Abstract
Transcatheter aortic valve replacement has emerged as a safe and effective alternative to surgical aortic valve replacement for patients with severe symptomatic aortic stenosis across the spectrum of surgical risks based on a series of foundational randomized clinical trials. Of note, patients with bicuspid aortic valve (BAV) disease were excluded from all these pivotal randomized trials, leaving a significant knowledge gap because BAVs are commonly encountered in patients referred for aortic valve surgery or intervention. In this comprehensive review, we aim to provide heart teams with a detailed insight into how to approach patients with BAV disease, focusing on imaging and characterization of bicuspid valves, an overview of surgical approaches, and an understanding of the current data behind the role of transcatheter aortic valve replacement for patients with BAV disease.
Keywords: bicuspid aortic valve, severe aortic stenosis, surgical aortic valve replacement, transcatheter aortic valve replacement
Central Illustration
Highlights
-
•
A comprehensive review of bicuspid valve disease, focusing on imaging and therapeutic options.
-
•
Detailed discussion of multimodality imaging for bicuspid aortic valve disease.
-
•
Approaches to surgery and transcatheter aortic valve replacement.
-
•
Discussion of available data and technical considerations.
-
•
Emphasis on multidisciplinary heart team evaluation at dedicated valve centers.
Introduction
The past decade has witnessed a transformation in the care and management of aortic valve disease.1, 2, 3, 4, 5 During this time, the most common therapeutic treatment option for severe symptomatic aortic stenosis in the United States has shifted from surgical aortic valve replacement (SAVR) to transcatheter aortic valve replacement (TAVR).6 In addition, the role of TAVR is now being explored beyond the management of severe symptomatic disease, with ongoing randomized clinical trials evaluating the role of TAVR in asymptomatic patients with severe aortic stenosis (NCT03042104) and patients with moderate aortic stenosis and cardiac dysfunction (NCT02661451 and NCT04889872). Notably, however, in all major randomized studies that have been conducted to date, and in the majority of the ongoing studies mentioned above, patients with bicuspid aortic valve (BAV) disease have been excluded. This omission leaves a significant gap in knowledge because although only 1% to 2% of the population has BAV, patients with BAV represent up to 50% of patients who have historically needed SAVR.7 As a result, patients as well as cardiologists, cardiac surgeons, and other members of collaborative multidisciplinary heart teams are often left uncertain of how to best characterize BAV disease and furthermore how to make an informed decision about the best options for definitive management of BAV disease. The goal of this review is to provide heart teams with an understanding of how to approach patients with BAV disease, focusing on imaging and characterization of bicuspid valves, an understanding of surgical approaches to BAV disease, and, lastly, understanding the current data behind the role of TAVR for patients with BAV disease.
Imaging of bicuspid valves
For patients with BAV disease, imaging plays a critical role in early identification, accurate assessment of aortic pathophysiology, and planning of timely intervention. Partly because of the higher incidence of valvular dysfunction and aortopathy in patients with BAV, multimodality imaging serves as the cornerstone of a comprehensive approach to the evaluation of patients with BAV disease. Given the unique anatomic characteristics of patients with BAV disease, it is necessary for heart teams to understand the information that may be distilled by the multimodality imaging interrogation of this complex disease process.
Echocardiography
Transthoracic echocardiography (TTE) serves as the primary tool for the initial diagnosis of BAV morphology and its associated aortic pathophysiology. From the perspective of the multidisciplinary heart team, there are 3 general categorical approaches to classification of patients with BAV pathologies. These are as follows: (1) complex valvuloaortopathy: patients diagnosed at earlier stages of life when aortopathy and associated disease conditions present sooner than valvular pathology, (2) typical valvulopathy: commonly diagnosed in patients presenting with BAV disease-associated valvular pathology with or without progressive aortopathy, and (3) acquired bicuspid valve morphologies presenting at later stages in patients’ lives.8
Aortic valve morphology
There are multiple echocardiographic views that help with the baseline interrogation of aortic pathophysiology. Short-axis aortic valve cross sections are commonly the first line of evaluation for valvular morphology using 2-dimensional echocardiography. The accuracy of echocardiographic delineation of patients’ aortic valve morphology is highly dependent on echogenic imaging windows and the presence or absence of heavy calcific artifacts in the area of interest.2 Systolic doming of the aortic valve leaflets, coexisting aortopathy in parasternal long-axis views, the presence of a raphe or asymmetric sinuses of Valsalva in parasternal short-axis views are indicators for a more thorough examination of the aortic valve (Figure 1). An asymmetric aortic root is commonly noted with an incidence of 48% in patients with BAV with fused right and noncoronary cusp morphology and in 50% of the population with Sievers type 0 morphotype.9 TTE is an excellent tool for morphologic classification and quantification of affiliated valvular pathology, ie, regurgitation, and stenosis with morphologic phenotypes commonly identified using short-axis imaging based on the position of raphae (Figure 2).10
Figure 1.
(A) Parasternal echocardiographic imaging depicting systolic doming of the aortic valve leaflets (arrow). (B) Unequal sinuses of Valsalva observed using parasternal long-axis imaging. (C) Unequal sinuses observed using aortic short-axis imaging
Figure 2.
Sievers and Schmidtke classification of the morphotypes of the bicuspid valve. Based on the presence and number of raphae, bicuspid aortic valves are classified into 3 categories. Type 0, no raphe (5%-7%); type 1, presence of 1 raphe with fusion of any 2 cusps (right-left cusp fusion [70%-80%], right-noncusp fusion [20%-30%] left-noncusp fusion [3%-6%]); and type 2, presence of 2 raphae. Partial-fusion bicuspid aortic valves (forme fruste) with small or mini raphae with partial fusion of the cusps, which is not part of the Sievers classification but is in the International Consensus Statement classification.8
Assessment of the degree of aortic stenosis
Stenosis is observed more often in patients with BAV in the later stages of life and is more often seen in men. The following methods are used to quantify the severity of aortic stenosis:
-
•
Hemodynamic assessment using peak and mean transaortic gradients and peak transaortic velocity
-
•
Calculation of aortic valve area (AVA) and indexed AVA using the continuity equation
-
•
Direct planimetry of AVA at the leaflet tips on the short axis using parasternal long-axis imaging with orthogonal short-axis view
-
•
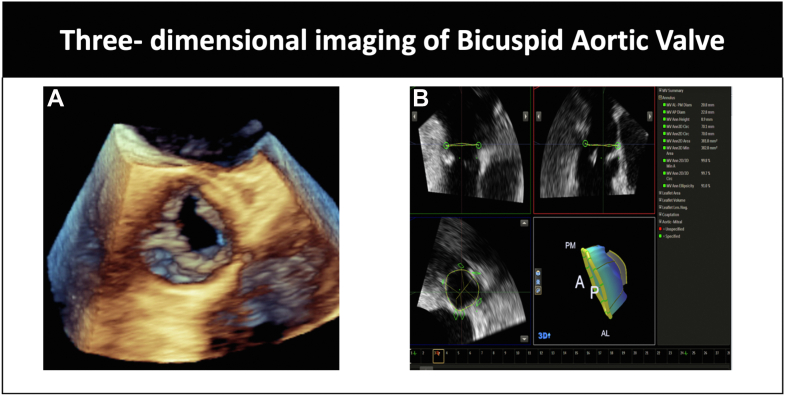
Three-dimensional AVA via planimetry using multiplanar imaging of the aortic valve (Figure 3).
Figure 3.
(A) Three-dimensional imaging of bicuspid aortic valve. (B) Multiplanar reconstruction of the aortic annulus with annular measurements.
Assessment of aortic regurgitation
Aortic regurgitation (AR) is more prevalent in the patient population with BAV, with moderate-to-severe AR observed in ∼30% of patients with bicuspid disease.9,10 Eccentric regurgitant jets are more commonly seen with the origin of the jet closer to raphae. The assessment of AR includes the following:
-
•
Qualitative parameters such as jet width, jet area, and vena contracta
-
•
Quantitative parameters that require the calculation of regurgitant volume, regurgitant fraction, and effective regurgitant orifice area using the principle of the continuity equation
-
•
AVA using pressure half time
-
•
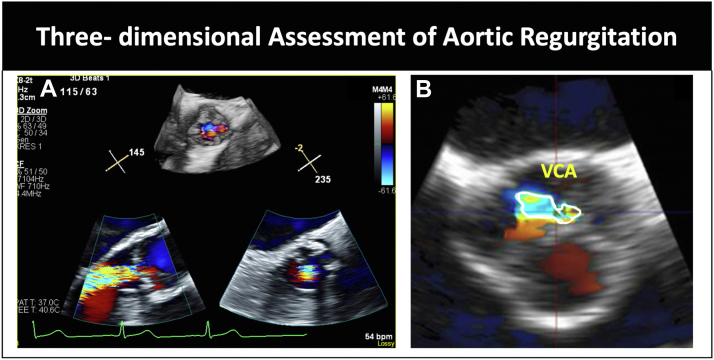
Measurement of vena contracta area using 3-dimensional multiplanar imaging (Figure 4).
Figure 4.
(A) Three-dimensional assessment of aortic regurgitation. (B) Multiplanar alignment and measurement of 3-dimensional vena contracta area. VCA, vena contracta area.
There are potential limitations of some of these quantitative metrics because certain metrics (such as regurgitant jet width and ratio to left ventricular outflow tract [LVOT] diameter) assume a circular regurgitant orifice, which is often not the case in patients with BAV. Therefore, in patients with BAV, it is recommended to rely more on vena contracta width and the quantification of effective regurgitant orifice area and regurgitant volume using the proximal isovelocity surface area method (although this method can also have limitations in patients with eccentric regurgitant jets).
Assessment of aorta
Aortic dilatation is commonly encountered in patients with BAV. Studies have shown that ∼30% of the population with BAV with no significant valvular dysfunction may have aortic dilatation.11 TTE serves as an initial screening tool for identification of the presence of aortic dilatation because it allows imaging of the sinuses of Valsalva and the ascending aorta. However, it is limited in terms of detailed evaluation. TTE provides the following information:
-
•
Dimensions of the sinus of Valsalva, sinotubular, and ascending aorta measured in end diastole stage using parasternal long-axis imaging using the leading-edge-to-leading-edge method
-
•
Measurement of the aortic annulus using inner-wall-to-inner-wall measurement in mid systole
-
•
Three-dimensional aortic annular measurement via direct planimetry using multiplanar reconstruction provides annular measurement, which is more accurate than measurement using a single plane 2-dimensional image12
-
•
Doppler imaging of the descending and ascending aortas for identification of holodiastolic flow reversal (for AR)
-
•
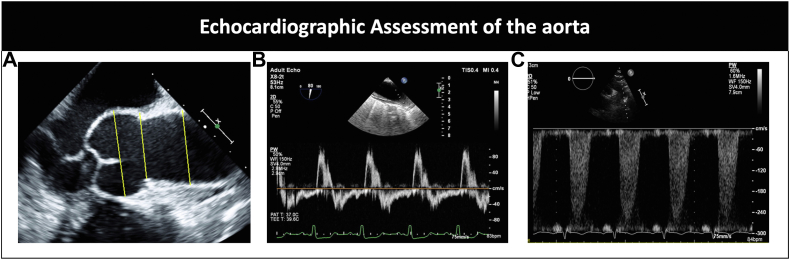
Peak systolic gradient, long time to half peak diastolic velocity, and descending aorta measurement for identification of coexisting coarctation of the aorta (Figure 5).
Figure 5.
(A) Parasternal imaging with measurement of the aortic root at the sinus, sinotubular junction, and ascending aorta using leading-edge-to-leading-edge measurement (yellow lines). (B) Pulse wave Doppler of the ascending aorta showing diastolic flow reversal in a patient with aortic regurgitation. (C) Doppler showing flow acceleration in the descending aorta in a patient with concomitant coarctation of the aorta.
Transesophageal echocardiogram (TEE) allows detailed, invasive assessment of the morphologic phenotype of the valve, assessment of valvular dysfunction, and detection of proximal aortopathy. TEE can also be utilized for hemodynamic assessment of aortic stenosis using the same parameters as those described for TTE. Three-dimensional TEE is a useful tool for the assessment and sizing of the aortic annulus for TAVR sizing and has been shown to be reproducible and comparable with multidetector computed tomography (MDCT).13, 14, 15
Limitations of echocardiography
Suboptimal imaging windows may preclude precise assessment of the valve and aorta. Improper Doppler alignment may lead to underestimation of valvular stenosis and regurgitation. The interpretation of aortic valve dysfunction requires careful attention for accurate measurement of the LVOT and calculation of AVA. Modern 3-dimensional TEE and multidetector slice computed tomography (CT) data sets have demonstrated that the LVOT is not a circular structure. The calculation of AVA may be erroneous based on assumptions and errors in the constituent parameters of the equation, and AVA estimation using the continuity equation may result in underestimation of the degree of valvular pathology if the true dimensions of the LVOT are not accurately accounted for by 3-dimensional imaging and can also result in overestimation of the degree of stenosis.
CT
In recent years, MDCT has become a powerful tool for the assessment of aortic valve pathology. The higher spatial resolution of MDCT makes it an efficient and accurate means for evaluating the aortic valve. This is particularly true for BAV pathologies. Acquisition of artifact-free, electrocardiogram (ECG)-gated CT angiographic images of the cardiac and thoracic aortic vasculatures is quintessential for providing precise diagnosis. CT is helpful in determining the morphologic classification of the valve and quantifying the degree of calcification, thereby aiding in the assessment of the degree of aortic stenosis.16 Multiplanar reconstruction of the aortic valve may allow for planimetry of the aortic valve orifice with greater spatial resolution than that afforded by traditional 2-dimensional echocardiographic windows (Figure 6). CT provides not only high-quality information about the morphology of the aortic valve but also important data regarding the cardiac chambers, the status of coronary artery disease, and additional periprocedural device access case planning. The acquisition of multidetector retrospective ECG-gated CT images provides a wealth of data on valvular morphology in the systolic and diastolic phases of the cardiac cycle.
Figure 6.
Imaging demonstrating key measurements obtained during computed tomographic assessment for baseline and before the procedure.
Assessment of aortic stenosis using CT
-
•
Planimetry of the aortic valve is performed during maximal valve opening. This occurs at ∼50 ms after the R wave. The reconstructed peak systolic phase (50-150 ms) allows optimal imaging for this measurement. A good correlation has been demonstrated between planimetry of AVA using MDCT and that of AVA using TEE compared with transaortic gradients (r = 0.99, P < .001 using MDCT and r = 0.74, P < .01 using TEE).13 CT is superior to TEE in cases in which acoustic shadowing due to calcified leaflets prevents visualization of the valve orifice. More recently, it has been shown that the AVA measured using CT is higher (both via planimetry and calculation using CT-derived LVOT area) than the AVA measured using echocardiographic modalities. This is likely, in part, because of the larger (and more accurate) LVOT measurement using CT than that using LVOT assessment routinely performed using 2-dimensional imaging.14 It has, hence, been suggested that a higher cutoff of 1.2 cm2 versus the traditional cutoff of 1 cm2 be used for defining severe aortic stenosis when AVA measured using CT is being used.15,16
-
•
Cusp calcification is a complex and dynamic process that is mediated by multiple factors, including genetic, inflammatory, and hemodynamic variables. This is much more relevant in patients with BAV because of their abnormal valvular morphology and associated aortopathy. It carries not only morphologic but also prognostic information. The main location of calcification in patients with BAV is raphae, which, over time, leads to valvular degeneration and stenosis. This is noted to occur much earlier than in a normal tricuspid aortic valve. The degree of cusp calcification in BAVs using the Agatston calcium score method is much higher than in those with trileaflet severe aortic stenosis.17 However, the degree of calcification does not correlate with a particular phenotype. The suggested cutoff for the Agatston calcium score for severe stenosis is 2065 AU in men and 1275 in women. CT calcium scoring, like in tricuspid valves, is extremely helpful for patients with borderline or discordant echocardiographic parameters and low flow states.18,19
Assessment of AR using CT
Axial imaging through the aortic root allows identification and detection of aortic leaflet malcoaptation. CT is sensitive and specific in identifying patients with moderate and severe AR.20 Diastolic phase data sets provide qualitative assessment of the degree of AR. Associated features, such as compensatory left ventricular (LV) hypertrophy and LV dilatation, are well visualized and assessed using TAVR CT protocols.
Assessment of the aorta
Aortic root
Multidetector computed tomography provides multiplanar visualization and assessment of the aorta. Multiplanar imaging overcomes the limitations of traditional off-axis 2-dimensional imaging, providing more accurate anatomic assessment of the aortic anatomy. The population with BAV has been noted to have associated aorta pathophysiologies. Mid-ascending aortic aneurysms are seen in 70% to 80% of the population with BAV and aortopathy with sparing of the aortic root. Moreover, 20% to 30% of patients have been noted to have root dilatation (Figure 7). Patients with BAV with fused right-noncoronary cusp morphotype are commonly noted to have a higher incidence of ascending aortic aneurysms, and those with BAV with fused right-left coronary cusp are more commonly associated with involvement of the aortic root.
Figure 7.
Computed tomography imaging of the aorta demonstrating (A) aortopathy affecting the tubular ascending aorta, (B) coarctation of the aorta (arrow), and (C) aneurysmal dilatation of both the aortic root and ascending aorta.
Descending aorta
Seven percent of patients with BAV have coarctation, and this is more common in those with the BAV with the right-left coronary cusp morphotype. The presence and severity of coarctation of the aorta are well assessed using CT.
Limitations of MDCT
Radiation
Computed tomography is unique in its provision of a quick, noninvasive test that provides exceptional anatomic details and spatial resolution. However, it requires contrast and radiation exposure. Tremendous efforts have been made over the last few years to reduce the radiation dose using specific acquisition protocols that allow for all the required information with as little radiation as possible following the as-low-as-reasonably-achievable principle. The goal of computed tomography angiography (CTA) is to obtain diagnostic quality images and minimize the need for repeat imaging and repeat contrast administration. The average age of the population with bicuspid disease being evaluated for aortic valvulopathy is lower than that of their trileaflet counterparts, who are usually in the seventh and eighth decades of life. Minimization of ECG-synchronized scan time and limiting the peak dose coverage while using dose modulation are ways to reduce overall radiation in this population.
Iodinated contrast
Intravenous administration of contrast agents is needed to accurately identify the annular plane, assess aortic root dimensions, and assess the proximal and distal aortic vasculatures. The volume of the contrast agent used depends on the patient’s body size, with larger patients requiring a higher dose for adequate visualization of a structure. Usually, the dose varies between 60 and 100 mL and is dependent on the exact imaging protocol used. The use of intravenous contrast agents may be challenging in patients with impaired renal function and advanced chronic kidney disease. In cases in which the estimated glomerular filtration rate is ≤30 mL/min/1.73m2, TEE may be useful. Noncontrast CT is used for the assessment of calcium scores.
Patient characteristics
Tachycardia and atrial fibrillation pose challenges with ECG synchronization and may lead to artifacts. Beta blockade is not routinely used in these patients. Patient cooperation with breath hold is needed. The presence of prosthetic material in the heart may lead to artifacts, which may affect the overall accuracy of the test.
Cardiac magnetic resonance
Transesophageal echocardiogram, MDCT, and cardiac magnetic resonance (CMR) imaging provide comparable sensitivity in anatomic assessment of BAV. Besides providing information analogous to CT with regard to aortic dimensions, CMR provides additional functional information for the evaluation of the severity of valve pathology and compensatory effects on the left ventricle. It is the preferred imaging modality for pediatric patients both for initial evaluation and follow-up. Aortic dimensions are obtained either using contrast-enhanced or noncontrast-enhanced imaging using inner-to-inner measurement. Balanced steady-state free-precision or cine imaging is useful for the assessment of the aorta and evaluation of the morphology and movement of the aortic valve (Figure 8). Four-dimensional flow CMR is used to visualize abnormal hemodynamic flow patterns (helical or vortical flow) across the aortic valve and into the aorta (Figure 9). Characterization of eccentric regurgitant jets can be performed using CMR.21 LV function and volumes are estimated using ECG-gated balanced steady-state free-precision CMR imaging. Quantitative assessment of AR can be performed using a volumetric assessment.22
Figure 8.
Magnetic resonance imaging of aortic root thickness, aortic dimensions, and coronary height assessment.
Figure 9.
Four-dimensional flow model (time-resolved, phase-contrast, 3-dimensional flow). (A) Flow through trileaflet aortic valve stenosis. (B) Turbulent flow through a bicuspid aortic valve with an aneurysm.
Limitations of CMR
Severe claustrophobia is noted in ∼5% of the population that undergoes these tests. CMR is a considerably longer procedure and requires the patient’s cooperation during the test. In the patient population with valvular heart disease, optimal fluid management must be performed prior to the initiation of a CMR study to ensure that the patients can tolerate the duration of the entire study. The presence of prosthetic material in the heart may additionally lead to creation of artifacts, which may affect the overall accuracy of the test.
Identification of high-risk features for TAVR using multimodality imaging
Transcatheter aortic valve replacement in BAVs presents unique challenges compared with TAVR in tricuspid aortic valves. Screening using multimodality imaging (echocardiography with CT or CMR with CT) is essential to thoroughly evaluate the morphology of the aortic valve apparatus prior to sizing and selection of the type of valve. The important differences between BAVs and tricuspid aortic valves pertinent to TAVR include the following:
-
•
Eccentric orifice
-
•
Annular and supra-annular size mismatch
-
•
Nonuniform and bulky focal calcification
-
•
Presence of raphae
-
•
Concomitant aortopathy
Echocardiography helps identify some of these features, which is then followed by CT evaluation (Central Illustration depicts a high-risk feature algorithm for consideration of TAVR for BAV stenosis).
Central Illustration.
Algorithm highlighting high-risk features on preassessment and the need for careful consideration prior to interventional planning. AV, aortic valve; CMR, cardiac magnetic resonance; CT, computed tomography; LVOT, left ventricular outflow tract; TAVR, transcatheter aortic valve replacement; TEE, transesophageal echocardiography.
Surgical interventions for patients with BAV disease
Surgeons view BAV disease as both a valvular and an aortic disease, with heterogeneity in presentation. BAV disease is common among surgical series, and patients with bicuspid disease can represent ∼50% of aortic valve replacements at surgical centers.23 Most patients who undergo SAVR for bicuspid valve disease have severe aortic stenosis. Aortic stenosis has been reported as the primary cause of valve replacement in patients with bicuspid disease in 61% to 88% of population-based studies and those from tertiary referral centers. Conversely, aortic insufficiency is the reason for aortic valve surgery in 15% to 29% of patients.11,24,25 The most common presentation of BAV disease to cardiac surgeons is isolated aortic valve dysfunction that necessitates either valve replacement or valve repair. Valve repair for bicuspid valve disease can be utilized in experienced hands for pure AR but is beyond the scope of this review. A significant minority of patients who are treated with SAVR for aortic stenosis also undergo operations for ascending or root aneurysms. Associated aortopathy is common in patients with bicuspid valve disease, with aortic dilation noted in up to 40% of patients.26 While considering TAVR versus SAVR for BAV disease, the high proportion of patients with aortic dilation can complicate decision making.
First performed in 1960 by Dwight Harken21 in Boston, removal and replacement of the aortic valve is a mature therapy, with many thousands of patient outcomes reported in single-center reports,22,27, 28, 29 large clinical databases,30,31 and clinical trials of new prostheses (see Table 1).32, 33, 34 Historically, it has been rare to report outcomes specifically for patients with bicuspid versus tricuspid aortic stenosis who underwent aortic valve replacement; the conventional wisdom among surgeons appears to be that differences in outcomes in patients with bicuspid versus tricuspid aortic stenosis are so unlikely that it is not worth investigating. This is likely mainly related to the fact that all BAV anatomies can be easily handled with open SAVR; so, procedural outcomes are unlikely to vary. Given the reported differences for TAVR for bicuspid valve disease and the exclusion of patients with BAV from randomized controlled trials (RCTs) comparing SAVR with TAVR,35,36 it is likely that SAVR for bicuspid stenotic disease will receive closer scrutiny soon. Until that time, we are left to infer the results of SAVR for patients with bicuspid valve aortic stenosis based on the current series of notable SAVR trials and registry data.
Table 1.
Outcomes with different valves with surgical aortic valve replacement with proportion of patients with bicuspid aortic valve disease shown
| N | Operative mortality, % | Stroke, % | Bicuspid proportion, % | Pacemaker rate, % | Mean gradient | Paravalvular leak, % | |
|---|---|---|---|---|---|---|---|
| Trifecta | 1014 | 1.80 | 0.80 | 29.00 | NR | 4.1-9.3 | 0.10 |
| Inspiris | 689 | 1.20 | 1.60 | 30 | 4.70 | 10.1 ± 4.3 | 0.30 |
| Ghorieshi | 122,474 | 1.90 | 1.20 | NR | NR | NR | NR |
| Mosaic | 1260 | 3.30 | 0.60 | NR | NR | 10.0-16.0 | 0.10 |
NR, not reported.
When a patient with a BAV develops an indication for valve replacement for aortic stenosis and no indication for an aortic operation, typically isolated aortic valve replacement is performed, although a minority of surgeons perform a pulmonary autograft (Ross procedure) in select, typically young patients. Aortic valve bioprostheses can include sutureless stented valves (Perceval and Intuity valves), standard stented valves such as the Carpentier-Edwards pericardial valves or porcine valves such as the Medtronic Mosaic valve, and stentless valves (homografts or porcine roots). Most stentless valves are performed as a full root replacement, although a subcoronary implantation technique is possible. Mechanical valves, used less commonly today, provide excellent durability but require lifelong anticoagulation with warfarin. The most common (>60%) prosthesis utilized in North America today is the stented bioprosthetic valve.37 These valves are selected by surgeons most often because of excellent hemodynamics, ease of implantation, and documented durability.
A comparison of TAVR results with currently published general data on bioprosthetic valves probably unfairly penalizes SAVR because endocarditis is an indication for SAVR that would largely exclude the use of TAVR, and much more morbidity and mortality occur with endocarditis than with other indications for SAVR.38 Nonetheless, recent results from clinical registries (Society of Thoracic Surgeons Adult Cardiac Surgery Database) and clinical trials have provided the best current data until bicuspid-specific aortic stenosis series are reported. The mean gradients are higher than for TAVR, with lower rates of paravalvular insufficiency. Importantly, data regarding the long-term durability of TAVR valves remain limited. The durability of stented bioprosthetic valves has been described. For patients aged >60 years, the 20-year freedom from valve deterioration has been documented to exceed 80%.39,40 The 15-year freedom from valve deterioration among patients aged ≤60 years treated with a pericardial valve was 70%.41 The durability of surgical aortic bioprostheses is not monolithic: early failure has been seen with the Mitroflow, Trifecta, Mosaic, and Toronto valves, and Toronto valves are no longer on the market. The durability of SAVR valves should be considered on an individual rather than a group basis.
Further complicating the picture, aneurysms frequently accompany BAV disease. From referral centers, as many as 30% of patients with bicuspid disease who have an indication for aortic valve replacement also have current indications for aortic replacement.42 BAV disease aortopathy is a heterogeneous disease, with aortic dilation, which can occur in the aortic root, tubular ascending aorta, and/or proximal aortic arch. The current recommendations by several societies are to replace the aorta in patients who have another valvular indication for surgery at 4.5 cm in patients undergoing SAVR for BAV. This is a IIA indication, level of evidence C in American Heart Association/American College of Cardiology 2014 guidelines,43 European Society of Cardiology aortic guidelines,44 and European Society of Cardiology valvular guidelines.45 The evidence in support of these guidelines includes the finding that patients with BAV who underwent aortic valve replacement had a higher rate of events when the aortic size exceeded 4.5 cm46 and a study that showed that a majority of patients with BAV who experienced an aortic dissection did so at an aortic size of ≥4.5 cm (although this was still a small number of patients).47 It should be acknowledged that these guideline recommendations are at least, in part, based on the desire to avoid later redo surgery and that this paradigm does not apply to TAVR. In theory, a patient could receive a TAVR valve that does not extend beyond the sinotubular junction, and if the patient’s ascending aorta eventually dilates to the point that isolated surgical repair is indicated, then first-time surgery could be performed at that point.
Root replacement is also recommended for aortic root sizes >4.5 cm.11,48 It may be reasonable to leave a mildly dilated root (4.5-5 cm) in young patients who have opted for a biologic prosthesis because the risk of root replacement after aortic valve replacement is lower than that of true redo root replacement.
In summary, patients with BAV stenosis commonly undergo SAVR. The conventional wisdom is that the results are not very different for patients who undergo SAVR for aortic stenosis in the trileaflet valve; however, data thus far are lacking. It is likely that reports that detail specific outcomes of SAVR for bicuspid stenosis will be forthcoming soon to serve as a benchmark to compare it with TAVR. Clinicians should also examine the aorta using noninvasive imaging, such as CT or magnetic resonance imaging, once a patient with a BAV meets the criteria for valve replacement because all parts of the ascending aorta cannot be imaged using standard TTE and aortic aneurysms, which can occur anywhere along the aorta, are a common finding in this patient population. Controversy remains about the best recommendation for patients with bicuspid disease who have indications for valve replacement because of stenosis and prefer TAVR, and this discussion follows. Particularly when patients have an aortic aneurysm that exceeds 4.5 cm, we generally favor open operation to decrease the risk of future aortic catastrophes; however, level I evidence does not guide this decision.
Transcatheter interventions for patients with BAV disease
Although the first patient who ever underwent TAVR had a BAV, since the procedure by Dr Alain Cribier in 2002, the vast majority of patients who have undergone TAVR have had trileaflet aortic valves. Compared with calcific trileaflet aortic stenosis, BAV disease represents a much more heterogeneous group. Moreover, there are both specific anatomic and clinical features of BAV disease that make TAVR in this patient population inherently more challenging than in patients with trileaflet aortic valves.
Anatomic considerations
In patients with BAVs, the aortic valve complex is often eccentrically sized, with either a “flare” or “taper” upward from the aortic valve annulus.49,50 This is in contrast to trileaflet aortic valve anatomy, in which the most common configuration of the aortic valve complex is tubular, with consistent sizing through most elements. This anatomic feature of bicuspid anatomy can impede circularization of the transcatheter heart valve51 and can present dilemmas because it relates to valve sizing.52 This is a unique issue for TAVR: although the sizing of surgical aortic prostheses is performed at the time of surgery using direct visualization and measurements of the physical anatomy, sizing for modern-day transcatheter valves is almost entirely determined prior to the procedure based on CT measurements. Appropriate sizing for TAVR in patients with bicuspid disease is also impacted by the fact that both the annular and supra-annular portions of the valve complex are typically more elliptical (ie, less circular).53 These challenges are accompanied by attendant risks either of undersizing and underexpansion (which can lead to significant paravalvular regurgitation) or of oversizing (which can lead to annular injury) or valve dysfunction.
Additional challenges with sizing are presented by the fact that the annular dimensions of patients with BAVs are typically much larger than those of patients with trileaflet aortic stenosis.54,55 These annular dimensions can often fall outside the range of those recommended for treatment with commercial TAVR valves. Off-label treatment of larger annuli with conventional TAVR valves can again risk undersizing and underexpansion, with consequent paravalvular regurgitation. If this is mitigated by either additional volume within a balloon-expandable valve or aggressive postdilatation of a self-expanding valve, this may theoretically lead to damage of the leaflets, with a risk of transvalvular regurgitation, or an increased long-term risk of structural valve deterioration.
Besides increased dimensions of the annulus and entire aortic valve complex, patients with BAV disease have increased aortic dimensions and aortopathy.56,57 This is relevant for patients being considered for TAVR for 2 broad reasons: first, the aortopathy itself cannot be treated by TAVR and may well progress after TAVR is performed to the extent that root replacement would later be indicated based on anatomic criteria58; second, the dilated aorta itself is more prone to injury during instrumentation of a TAVR procedure, with an increased risk of dissection and rupture.59 The aorta is also more likely to take a horizontal orientation, which poses challenges in both advancing and positioning the transcatheter heart valve.60
Patients with BAV disease have a greater degree of calcification than those with trileaflet valves,7,49,61 and this calcification is often bulky and asymmetric. This bulky eccentric calcification can impede crossing and expansion of the transcatheter heart valve; this can increase the risk of paravalvular regurgitation, annular injury or rupture, and, potentially, embolism of calcific material, causing stroke or other clinical sequelae. The presence of a raphe in patients with Sievers types 1 and 2 bicuspid disease62 is also distinct from patients with trileaflet valves, and these raphae are often calcified, which can be associated with adverse clinical outcomes.63
Finally, patients with BAV disease more commonly have some forms of coronary artery anomalies,64 such as separate ostia of the left anterior descending and left circumflex arteries and left-dominant coronary circulation.53,55 The coronary ostia also more commonly lie close to the commissures,64 which can increase the risk of coronary obstruction during TAVR. This is exacerbated by the fact that the native aortic valve leaflets are typically longer and with calcification, which can also lead to an increased risk of coronary obstruction despite conventionally adequate coronary heights.
Clinical considerations
Patients with BAV disease typically present with severe valvular stenosis at a much earlier time point than patients with trileaflet valves because of more rapid disease progression65 causing earlier onset of clinically apparent, hemodynamically significant valvular dysfunction. This has been estimated to be 10 years earlier,66 with the attendant implications for valve durability and potential feasibility of future procedures within that patient’s lifetime. This is a potential challenge for both transcatheter and surgical prostheses because the available data have suggested that the surgical valve longevity is shortest in younger patients67; data on long-term valve longevity are not available for transcatheter heart valves. Patients with BAVs are also more likely to have mixed aortic valve disease or predominant AR.68,69 Patients with isolated or predominant AR are less suited to treatment with TAVR because of absent or mild calcification and frequent coexistent aortopathy.
Observational data on TAVR for BAV disease
Because of the aforementioned complexities of BAVs, patients with bicuspid aortic stenosis were excluded from all foundational RCTs comparing TAVR with SAVR for the treatment of severe symptomatic aortic stenosis. The available data are, therefore, limited to observational studies with various study designs; however, all are inevitably confounded by selection biases.
Early retrospective observational data on TAVR for bicuspid aortic stenosis
The early published experience of performing TAVR for bicuspid aortic stenosis established its feasibility.70 These early studies utilized older versions of the transcatheter heart valve technology (predominantly the balloon-expandable Sapien XT; Edwards Lifesciences, and the self-expanding CoreValve; Medtronic). In this initial retrospective report of 139 patients from 12 centers, the procedural mortality was 3.6%, significant post-TAVR AR was seen in over one-quarter of the patients, and other severe complications, such as conversion to open surgery and valve embolization, were observed in approximately 2% of the patients. Apart from representing early versions of the technology, routine CTA for valve sizing was not incorporated into clinical practice at that time and certainly contributed to some of the adverse events. This is supported by the fact that in this study, the use of CTA for sizing was associated with a reduced risk of AR in a multivariate analysis.
Another early retrospective registry demonstrated similar results. This study compared the outcomes of TAVR for bicuspid aortic stenosis with those of TAVR for tricuspid aortic stenosis in 546 propensity-matched pairs.71 The risk of conversion to early surgery was again 2%, and the risk of requiring implantation of a second transcatheter heart valve was ∼5%. Aortic root injury and significant AR occurred in 1.6% and 10.4% of patients, respectively, and all of these adverse outcomes occurred significantly more frequently than in patients who underwent TAVR for trileaflet valves. However, when this analysis was restricted to patients treated with newer-generation valve platforms, there were no longer significant differences in any of these adverse events between the patients with bicuspid aortic stenosis and those with tricuspid aortic stenosis.
Advances in TAVR technology and techniques as well as retrospective observational data on newer-generation TAVR valves
The early experience of performing TAVR for BAV disease highlighted the need for both technological advances and refinements in procedural planning and techniques. Newer valve platforms include the presence of sealing skirts to increase contact with the native valve anatomy and, therefore, reduce paravalvular regurgitation. CTA for procedural planning and valve sizing is now routine for all TAVR procedures,72 and for patients with bicuspid disease, there is now a focus on annular sizing (as opposed to supra-annular), which has proven to be more reliably reproducible.50 The use of cerebral embolic protection may help to reduce the risk of stroke in patients with bicuspid disease with heavy valve calcification,73 although large-scale RCTs are awaited to prove clinical efficacy. Procedural experience has also evolved to recommend more liberal use of predilatation of the valve in cases with bicuspid disease to facilitate crossing and complete expansion of the transcatheter valve.
These advances in technology and techniques as well as accrual of procedural experience have been shown to be associated with improved outcomes in large-scale observational studies. In a propensity-matched analysis of participants from the STS/American College of Cardiology Transcatheter Valve Therapies Registry, patients who underwent TAVR for bicuspid aortic stenosis and those who underwent TAVR for tricuspid aortic stenosis with the third-generation balloon-expandable Sapien 3 valve were studied.74 In this study of 2691 matched pairs, no difference in paravalvular regurgitation was found between the patients with bicuspid aortic stenosis and those with tricuspid aortic stenosis, although the bicuspid group did have an increased risk of open surgery and stroke at 30 days (it should be noted that the rate of conversion to open surgery was still lower than that in previous studies, at 0.9%). A newer publication from the same registry focused on patients considered to be at a low surgical risk (STS-predicted risk of mortality of <3%) and included some patients who received the fourth-generation Sapien ultra balloon-expandable valve (with the addition of a sealing skirt), along with Sapien 3.75 In this analysis of 3168 propensity-matched pairs, no difference was found between TAVR for bicuspid aortic stenosis and that for tricuspid aortic stenosis in terms of the outcomes of stroke, mortality, paravalvular regurgitation, and any procedural complications, including conversion to open surgery.
In a similar propensity-matched analysis of 929 pairs from the STS/American College of Cardiology Transcatheter Valve Therapies Registry, the authors focused on TAVR with the self-expanding Evolut-R and Evolut-PRO devices (third- and fourth-generation valve platforms).76 There was no difference in the outcomes of stroke, mortality, and paravalvular regurgitation at 1 year between the bicuspid and tricuspid groups.
However, these large retrospective studies are inherently limited by selection biases as well as dependence on site reporting of outcomes and the absence of echocardiographic core laboratories.
Imaging phenotyping using advanced CT
Apart from improved technology and technical experience, part of the improvement observed in the outcomes of TAVR for bicuspid aortic stenosis can be attributed to a more sophisticated understanding of which bicuspid anatomies are more and less suitable for treatment with TAVR. This is based on advanced CT analyses, with an understanding that the morphology of bicuspid valves can predict the clinical outcomes of TAVR. An international multicenter registry of consecutive patients with bicuspid disease undergoing TAVR focused on the phenotyping of this valve using a core laboratory analysis of all CT scans. The patients were classified according to the Sievers classification system; patients who had the presence of a raphe (Sievers types 1 and 2) were classified according to whether the raphe was calcified. Finally, the severity of calcification of the LVOT and the valve leaflets themselves was examined. This study demonstrated that the presence of a raphe itself conferred an adverse prognosis, with higher mortality for patients with Sievers types 1 and 2 than for patients with Sievers type 0, and those with a calcified raphe had higher mortality than those with a noncalcified raphe. It was also demonstrated that excess leaflet calcification was similarly associated with increased mortality (excess leaflet calcification was defined as leaflets with a calcium volume greater than the median of the entire cohort). Patients with both a calcified raphe and excess leaflet calcification had the worst prognosis, with the highest mortality compared with patients with either 1 or none of these adverse features. The mortality at 2 years with both the features present was 25.7%; with 1 feature present, it was 9.5%; and with none of these features present, it was 5.9% (log-rank P < .001). Besides mortality, patients with both the features also experienced worse AR after TAVR and an increased risk of aortic root injury. These data are limited by the inability to easily prospectively apply them while assessing patients for TAVR; excess leaflet calcification was determined by the population studied and defined as a volume greater than the median value.
Prospective observational data on TAVR for bicuspid aortic stenosis
Compared with previously described large-scale, retrospective registries, prospective observational studies benefit from independent clinical event committees to adjudicate clinical outcomes and centralized independent core laboratories to analyze echocardiographic data. Such an approach has been utilized in a handful of small prospective studies, the first of which was the Low-Risk TAVR trial (although there was no randomization despite the use of the word “trial”).77 This study included a mixture of balloon-expandable and self-expanding valve platforms, with a total of 61 patients with bicuspid disease treated with TAVR. There were no deaths, strokes, or conversions to open surgery within the first 30 days, and only 1 patient had moderate paravalvular regurgitation.
These excellent early outcomes were also seen in the Evolut Low-Risk prospective study.78 Among 150 included patients (all treated with the self-expanding Evolut platform), there was only 1 conversion to open surgery and 1 death; 3.3% of the patients had >1 valve inserted. The 1-year results were similarly encouraging,79 and when these patients were compared with a propensity-matched group of patients with tricuspid aortic stenosis from the Evolut Low-Risk trial, there was no difference in any clinical or echocardiographic outcomes. A similar propensity-matched analysis from the PARTNER 3 bicuspid registry demonstrated equivalent outcomes between patients with bicuspid aortic stenosis and those with tricuspid aortic stenosis treated with the balloon-expandable Sapien 3 valve platform.80
These prospective studies were also limited by selection biases, whereby the clinical teams and researchers utilize their experience and knowledge to select cases that are more favorable for TAVR with an expectation of obtaining excellent results; these, by definition, do not include any patients who were deemed unsuitable for TAVR.
Observational data comparing the outcomes of TAVR and SAVR for bicuspid aortic stenosis
All the previously described studies have 1 common key limitation: absence of a comparator group undergoing SAVR. There are, to our knowledge, only 2 observational studies comparing the outcomes of treatment with TAVR with those of treatment with SAVR for bicuspid aortic stenosis. The first was based on an analysis using the National Inpatient Sample database between the years 2012 and 2016.81 This analysis suggested similar inpatient survival after TAVR and SAVR for bicuspid aortic stenosis but was severely limited because of the absence of any information on outcomes after discharge from the hospital and because of epochal advances in TAVR technology and techniques since the study period, making the study particularly susceptible to claims of obsolescence. A more recent study utilized the Nationwide Readmission Database to provide 6-month follow-up data on 848 propensity-matched pairs of patients with bicuspid disease who underwent TAVR and SAVR.82 This study utilized the study period from 2016 to 2018 and suggested that TAVR was associated with reduced in-hospital mortality compared with SAVR, with similar rates of major adverse cardiac events at 30 days and 6 months. However, both these studies were also severely limited by their dependence on administrative databases, with susceptibility to coding errors, omissions, and inaccuracies. They also had no data on echocardiographic parameters beyond hospital discharge or any quality-of-life metrics. Propensity-matched analyses of observational data are of course also vulnerable to biases due to residual unknown confounders and biases due to indications: it cannot be assumed that included patients are deemed potentially suitable for both the therapies.
Technical considerations for performing TAVR in patients with BAV disease
There are specific technical aspects that are distinct to performing TAVR in patients with bicuspid aortic stenosis compared with those for performing TAVR in patients with trileaflet valves. We suggest the following specific considerations while performing TAVR in patients with bicuspid disease.
Preprocedural planning using CTA
Particular attention should be paid to characterization of the morphology of the valve, including the presence of raphae and whether they are calcified, because this has been identified as an adverse prognostic marker. The pattern of calcification should also be examined, including whether the leaflets themselves are severely calcified and whether the calcification extends to the LVOT. Coronary heights should be interpreted in the context of patients with bicuspid disease often having longer leaflets with bulky calcification, which place them at the risk of coronary obstruction even with conventionally adequate or borderline coronary heights. Valve sizing should generally be performed at the annular level because this has been demonstrated to be more reliable and reproducible than supra-annular sizing. If the valve size is borderline between 2 sizes and the valve complex is severely calcified, consideration should be given to choosing the smaller size to avoid potential risks associated with annular rupture. For patients being treated with a balloon-expandable platform, we would advocate removing some volume from the nominal deployment and then assessing the need for further dilatations to aid in complete expansion and eliminating any recoil from an underdeployed valve.
For patients with multiple adverse features detected using CTA (excess leaflet calcium, calcified raphe, severe LVOT calcification, and sizing outside the recommended range for commercially available valves), consideration should be given as to whether TAVR can truly be safely performed and whether surgery is preferable. While analyzing CTA images, consideration should also be given to the feasibility of performing future procedures (TAVR-in-TAVR) because patients with bicuspid disease are generally younger than patients with trileaflet aortic stenosis.
More liberal use of TEE
The use of TEE can aid in the assessment of the result of the valve implant acutely in catheterization laboratories and is more sensitive than TTE in the detection of paravalvular regurgitation. This can then impact the procedural strategy in terms of valve position and by prompting further dilatation of the valve to ensure that the patient does not leave the catheterization laboratory with greater than mild paravalvular regurgitation.
More liberal use of predilatation
The routine use of predilatation with balloon aortic valvuloplasty is less common in TAVR for patients with trileaflet aortic stenosis; however, there are several reasons to consider a more liberal approach to predilatation in patients with bicuspid disease. Firstly, if there is severe valvular calcification, then performing predilatation will aid in crossing and expansion of the transcatheter heart valve. This is also particularly important for patients with a horizontal aorta, in whom crossing the valve is challenging and may be aided by predilatation of the valve as well as the use of stiffer wires in the left ventricle (such as the double-curve Lunderquist wire). Furthermore, patients with concomitant aortopathy may be more prone to aortic injury, and facilitating easy, smooth passage of the transcatheter valve without pushing or straining against a dilated, diseased aorta may enhance the overall safety of the procedure.
More liberal use of cerebral embolic protection
In patients with a heavily calcified valve complex and in whom multiple dilatations of the valve (both predilatation and postdilatation) are anticipated, there may be a role of cerebral embolic protection. Earlier, observational data had suggested an increased risk of stroke after TAVR for bicuspid aortic stenosis compared with that after TAVR for trileaflet aortic stenosis, although this has not been consistently reported in newer studies. The role of cerebral protection will be defined by the results of ongoing large-scale randomized trials powered for stroke and other clinical end points. Accepting this, our current pragmatic approach would be to perform cerebral embolic protection in cases of bicuspid disease with heavy calcification of the aortic valve complex if their aortic arch anatomy is suitable, taking into consideration our anticipated predilatation and potential postdilatation of the valve.
Valve choice
There are no head-to-head randomized comparators of valve type (ie, balloon-expandable or self-expanding) for patients with bicuspid valves undergoing TAVR. A meta-analysis of observational studies suggested no difference in mortality with the 2 valve platforms, albeit with all included studies having significant susceptibility to biases, and the overall quality of evidence was found to be of low or very low quality.83 Some other observational data have suggested an increased risk of annular rupture with balloon-expandable valves, weighed against an increased risk of moderate-severe paravalvular regurgitation with self-expanding valves.84 We would generally advocate that operators use the valve platform they are most comfortable with and focus on the above-described aspects of preprocedural planning and procedural considerations that are uniform across valve platforms. Excellent results can be obtained with both balloon-expandable and self-expanding platforms in patients with bicuspid aortic stenosis with appropriate anatomy.
Clinical trial considerations
Patients with bicuspid aortic stenosis were excluded from foundational RCTs comparing TAVR with SAVR, and there are specific challenges to consider while contemplating performing randomized trials in patients with bicuspid aortic stenosis. First, in contrast to trileaflet aortic stenosis, patients with bicuspid aortic stenosis have numerous anatomic and clinical phenotypes and represent a much more heterogeneous group of patients. This then raises questions regarding inclusion and exclusion criteria for a proposed clinical trial and how narrow or broad these should be. There is also the specific issue of concomitant aortopathy, which cannot be treated with TAVR. Patients presenting with bicuspid aortic stenosis also tend to be younger than those with trileaflet aortic stenosis, which might obligate even longer-term follow-up of trials with implications for the cost of conducting such a trial. Young patients may also require future aortic valve procedures, which can further complicate the trial’s design, particularly when the goal is to assess the overall therapeutic strategy over a patient’s lifetime. Finally, there is the challenge of recruiting patients into trials of TAVR when the procedure has already been approved by the Food and Drug Administration and fully reimbursed for patients with bicuspid disease. Such key methodologic issues and challenges are being considered by Interventional Cardiology and Cardiac Surgery communities; however, as yet, no definitive plans have been made to conduct a randomized trial for patients with bicuspid aortic stenosis.
Suggestions for therapeutic decision making
Because of the absence of RCT data, clinicians must weigh the available evidence and apply it as best as possible to patients with BAV disease. There are certain factors that might favor one therapy or the other. For example, very young patients and those with aortopathy at a low surgical risk should be considered for open surgery. We believe that it is reasonable to consider TAVR in older patients without significant aortic dilatation, those with iliofemoral anatomy suitable for transfemoral access, and those with favorable anatomy of the aortic valve complex. Certain anatomic phenotypes have been identified as less favorable for TAVR, such as calcified raphae and excess leaflet calcification; in these cases consideration should be given to whether surgery is preferred. Other adverse anatomic features for TAVR that should be considered in the decision-making process include heavy calcification of the LVOT, very large annulus beyond the capabilities of most TAVR devices, and low coronary heights with narrow sinuses of Valsalva, which may increase the risk of coronary occlusion. Finally, consideration should be given to concomitant valvular heart disease or coronary artery disease, which could also be corrected with cardiac surgery, and to the comorbid state of patients, which might discourage surgery. These decisions should be made in the context of a multidisciplinary heart team at dedicated valve centers with emphasis on shared decision making with patients.
Conclusion
Bicuspid aortic valve disease is common, affecting 1% to 2% of the population, up to 50% of patients referred for SAVR, and ∼10% of patients currently treated with TAVR. Patients with BAV disease were excluded from foundational randomized studies comparing the outcomes of TAVR with those of SAVR, and there is, therefore, often uncertainty regarding the optimal therapeutic strategy for these patients. Furthermore, BAV disease is a heterogeneous condition and, commonly, a disease of the aorta and aortic valve. The cornerstone of management of these patients should be a multidisciplinary heart team approach with a focus on multimodality imaging both for the diagnosis and characterization of the valve in each patient and for therapeutic decision making. TAVR is feasible in certain patient subsets with bicuspid aortic stenosis, with suitability determined using meticulous analysis of preprocedure CTA images and procedural success dependent on modifications of standard techniques employed for patients with trileaflet aortic stenosis. We believe that these patients should be assessed and treated at dedicated valve centers where imaging cardiologists, cardiac surgeons, and interventional cardiologists all have experience in assessing and treating patients with BAV disease. Because these patients are typically younger than those with trileaflet aortic stenosis, there should be particular emphasis on shared decision making and lifetime planning for each patient. Finally, there is a hope that randomized trials will be performed in this patient population to further guide therapeutic decision making.
Acknowledgments
Declaration of competing interest
Dr Wang has served as a consultant to Edwards Lifesciences, Abbott, NeoChord, and Boston Scientific and has received research grant support from Boston Scientific assigned to her employer, the Henry Ford Health System. Dr Reardon served as national surgical principal investigator on SURTAVI, Evolut Low-Risk, Reprise III, Acurate, Portico NG, and Vantage and received research support from Medtronic, Boston Scientific, Abbott Medical, and Gore. Dr Cavalvante has received consulting fees from Boston Scientific and Abbott Vascular and has received research grant support from Circle Cardiovascular Imaging, Edwards Lifesciences, Medtronic, Boston Scientific, and Abbott Vascular. He has been a speaker for Medtronic, Circle Cardiovascular Imaging, and Siemens Healthineers. Dr Makkar has received research grants from Edwards Lifesciences, Abbott, Medtronic, and Boston Scientific and has served as national principal investigator for Portico (Abbott) and Acurate (Boston Scientific) US investigation device exemption trials. In addition, he has received personal proctoring fees from Edwards Lifesciences and travels support from Edwards Lifesciences, Abbott, and Boston Scientific. Dr Forrest is a consultant for Edwards Lifesciences and Medtronic and receives grant support from Edwards Lifesciences and Medtronic. Drs Ahmad, Agarwal, and Williams reported no financial interests.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics statement
The research reported has adhered to the relevant ethical guidelines.
References
- 1.Leon M.B., Smith C.R., Mack M., et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 2.Mack M.J., Leon M.B., Smith C.R., et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385(9986):2477–2484. doi: 10.1016/S0140-6736(15)60308-7. [DOI] [PubMed] [Google Scholar]
- 3.Mack M.J., Leon M.B., Thourani V.H., et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380(18):1695–1705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 4.Adams D.H., Popma J.J., Reardon M.J., et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370(19):1790–1798. doi: 10.1056/NEJMoa1400590. [DOI] [PubMed] [Google Scholar]
- 5.Popma J.J., Deeb G.M., Yakubov S.J., et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380(18):1706–1715. doi: 10.1056/NEJMoa1816885. [DOI] [PubMed] [Google Scholar]
- 6.Carroll J.D., Mack M.J., Vemulapalli S., et al. STS-ACC TVT registry of transcatheter aortic valve replacement. J Am Coll Cardiol. 2020;76(21):2492–2516. doi: 10.1016/j.jacc.2020.09.595. [DOI] [PubMed] [Google Scholar]
- 7.Roberts W.C., Janning K.G., Ko J.M., Filardo G., Matter G.J. Frequency of congenitally bicuspid aortic valves in patients ≥80 years of age undergoing aortic valve replacement for aortic stenosis (with or without aortic regurgitation) and implications for transcatheter aortic valve implantation. Am J Cardiol. 2012;109(11):1632–1636. doi: 10.1016/j.amjcard.2012.01.390. [DOI] [PubMed] [Google Scholar]
- 8.Michelena H.I., Della Corte A., Evangelista A., et al. International consensus statement on nomenclature and classification of the congenital bicuspid aortic valve and its aortopathy, for clinical, surgical, interventional and research purposes. Ann Thorac Surg. 2021;112(3):e203–e235. doi: 10.1016/j.athoracsur.2020.08.119. [DOI] [PubMed] [Google Scholar]
- 9.Masri A., Svensson L.G., Griffin B.P., Desai M.Y. Contemporary natural history of bicuspid aortic valve disease: a systematic review. Heart. 2017;103(17):1323–1330. doi: 10.1136/heartjnl-2016-309916. [DOI] [PubMed] [Google Scholar]
- 10.Evangelista A., Gallego P., Calvo-Iglesias F., et al. Anatomical and clinical predictors of valve dysfunction and aortic dilation in bicuspid aortic valve disease. Heart. 2018;104(7):566–573. doi: 10.1136/heartjnl-2017-311560. [DOI] [PubMed] [Google Scholar]
- 11.Michelena H.I., Desjardins V.A., Avierinos J.F., et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation. 2008;117(21):2776–2784. doi: 10.1161/CIRCULATIONAHA.107.740878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hahn R.T., Little S.H., Monaghan M.J., et al. Recommendations for comprehensive intraprocedural echocardiographic imaging during TAVR. JACC Cardiovasc Imaging. 2015;8(3):261–287. doi: 10.1016/j.jcmg.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Alkadhi H., Wildermuth S., Plass A., et al. Aortic stenosis: comparative evaluation of 16-detector row CT and echocardiography. Radiology. 2006;240(1):47–55. doi: 10.1148/radiol.2393050458. [DOI] [PubMed] [Google Scholar]
- 14.Blanke P., Weir-McCall J.R., Achenbach S., et al. Computed tomography imaging in the context of transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR): an expert consensus document of the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr. 2019;12(1):1–24. doi: 10.1016/j.jcct.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Clavel M.A., Malouf J., Messika-Zeitoun D., Araoz P.A., Michelena H.I., Enriquez-Sarano M. Aortic valve area calculation in aortic stenosis by CT and Doppler echocardiography. JACC Cardiovasc Imaging. 2015;8(3):248–257. doi: 10.1016/j.jcmg.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Halpern E.J., Mallya R., Sewell M., Shulman M., Zwas D.R. Differences in aortic valve area measured with CT planimetry and echocardiography (continuity equation) are related to divergent estimates of left ventricular outflow tract area. AJR Am J Roentgenol. 2009;192(6):1668–1673. doi: 10.2214/AJR.08.1986. [DOI] [PubMed] [Google Scholar]
- 17.Choi B.H., Ko S.M., Shin J.K., Chee H.K., Kim J.S., Kim J. Association between aortic valvular calcification and characteristics of the aortic valve in patients with bicuspid aortic valve stenosis. Acta Radiol. 2019;60(4):468–477. doi: 10.1177/0284185118787359. [DOI] [PubMed] [Google Scholar]
- 18.Pawade T., Clavel M.A., Tribouilloy C., et al. Computed tomography aortic valve calcium scoring in patients with aortic stenosis. Circ Cardiovasc Imaging. 2018;11(3):e007146–e007147. doi: 10.1161/CIRCIMAGING.117.007146. [DOI] [PubMed] [Google Scholar]
- 19.Clavel M.A., Pibarot P., Messika-Zeitoun D., et al. Impact of aortic valve calcification, as measured by MDCT, on survival in patients with aortic stenosis: results of an international registry study. J Am Coll Cardiol. 2014;64(12):1202–1213. doi: 10.1016/j.jacc.2014.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feuchtner G.M., Dichtl W., Muller S., et al. 64-MDCT for diagnosis of aortic regurgitation in patients referred to CT coronary angiography. AJR Am J Roentgenol. 2008;191(1):80. doi: 10.2214/AJR.07.3432. [DOI] [PubMed] [Google Scholar]
- 21.Harken D.E., Soroff H.S., Taylor W.J., Lefemine A.A., Gupta S.K., Lunzer S. Partial and complete prostheses in aortic insufficiency. J Thorac Cardiovasc Surg. 1960;40(6):744–762. [PubMed] [Google Scholar]
- 22.Attia T., Yang Y., Svensson L.G., et al. Similar long-term survival after isolated bioprosthetic versus mechanical aortic valve replacement: a propensity-matched analysis. Preprint. Posted online January 20, 2021 doi: 10.1016/j.jtcvs.2020.11.181. J Thorac Cardiovasc Surg. [DOI] [PubMed] [Google Scholar]
- 23.Roberts W.C., Ko J.M. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation. 2005;111(7):920–925. doi: 10.1161/01.CIR.0000155623.48408.C5. [DOI] [PubMed] [Google Scholar]
- 24.Tzemos N., Therrien J., Yip J., et al. Outcomes in adults with bicuspid aortic valves. JAMA. 2008;300(11):1317–1325. doi: 10.1001/jama.300.11.1317. [DOI] [PubMed] [Google Scholar]
- 25.McKellar S.H., Michelena H.I., Li Z., Schaff H.V., Sundt T.M. Long-term risk of aortic events following aortic valve replacement in patients with bicuspid aortic valves. Am J Cardiol. 2010;106(11):1626–1633. doi: 10.1016/j.amjcard.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 26.Masri A., Kalahasti V., Alkharabsheh S., et al. Characteristics and long-term outcomes of contemporary patients with bicuspid aortic valves. J Thorac Cardiovasc Surg. 2016;151(6):1650–1659. doi: 10.1016/j.jtcvs.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 27.Ganapathi A.M., Englum B.R., Keenan J.E., et al. Long-term survival after bovine pericardial versus porcine stented bioprosthetic aortic valve replacement: does valve choice matter? Ann Thorac Surg. 2015;100(2):550–559. doi: 10.1016/j.athoracsur.2015.02.067. [DOI] [PubMed] [Google Scholar]
- 28.Gaca J.G., Clare R.M., Rankin J.S., et al. Risk-adjusted survival after tissue versus mechanical aortic valve replacement: a 23-year assessment. J Heart Valve Dis. 2013;22(6):810–816. [PMC free article] [PubMed] [Google Scholar]
- 29.Neely R.C., Boskovski M.T., Gosev I., et al. Minimally invasive aortic valve replacement versus aortic valve replacement through full sternotomy: the Brigham and Women’s Hospital experience. Ann Cardiothorac Surg. 2015;4(1):38–48. doi: 10.3978/j.issn.2225-319X.2014.08.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghoreishi M., Thourani V.H., Badhwar V., et al. Less-invasive aortic valve replacement: trends and outcomes from the Society of Thoracic Surgeons database. Ann Thorac Surg. 2021;111(4):1216–1223. doi: 10.1016/j.athoracsur.2020.06.039. [DOI] [PubMed] [Google Scholar]
- 31.Thourani V.H., Brennan J.M., Edelman J.J., et al. Association of volume and outcomes in 234 556 patients undergoing surgical aortic valve replacement. Ann Thorac Surg. 2021;114(4):1299–1306. doi: 10.1016/j.athoracsur.2021.06.095. [DOI] [PubMed] [Google Scholar]
- 32.Bavaria J.E., Desai N.D., Cheung A., et al. The St Jude Medical Trifecta aortic pericardial valve: results from a global, multicenter, prospective clinical study. J Thorac Cardiovasc Surg. 2014;147(2):590–597. doi: 10.1016/j.jtcvs.2012.12.087. [DOI] [PubMed] [Google Scholar]
- 33.Puskas J.D., Bavaria J.E., Svensson L.G., et al. The COMMENCE trial: 2-year outcomes with an aortic bioprosthesis with RESILIA tissue. Eur J Cardiothorac Surg. 2017;52(3):432–439. doi: 10.1093/ejcts/ezx158. [DOI] [PubMed] [Google Scholar]
- 34.Fradet G.J., Bleese N., Burgess J., Cartier P.C. Mosaic valve international clinical trial: early performance results. Ann Thorac Surg. 2001;71(5):S273–S277. doi: 10.1016/s0003-4975(01)02539-5. [DOI] [PubMed] [Google Scholar]
- 35.Forrest J.K., Deeb G.M., Yakubov S.J., et al. 2-year outcomes after transcatheter versus surgical aortic valve replacement in low-risk patients. J Am Coll Cardiol. 2022;79(9):882–896. doi: 10.1016/j.jacc.2021.11.062. [DOI] [PubMed] [Google Scholar]
- 36.Leon M.B., Mack M.J., Hahn R.T., et al. Outcomes 2 years after transcatheter aortic valve replacement in patients at low surgical risk. J Am Coll Cardiol. 2021;77(9):1149–1161. doi: 10.1016/j.jacc.2020.12.052. [DOI] [PubMed] [Google Scholar]
- 37.Bartus K., Sadowski J., Litwinowicz R., et al. Changing trends in aortic valve procedures over the past ten years-from mechanical prosthesis via stented bioprosthesis to TAVI procedures-analysis of 50,846 aortic valve cases based on a Polish National Cardiac Surgery Database. J Thorac Dis. 2019;11(6):2340–2349. doi: 10.21037/jtd.2019.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Brien S.M., Shahian D.M., Filardo G., et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2—isolated valve surgery. Ann Thorac Surg. 2009;88(1):S23–S42. doi: 10.1016/j.athoracsur.2009.05.056. [DOI] [PubMed] [Google Scholar]
- 39.Anselmi A., Flécher E., Ruggieri V.G., et al. Long-term results of the Medtronic Mosaic porcine bioprosthesis in the aortic position. J Thorac Cardiovasc Surg. 2014;147(6):1884–1891. doi: 10.1016/j.jtcvs.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 40.Johnston D.R., Soltesz E.G., Vakil N., et al. Long-term durability of bioprosthetic aortic valves: implications from 12,569 implants. Ann Thorac Surg. 2015;99(4):1239–1247. doi: 10.1016/j.athoracsur.2014.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bourguignon T., El Khoury R., Candolfi P., et al. Very long-term outcomes of the Carpentier-Edwards perimount aortic valve in patients aged 60 or younger. Ann Thorac Surg. 2015;100(3):853–859. doi: 10.1016/j.athoracsur.2015.03.105. [DOI] [PubMed] [Google Scholar]
- 42.Borger M.A., Fedak P.W., Stephens E.H., et al. The American Association for Thoracic Surgery consensus guidelines on bicuspid aortic valve-related aortopathy: full online-only version. J Thorac Cardiovasc Surg. 2018;156(2):e41–e74. doi: 10.1016/j.jtcvs.2018.02.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishimura R.A., Otto C.M., Bonow R.O., et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(23):e521–e643. doi: 10.1161/CIR.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 44.Erbel R., Aboyans V., Boileau C., et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the diagnosis and treatment of aortic Diseases of the European Society of Cardiology (ESC) Eur Heart J. 2014;35(41):2873–2926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- 45.Vahanian A., Beyersdorf F., Praz F., et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2021;43(7):561–632. doi: 10.1093/eurheartj/ehab395. [DOI] [PubMed] [Google Scholar]
- 46.Borger M.A., Preston M., Ivanov J., et al. Should the ascending aorta be replaced more frequently in patients with bicuspid aortic valve disease? J Thorac Cardiovasc Surg. 2004;128(5):677–683. doi: 10.1016/j.jtcvs.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 47.Eleid M.F., Forde I., Edwards W.D., et al. Type A aortic dissection in patients with bicuspid aortic valves: clinical and pathological comparison with tricuspid aortic valves. Heart. 2013;99(22):1668–1674. doi: 10.1136/heartjnl-2013-304606. [DOI] [PubMed] [Google Scholar]
- 48.Michelena H.I., Della Corte A., Prakash S.K., Milewicz D.M., Evangelista A., Enriquez-Sarano M. Bicuspid aortic valve aortopathy in adults: incidence, etiology, and clinical significance. Int J Cardiol. 2015;201:400–407. doi: 10.1016/j.ijcard.2015.08.106. [DOI] [PubMed] [Google Scholar]
- 49.Tchetche D., De Biase C., van Gils L., et al. Bicuspid aortic valve anatomy and relationship with devices: the BAVARD multicenter registry. Circ Cardiovasc Interv. 2019;12(1):e007107–e007108. doi: 10.1161/CIRCINTERVENTIONS.118.007107. [DOI] [PubMed] [Google Scholar]
- 50.Weir-McCall J.R., Attinger-Toller A., Blanke P., et al. Annular versus supra-annular sizing for transcatheter aortic valve replacement in bicuspid aortic valve disease. J Cardiovasc Comput Tomogr. 2020;14(5):407–413. doi: 10.1016/j.jcct.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 51.Xiong T.Y., Li Y.J., Feng Y., et al. Understanding the interaction between transcatheter aortic valve prostheses and supra-annular structures from post-implant stent geometry. JACC Cardiovasc Interv. 2019;12(12):1164–1171. doi: 10.1016/j.jcin.2019.02.051. [DOI] [PubMed] [Google Scholar]
- 52.Kim W.K., Renker M., Rolf A., et al. Annular versus supra-annular sizing for TAVI in bicuspid aortic valve stenosis. EuroIntervention. 2019;15(3):e231–e238. doi: 10.4244/EIJ-D-19-00236. [DOI] [PubMed] [Google Scholar]
- 53.Shibayama K., Harada K., Berdejo J., et al. Comparison of aortic root geometry with bicuspid versus tricuspid aortic valve: real-time three-dimensional transesophageal echocardiographic study. J Am Soc Echocardiogr. 2014;27(11):1143–1152. doi: 10.1016/j.echo.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 54.Watanabe Y., Chevalier B., Hayashida K., et al. Comparison of multislice computed tomography findings between bicuspid and tricuspid aortic valves before and after transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2015;86(2):323–330. doi: 10.1002/ccd.25830. [DOI] [PubMed] [Google Scholar]
- 55.Philip F., Faza N.N., Schoenhagen P., et al. Aortic annulus and root characteristics in severe aortic stenosis due to bicuspid aortic valve and tricuspid aortic valves: implications for transcatheter aortic valve therapies. Catheter Cardiovasc Interv. 2015;86(2):E88–E98. doi: 10.1002/ccd.25948. [DOI] [PubMed] [Google Scholar]
- 56.Keane M.G., Wiegers S.E., Plappert T., Pochettino A., Bavaria J.E., Sutton M.G. Bicuspid aortic valves are associated with aortic dilatation out of proportion to coexistent valvular lesions. Circulation. 2000;102(suppl 3):Iii35–39. doi: 10.1161/01.cir.102.suppl_3.iii-35. [DOI] [PubMed] [Google Scholar]
- 57.Verma S., Siu S.C. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med. 2014;370(20):1920–1929. doi: 10.1056/NEJMra1207059. [DOI] [PubMed] [Google Scholar]
- 58.He Y.X., Fan J.Q., Zhu Q.F., et al. Ascending aortic dilatation rate after transcatheter aortic valve replacement in patients with bicuspid and tricuspid aortic stenosis: a multidetector computed tomography follow-up study. World J Emerg Med. 2019;10(4):197–204. doi: 10.5847/wjem.j.1920-8642.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kong W.K., Delgado V., Poh K.K., et al. Prognostic implications of raphe in bicuspid aortic valve anatomy. JAMA Cardiol. 2017;2(3):285–292. doi: 10.1001/jamacardio.2016.5228. [DOI] [PubMed] [Google Scholar]
- 60.Jilaihawi H., Wu Y., Yang Y., et al. Morphological characteristics of severe aortic stenosis in China: imaging corelab observations from the first Chinese transcatheter aortic valve trial. Catheter Cardiovasc Interv. 2015;85(suppl 1):752–761. doi: 10.1002/ccd.25863. [DOI] [PubMed] [Google Scholar]
- 61.van Rosendael P.J., Kamperidis V., Kong W.K., et al. Comparison of quantity of calcific deposits by multidetector computed tomography in the aortic valve and coronary arteries. Am J Cardiol. 2016;118(10):1533–1538. doi: 10.1016/j.amjcard.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 62.Sievers H.H., Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg. 2007;133(5):1226–1233. doi: 10.1016/j.jtcvs.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 63.Yoon S.H., Kim W.K., Dhoble A., et al. Bicuspid aortic valve morphology and outcomes after transcatheter aortic valve replacement. J Am Coll Cardiol. 2020;76(9):1018–1030. doi: 10.1016/j.jacc.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 64.Lerer P.K., Edwards W.D. Coronary arterial anatomy in bicuspid aortic valve. Necropsy study of 100 hearts. Br Heart J. 1981;45(2):142–147. doi: 10.1136/hrt.45.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shen M., Tastet L., Capoulade R., et al. Effect of bicuspid aortic valve phenotype on progression of aortic stenosis. Eur Heart J Cardiovasc Imaging. 2020;21(7):727–734. doi: 10.1093/ehjci/jeaa068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Otto C.M., Newby D.E. Transcatheter valve replacement for bicuspid aortic stenosis. JAMA. 2021;326(11):1009–1010. doi: 10.1001/jama.2021.13229. [DOI] [PubMed] [Google Scholar]
- 67.Bourguignon T., Bouquiaux-Stablo A.L., Candolfi P., et al. Very long-term outcomes of the Carpentier-Edwards Perimount valve in aortic position. Ann Thorac Surg. 2015;99(3):831–837. doi: 10.1016/j.athoracsur.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 68.Sabet H.Y., Edwards W.D., Tazelaar H.D., Daly R.C. Congenitally bicuspid aortic valves: a surgical pathology study of 542 cases (1991 through 1996) and a literature review of 2,715 additional cases. Mayo Clin Proc. 1999;74(1):14–26. doi: 10.4065/74.1.14. [DOI] [PubMed] [Google Scholar]
- 69.Egbe A.C., Luis S.A., Padang R., Warnes C.A. Outcomes in moderate mixed aortic valve disease: is it time for a paradigm shift? J Am Coll Cardiol. 2016;67(20):2321–2329. doi: 10.1016/j.jacc.2016.03.509. [DOI] [PubMed] [Google Scholar]
- 70.Mylotte D., Lefevre T., Søndergaard L., et al. Transcatheter aortic valve replacement in bicuspid aortic valve disease. J Am Coll Cardiol. 2014;64(22):2330–2339. doi: 10.1016/j.jacc.2014.09.039. [DOI] [PubMed] [Google Scholar]
- 71.Yoon S.H., Bleiziffer S., De Backer O., et al. Outcomes in transcatheter aortic valve replacement for bicuspid versus tricuspid aortic valve stenosis. J Am Coll Cardiol. 2017;69(21):2579–2589. doi: 10.1016/j.jacc.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 72.Jilaihawi H., Kashif M., Fontana G., et al. Cross-sectional computed tomographic assessment improves accuracy of aortic annular sizing for transcatheter aortic valve replacement and reduces the incidence of paravalvular aortic regurgitation. J Am Coll Cardiol. 2012;59(14):1275–1286. doi: 10.1016/j.jacc.2011.11.045. [DOI] [PubMed] [Google Scholar]
- 73.Ahmad Y., Howard J.P. Meta-analysis of usefulness of cerebral embolic protection during transcatheter aortic valve implantation. Am J Cardiol. 2021;146:69–73. doi: 10.1016/j.amjcard.2021.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Makkar R.R., Yoon S.H., Leon M.B., et al. Association between transcatheter aortic valve replacement for bicuspid vs tricuspid aortic stenosis and mortality or stroke. JAMA. 2019;321(22):2193–2202. doi: 10.1001/jama.2019.7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Makkar R.R., Yoon S.H., Chakravarty T., et al. Association between transcatheter aortic valve replacement for bicuspid vs tricuspid aortic stenosis and mortality or stroke among patients at low surgical risk. JAMA. 2021;326(11):1034–1044. doi: 10.1001/jama.2021.13346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Forrest J.K., Kaple R.K., Ramlawi B., et al. Transcatheter aortic valve replacement in bicuspid versus tricuspid aortic valves from the STS/ACC TVT registry. JACC Cardiovasc Interv. 2020;13(15):1749–1759. doi: 10.1016/j.jcin.2020.03.022. [DOI] [PubMed] [Google Scholar]
- 77.Waksman R., Corso P.J., Torguson R., et al. TAVR in low-risk patients: 1-year results from the LRT trial. JACC Cardiovasc Interv. 2019;12(10):901–907. doi: 10.1016/j.jcin.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 78.Forrest J.K., Ramlawi B., Deeb G.M., et al. Transcatheter aortic valve replacement in low-risk patients with bicuspid aortic valve stenosis. JAMA Cardiol. 2021;6(1):50–57. doi: 10.1001/jamacardio.2020.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Deeb G.M., Reardon M.J., Ramlawi B., et al. Propensity-matched 1-year outcomes following transcatheter aortic valve replacement in low-risk bicuspid and tricuspid patients. JACC Cardiovasc Interv. 2022;15(5):511–522. doi: 10.1016/j.jcin.2021.10.027. [DOI] [PubMed] [Google Scholar]
- 80.Williams M.R., Jilaihawi H., Makkar R., et al. The PARTNER 3 bicuspid registry for transcatheter aortic valve replacement in low-surgical-risk patients. JACC Cardiovasc Interv. 2022;15(5):523–532. doi: 10.1016/j.jcin.2022.01.279. [DOI] [PubMed] [Google Scholar]
- 81.Elbadawi A., Saad M., Elgendy I.Y., et al. Temporal trends and outcomes of transcatheter versus surgical aortic valve replacement for bicuspid aortic valve stenosis. JACC Cardiovasc Interv. 2019;12(18):1811–1822. doi: 10.1016/j.jcin.2019.06.037. [DOI] [PubMed] [Google Scholar]
- 82.Majmundar M., Kumar A., Doshi R., et al. Early outcomes of transcatheter versus surgical aortic valve implantation in patients with bicuspid aortic valve stenosis. EuroIntervention. 2022;18(1):23–32. doi: 10.4244/EIJ-D-21-00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sá M.P., Simonato M., Van den Eynde J., et al. Balloon versus self-expandable transcatheter aortic valve implantation for bicuspid aortic valve stenosis: a meta-analysis of observational studies. Catheter Cardiovasc Interv. 2021;98(5):E746–E757. doi: 10.1002/ccd.29538. [DOI] [PubMed] [Google Scholar]
- 84.Mangieri A., Tchetchè D., Kim W.K., et al. Balloon versus self-expandable valve for the treatment of bicuspid aortic valve stenosis: insights from the BEAT international collaborative registrys. Circ Cardiovasc Interv. 2020;13(7):e008714–e008715. doi: 10.1161/CIRCINTERVENTIONS.119.008714. [DOI] [PubMed] [Google Scholar]