fetal considerations. Beyond 20 weeks of gestation, due to uterine aortocaval compression, manual left uterine displacement is mandatory to ensure adequate venous return. Thus, the patient should be placed in a slightly left-sided tilt position, if possible, to relieve the pressure on venous return through the inferior vena cava. The same basic life support and advanced cardiovascular life support principles apply to pregnant patients as to nonpregnant patients. Maternal defibrillation is safe for the fetus, and defibrillation pads should be positioned in the front and back.14 When performing procedures in patients beyond 20 weeks of gestation (or fundal height at or above the level of the umbilicus), a cesarean delivery tray should be readily available in the rare case of intraprocedural hemodynamic collapse and advanced cardiovascular life support, as the return of maternal circulation should occur within 5 minutes or the uterus should be evacuated.14 The initiation of perimortem cesarean section should begin at 4 minutes into advanced cardiovascular life support but may occur earlier if maternal resuscitation is felt to be futile.
Acute cardiovascular conditions that may require interventional expertise
Myocardial infarction during pregnancy
Acute myocardial infarction (MI) is an uncommon complication reported in 2.8 to 8.1 per 100,000 pregnancies.15, 16, 17, 18 Pregnant patients are at a 3- to 4-fold greater risk of MI than nonpregnant, reproductive-aged patients.15 Risk factors for MI in pregnancy include older maternal age, Black race, tobacco use, and traditional cardiovascular risk factors, including hypertension, hyperlipidemia, and diabetes mellitus.15, 16, 17,19 Approximately 20% of pregnancy-associated MI occur antepartum, approximately 25% occur during hospitalization for labor and delivery, and approximately 55% occur postpartum.15 The outcomes of pregnancy-associated MI are poor, with 5% in-hospital maternal mortality.17,18 Pregnancy-associated MI may be caused by flow-limiting, obstructive coronary artery disease, or in approximately 10% of cases, MI without obstructive coronary arteries at the time of coronary angiography.19,20 The mechanisms of MI during pregnancy can include atherosclerotic coronary artery disease and plaque rupture, plaque erosion and thrombosis, spontaneous coronary artery dissection (SCAD), coronary artery spasm, or coronary embolism.19,21,22 Although mechanistically distinct from MI, Takotsubo syndrome, myocarditis, and pulmonary embolism during pregnancy can mimic acute MI and should be considered in the differential diagnosis.
Spontaneous coronary artery dissection, a separation of the layers of the coronary artery wall due to intramural hematoma, with or without an intimal tear, is the most common cause of pregnancy-associated MI, reported in approximately 40% of cases.19 By definition, SCAD occurs independently of atherosclerosis, iatrogenic causes, or trauma. Although the exact mechanisms are unknown, SCAD is associated with fibromuscular dysplasia and other arteriopathies, and changes in sex hormones during pregnancy are postulated to play a role in SCAD pathogenesis.23 Pregnancy-associated SCAD (P-SCAD) occurs most commonly in the first month postpartum and is associated with high-risk features, including left main and multivessel involvement and presentation with ST-segment elevation MI, compared with patients with nonpregnancy-associated SCAD.24,25
Atherosclerotic coronary artery disease with plaque disruption is identified in 30% of pregnancy-associated acute MI.15 As in nonpregnant adults, the rupture of a thin fibrous cap overlying a lipid-rich atheroma can expose the necrotic core and initiate thrombus formation. Plaque erosion, defined by thrombus formation in an area of disrupted endothelium with exposed smooth muscle cells and proteoglycans in the absence of lipid-rich plaque, can also occur. Coronary spasm is infrequently reported, but given its dynamic nature, the prevalence of spasm in pregnancy may be underreported as a cause of MI.19
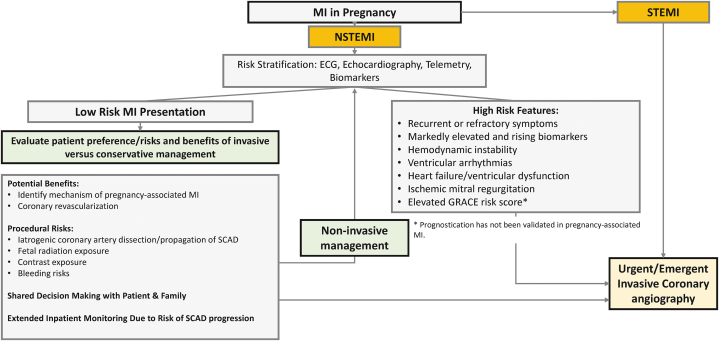
Given the risks of maternal mortality, prompt management of MI in pregnancy is indicated, and invasive coronary angiography should be considered unless the risks outweigh the anticipated benefits. An ischemia-guided management strategy without routine invasive coronary angiography may be appropriate for low-risk patients with resolved symptoms, mildly elevated cardiac biomarkers, and normal ventricular function.26 However, in patients with higher-risk MI features, ongoing symptoms, or evidence of ST-segment elevation MI, urgent invasive angiography should be performed (Figure 3). An invasive management approach does carry some risks, such as iatrogenic coronary dissection, which is more common in pregnancy.19 Measures to reduce maternal and fetal risk during coronary angiography and percutaneous coronary intervention (PCI), such as efforts to reduce radiation exposure and minimize the risk of iatrogenic coronary dissection, are listed in Figure 2. Although the management of pregnancy-associated MI is challenging and a multidisciplinary cardio-obstetrics team should guide decision-making, invasive management should not be delayed in patients with hemodynamic instability or ST-segment elevation MI. Cardio-obstetrics team management is also essential to discuss subsequent plans for delivery both with regard to timing and mode of delivery based on hemodynamic factors, need for antiplatelet therapy as discussed previously, and other patient-specific factors. Consideration for termination in cases with acute coronary syndrome is exceedingly rare, but discussion may occur in the setting of excessive fetal radiation exposure or associated severe ischemic cardiomyopathy.
Figure 3.
Management of MI in pregnancy. Suggested algorithm to guide assessment and management of MI in pregnancy. ECG, electrocardiography; GRACE, Global Registry of Acute Coronary Events; MI, myocardial infarction; NSTEMI, non–ST-elevation myocardial infarction; SCAD, spontaneous coronary artery dissection; STEMI, ST-elevation myocardial infarction.
Considerations for medical therapy in MI during pregnancy
The initiation of medical therapy depends on the etiology and timing of MI with respect to delivery. Aspirin may be safely administered during pregnancy. Heparin is the preferred anticoagulant because it does not cross the placenta, is short-acting, and has an excellent safety profile in pregnancy.27 When dual antiplatelet therapy is indicated, clopidogrel is the preferred P2Y12 inhibitor. Beta blockers and nitroglycerin are generally considered safe in pregnancy, whereas angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are contraindicated because of risks to the developing fetus.27,28
Considerations for PCI in non-SCAD MI in pregnancy
If an atherosclerotic lesion is present, then PCI should be performed according to standard revascularization guidelines.26 When coronary stent placement is indicated, drug-eluting stents should be preferred to ensure low restenosis rates and long-term coronary patency. This is particularly important in pregnant patients who are younger than the population typically undergoing revascularization. The short duration of dual antiplatelet therapy after drug-eluting stent placement is feasible with second and third-generation platforms, with rates of ischemic events comparable with those of bare-metal stents.29,30 The timing of delivery is important after stent implantation because dual antiplatelet therapy increases bleeding risks, particularly in the context of neuraxial anesthesia and the risk of epidural hematoma.27 Therefore, clopidogrel should be discontinued 7 days before planned delivery, if appropriate, and consideration for bridging with intravenous antiplatelet or anticoagulant agents should be discussed with the cardio-obstetrics team and interventionalist. Individualized bleeding risk at the time of delivery should be weighed against the risk of stent thrombosis with cessation of dual antiplatelet therapy based on patient-specific PCI outcomes on a case-by-case basis. Patients who present with acute MI secondary to coronary thrombus in the absence of a significant atherosclerotic lesion can be managed with balloon dilatation and aspiration thrombectomy without stent implantation if coronary flow is restored.31
Considerations for PCI in P-SCAD
The optimal treatment strategy for SCAD remains controversial, although conservative therapy is generally the preferred strategy, particularly for stable patients with no further evidence of ischemia. Considerations for diagnostic angiography and management of suspected P-SCAD are highlighted in Figure 4. PCI should be avoided in stable patients with P-SCAD to reduce the propagation of dissection and intramural hematoma, which can further compromise flow.23,32 Revascularization is typically pursued in unstable patients with ongoing ischemia, hemodynamic instability, or left main involvement.33,34 Patients with P-SCAD tend to have an increased risk of left main, proximal left anterior descending, or multivessel involvement along with larger troponin elevations and infarct sizes, lower left ventricular ejection fractions, and more often present with CS than nonpregnant patients with SCAD.24,25,35,36 All these factors increase the likelihood that patients with P-SCAD will need revascularization with PCI or coronary artery bypass grafting rather than conservative management.37
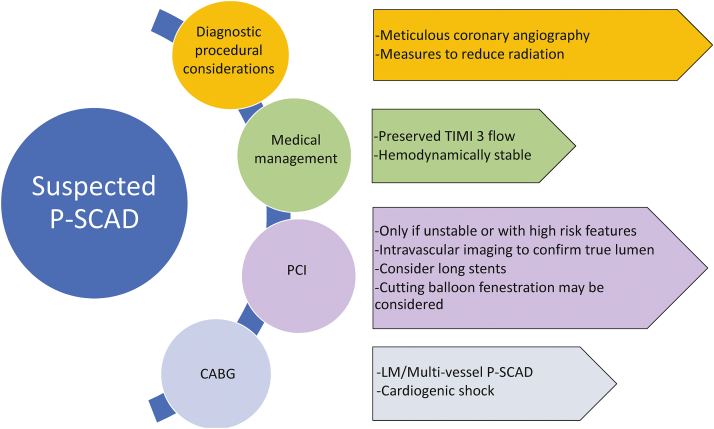
Figure 4.
Management of suspected P-SCAD. Pregnant patients with suspected P-SCAD should be carefully managed with close attention to procedural techniques for diagnostic angiography and intervention with either PCI or surgery, as noted here. CABG, coronary artery bypass graft surgery; LM, left main; PCI, percutaneous coronary intervention; P-SCAD, pregnancy-associated spontaneous coronary artery dissection; TIMI, thrombolysis in myocardial infarction.
When PCI is required in patients with P-SCAD, there are specific considerations to maximize the chances of a successful outcome and minimize risk (Figure 4). The approach to P-SCAD PCI should be similar to that in nonpregnant patients with SCAD.38 The coronary vasculature in SCAD is fragile and prone to iatrogenic dissection; therefore, catheter manipulation should be minimized.39 Intracoronary imaging is important to confirm wire positioning within the true lumen along with the consideration of implantation of longer stents to avoid further propagation of subintimal hematoma and dissection. Cutting balloon fenestration may also be considered.40
Management of CS in pregnancy
Although CS in the pregnant patient can arise from a number of possible etiologies, a chief consideration should be peripartum cardiomyopathy (PPCM). PPCM is present in more than half of all CS cases involving pregnant or recently pregnant patients and accounts for >80% of postpartum cases.41 Recommendations for the evaluation and management of CS in pregnancy are shown in Figure 5.42, 43, 44 The key to management of CS in pregnancy is rapid identification and early initiation of therapies. In less severe cases of CS, inotropic support can be used; however, such interventions have not been demonstrated to improve outcomes, and the currently available literature regarding the use of inotropic support is limited by ascertainment bias and a focus on PPCM rather than overt CS.45, 46, 47
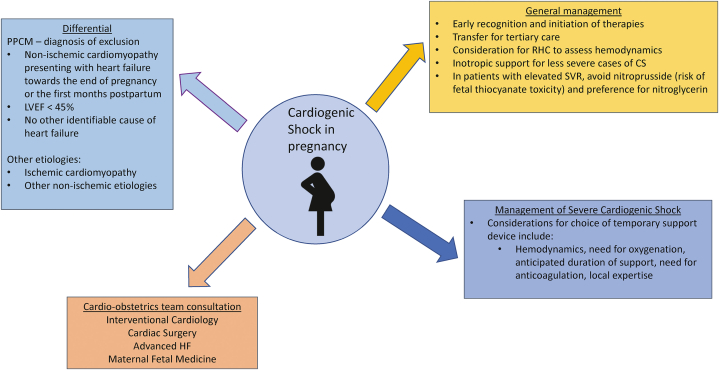
Figure 5.
Evaluation and management of CS in pregnancy. Management of cardiogenic shock in pregnancy should involve the interdisciplinary cardio-obstetrics team with an indication for hemodynamic support based on patient-specific characteristics and institutional expertise. CS, cardiogenic shock; HF, heart failure; LVEF, left ventricular ejection fraction; PPCM, peripartum cardiomyopathy; RHC, right heart catheterization; SVR, systemic vascular resistance.
More severe cases of CS should be managed with temporary mechanical circulatory support (tMCS) and/or extracorporeal life support, including extracorporeal membrane oxygenation (ECMO), as in the nonpregnant population. Pregnant patients with CS who survive to discharge are more likely to have received early support from an intraaortic balloon pump, percutaneous ventricular assist device, or ECMO than nonsurvivors. Furthermore, early tMCS, defined as within 6 days from the onset of CS, has been associated with lower mortality. Overall, survival associated with tMCS in this population is >80%, suggesting safety and efficacy, although overall data are limited.41 The decision to proceed with tMCS should be based on the degree of CS, needs, and duration of hemodynamic support, and with the guidance of a multidisciplinary team in the context of institutional expertise as intra-aortic balloon pumps, percutaneous ventricular assist devices, and ECMO have all been described in the literature.41 Consideration for potential complications such as bleeding, limb ischemia, and adverse neurologic events should also be weighed carefully. Additionally, invasive hemodynamic data can not only guide this and other management strategies but also have been demonstrated to improve outcomes across patients with CS.48 Anticoagulation considerations need to be discussed with the cardio-obstetrics team to carefully assess the risk and benefits based on tMCS used. A recent systematic review and meta-analysis of the use of extracorporeal life support in pregnancy for respiratory and cardiac decompensation assessed approximately 350 patients with 75% maternal survival at 30 days and 74% at 1 year with venoarterial ECMO specifically associated with 72% maternal survival. Fetal survival was 65%. The most common maternal complication was bleeding, ranging from mild to moderate (18%) to severe (13%).49 The use of bromocriptine with prolonged treatment of several weeks may be considered in cases of PPCM-associated CS per the European Society of Cardiology guidelines.26 However, because of the risk of thrombosis, anticoagulation should be concomitantly used while administering bromocriptine.
Valvular heart disease intervention during pregnancy
Regurgitant valvular lesions are generally well-tolerated during pregnancy in asymptomatic patients with normal exercise tolerance, preserved ejection fraction, and normal pulmonary artery pressures. However, asymptomatic patients may present with acute volume overload in the first week postpartum.3 Higher risks of complications during pregnancy occur in the setting of underlying ventricular dysfunction, heart failure symptoms prior to conception, and/or pulmonary hypertension.3,50 Stenotic lesions are generally poorly tolerated during pregnancy. As cardiac output increases during pregnancy, pressure gradients across fixed obstructive valve lesions also increase and often precipitate heart failure in the late second to early third trimester.3 Although pulmonary stenosis is typically well-tolerated during pregnancy, left-sided obstructive lesions pose a significant risk of maternal morbidity.3 Labor and delivery may be associated with cardiovascular complications due to acute reduction in preload due to postpartum hemorrhage or acute volume overload following the “autotransfusion” of blood from the placenta and uterus with delivery and relief of inferior vena cava obstruction.2 The discussion here will focus on the most commonly encountered significant valvular lesions of aortic and mitral valve stenosis with a general discussion of regurgitant lesions. Table 3 discusses indications for medical management and/or percutaneous treatment by valve condition. If open surgical replacement is considered, elevated risk of fetal loss up to 19% and 29% in various studies should be discussed as part of a heart team approach and shared decision-making with the patient and family.5,6
Table 3.
Indications for medical management and/or percutaneous treatment by valve condition.
| Valvular lesion | Risk stratification | Management options | |
|---|---|---|---|
| Mitral stenosis | Moderate risk | Mild (area >1.5 cm2) | Beta blockers/diuretics Consider anticoagulationa Consider limiting activity |
| High risk | Moderate to severe (area <1.5 cm2) | Beta blockers/diuretics Consider anticoagulationa Limit activity Percutaneous mitral valvuloplasty for severe heart failure/pulmonary pressures >50 mm Hg and acceptable anatomyb |
|
| Aortic stenosis | Moderate risk | Severe asymptomatic | Beta blockers/diuretics/limit exercise and activity |
| High risk | Severe symptomatic and/or LV dysfunction | Aortic valvuloplasty if acceptable anatomy or consider TAVR | |
| Mitral regurgitation | Moderate risk | Moderate to severe with normal LV function | Diuretics/afterload reduction |
| High risk | Acute severe Severe with LV dysfunction Symptomatic |
Diuretics/afterload reduction Refractory symptoms consider percutaneous mitral clip vs surgical repair/replacement |
|
| Aortic regurgitation | Moderate risk | Moderate to severe with normal LV function | Diuretics/afterload reduction |
| High risk | Acute severe Severe with LV dysfunction |
Diuretics/afterload reduction Consider TAVR vs surgical replacement if refractory symptoms |
|
| Tricuspid regurgitation | Low risk | Moderate to severe with normal PA pressures and RV function | Diuretics |
| High risk | Severe/torrential with impaired RV function and/or severe pulmonary hypertension | Diuretics/inotropic support | |
| Pulmonary stenosis | Low risk | Severe asymptomatic | Diuretics |
| Moderate risk | Severe symptomatic RV dysfunction |
Diuretics Consider percutaneous balloon valvuloplasty or percutaneous pulmonary valve replacement for refractory symptoms |
|
| Pulmonary regurgitation | Low risk | Severe asymptomatic | Diuretics |
| Moderate risk | Severe symptomatic RV dysfunction |
Diuretics Consider percutaneous pulmonary valve replacement for refractory symptoms |
|
| Bioprosthetic valve dysfunction | Low risk | Regurgitation, asymptomatic with normal LV function | Beta blockers/diuretics |
| High risk | Stenosis with LV dysfunction and/or symptoms | Consider percutaneous valve-in-valve procedure vs surgical valve replacement | |
LV, left ventricular; PA, pulmonary artery; RV, right ventricular; TAVR, transcatheter aortic valve replacement.
Anticoagulation for mitral stenosis in pregnancy is recommended for patients with atrial fibrillation, left atrial thrombus, prior embolism, severe mitral stenosis, spontaneous echo contrast in the left atrium, left atrial volume index of ≥60 mL/m2, or heart failure.
Wilkins score of <8; for poor anatomy, consider surgical commissurotomy; more than mild insufficiency would be poor anatomy for valvuloplasty.
Aortic stenosis
Severe aortic stenosis may be poorly tolerated with the hemodynamic changes of pregnancy leading to heart failure, arrhythmias, and syncope, and symptomatic patients should strongly be considered for treatment before pregnancy.51 Considering the high-risk nature of such patients, if early in pregnancy, termination is recommended, particularly in patients with left ventricular dysfunction, as this is considered modified WHO (mWHO) class IV. However, decisions regarding the continuation of pregnancy versus termination should be made in conjunction with a heart team with shared decision-making between patient, family, and clinicians. If late in pregnancy, early delivery followed by surgical aortic valve replacement may be considered. Most patients who develop symptoms can be medically managed with exercise restriction, beta-blockade, and diuretics. However, as native valve aortic stenosis is commonly congenital (bicuspid) or related to rheumatic heart disease, percutaneous balloon aortic valvuloplasty (pBAV) is a reasonable option for treatment if symptoms remain refractory to medical therapy. pBAV has been reported in limited cases in select patients with severe aortic stenosis during pregnancy52,53 and can be performed with minimal radiation exposure at experienced centers. As pBAV may lead to aortic insufficiency, a “minimalistic” approach to valvuloplasty should be considered, with smaller diameter balloons used to avoid this risk. In patients with impaired left ventricular function, or if rapid ventricular pacing needs to be avoided, a valvuloplasty balloon with an inner lumen to allow continued cardiac output during inflation may be considered. Transcatheter valve replacement (TAVR) is an additional treatment option, in particular in patients with poor pBAV anatomy, concomitant aortic regurgitation, or bioprosthetic valve dysfunction. Both native TAVR and valve-in-valve TAVR have been reported with successful outcomes, but data are limited.54,55 However, the long-term durability of TAVR in young patients with bicuspid aortic valve disease is unknown, and the associated potential risk for permanent pacing should be weighed carefully when considering an approach to intervention. If TAVR is pursued, planning requires computed tomography imaging with intravenous contrast of the chest, abdomen, and pelvis for procedural planning, but interventional procedures can be performed without general anesthesia and with limited additional contrast and fluoroscopy time. Valve-in-valve TAVR without computed tomography may be considered in patients who are otherwise at low risk of peripheral arterial disease. In patients with concomitant dilation of the aorta, surgical intervention should be considered to address both aortic stenosis and aneurysm repair. The timing of surgery in patients with aortic dilation in addition to aortic stenosis should be individualized based on gestational age and rate of progression of aortic dilation, as well as symptoms and valve hemodynamics and left ventricular dysfunction. Progressive aortic dilation of >5 mm or dimension of >50 mm should prompt the consideration of cesarean delivery followed by ascending aorta and valve surgery soon after delivery.
Mitral stenosis
Severe mitral stenosis is most commonly the result of rheumatic fever and is a frequently encountered valve lesion during pregnancy worldwide.56 Patients with severe mitral stenosis may become symptomatic with heart failure, arrhythmias, and pulmonary hypertension during pregnancy.57 Symptom-based interventions, including exercise restriction, beta-blockade, and diuresis, are often successful, and intervention is rarely needed. However, should refractory symptoms occur despite medical management, percutaneous balloon valvuloplasty has been widely used to address mitral stenosis,58 has successfully been performed during pregnancy,59 and is the procedure of choice for those with suitable anatomy. Anatomy unsuitable for valvuloplasty would include the presence of severe mitral regurgitation, and caution is advised in valves with valvular or subvalvular thickening, calcification, or restricted leaflet mobility, and a Wilkins score of ≥8.60 Decisions regarding the surgical or percutaneous treatment of severe mitral stenosis should be made in conjunction with the heart team approach considering anatomic and clinical considerations for both mother and fetus and fetal risk with cardiac surgery. If the patient is not a candidate for valvuloplasty, consider cesarean delivery if symptomatic heart failure and/or very severe mitral stenosis with surgical valve intervention occuring shortly after delivery. If the decision is made to proceed with valvuloplasty, a cardiac surgeon and mechanical circulatory support should be on standby. For patients with bioprosthetic mitral valve stenosis, valve-in-valve transcatheter mitral valve replacement may be considered in discussion with cardiothoracic surgery based on individualized patient characteristics and anatomy. Anticoagulation during the procedure should be titrated appropriately for the hypercoagulable state during pregnancy, particularly when interventional equipment is present in the left atrium.
Regurgitant lesions
As mentioned earlier and noted in Table 3, regurgitant lesions are generally well-tolerated in pregnancy because of a decrease in afterload in the setting of peripheral vasodilation. If symptoms of volume overload occur and remain refractory despite afterload reduction and diuresis, transcatheter options such as transcatheter edge-to-edge repair for mitral regurgitation and TAVR for aortic regurgitation can be considered. However, there are no systematic data assessing the short- or long-term outcomes of such interventions with TAVR and no reported cases of transcatheter mitral valve repair in the literature. Heart team discussion with cardiothoracic surgery is critical in assessing the best course of action for these patients, with consideration for fetal outcomes should surgery be considered, as mentioned previously.
Pulmonary embolism
Venous thromboembolism is the fourth leading cause of pregnancy-related mortality, with the primary mechanism of acute mortality being a right ventricular failure.61 Pregnancy is associated with an elevated risk of venous thromboembolism because of a combination of stasis and hypercoagulability and vascular trauma during delivery in particular.62,63 Overall risk begins during the first trimester, peaking in the immediate postpartum period, and then tapering off 6 weeks after delivery.64 The gold standard for the diagnosis of pulmonary embolism remains computed tomographic pulmonary angiography.
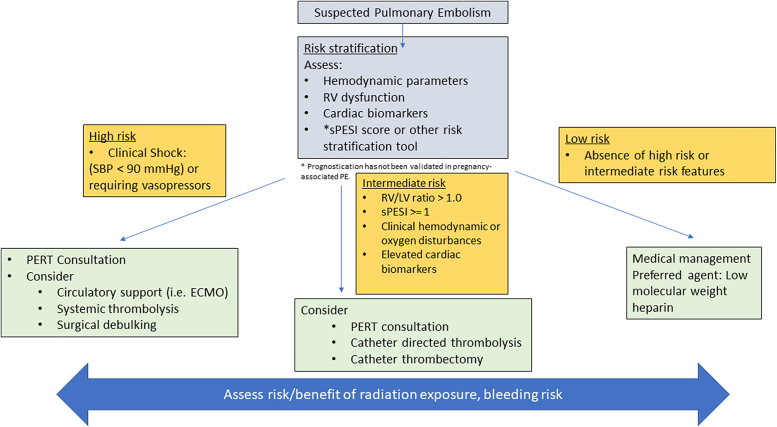
The European Society of Cardiology 2019 guideline divides patients into 4 major risk categories.65 Treatment strategies are guided by the clinical, laboratory, and echocardiographic parameters, with particular attention to bleeding and radiation risks in pregnancy (Figure 6). Medical therapy is sufficient for low-risk patients with anticoagulation with either low-molecular-weight heparin or intravenous heparin.66 Intermediate- to high-risk patients should be considered for advanced therapies, depending on clinical stability, echocardiography parameters, risk/benefit relative to radiation exposure, and bleeding risk (Figure 6). Hemodynamic parameters, oxygenation status, right ventricular dysfunction and dimensions (right ventricle/left ventricle ratio of >1 and tricuspid annular plane excursion of <16 mm being most predictive of adverse outcomes), and cardiac biomarkers should be assessed for risk stratification. Additionally, the simplified Pulmonary Embolism Severity Index is a useful risk assessment tool for 30-day outcomes, albeit this has not been specifically validated in pregnancy. The simplified Pulmonary Embolism Severity Index includes an age of >80 years, history of malignancy, history of cardiopulmonary disease, heart rate of ≥110 beats/min, systolic blood pressure of <100 mm Hg, and oxygen saturation of <90%. Fibrinolytic therapy has an inherent bleeding risk to the mother, and fetus and should be used judiciously.67 Catheter-based thrombectomy or thrombolysis procedures have not been studied in pregnancy, and the literature on this population is limited to case reports. Where available, pulmonary embolism response teams should assist in the complex decision-making process for these high-risk patients in conjunction with a cardio-obstetrics team.68 Consideration for catheter-based therapies will rely on an interdisciplinary team and patient-centered decision-making and operator and institutional experience and expertise but should generally be reserved for those with intermediate to high risk.
Figure 6.
Management of PE in pregnancy and considerations for intervention. Suggested algorithm to guide the management of PE and indications for potential intervention. ECMO, extracorporeal membrane oxygenation; LV, left ventricle; PE, pulmonary embolism; PERT, pulmonary embolism response team; RV, right ventricle; SBP, systolic blood pressure; sPESI, simplified Pulmonary Embolism Severity Index.
Conclusion
Acute conditions requiring intervention in the catheterization laboratory are rare, but such conditions are associated with elevated morbidity and mortality for both mother and baby. The treatment of conditions such as MI, CS, valvular disease, and pulmonary embolism during pregnancy requires careful discussion of maternal and fetal risks and benefits. Cardio-obstetrics teams, including interdisciplinary team care, should be involved to individualize treatment decisions relevant to local expertise and shared decision-making.
Acknowledgments
The authors would like to thank Dr Rohit Samuel.
Declaration of competing interest
Dr Park reports consulting with Abbott and CSI. Dr Voeltz reports consulting with Abiomed, Phillips, and Medtronic. Dr Bortnick serves as site principal investigator for multicenter trials sponsored by Abbott, Inc, and CSL-Behring, Inc, for which her institution received compensation, and reports honoraria from Clearview Healthcare Partners, LLC, and S2N Healthcare, LLC, outside the submitted work. Dr Vidovich reports royalty payments from Merit and grant funding from Boston Scientific. Drs Lindley, Sintek, Sethi, Choi, Davis, Walsh, Bello, Saw, Ahmed, and Smilowitz reported no financial interests.
Funding sources
Dr Bello was supported by the National Institute of Health [grant numbers NHLBI K23 HL136853, R01153382]. Dr Smilowitz was supported by the National Institute of Health [grant number NHLBI K23 HL150315]. Dr Bortnick was supported by the American Heart Association [grant number 17MCPRP33630098] and the National Institute of Health [grant number NHLBI K23 HL146982].
References
- 1.Petersen E.E., Davis N.L., Goodman D., et al. Vital Signs: Pregnancy-related deaths, United States, 2011-2015, and strategies for prevention, 13 states, 2013-2017. MMWR Morb Mortal Wkly Rep. 2019;68(18):423–429. doi: 10.15585/mmwr.mm6818e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis M.B., Arendt K., Bello N.A., et al. Team-based care of women with cardiovascular disease from pre-conception through pregnancy and postpartum: JACC Focus Seminar 1/5. J Am Coll Cardiol. 2021;77(14):1763–1777. doi: 10.1016/j.jacc.2021.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindley K.J., Bairey Merz C.N., Asgar A.W., et al. Management of women with congenital or inherited cardiovascular disease from pre-conception through pregnancy and postpartum: JACC Focus Seminar 2/5. J Am Coll Cardiol. 2021;77(14):1778–1798. doi: 10.1016/j.jacc.2021.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolfe D.S., Hameed A.B., Taub C.C., Zaidi A.N., Bortnick A.E. Addressing maternal mortality: the pregnant cardiac patient. Am J Obstet Gynecol. 2019;220(2):167.e1–167.e8. doi: 10.1016/j.ajog.2018.09.035. [DOI] [PubMed] [Google Scholar]
- 5.Weiss B.M., von Segesser L.K., Alon E., Seifert B., Turina M.I. Outcome of cardiovascular surgery and pregnancy: a systematic review of the period 1984-1996. Am J Obstet Gynecol. 1998;179(6 Pt 1):1643–1653. doi: 10.1016/s0002-9378(98)70039-0. [DOI] [PubMed] [Google Scholar]
- 6.Parry A.J., Westaby S. Cardiopulmonary bypass during pregnancy. Ann Thorac Surg. 1996;61(6):1865–1869. doi: 10.1016/0003-4975(96)00150-6. [DOI] [PubMed] [Google Scholar]
- 7.Toppenberg K.S., Hill D.A., Miller D.P. Safety of radiographic imaging during pregnancy. Am Fam Physician. 1999;59(7):1813–1818. 1820. [PubMed] [Google Scholar]
- 8.Committee Opinion No. 723: guidelines for diagnostic imaging during pregnancy and lactation. Obstet Gynecol. 2017;130(4):e210–e216. doi: 10.1097/AOG.0000000000002355. [DOI] [PubMed] [Google Scholar]
- 9.Briggs G.G., Freeman R.K., Towers C.V., Forinash A.B. 11th ed. Wolters Kluwer; 2017. Drugs in Pregnancy and Lactation: a Reference Guide to Fetal and Neonatal Risk. [Google Scholar]
- 10.Webb J.A., Thomsen H.S., Morcos S.K., Members of Contrast Media Safety Committee of European Society of Urogenital Radiology (ESUR) The use of iodinated and gadolinium contrast media during pregnancy and lactation. Eur Radiol. 2005;15(6):1234–1240. doi: 10.1007/s00330-004-2583-y. [DOI] [PubMed] [Google Scholar]
- 11.Cova M.A., Stacul F., Quaranta R., et al. Radiological contrast media in the breastfeeding woman: a position paper of the Italian Society of Radiology (SIRM), the Italian Society of Paediatrics (SIP), the Italian Society of Neonatology (SIN) and the Task Force on Breastfeeding, Ministry of Health. Italy. Eur Radiol. 2014;24(8):2012–2022. doi: 10.1007/s00330-014-3198-6. [DOI] [PubMed] [Google Scholar]
- 12.Horlocker T.T., Vandermeuelen E., Kopp S.L., Gogarten W., Leffert L.R., Benzon H.T. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-based Guidelines (fourth edition) Reg Anesth Pain Med. 2018;43(3):263–309. doi: 10.1097/AAP.0000000000000763. [DOI] [PubMed] [Google Scholar]
- 13.Facco F.L., Ouyang D.W., Zee P.C., Grobman W.A. Sleep disordered breathing in a high-risk cohort prevalence and severity across pregnancy. Am J Perinatol. 2014;31(10):899–904. doi: 10.1055/s-0033-1363768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeejeebhoy F.M., Zelop C.M., Lipman S., et al. Cardiac arrest in pregnancy: a scientific statement from the American Heart Association. Circulation. 2015;132(18):1747–1773. doi: 10.1161/CIR.0000000000000300. [DOI] [PubMed] [Google Scholar]
- 15.James A.H., Jamison M.G., Biswas M.S., Brancazio L.R., Swamy G.K., Myers E.R. Acute myocardial infarction in pregnancy: a United States population-based study. Circulation. 2006;113(12):1564–1571. doi: 10.1161/CIRCULATIONAHA.105.576751. [DOI] [PubMed] [Google Scholar]
- 16.Ladner H.E., Danielsen B., Gilbert W.M. Acute myocardial infarction in pregnancy and the puerperium: a population-based study. Obstet Gynecol. 2005;105(3):480–484. doi: 10.1097/01.AOG.0000151998.50852.31. [DOI] [PubMed] [Google Scholar]
- 17.Smilowitz N.R., Gupta N., Guo Y., et al. Acute myocardial infarction during pregnancy and the puerperium in the United States. Mayo Clin Proc. 2018;93(10):1404–1414. doi: 10.1016/j.mayocp.2018.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibson P., Narous M., Firoz T., et al. Incidence of myocardial infarction in pregnancy: a systematic review and meta-analysis of population-based studies. Eur Heart J Qual Care Clin Outcomes. 2017;3(3):198–207. doi: 10.1093/ehjqcco/qcw060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elkayam U., Jalnapurkar S., Barakkat M.N., et al. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation. 2014;129(16):1695–1702. doi: 10.1161/CIRCULATIONAHA.113.002054. [DOI] [PubMed] [Google Scholar]
- 20.Tamis-Holland J.E., Jneid H., Reynolds H.R., et al. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American Heart Association. Circulation. 2019;139(18):e891–e908. doi: 10.1161/CIR.0000000000000670. [DOI] [PubMed] [Google Scholar]
- 21.Roth A., Elkayam U. Acute myocardial infarction associated with pregnancy. J Am Coll Cardiol. 2008;52(3):171–180. doi: 10.1016/j.jacc.2008.03.049. [DOI] [PubMed] [Google Scholar]
- 22.Thygesen K., Alpert J.S., Jaffe A.S., et al. Fourth universal definition of myocardial infarction (2018) Circulation. 2018;138(20):e618–e651. doi: 10.1161/CIR.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 23.Saw J., Aymong E., Sedlak T., et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv. 2014;7(5):645–655. doi: 10.1161/CIRCINTERVENTIONS.114.001760. [DOI] [PubMed] [Google Scholar]
- 24.Tweet M.S., Hayes S.N., Codsi E., Gulati R., Rose C.H., Best P.J.M. Spontaneous coronary artery dissection associated with pregnancy. J Am Coll Cardiol. 2017;70(4):426–435. doi: 10.1016/j.jacc.2017.05.055. [DOI] [PubMed] [Google Scholar]
- 25.Havakuk O., Goland S., Mehra A., Elkayam U. Pregnancy and the risk of spontaneous coronary artery dissection: an analysis of 120 contemporary cases. Circ Cardiovasc Interv. 2017;10(3) doi: 10.1161/CIRCINTERVENTIONS.117.004941. [DOI] [PubMed] [Google Scholar]
- 26.Regitz-Zagrosek V., Roos-Hesselink J.W., Bauersachs J., et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39(34):3165–3241. doi: 10.1093/eurheartj/ehy340. [DOI] [PubMed] [Google Scholar]
- 27.Halpern D.G., Weinberg C.R., Pinnelas R., Mehta-Lee S., Economy K.E., Valente A.M. Use of medication for cardiovascular disease during pregnancy: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73(4):457–476. doi: 10.1016/j.jacc.2018.10.075. [DOI] [PubMed] [Google Scholar]
- 28.Park K., Bairey Merz C.N., Bello N.A., et al. Management of women with acquired cardiovascular disease from pre-conception through pregnancy and postpartum: JACC Focus Seminar 3/5. J Am Coll Cardiol. 2021;77(14):1799–1812. doi: 10.1016/j.jacc.2021.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Windecker S., Latib A., Kedhi E., et al. Polymer-based or polymer-free stents in patients at high bleeding risk. N Engl J Med. 2020;382(13):1208–1218. doi: 10.1056/NEJMoa1910021. [DOI] [PubMed] [Google Scholar]
- 30.Kandzari D.E., Kirtane A.J., Windecker S., et al. One-month dual antiplatelet therapy following percutaneous coronary intervention with zotarolimus-eluting stents in high-bleeding-risk patients. Circ Cardiovasc Interv. 2020;13(11) doi: 10.1161/CIRCINTERVENTIONS.120.009565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tweet M.S., Lewey J., Smilowitz N.R., Rose C.H., Best P.J.M. Pregnancy-associated myocardial infarction: prevalence, causes, and interventional management. Circ Cardiovasc Interv. 2020;13:e008687. doi: 10.1161/CIRCINTERVENTIONS.120.008687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tweet M.S., Eleid M.F., Best P.J., et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv. 2014;7(6):777–786. doi: 10.1161/CIRCINTERVENTIONS.114.001659. [DOI] [PubMed] [Google Scholar]
- 33.Hayes S.N., Kim E.S.H., Saw J., et al. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation. 2018;137(19):e523–e557. doi: 10.1161/CIR.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adlam D., Alfonso F., Maas A., Vrints C., Writing Committee European Society of Cardiology, acute cardiovascular care association, SCAD study group: a position paper on spontaneous coronary artery dissection. Eur Heart J. 2018;39(36):3353–3368. doi: 10.1093/eurheartj/ehy080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheikh A.S., O’Sullivan M. Pregnancy-related spontaneous coronary artery dissection: two case reports and a comprehensive review of literature. Heart Views. 2012;13(2):53–65. doi: 10.4103/1995-705X.99229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cade J.R., Szarf G., de Siqueira M.E., et al. Pregnancy-associated spontaneous coronary artery dissection: insights from a case series of 13 patients. Eur Heart J Cardiovasc Imaging. 2017;18(1):54–61. doi: 10.1093/ehjci/jew021. [DOI] [PubMed] [Google Scholar]
- 37.Saw J. Pregnancy-associated spontaneous coronary artery dissection represents an exceptionally high-risk spontaneous coronary artery dissection cohort. Circ Cardiovasc Interv. 2017;10(3) doi: 10.1161/CIRCINTERVENTIONS.117.005119. [DOI] [PubMed] [Google Scholar]
- 38.Saw J. Natural history of spontaneous coronary artery dissection: to stent or not to stent? EuroIntervention. 2019;14(13):1353–1356. doi: 10.4244/EIJV14I13A245. [DOI] [PubMed] [Google Scholar]
- 39.Prakash R., Starovoytov A., Heydari M., Mancini J., Saw J. TCT-386 Iatrogenic catheter-induced dissection during angiography of patients with spontaneous coronary artery dissection. J Am Coll Cardiol. 2015;66(15):B155–B156. doi: 10.1016/j.jacc.2015.08.1002. [DOI] [Google Scholar]
- 40.Saw J., Mancini G.B.J., Humphries K.H. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol. 2016;68(3):297–312. doi: 10.1016/j.jacc.2016.05.034. [DOI] [PubMed] [Google Scholar]
- 41.Banayan J., Rana S., Mueller A., et al. Cardiogenic shock in pregnancy: analysis from the National Inpatient Sample. Hypertens Pregnancy. 2017;36(2):117–123. doi: 10.1080/10641955.2016.1242606. [DOI] [PubMed] [Google Scholar]
- 42.Davis M.B., Arany Z., McNamara D.M., Goland S., Elkayam U. Peripartum cardiomyopathy: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75(2):207–221. doi: 10.1016/j.jacc.2019.11.014. [DOI] [PubMed] [Google Scholar]
- 43.Hibbard J.U., Lindheimer M., Lang R.M. A modified definition for peripartum cardiomyopathy and prognosis based on echocardiography. Obstet Gynecol. 1999;94(2):311–316. doi: 10.1016/s0029-7844(99)00293-8. [DOI] [PubMed] [Google Scholar]
- 44.Sliwa K., Hilfiker-Kleiner D., Petrie M.C., et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail. 2010;12(8):767–778. doi: 10.1093/eurjhf/hfq120. [DOI] [PubMed] [Google Scholar]
- 45.Abdel Hamid H.A., El-Tohamy S.A. Comparison between milrinone and levosimendan infusion in patients with peripartum cardiomyopathy. Ain-Shams J Anaesthesiol. 2014;7(2):114–120. doi: 10.4103/1687-7934.133308. [DOI] [Google Scholar]
- 46.Biteker M., Duran N.E., Kaya H., et al. Effect of levosimendan and predictors of recovery in patients with peripartum cardiomyopathy, a randomized clinical trial. Clin Res Cardiol. 2011;100(7):571–577. doi: 10.1007/s00392-010-0279-7. [DOI] [PubMed] [Google Scholar]
- 47.Stapel B., Kohlhaas M., Ricke-Hoch M., et al. Low STAT3 expression sensitizes to toxic effects of beta-adrenergic receptor stimulation in peripartum cardiomyopathy. Eur Heart J. 2017;38(5):349–361. doi: 10.1093/eurheartj/ehw086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hernandez G.A., Lemor A., Blumer V., et al. Trends in utilization and outcomes of pulmonary artery catheterization in heart failure with and without cardiogenic shock. J Card Fail. 2019;25(5):364–371. doi: 10.1016/j.cardfail.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 49.Naoum E.E., Chalupka A., Haft J., et al. Extracorporeal life support in pregnancy: a systematic review. J Am Heart Assoc. 2020;9(13) doi: 10.1161/JAHA.119.016072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pfaller B., Dave Javier A., Grewal J., et al. Risk associated with valvular regurgitation during pregnancy. J Am Coll Cardiol. 2021;77(21):2656–2664. doi: 10.1016/j.jacc.2021.03.327. [DOI] [PubMed] [Google Scholar]
- 51.Orwat S., Diller G.P., van Hagen I.M., et al. Risk of pregnancy in moderate and severe aortic stenosis: from the Multinational ROPAC Registry. J Am Coll Cardiol. 2016;68(16):1727–1737. doi: 10.1016/j.jacc.2016.07.750. [DOI] [PubMed] [Google Scholar]
- 52.Banning A.P., Pearson J.F., Hall R.J. Role of balloon dilatation of the aortic valve in pregnant patients with severe aortic stenosis. Br Heart J. 1993;70(6):544–545. doi: 10.1136/hrt.70.6.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McIvor R.A. Percutaneous balloon aortic valvuloplasty during pregnancy. Int J Cardiol. 1991;32(1):1–3. doi: 10.1016/0167-5273(91)90037-p. [DOI] [PubMed] [Google Scholar]
- 54.Hodson R., Kirker E., Swanson J., Walsh C., Korngold E.C., Ramelli S. Transcatheter aortic valve replacement during pregnancy. Circ Cardiovasc Interv. 2016;9(10) doi: 10.1161/CIRCINTERVENTIONS.116.004006. [DOI] [PubMed] [Google Scholar]
- 55.Berry N., Sawlani N., Economy K., et al. Transcatheter aortic valve replacement for bioprosthetic aortic stenosis in pregnancy. JACC Cardiovasc Interv. 2018;11(19):e161–e162. doi: 10.1016/j.jcin.2018.07.046. [DOI] [PubMed] [Google Scholar]
- 56.Sliwa K., Johnson M.R., Zilla P., Roos-Hesselink J.W. Management of valvular disease in pregnancy: a global perspective. Eur Heart J. 2015;36(18):1078–1089. doi: 10.1093/eurheartj/ehv050. [DOI] [PubMed] [Google Scholar]
- 57.van Hagen I.M., Thorne S.A., Taha N., et al. Pregnancy outcomes in women with rheumatic mitral valve disease: results from the Registry of Pregnancy and Cardiac Disease. Circulation. 2018;137(8):806–816. doi: 10.1161/CIRCULATIONAHA.117.032561. [DOI] [PubMed] [Google Scholar]
- 58.Vahanian A., Michel P.L., Cormier B., et al. Results of percutaneous mitral commissurotomy in 200 patients. Am J Cardiol. 1989;63(12):847–852. doi: 10.1016/0002-9149(89)90055-6. [DOI] [PubMed] [Google Scholar]
- 59.Mangione J.A., Zuliani M.F., Del Castillo J.M., Nogueira E.A., Arie S. Percutaneous double balloon mitral valvuloplasty in pregnant women. Am J Cardiol. 1989;64(1):99–102. doi: 10.1016/0002-9149(89)90663-2. [DOI] [PubMed] [Google Scholar]
- 60.Abascal V.M., Wilkins G.T., Choong C.Y., et al. Echocardiographic evaluation of mitral valve structure and function in patients followed for at least 6 months after percutaneous balloon mitral valvuloplasty. J Am Coll Cardiol. 1988;12(3):606–615. doi: 10.1016/s0735-1097(88)80045-7. [DOI] [PubMed] [Google Scholar]
- 61.Creanga A.A., Syverson C., Seed K., Callaghan W.M. Pregnancy-related mortality in the United States, 2011-2013. Obstet Gynecol. 2017;130(2):366–373. doi: 10.1097/AOG.0000000000002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bourjeily G., Paidas M., Khalil H., Rosene-Montella K., Rodger M. Pulmonary embolism in pregnancy. Lancet. 2010;375(9713):500–512. doi: 10.1016/S0140-6736(09)60996-X. [DOI] [PubMed] [Google Scholar]
- 63.Nichols K.M., Henkin S., Creager M.A. Venous thromboembolism associated with pregnancy: JACC Focus Seminar. J Am Coll Cardiol. 2020;76(18):2128–2141. doi: 10.1016/j.jacc.2020.06.090. [DOI] [PubMed] [Google Scholar]
- 64.Jacobsen A.F., Skjeldestad F.E., Sandset P.M. Incidence and risk patterns of venous thromboembolism in pregnancy and puerperium—a register-based case-control study. Am J Obstet Gynecol. 2008;198(2):233.e1–233.e7. doi: 10.1016/j.ajog.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 65.Konstantinides S.V., Meyer G., Becattini C., et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): the Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC) Eur Respir J. 2019;54(3) doi: 10.1183/13993003.01647-2019. [DOI] [PubMed] [Google Scholar]
- 66.Mehta L.S., Warnes C.A., Bradley E., et al. Cardiovascular considerations in caring for pregnant patients: a scientific statement from the American Heart Association. Circulation. 2020;141(23):e884–e903. doi: 10.1161/CIR.0000000000000772. [DOI] [PubMed] [Google Scholar]
- 67.Martillotti G., Boehlen F., Robert-Ebadi H., Jastrow N., Righini M., Blondon M. Treatment options for severe pulmonary embolism during pregnancy and the postpartum period: a systematic review. J Thromb Haemost. 2017;15(10):1942–1950. doi: 10.1111/jth.13802. [DOI] [PubMed] [Google Scholar]
- 68.Rivera-Lebron B.N., Rali P.M., Tapson V.F. The PERT concept: a step-by-step approach to managing pulmonary embolism. Chest. 2021;159(1):347–355. doi: 10.1016/j.chest.2020.07.065. [DOI] [PubMed] [Google Scholar]