The transradial artery approach is the preferred access route for coronary angiography. The risk of major vascular complications is 0.2% and typically involves hematomas arising from the radial, brachial, or subclavian artery, with pseudoaneurysms of the radial artery previously described.1,2 Brachiocephalic pseudoaneurysm is a rare and anatomically challenging clinical entity from a diagnostic and therapeutic perspective.3 To date, brachiocephalic artery pseudoaneurysms with carotid artery involvement as a complication of coronary angiography have been unreported, with a paucity of literature to guide management. In this report, we describe a case of brachiocephalic pseudoaneurysm and associated carotid artery dissection diagnosed 2 weeks following coronary angiography. This required a hybrid exclusion procedure with open surgical bypass of the carotid dissection and retrograde brachiocephalic stenting.
Case report
An 81-year-old woman presented with chest pain and paroxysmal nocturnal dyspnea. The relevant background included chronic kidney disease, hypertension, hypothyroidism, severe obstructive sleep apnea, morbid obesity, and gastroesophageal reflux disease. Transthoracic echocardiography revealed normal left ventricular function, mild aortic regurgitation, and a severely dilated ascending aorta (47 mm; SD, 2.9 from normal for age and body surface area) and aortic root.
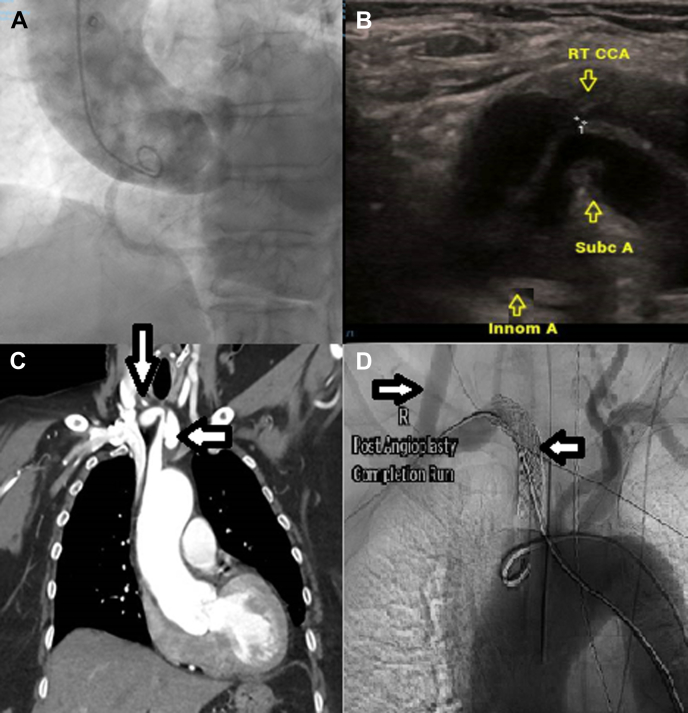
The patient underwent diagnostic coronary angiography via the right transradial artery. There was marked tortuosity of the brachiocephalic artery. There was difficulty engaging the coronary arteries because of the patient’s aortic anatomy; therefore, several catheters were trialed for selective engagement of both the left and right coronary arteries. Coronary angiography confirmed minor coronary artery disease, with only mild aortic regurgitation confirmed on an aortogram (Figure 1A). The patient was asymptomatic after the procedure and was discharged for home.
Figure 1.
(A) An aortogram demonstrated a severely dilated aortic root and mild aortic regurgitation. (B) Vascular ultrasound showed severe ostial stenosis of the right common carotid artery (marked + +1) because of an intramural hematoma (marked with “↓”) and compression by the false lumen. (C) Computed tomography of the chest revealed a pseudoaneurysm at the level of the brachiocephalic artery (marked with “←”) and dissection extending into the common carotid artery (marked with “↓”). (D) Fluoroscopy demonstrated well-deployed stents and exclusion of the pseudoaneurysm, with patency of the right common carotid bypass graft. Innom A, innominate (brachiocephalic) artery; RT CCA, right common carotid artery; Subc A, subclavian artery.
Two days later, the patient re-presented to the hospital with chest pain, dyspnea, and dysphagia. Her troponin levels were within normal limits, and 12-lead electrocardiography, chest radiography, and ventilation-perfusion scan of the lungs yielded normal results. Subsequent gastroscopy revealed only mild tortuosity of the upper esophagus. The patient was subsequently discharged for home.
Dysphagia persisted, and 2 weeks after coronary angiography, the patient underwent computed tomography (CT) angiography of the chest, which revealed a 20 × 26 × 12-mm3 pseudoaneurysm of the brachiocephalic artery with an associated mural hematoma. There was arterial dissection with an intramural hematoma of the right common carotid artery that extended to the carotid bifurcation (Figure 1C). Vascular ultrasound showed severe stenosis at the ostium of the right common carotid artery due to compression by the intramural hematoma (Figure 1B).
The patient underwent open repair with ligation of the proximal common carotid artery using a subclavian-carotid artery Dacron Gelsoft Plus graft (Terumo Corporation). The pseudoaneurysm was visualized under fluoroscopic guidance from the femoral approach.
Utilizing the right brachial artery retrograde approach, an 8F sheath was inserted and over a Rosen wire (Cook Medical); sequential 11 × 38-mm2 and 11 × 29-mm2 VBX stent grafts (Gore Medical) were deployed and postdilated using an Armada 35 balloon (Abbott).
Digital subtraction angiography (Figure 1D) and postoperative CT angiography confirmed satisfactory exclusion of the pseudoaneurysm, with patency of the right subclavian artery and bypass graft. The patient tolerated the procedure and was discharged.
Discussion
We describe brachiocephalic artery pseudoaneurysm as an unusual complication of diagnostic angiography that ultimately requires open surgical and endovascular therapy. Most reports of brachiocephalic pseudoaneurysm occurred after major chest trauma, with iatrogenic causes, such as coronary angiography, less frequently reported.3,4, 5, 6, 7, 8, 9 Uniquely, this case was associated with vessel dissection extending into the right carotid artery. All previously reported cases were associated with an abrupt presentation characterized by various symptoms, including chest pain, back pain, airway compromise, and dysphonia, and were managed urgently; in this case, the patient was not diagnosed for 2 weeks following the precipitating event due to the ongoing atypical symptoms.
We suspect that patient factors, including weakened arterial walls caused by increased shear wall stress due to a tortuous, dilated artery (in a comorbid patient), contributed to vessel trauma. It is also possible that the pseudoaneurysm originated at the site of a calcific nodule or ulcerative plaque, both of which can contribute to peripheral pseudoaneurysm formation.10,11 Subsequent imaging did not show evidence of atherosclerotic disease at the site of the pseudoaneurysm. Guide wire manipulation and subsequent multiple catheter exchanges likely contributed to trauma to the brachiocephalic artery. This emphasizes the importance of fluoroscopic imaging guidance to facilitate safe advancement of equipment, particularly in the setting of tortuous anatomy, in which the extent of arterial disease is unknown. Direct visualization of wire passage hopefully limits inadvertent arterial wall trauma, which may result in vessel perforation or dissection. The use of angiography wires, smaller caliber catheters, and balloon-assisted tracking should limit trauma to the vessel wall.12 When difficulty is encountered while negotiating tortuous vasculature, alternate vascular access sites should be considered.
Delayed injury can lead to an array of atypical signs and symptoms, including diminished peripheral pulse, superior vena cava syndrome, dysphagia, bruits, and pulsatile suprasternal mass.
This case involved a hybrid operation, with both open and endovascular techniques employed. The brachiocephalic artery is problematic to access directly, and it was thought that the patient was not suitable for open repair via thoracotomy.
The intramural hematoma and dissection extended along the common carotid artery and required ligation, with the carotid subclavian bypass graft providing perfusion to the ipsilateral internal carotid artery. The common carotid artery was inflamed and friable at the time of the operation as a result of the intramural hematoma, making repair challenging. This type of operative approach is more commonly performed for aortic arch-debranching dissections (such as Stanford type B aortic dissections).13
A covered stent was used to exclude the area of the pseudoaneurysm; the polytetrafluoroethylene covering effectively excludes the aneurysm and promotes thrombosis of the aneurysmal sac. Fenestrated stent grafts are custom designed based on patients’ anatomic factors; however, they typically take up to 6 weeks to be manufactured. Given their size and symptomatology, it was thought inappropriate to wait 6 weeks for such a graft. An alternate option would be kissing covered stents; however, these would still require exposure of the common carotid or brachial artery.
Our case highlights the importance of continuing to be vigilant for vascular complications following coronary angiography using the transradial approach. New-onset, nonspecific thoracic symptoms, including atypical chest discomfort as in this case, should prompt consideration of vascular injury following cardiac catheterization.
Conclusion
Although injuries to the brachiocephalic artery are extremely rare, particularly those that extend into the carotid artery, the potentially fatal risk of hemorrhage or cerebral ischemia in this anatomically challenging location should prompt CT angiography following concerning thoracic symptoms after transradial arterial interventions.
Acknowledgments
Declaration of competing interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics statement and patient consent
This case report adhered to the local ethical guidelines, and written consent was gained for publication from the patient. The patient provided written consent for the publication of this article and was given the opportunity to review the article prior to its publication.
References
- 1.Rao S.V., Ou F.S., Wang T.Y., et al. Trends in the prevalence and outcomes of radial and femoral approaches to percutaneous coronary intervention: a report from the National Cardiovascular Data Registry. JACC Cardiovasc Interv. 2008;1(4):379–386. doi: 10.1016/j.jcin.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Collins N., Wainstein R., Ward M., Bhagwandeen R., Dzavik V. Pseudoaneurysm after transradial cardiac catheterization: case series and review of the literature. Catheter Cardiovasc Interv. 2012;80(2):283–287. doi: 10.1002/ccd.23216. [DOI] [PubMed] [Google Scholar]
- 3.Hirose H., Gill I.S. Blunt injury of the innominate artery: a case report and review of literature. Ann Thorac Cardiovasc Surg. 2004;10(4):218–223. [PubMed] [Google Scholar]
- 4.Farooqi F., Alexander J., Sarma A. Rare vascular perforation complicating radial approach to percutaneous coronary angioplasty. BMJ Case Rep. 2013;2013 doi: 10.1136/bcr-2012-007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdool M.A., Morrison S., Sullivan H. Iatrogenic perforation of subclavian artery as a complication of coronary angiography from the radial route, endovascularly repaired with a covered stent-graft. BMJ Case Rep. 2013;2013 doi: 10.1136/bcr-2012-007602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi S., Joh J.H., Choe J.W. Fatal vascular complications during transradial percutaneous coronary intervention: a case report. Medicine. 2020;99(28) doi: 10.1097/MD.0000000000021205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Habib N., Jerzewski A., Koomen E.M., et al. Subclavian artery perforation complicating coronary angiography. Neth Heart J. 2012;20(6):288–290. doi: 10.1007/s12471-011-0164-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merkle J., Hohmann C., Sabashnikov A., Wahlers T., Wippermann J. Central vascular complications following elective catheterization using transradial percutaneous coronary intervention. J Investig Med High Impact Case Rep. 2017;5(1) doi: 10.1177/2324709617698717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smilowitz N.R., Saric M., Attubato M.J., Slater J.N. Mediastinal hematoma and tracheal compression following transradial percutaneous coronary intervention. Case Rep Cardiol. 2018;2018 doi: 10.1155/2018/6790120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stone P.A., Campbell J.E., AbuRahma A.F. Femoral pseudoaneurysms after percutaneous access. J Vasc Surg. 2014;60(5):1359–1366. doi: 10.1016/j.jvs.2014.07.035. [DOI] [PubMed] [Google Scholar]
- 11.Lee S., Cho S.H. Huge ascending aortic pseudoaneurysm caused by a penetrating atherosclerotic ulcer. Circ Cardiovasc Imaging. 2008;1(3):e19–e20. doi: 10.1161/CIRCIMAGING.108.788133. [DOI] [PubMed] [Google Scholar]
- 12.Patel T., Shah S., Pancholy S., Rao S., Bertrand O.F., Kwan T. Balloon-assisted tracking: a must-know technique to overcome difficult anatomy during transradial approach. Catheter Cardiovasc Interv. 2014;83(2):211–220. doi: 10.1002/ccd.24959. [DOI] [PubMed] [Google Scholar]
- 13.Daily P.O., Trueblood H.W., Stinson E.B., Wuerflein R.D., Shumway N.E. Management of acute aortic dissections. Ann Thorac Surg. 1970;10(3):237–247. doi: 10.1016/s0003-4975(10)65594-4. [DOI] [PubMed] [Google Scholar]