Abstract
Background and Aims
Acute liver injury (ALI) due to autoimmune hepatitis (AIH) can be treated by immunosuppression. In contrast, idiosyncratic drug-induced liver injury (DILI) had a poor prognosis. DILI thus needs to be distinguished from non-DILI.
Methods
Twenty-nine patients with DILI and 77 with non-DILI (42 of AIH and 35 with undetermined cause) diagnosed during 2005–2017 comprised the derivation cohort. 110 patients with ALI due to either AIH, DILI, or obscure causes at 6 liver centers during 2010–2015 were the validation cohort. Revised international AIH group scores (IAIHGs) and the Roussel-Uclaf Causality Assessment Method (RUCAM) were modified to calculate results using medical interviews and laboratory data without chronological changes. Diagnostic accuracy for the distinction of DILI and non-DILI was evaluated by receiver operating characteristic analysis and results were expressed as the area under the curve (AUC). This study received institutional institutional review board approval (MH2020-205).
Results
The AUCs of modified IAIHGs and RUCAM scores for the diagnosis of DILI were 0.96 and 1.00 when cut-off values were set at 3 for the modified RUCAM and 5 for the modified IAIHGs in the derivation cohort. In the validation cohort, the AUCs of modified IAIHGs and RUCAM scores for the diagnosis of DILI were 0.95 and 0.97, respectively. The accuracy of the combination of the modified scores was 81% (89/110).
Conclusion
Modified diagnostic scores based on detailed medical interviews and routine laboratory data can distinguish DILI from non-DILI in patients with ALI.
Keywords: ALI, RUCAM, AIH, DILI
Graphical abstract
Introduction
Severe liver injury (SLI) can develop into hepatic encephalopathy depending on the progression of the disease.1 Patients with SLI who complicate hepatic encephalopathy are diagnosed with acute liver failure (ALF). Because liver transplantation is a curative treatment for patients with ALF, a referral to a liver center may reduce the mortality of ALF by recommending a patient for liver transplantation.2,3 To properly make a referral, it is necessary to appropriately assess the severity of the acute liver injury (ALI) with an accurate diagnosis of the etiology.4,5 A prospective, observational cohort study of ALI patients has shown that hepatitis B virus exacerbations or idiosyncratic drug-induced liver injury (DILI) are at higher risks for poor prognosis.6 In contrast, ALI due to an oral virus, autoimmune hepatitis (AIH), or an undetermined cause (UC) responds well to the treatment.6
To optimize treatment strategy, ALI should be classified according to etiology as soon as possible. Because most of viral infection is self-limiting and diagnostic markers are available through blood examination,7 AIH and DILI are remaining causes, which should be comprehensively diagnosed using detailed medical interviews, laboratory data, and histological examination.8 It has been previously reported that AIH and DILI can be diagnosed by way of 2 scoring systems as follows: the Revised International AIH Group Scoring System for the Diagnosis of AIH (IAIHG) score for AIH and the Roussel–Uclaf Causality Assessment Method (RUCAM) for DILI.9,10 Because the 2 scoring systems contain items regarding chronological and histological data, the scores that are available in the first assessment of the ALI patients need to be established to make prompt clinical decisions for the treatment of ALI, although usefulness of histological evaluation at the diagnosis of AIH and DILI has been reported.11,12 In addition, DILI should be distinguished from liver injury of UC as well as AIH. We thus evaluated whether modified (m-) RUCAM and m-IAIHG scores at the time of the first assessment can distinguish DILI from ALI due to AIH or UC.
To diagnose etiologies of ALI, history taking has been strictly performed. For DILI, extensive review for medication lists and timing of medication administration to onset of liver enzyme elevation should be evaluated. In addition, viral and alcohol associated hepatitis were excluded via serological marker testing and interview about alcohol use.
Methods
Subjects for derivation cohort
We have prospectively and consecutively collected data on 905 patients with liver injury from April 2005 to December 2017 at a single center.13 Among those patients, 106 patients who satisfied the following criteria were selected for the derivation cohort; absence of chronic hepatitis and liver cirrhosis, ALI (aspartate transaminase [AST] > 200 IU/L or alanine transaminase [ALT] > 300 IU/L), established diagnosis of AIH, DILI, or UC with the assessment of liver histology (Table 1). The diagnosis of AIH was made based on Japanese diagnostic guidelines.14
Table 1.
Characteristics of the Patients for Derivation Cohort
| Total (n = 106) | DILI (n = 29) | AIH (n = 42) | Undetermined (n = 35) | |
|---|---|---|---|---|
| Sex (M:F) | 29:77 | 11:18 | 8:34 | 10:25 |
| Age (Years-old) | 56 (45–67) | 56 (47–69) | 56 (47–66) | 52 (33–65) |
| Prognosis | ||||
| Survival:Deceased:Transplantation | 98:5:3 | 27:2:0 | 39:2:1 | 32:1:2 |
| Daily alcohol intake | ||||
| <25 g, 25–60 g, 60g< | 84:18:4 | 21:6:2 | 34:6:2 | 29:6:0 |
| WBC (/μL) | 5510 (4178–6890) | 5740 (4660–6890) | 5390 (4103–6875) | 5250 (4075–6870) |
| Platelet (103/μLa) | 12.2 (7.0–16.0) | 18.7 (16.0–24.2) | 16.0 (12.2–21.1) | 18.9 (13.7–25.4) |
| Alb (mg/dL) | 3.8 (2.9–3.2) | 3.5 (3.0–3.9) | 3.2 (2.9–3.8) | 3.7 (3.3–4.1)a |
| Cre (mg/dL) | 0.57 (0.49–0.68) | 0.60 (0.52–0.66) | 0.55 (0.50–0.63) | 0.57 (0.44–0.71) |
| AST (U/L) | 926 (465–1357) | 909 (514–1234) | 880 (452–1061) | 1053 (505–1667) |
| ALT (U/L) | 1059 (584–1585) | 1054 (644–1399) | 955 (451–1177) | 1198 (778–2229)a |
| ALP (U/L) | 501 (379–651) | 514 (383–759) | 519 (413–648) | 426 (302–545) |
| T-Bil (mg/dL) | 8.2 (2.9–14.3) | 8.5 (3.6–17.4) | 10.4 (4.9–14.8) | 4.7 (2.4–11.3) |
| D-Bil (mg/dL) | 6.1 (1.9–10.8) | 6.1 (2.9–10.8) | 7.8 (3.9–11.0) | 4 (1.8–8.8) |
| IgG (mg/dL) | 1520 (1194–2032) | 1365 (1103–1938) | 2114 (1535–2491) | 1317 (1123–1562)ab |
| IgM (mg/dL) | 126 (82–175) | 125 (80–170) | 133 (97–176) | 117 (69–180) |
| PT-INR | 1.27 (1.13–1.56) | 1.28 (1.16–1.45) | 1.24 (1.14–1.55) | 1.35 (1.07–1.66) |
AIH, autoimmune hepatitis; Alb, Albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Cre, creatinine; D-Bil, direct bilirubin; DILI, drug-induced liver injury; Ig, immunoglobulin; PT-INR, prothrombin time international ratio; T-Bil, total bilirubin; WBC, white blood cell.
P < .05, AIH, vs Undetermined cause.
P<.05, AIH, vs DILI.
Patients were suspected as having AIH when one or more of the serological findings were fulfilled; presence of antinuclear antibodies or antismooth muscle antibodies and high serum immunoglobulin (Ig) G levels (>1.1 times the upper limit of normal). Patients were diagnosed with AIH when a simplified scoring system of the international AIH group was more than 7.15 DILI was diagnosed by 2 or more senior hepatologists, who assessed both the clinical course and present illness with a careful medical interview. A diagnosis of UC was made after the exclusion of viral hepatitis, AIH, and DILI. We excluded cases of drug-induced AIH, overlap syndrome of AIH and either primary biliary cholangitis or primary sclerosing cholangitis, and DILI in patients with a previous diagnosis of AIH.
Both RUCAM and IAIHG scores of 106 patients were calculated. Detailed formulae for these scores were reported elsewhere.9,10 Because this study was an observational study, the treatment applied to each patient was decided by their physician.
Subjects for validation cohort
For external validation of the results obtained by derivation cohort, 110 patients with ALI due to any one of AIH, DILI or a UC were retrospectively accumulated from 6 liver centers in Japan. The institutions included Saitama Medical University, Niigata University, Chiba University, Juntendo University Shizuoka hospital, Yamaguchi University, and Kagoshima University (Table 2). All patients were diagnosed by hepatologists in each center from January 2010 to December 2015. Diagnoses were confirmed by histological analysis. The study procedures were approved by the institutional ethical board at each institution (MH2020-205), and informed consent was obtained from all subjects or indicated guardians by an opt-out manner.
Table 2.
Characteristics of the Patients for Validation Cohort
| Total (n = 110) | DILI (n = 34) | AIH (n = 67) | Undetermined (n = 9) | |
|---|---|---|---|---|
| Sex (M:F) | 29:81 | 17:17 | 9:58 | 3:6 |
| Age (Years-old) | 60 (47–68) | 54 (47–64) | 61 (51–69) | 55 (38–66) |
| Prognosis | ||||
| Survival:Deceased:Transplantation | 105:1:4 | 64:1:2 | 39:2:1 | 9:0:0 |
| Daily alcohol intake | ||||
| <25 g, 25–60 g, 60g< | 100:8:2 | 26:6:2 | 66:1:0 | 8:1:0 |
| WBC (/μL) | 5645 (4415–7793) | 6685 (5380–9243) | 5270 (4300–6545) | 6550 (4490–6850)b |
| Platelet (103/μLa) | 19.8 (15.0–24.5) | 21.0 (14.4–25.6) | 19.3 (15.7–24.1) | 19.0 (16.7–24.5) |
| Alb (mg/dL) | 3.6 (3.1–4.0) | 3.8 (3.3–4.1) | 3.5 (3.1–4.0) | 3.5 (3.2–3.8) |
| Cre (mg/dL) | 0.63 (0.53–0.81) | 0.80 (0.61–1.03) | 0.60 (0.52–0.66) | 0.63 (0.51–0.89) |
| AST (U/L) | 545 (328–933) | 496 (275–849) | 736 (434–1149) | 388 (275–514) |
| ALT (U/L) | 641 (424–1085) | 658 (429–1041) | 707 (558–1210) | 516 (390–978) |
| ALP (U/L) | 505 (364–734) | 484 (357–842) | 595 (400–764) | 453 (302–521) |
| T-Bil (mg/dL) | 4.6 (1.6–11.0) | 2.9 (1.2–14.7) | 6.2 (2.4–11.6) | 6.3 (2.3–7.9) |
| D-Bil (mg/dL) | 2.9 (0.7–8.9) | 1.8 (0.5–11.5) | 4.7 (1.6–9.9) | 4.0 (0.7–6.0) |
| IgG (mg/dL) | 1590 (1270–2119) | 1416 (1154–1595) | 1785 (1457–2305) | 1191 (1066–1300)a,b |
| IgM (mg/dL) | 115 (79–181) | 96 (67–149) | 113 (87–213) | 140 (102–165) |
AIH, autoimmune hepatitis; Alb, Albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Cre, creatinine; D-Bil, direct bilirubin; DILI, drug-induced liver injury; Ig, immunoglobulin; PT-INR, prothrombin time international ratio; T-Bil, total bilirubin; WBC, white blood cell.
P < .05, AIH, vs Undetermined cause.
P<.05, AIH, vs DILI.
Modified RUCAM and IAIHG scores and laboratory data
To refrain from calculation of histological findings and chronological data, we modified the RUCAM and IAIHG scores. The m-RUCAM score was calculated by age, time until onset of liver injury, alcoholic intake, and previous information on the hepatotoxicity of the drug (Table A1). The m-RUCAM values ranged from 0 to 6. The m-IAIHG score was calculated by sex, IgG level, ratio of alkaline phosphatase to ALT/AST, medication history, presence of autoantibodies, absence of antimitochondrial antibody, and alcoholic intake, and the value ranged from −12 to 13 (Table A1). Daily alcohol intake in each patient was graded according to 3 categories for IAIHG score (<25 g/d, 25–60 g/d or >60 g/d) and 2 for RUCAM (<20 g/d or ≥20 g/d).
White blood cell counts , plasma prothrombin time (PT), international normalized ratio (PT-INR), and serum levels of albumin , alkaline phosphatase, ALT, AST, creatinine, IgG, IgM, and total bilirubin were analyzed using an autoanalyzer (JCA-BM2250; JEOL, Tokyo, Japan).
Statistical analysis
The results are expressed as the median value and range. All statistical analyses were performed using the SPSS 17.0 software program (SPSS Inc, Chicago, IL). One-way analysis of variance with Dunnet T3 tests were used to evaluate the statistical significance of the results. A 2-sided P-value of <.05 was considered statistically significant. A receiver operating characteristic curve (ROC) was used to assess the diagnostic performance of each etiology. The cut-off values for each diagnosis were estimated using the Youden index. The primary endpoint of the derivation cohort was the diagnosis of DILI. Proportion of DILI among the derivation cohort was 27.3% (29/106), whereas that was 29.0% (216/746) in the national survey of SLI/ALF in Japan.16 Therefore, we assumed proportion of DILI in the validation cohort was about 27%–29%.
Results
Demography, laboratory data, and prognosis in 2 cohorts
According to the inclusion criteria in this study, all patients were diagnosed with ALI due to either DILI, AIH, or a UC. Age, laboratory data, and prognosis of the derivation cohort are summarized and compared in Table 1. There was no significant difference in sex among DILI, AIH, and UC groups. The serum level of albumin was significantly lower in AIH group than in UC group, and IgG was significantly higher in AIH group than in DILI and UC group. However, there was no parameter with a statistically significant difference in DILI group compared with the other 2 groups.
Table 2 summarizes demographic and clinical data and prognosis in the validation cohort. There was no significant difference in the proportion of females among the 3 groups. White blood cell count was significantly lower in AIH group than in DILI group and IgG was significantly higher in AIH group than in DILI and UC groups. However, there were no other significant parameters which were significantly different among the 3 groups. There were no pregnant patients in the derivation and validation cohorts.
Causal drugs of DILI were summarized in Table A2. The most frequent causal agent was dietary supplement in the derivation and validation cohorts. Chinese herb was also found as the second causal drug in both cohorts.
Diagnostic accuracy of RUCAM and IAIHG for the diagnosis of DILI
We first examined the diagnostic accuracy of RUCAM and IAIHG scores for ALI due to DILI in the derivation cohort. The area under ROC (AUROC) of the RUCAM and IAIHG scores were 0.942 and 0.999, respectively. Thus, the RUCAM and IAIHG score were found to be useful for the distinction of DILI from non-DILI, AIH, and a UC. Alcohol intake and delayed improvement of transaminase after discontinuation of causative drug use for RUCAM score and results in viral markers, alcohol intake, and a high IgG level for IAIHG score contributed negatively to the accuracy of each score (Figures A1 and A2).
Modified RUCAM and IAIHG scores for the diagnosis of DILI in derivation cohort
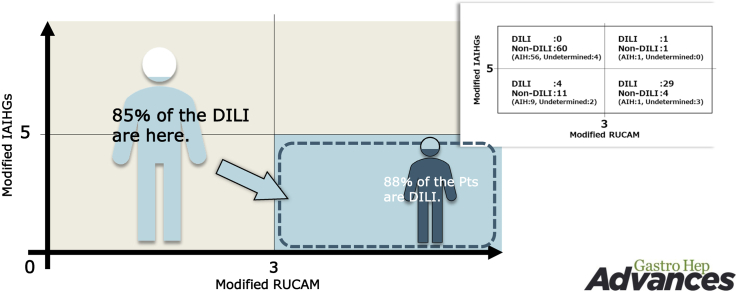
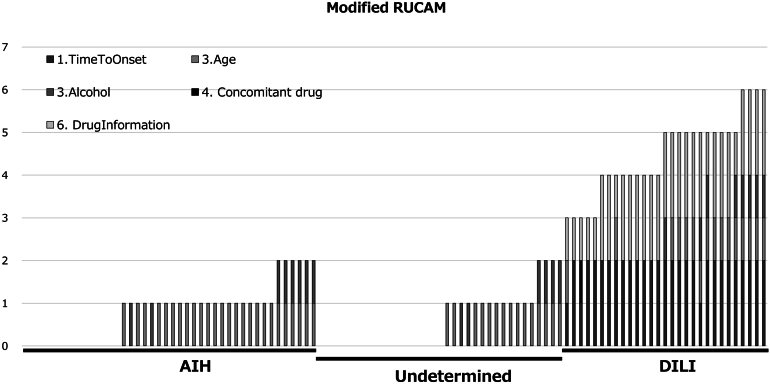
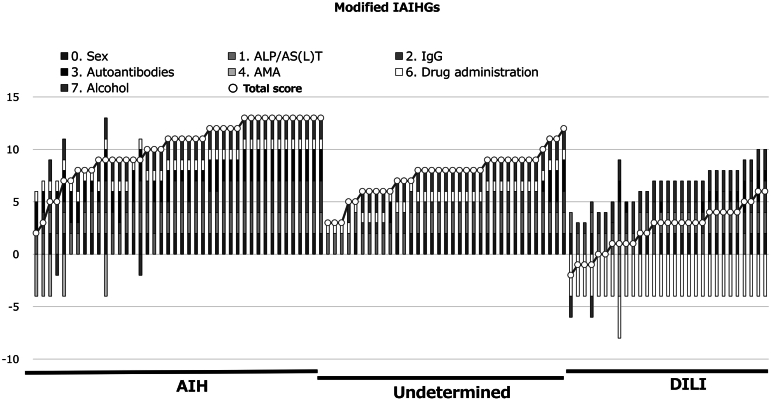
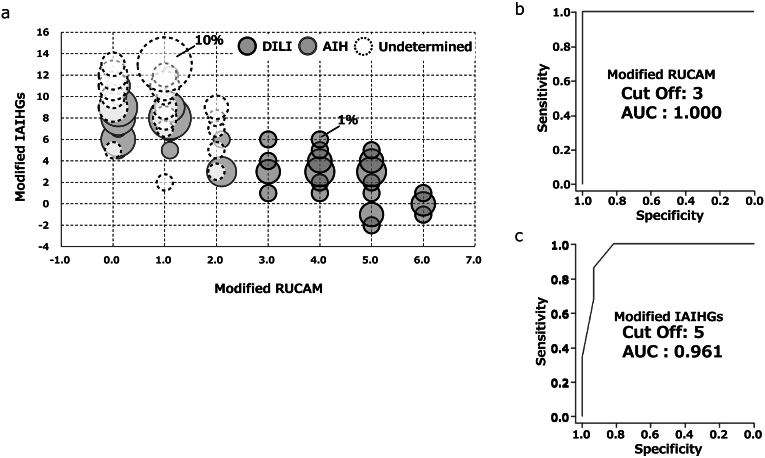
Figures 1 and 2 indicate values of m-RUCAM and IAIHG in patients in AIH, UC, and DILI groups. As shown in the figures, there were trends toward lower score for the m- RUCAM and the m-IAIHG in patients with DILI than those with AIH and a UC. Figure 3A indicates relations between the m-RUCAM and m-IAIHG scores. As shown in Figure 3B and C, the AUROCs of the m-RUCAM score and IAIHG score for the diagnosis of DILI were calculated to be 1.00 and 0.96 (Figure 3B and C), respectively, when each cut-off value was determined to be 3 for m-RUCAM and 5 for m-IAIHG.
Figure 1.
Distribution of modified RUCAM scores in patients. Horizontal axis indicates each patient. Diagnosis is presented in boxes in the upper part of the graph. Squares in each bar indicate the parameters of the modified RUCAM scores.
Figure 2.
Distribution of modified IAIHGs scores in patients. Horizontal axis indicates each patient. Diagnosis is presented in boxes in the upper part of the graph. Squares in each bar indicate the parameters of the modified IAIHGs scores.
Figure 3.
Scatter diagram representing the relationship between the modified scores of RUCAM and IAIHG in the derivation cohort. Horizontal axis indicates the modified RUCAM score and vertical axis indicates the modified international AIH group (IAIHG) score. Dark gray, gray, and white circles indicate DILI, AIH, or UC (undetermined) patients, respectively. Size of circle indicates number of patients (A). ROC analysis of modified scores of RUCAM (B) and IAIHG (C).
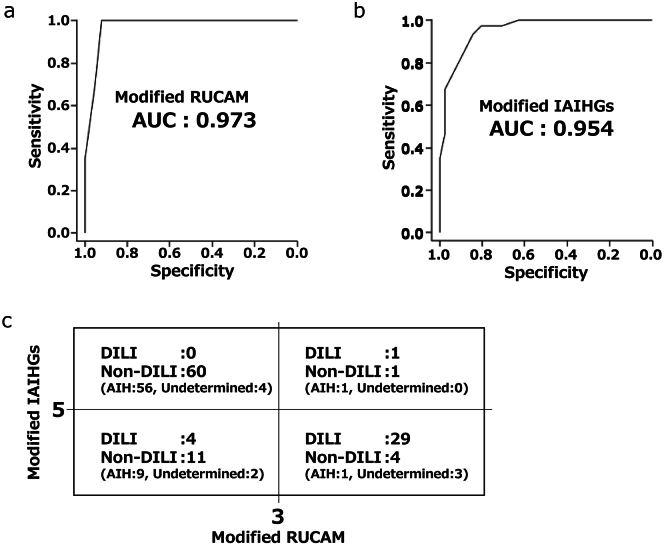
Modified RUCAM and IAIHG scores for the diagnosis of DILI in validation cohort
The AUROC of the m-RUCAM score and m-IAIHG score for the diagnosis of DILI were 0.973 and 0.954, respectively (Figure 4B and C). To assess the clinical utility of the combination of the 2 scores in identifying patients with DILI, the diagnostic accuracy was calculated and shown in Figure 4D. When the cut-off value of 3 for the m-RUCAM score and the value of 5 for the m-IAIHG score were applied, the accuracy for the diagnosis of DILI was calculated to be 81% (89/110).
Figure 4.
Combination with modified scores of RUCAM and IAIHG were accurately diagnosed DILI in the validation cohort. ROC analysis of modified scores of RUCAM (A) and IAIHG (B). Proportion of patients with DILI or other in each group according to the 2 modified scores (C).
Discussion
The aim of the present study was to evaluate modified scores using data from the first assessment of patients with ALI to distinguish DILI from ALI due to either AIH or an UC. The results showed that RUCAM and IAIHG scores were useful for the diagnosis of DILI, and that m-RUCAM and m-IAIHG scores that were calculated using data at the time of the first assessment were able to distinguish DILI from ALI/SLI due to AIH or to an UC. m-RUCAM and m-IAIHG scores can be calculated using routine laboratory data and data from medical interviews. Thus, the results of the present study suggest that medical interviews concerning DILI and laboratory data are useful in diagnosing patients with ALI due to DILI.
A previous study reported that DILI and hepatitis B virus complications are etiologies associated with a poor prognosis in patients with ALI. In addition, an effective medical therapy has not been established for patients with DILI, while a nucleotide analog can be used as a treatment for patients with hepatitis B virus complications.17 Thus, DILI should be considered a risk factor for a poor prognosis in patients with ALI/SLI. In contrast, acute viral hepatitis, an UC, and AIH are favorable etiologies in patients with ALI/SLI. Acute viral hepatitis is self-limiting, and AIH is controllable with appropriate immunosuppression. Although the detailed mechanisms behind it remain unclear, a proportion of hepatic encephalopathy development drastically decreased after early intervention through the referral system.18 Therefore, DILI should be distinguished from ALI due to AIH or a UC. The present study demonstrated that careful medical interviews provide useful information for the diagnosis of DILI. In line with these results, a previous report established that expert opinion is very useful for the diagnosis of DILI.19 Furthermore, additional laboratory data can help physicians to diagnosis AIH, which should be excluded during the diagnostic process in ALI/SLI patients. With this regard, the modified scores seem inevitable in identifying patients with DILI.
The modified scores revealed the diagnostic difficulty associated with specific patient characteristics. Alcoholic intake is a common risk factor in the 2 scores. A high level of IgG and a positive result for autoantibodies in DILI patients adds points to the IAIHG score. A low IgG level or negative result for autoantibodies in patients with AIH may also be missed by the m-IAIHG score. These factors decrease the accuracy of the modified scores. Considering the primary aim of the present study, however, it is crucial that DILI can be distinguished from ALI due to AIH or a UC at the first identification of ALI/SLI. Therefore, we propose the modified scores for the diagnosis of DILI, although we recognize that the scores may decrease diagnostic accuracy in a certain proportion of patients, who have confounding factors.
The treatment of patients with ALI/SLI must be based on the etiology of the ALI/SLI. Although the modified scores in the present study would be useful for a diagnosis of DILI, a comprehensive diagnosis is not always achieved before the start of therapy. For safe management, prediction of the patients who progress to severe type of ALI (SLI/ALF) is also helpful in determining the time to start treatment. Two previous studies showed potential indicators for when to start treatment. One of the studies reported that a PT-INR score less than 1.3 at one week after ALI identification predicted transplant-free survival.20 Another study reported that a PT-INR score less than 1.32 could predict which patients with ALI/SLI would spontaneously recover.21 Both studies suggested that a 1.3 PT-INR score is an indication to start treatment. However, those studies did not take the clinical course of a specific etiology into consideration. Hence, ALI/SLI patients with DILI need to be observed under a careful assessment of disease severity.
There are several limitations to this study. First, the diagnosis of DILI by senior hepatologists could have been affected by several parameters of the RUCAM score because the hepatologists had already used the RUCAM score in clinics.22 Therefore, the RUCAM for patients with DILI might have scored high compared to those of patients with AIH. However, it is a novel finding that IAIHG scores are complementary to RUCAM scores among the study population. Second, there were no patients with drug-induced AIH in this study. Drug-induced AIH has been recognized as a liver injury due to autoimmunity triggered by administration of a drug.23 A previous study reported that patients with drug-induced AIH showed similar positivity for autoantibodies to those with AIH.24 However, a drug history subtracts 4 points from the IAIHG score. If drug-induced AIH were included in this study, the accuracy of the modified scores would have decreased. However, drug-induced AIH is a condition that should be considered a differential diagnosis for DILI when liver injury is sustained after the discontinuation of a causative drug. Finally, the results were not confirmed by prospective data. To generalize the findings of the present study, the utility of the modified scores needs to be validated in a future prospective study.
Conclusion
In conclusion, we found that a combination of m-RUCAM and m-IAIH based on detailed medical interviews and routine laboratory data could distinguish DILI from non-DILI in patients with ALI. Our present study also revealed that medical interview about drug administration is crucial for the early diagnosis of DILI in the ALI patients. The application of m-RUCAM and m-IAIH for the diagnosis and treatment of ALI should be validated in a prospective study.
Acknowledgments
Authors' Contributions:
Keisuke Kakisaka: Design of the research, drafting the manuscript, and final approval of the article. Naoya Kato, Kotaro Kumagai, Takuro Hisanaga, Takayuki Kondo, Toru Setsu, Shunsuke Sato, Yohei Kooka, Kei Endo, Takayoshi Oikawa, Hidekatsu Kuroda, Akio Miyasaka, Ryuzo Abe, Taka-aki Nakada, Yoshihiro Ikura, and Kenichi Harada: Acquisition of the data, and final approval of the article. Yuichi Yoshida and Hidekatsu Kuroda: Statistical analysis of the data, and final approval of the article. Takuya Genda, Shuji Terai, Naoya Kato, Taro Takami, and Akio Ido: Interpretation of the data, and final approval of the article. Satoshi Mochida: Conception of the research, interpretation of the data and final approval of the article. Takayuki Matsumoto: Interpretation of the data, revising the draft, and final approval of the article.
Footnotes
Conflicts of Interest: The authors disclose no conflicts.
Funding: This study was supported in part by a Health Labor Sciences Research Grant from the Ministry of Health, Labor, and Welfare of Japan as a project by the Intractable Hepato-Biliary Diseases Study Group of Japan (JPMH20FC1023).
Ethical Statement: The corresponding author, on behalf of all authors, jointly and severally, certifies that their institution has approved the protocol for any investigation involving humans or animals and that all experimentation was conducted in conformity with ethical and humane principles of research.
Data Transparency Statement: The dataset used and/or analyzed during the current study is available from the corresponding author on reasonable request.
Reporting Guidelines: STROBE, SAGER.
Material associated with this article can be found in the online version at 10.1016/j.gastha.2023.02.002.
Supplementary materials
and A2
Distribution of RUCAM scores in patients. Horizontal axis indicates each patient. Diagnosis is presented in boxes in the upper part of the graph. Squares in each bar indicate the parameters of the modified RUCAM scores.
Distribution of IAIHGs scores in patients. Horizontal axis indicates each patient. Diagnosis is presented in boxes in the upper part of the graph. Squares in each bar indicate the parameters of the modified IAIHGs scores.
References
- 1.Lee W.M., Stravitz R.T., Larson A.M. Introduction to the revised American Association for the Study of Liver Diseases position paper on acute liver failure 2011. Hepatology. 2012;55:965–967. doi: 10.1002/hep.25551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kakisaka K., Suzuki Y., Abe H., et al. Early identification using the referral system prolonged the time to onset for hepatic encephalopathy after diagnosing severe acute liver injury. Sci Rep. 2020;10:17280. doi: 10.1038/s41598-020-74466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koch D.G., Speiser J.L., Durkalski V., et al. The natural history of severe acute liver injury. Am J Gastroenterol. 2017;112:1389–1396. doi: 10.1038/ajg.2017.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patton H., Misel M., Gish R.G. Acute liver failure in adults: an evidence-based management protocol for clinicians. Gastroenterol Hepatol (N Y) 2012;8:161–212. [PMC free article] [PubMed] [Google Scholar]
- 5.Takikawa Y., Endo R., Suzuki K., et al. Early prediction of short-term development of hepatic encephalopathy in patients with acute liver disease unrelated to paracetamol. A prospective study in Japan. J Hepatol. 2009;51:1021–1029. doi: 10.1016/j.jhep.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Kakisaka K., Suzuki Y., Jinnouchi Y., et al. Unfavorable prognosis of patients with acute liver injury due to drug-induced liver injury and acute exacerbation of hepatitis B virus infection. Hepatol Res. 2019;49:1286–1293. doi: 10.1111/hepr.13397. [DOI] [PubMed] [Google Scholar]
- 7.Prasidthrathsint K., Stapleton J.T. Laboratory diagnosis and monitoring of viral hepatitis. Gastroenterol Clin North Am. 2019;48:259–279. doi: 10.1016/j.gtc.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki A., Brunt E.M., Kleiner D.E., et al. The use of liver biopsy evaluation in discrimination of idiopathic autoimmune hepatitis versus drug-induced liver injury. Hepatology. 2011;54:931–939. doi: 10.1002/hep.24481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roussel Uclaf causality assessment method (RUCAM) in drug induced liver injury. LiverTox: clinical and research information on drug-induced liver injury. National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2012. [PubMed] [Google Scholar]
- 10.Alvarez F., Berg P.A., Bianchi F.B., et al. International autoimmune hepatitis group report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929–938. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
- 11.Ahmad J., Barnhart H.X., Bonacini M., et al. Value of liver biopsy in the diagnosis of drug-induced liver injury. J Hepatol. 2022;76:1070–1078. doi: 10.1016/j.jhep.2021.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khalifa A., Lewin D.N., Sasso R., et al. The utility of liver biopsy in the evaluation of liver disease and abnormal liver function tests. Am J Clin Pathol. 2021;156:259–267. doi: 10.1093/ajcp/aqaa225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kakisaka K., Suzuki Y., Takahashi F., et al. Referral system has a diminished difference in the risk for hepatic encephalopathy development among each etiology in patients with acute liver injury. Hepatol Res. 2022;52:401–410. doi: 10.1111/hepr.13744. [DOI] [PubMed] [Google Scholar]
- 14.Onji M., Zeniya M., Yamamoto K., et al. Autoimmune hepatitis: diagnosis and treatment guide in Japan, 2013. Hepatol Res. 2014;44:368–370. doi: 10.1111/hepr.12300. [DOI] [PubMed] [Google Scholar]
- 15.Czaja A.J. Performance parameters of the diagnostic scoring systems for autoimmune hepatitis. Hepatology. 2008;48:1540–1548. doi: 10.1002/hep.22513. [DOI] [PubMed] [Google Scholar]
- 16.Nakao M., Nakayama N., Uchida Y., et al. Nationwide survey for acute liver failure and late-onset hepatic failure in Japan. J Gastroenterol. 2018;53:752–769. doi: 10.1007/s00535-017-1394-2. [DOI] [PubMed] [Google Scholar]
- 17.Wong V.W., Wong G.L., Yiu K.K., et al. Entecavir treatment in patients with severe acute exacerbation of chronic hepatitis B. J Hepatol. 2011;54:236–242. doi: 10.1016/j.jhep.2010.06.043. [DOI] [PubMed] [Google Scholar]
- 18.Kakisaka K., Kataoka K., Suzuki Y., et al. Necrotic cell death and suppression of T-cell immunity characterized acute liver failure due to drug-induced liver injury. Cytokine. 2016;86:21–28. doi: 10.1016/j.cyto.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Rockey D.C., Seeff L.B., Rochon J., et al. Causality assessment in drug-induced liver injury using a structured expert opinion process: comparison to the Roussel-Uclaf causality assessment method. Hepatology. 2010;51:2117–2126. doi: 10.1002/hep.23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mawatari S., Moriuchi A., Ohba F., et al. The recovery of the PT-INR to less than 1.3 predicts survival in patients with severe acute liver injury. J Gastroenterol. 2018;53:861–872. doi: 10.1007/s00535-017-1421-3. [DOI] [PubMed] [Google Scholar]
- 21.Kakisaka K., Suzuki Y., Kataoka K., et al. Predictive formula of coma onset and prothrombin time to distinguish patients who recover from acute liver injury. J Gastroenterol Hepatol. 2018;33:277–282. doi: 10.1111/jgh.13819. [DOI] [PubMed] [Google Scholar]
- 22.Teschke R. Idiosyncratic DILI: analysis of 46,266 cases assessed for causality by RUCAM and published from 2014 to early 2019. Front Pharmacol. 2019;10:730. doi: 10.3389/fphar.2019.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Boer Y.S., Kosinski A.S., Urban T.J., et al. Features of autoimmune hepatitis in patients with drug-induced liver injury. Clin Gastroenterol Hepatol. 2017;15:103–112.e2. doi: 10.1016/j.cgh.2016.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bjornsson E., Talwalkar J., Treeprasertsuk S., et al. Drug-induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatology. 2010;51:2040–2048. doi: 10.1002/hep.23588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
and A2
Distribution of RUCAM scores in patients. Horizontal axis indicates each patient. Diagnosis is presented in boxes in the upper part of the graph. Squares in each bar indicate the parameters of the modified RUCAM scores.
Distribution of IAIHGs scores in patients. Horizontal axis indicates each patient. Diagnosis is presented in boxes in the upper part of the graph. Squares in each bar indicate the parameters of the modified IAIHGs scores.