Abstract
Axillary artery access has become increasingly widespread as an alternative to the femoral route for large-bore transcatheter aortic valve replacement (TAVR), endovascular aortic repair (EVAR), and mechanical circulatory support (MCS) procedures. Advantages of percutaneous access include avoidance of a surgical incision, general anesthesia, and conduit graft infection. This statement aims to review the anatomic considerations and risks for percutaneous axillary artery access, suggest best practices for access techniques, hemostasis/closure strategies, and complication management, and recommend options for training and privileging.
This statement was endorsed by the American College of Cardiology (ACC), Heart Failure Society of America (HFSA), Society of Interventional Radiology (SIR), and Vascular & Endovascular Surgery Society (VESS) in March 2022.
Development methodology
This statement has been developed according to the Society for Cardiovascular Angiography and Interventions (SCAI) Publications Committee policies1 for writing group composition, disclosure and management of relationships with industry (RWI), internal and external review, and organizational approval.
The writing group has been organized to ensure diversity of perspectives and demographics, multi-stakeholder representation, and appropriate balance of RWI. Relevant author disclosures are included in Supplemental Table 1. Before appointment, members of the writing group were asked to disclose financial and intellectual relationships from the 12 months prior to their nomination. A majority of the writing group disclosed no relevant, significant financial relationships. Disclosures were periodically reviewed during document development and updated as needed. SCAI policy requires that writing group members with a current, relevant financial interest are recused from participating in related discussions or voting on recommendations. The work of the writing committee was supported exclusively by SCAI, a nonprofit medical specialty society, without commercial support. Writing group members contributed to this effort on a volunteer basis and did not receive payment from SCAI.
Literature searches were performed by group members designated to lead each section and initial section drafts were authored primarily by the section leads in collaboration with other members of the writing group. Recommendations were discussed by the full writing group until a majority of group members agreed on the text and qualifying remarks. All recommendations are supported by a short summary of the evidence or specific rationale.
The draft manuscript was peer reviewed in September 2021, and the document was revised to address pertinent comments. The writing group unanimously approved the final version of the document. The SCAI Publications Committee and Executive Committee endorsed the document as official society guidance in March 2022.
SCAI statements are primarily intended to help clinicians make decisions about treatment alternatives. Clinicians also must consider the clinical presentation, setting, and preferences of individual patients to make judgements about the optimal approach.
Introduction and background
Large-bore endovascular devices for the treatment of a variety of cardiac and aortic pathologies have become commonplace in modern cardiovascular disease management. The common femoral artery is the most frequent access site for percutaneous placement of large-bore arterial sheaths and devices.2 However, transfemoral access may be limited in 13-20% of patients due to prior surgical interventions or severe aortoiliac and/or iliofemoral atherosclerotic disease, tortuosity, or calcification. In these circumstances, axillary artery access is often a viable and beneficial alternative.2,3
Axillary arterial access is traditionally performed through open surgical exposure, which allows for direct puncture, primary arterial repair, or placement of a sidearm conduit. However, percutaneous axillary arterial access has become increasingly common and evolved from lessons learned from percutaneous access and closure of large-bore femoral arteriotomies.4,5 Advantages of percutaneous access include avoidance of a surgical incision, general anesthesia, and conduit graft infection.
The transaxillary approach facilitates long-term indwelling mechanical circulatory support (MCS) devices of varying dimensions, such as intra-aortic balloon pumps (IABP) and percutaneous left ventricular assist devices (pVAD). Such devices are frequently used in cardiogenic shock, high-risk percutaneous coronary interventions, and ventricular tachycardia ablation therapy, but also serve as a bridge to durable left ventricular assist device placement or heart transplantation after several days or weeks.6 In these circumstances, the prolonged presence of a large-bore femoral arterial sheath limits mobility, promotes deconditioning, and carries an increased risk of infection.4 Transaxillary access for prolonged-use MCS devices potentially avoids such complications.
Transaxillary access has also been shown to be a useful alternative access for transcatheter aortic valve replacement (TAVR) and as an adjunct during complex endovascular aortic repair (EVAR) of aneurysmal disease involving visceral and renal branches.7,8
While the need for transaxillary access is expected to increase, the published evidence base is limited. The goals of this SCAI expert consensus statement are to: 1) review the anatomic considerations and risks for percutaneous axillary artery access, 2) suggest best practices for access techniques, hemostasis/closure strategies, and complication management, and 3) recommend options for training and privileging.
Anatomic considerations
Understanding the relevant anatomy of both the axillary artery and subclavian artery is paramount when considering axillary artery access. The axillary arteries arise from the subclavian arteries that arise from the brachiocephalic artery on the right and directly from the aortic arch on the left (Figure 1).9 Variant arch anatomy may occasionally occur, with the more common including the bovine arch (common origin of the brachiocephalic and left common carotid arteries), arteria lusoria (right subclavian artery arises from the proximal descending thoracic aorta distal to the left subclavian artery origin) and left vertebral artery arising directly from the aortic arch.
Figure 1.
Axillary artery and surrounding structures including vessels, bones, nerve and muscle. The 3 segments of the axillary artery are marked relative to the pectoralis minor muscle.
The subclavian artery is mostly intrathoracic in location and supplies significant branches including the vertebral artery, the internal mammary artery, the thyrocervical trunk, costocervical trunk, and dorsal scapular artery. The subclavian artery is intimately related to the brachial plexus, and given its course and anatomy, injury to the subclavian artery may result in intrathoracic bleeding or compressive neck hematoma.10
Beyond the lateral border of the first rib, the subclavian artery continues as the axillary artery, which is now extrathoracic (Figure 1). This distinction is important as the extrathoracic location limits the risks of puncture-related pneumothorax and hemothorax. The axillary artery is divided into 3 segments defined by the pectoralis minor muscle. The first segment is medial to the pectoralis minor and has a single branch, the superior thoracic artery. The second segment lies directly posterior to the pectoralis minor and has 2 branches, the thoraco-acromial and lateral thoracic arteries. Lateral to pectoralis minor, the third segment is the origin of 3 branches, the subscapular, posterior humeral circumflex, and anterior humeral circumflex arteries. The third segment ends at the head of the humerus and continues as the brachial artery.
The axillary artery typically measures 6-7 mm in diameter, with a range of 5-8 mm.9 This corresponds to a relative French size of 15 to 24. The axillary artery is infrequently affected with atherosclerotic disease (2%) compared with the femoral artery, with unclear pathophysiology.9
The neurovascular relationship between the course of the axillary artery and the surrounding nerves favors the second segment for percutaneous puncture and closure. The phrenic nerve, sympathetic trunk, long thoracic nerve, and the proximal trunks of the brachial plexus are adjacent to the subclavian artery. They are unlikely to be injured during a percutaneous transaxillary approach. On the other hand, the posterior, medial, and lateral cords of the brachial plexus are intimately related to the axillary artery and named according to their positions relative to the first and second segments. The absence of a cord anterior to the second segment results in a safer window for percutaneous puncture and closure techniques. However, an unidentified expanding hematoma within the axillary fascia can potentially result in brachial plexus cord compression despite the absence of direct injury from a correct access path.
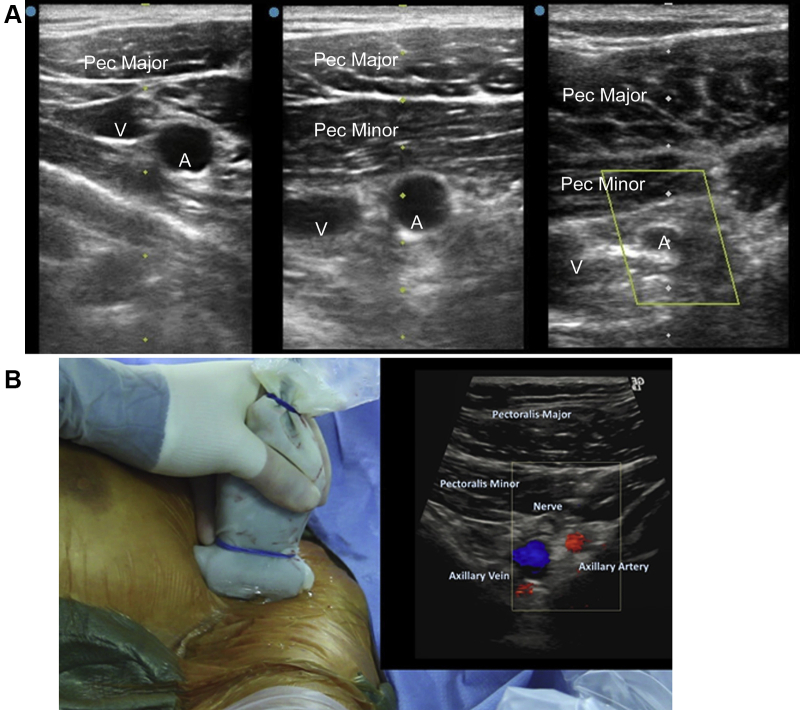
The median and ulnar nerves form from the brachial plexus cords and pass anterior to the third segment of the axillary artery, placing them at risk of injury if the third segment is chosen for access. The subscapular and circumflex humeral arteries mark this segment, and angiographic visualization can help the operator avoid that location. The median and ulnar nerve can also occasionally be visualized during ultrasound-guided access (Figure 2). Fluoroscopically, with the arm abducted, the inferior border of the glenoid cavity may represent a lateral marker distal to which the artery should not be punctured.
Figure 2.
Upper row: Representative cross-sectional left axillary ultrasound images at the first segment (left), second segment (center) and third segment (right) demonstrating the relationship between the pectoralis muscles (Pec), axillary vein (V), and axillary artery (A). Lower panel: Ultrasound probe with (inset) color Doppler ultrasound image showing a nerve anterior to third axillary artery segment.
Knowledge of the anatomy and branches of the axillary artery facilitates safer angiographic guidance of access and endovascular treatment of any access-related complications. Similarly, identification of the relationship of the axillary artery to the pectoralis minor enables accurate ultrasound-guided access of the second segment of the axillary artery. This panel recommends the second segment of the axillary artery as the preferred location for percutaneous puncture due to its distance from the chest cavity (which reduces the risk of accidental intrathoracic subclavian device placement and allows for proximal control if open surgical repair becomes necessary), absence of critical branches, potential compressibility against the chest wall, and decreased risk of brachial plexus injuries. Covered stent placement and closure device deployment are similarly safer at this location.
Patient selection and contraindications
The transaxillary route is typically chosen when femoral or other access sites are limited by atherosclerosis, calcification or tortuosity, or when ambulation with chronic device support is of importance. The suitability of the axillary artery can be assessed by computed tomographic angiography images if available, or with angiographic and ultrasound imaging at the time of the procedure. Absolute contraindications to transaxillary access include prior vascular procedures or surgical repairs which render the axillary artery unsuitable for percutaneous access such as prior covered stent placement. Relative contraindications include vessel calcification, stenosis, tortuosity, aneurysmal dilatation, or prior dissection. Patients on therapeutic anticoagulation may be at increased risk for bleeding complications after transaxillary access. Contralateral transaxillary access should be considered where an arteriovenous fistula or internal mammary bypass graft may be at risk of injury or decreased flow. The presence of a left sided pacemaker or defibrillator may physically limit access to the second segment of the axillary artery, though a shallow angle of needle entry often allows for success.
Insertion techniques
Numerous techniques have been described for obtaining percutaneous transaxillary access, with varying location, approach, and methods. All require a strong fundamental working knowledge of large-bore vascular access and the anatomy within the deltopectoral triangle as noted previously. The clavicle, second rib, and humeral head serve as useful bony fluoroscopic landmarks for the first, second, and third segments.
Selective angiography
Access may be facilitated by the concomitant use of selective angiography which may be performed from a secondary vascular access site such as the femoral, ipsilateral radial, or brachial arteries. Angiography provides essential information on vessel size, presence of stenosis, or severe tortuosity, and may be of particular benefit when pre-procedural imaging (such as CTA) cannot be performed. Digital subtraction angiography and roadmapping are useful tools to guide puncture. In addition, the lateral thoracic and subscapular arteries provide excellent landmarks for the second segment as they take perpendicular caudad courses off of the axillary artery, just outside of the rib cage and proximal to the head of the humerus, respectively (Figure 3).
Figure 3.

Angiographic anatomy of the axillary artery with branches.
Placement of a guide wire through a diagnostic catheter across the vessel may provide a fluoroscopic or ultrasound target for the needle. Such a wire may also reduce vessel tortuosity which may be misrepresented on CTA based on the positioning of the patients arms during that examination. If left in place during the procedure this wire can subsequently be used as a bailout or safety wire for balloon tamponade of the vessel.
Laterality and location
The left axillary is often the preferred side for access (Figure 2) due to avoidance of the origin of the right common carotid artery which theoretically reduces stroke risk. For TAVR, the left transaxillary approach more closely approximates femoral access.
Access is most commonly obtained with the needle being inserted directly through the pectoralis minor muscle into the second segment. Rarely, the access can be performed infra-pectorally, with the access point being in the axilla itself where the pulse is easily palpated. However, the transpectoral approach is typically favored due to device stability independent of the patient's arm position and lower risk of infection with prolonged implantation.
Needle angle
The single most important technical point in percutaneous transaxillary access relates to the angle of access. While a 45-degree needle access angle is desired for ideal femoral access, experience has shown that shallower access angles of 25 to 30 degrees parallel to the vessel improve vessel access success and decrease sheath malformation or kinking, bleeding or vessel perforation.
Needle size
As with any vascular access, micropuncture needles are likely to be helpful in minimizing trauma to adjacent tissues such as the pectoralis minor muscle. Micropuncture needles have a smaller crossing profile and require less force to puncture the artery. Fluoroscopy and care during advancement of the micropuncture wire is recommended reduce the risk of inadvertent dissection or perforation.
Wire and progressive dilation
The fascia and pectoralis minor muscle tend to resist sheath placement over a typical micropuncture wire. A small, tapered sheath can be passed more easily to confirm vessel cannulation, and a series of dilators exchanged over a stiff supportive 0.035ʺ wire enables the progressive enlargement of the tract to allow for large-bore access.
Ultrasound
Ultrasound imaging allows the operator to identify the pectoralis minor muscle, brachial plexus, and axillary artery and vein, which are distinguished with compression or Doppler flow (Figure 2). Ultrasound provides vessel size information and screening for atherosclerosis without radiation exposure or iodinated contrast. A vascular ultrasound probe (linear array, 5-13 MHz) can be used to image the artery in axial or longitudinal planes, and provide real-time guidance for needle cannulation. A smaller profile probe may be helpful to image between the bony structures. A single anterior wall stick is frequently possible and accuracy is often improved with experience or use of a needle guide.
Positioning
Abduction of the patient's arm to 45-90 degrees may decrease the tortuosity of the axillary artery and subclavian, improve alignment of the artery with the needle angle, aid in dissection of the pectoralis, and decrease the depth of the vessel due to the position of soft tissue and the pectoralis minor above it. While experienced operators are able to successfully place transaxillary devices with the arm in a neutral position, the consensus is that arm abduction facilitates transaxillary access.
Removal & closure techniques
The optimal technique for the removal of any transaxillary device is not known, but one must consider the size of the sheath, the insertion site's proximity to the second rib for possible compression, anticoagulation status, duration of access, and operator experience/comfort level.
We consider the safest fashion of removal to occur with balloon-tamponade proximal to the arteriotomy permitting “dry closure” (Figure 4). In brief, the subclavian or innominate artery is engaged from a secondary arterial access and a stiff exchange-length 0.035ʺ wire advanced past the large-bore axillary sheath into the brachial artery. To maximize angiographic visualization and delivery of covered stents, placement of a 7F or 8F long (90-110 cm) sheath from the femoral approach is recommended. If the radial approach is used, then a long sheath is recommended as passage of balloons and stents can induce spasm and entrapment. A 7-10 × 40 mm compliant peripheral balloon sized 1.0-1.1:1 to the artery is advanced over the wire proximal to the axillary sheath (ideally distal to the vertebral artery) and inflated at low pressure (2-4 atmospheres) under fluoroscopy to prevent traumatic injury to the vessel. Once endovascular hemostasis is achieved, the axillary sheath is removed and closure devices deployed. The balloon in the subclavian artery is then deflated and angiography performed via the secondary access to evaluate for extravasation from the arteriotomy site. In case of bleeding or extravasation at the access site, the operator can advance the same balloon to the arteriotomy site and inflate the balloon for 4-6 atmospheres for 15-20 min. Simultaneous manual compression and reversal of anticoagulation are helpful. Covered stents and surgical repair are generally last resorts reserved for large perforations or dissections as discussed in the next section of this document.
Figure 4.
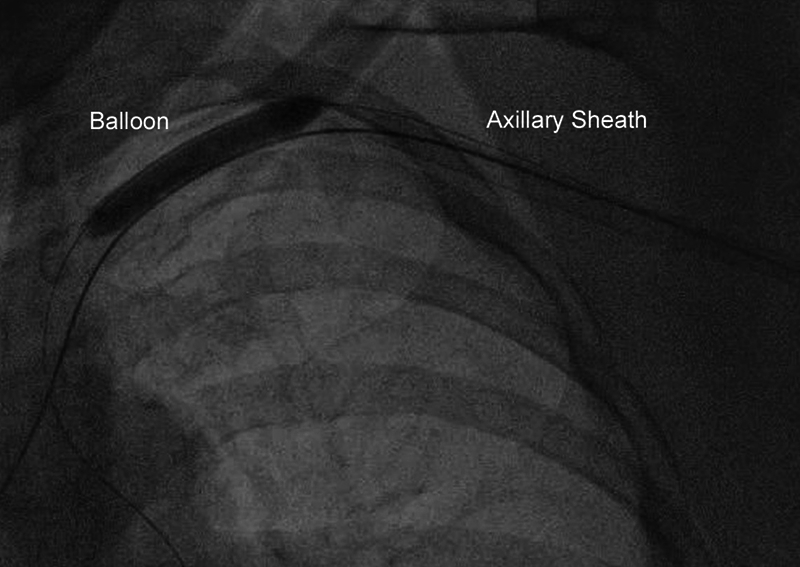
Dry closure of the axillary artery, using a balloon inserted from the femoral artery and inflated in the subclavian artery before removal of the axillary sheath.
Vascular closure devices (VCD) are frequently utilized “off-label” for axillary artery closure. Members of writing committee recommend the suture-mediated VCD (Perclose, Abbott), which permits rewiring and maintenance of access. The use of a collagen plug VCD (Angioseal, Terumo; Mynx, Cordis; Manta, Teleflex) has also been reported, but these devices do not allow preservation of access if the device fails. Two suture-mediated VCDs or a combination of a suture-mediated and collagen-plug VCD is usually necessary for large-bore closure. Operators should be aware that the axillary artery has more elastic lamina and less muscular lamina than the femoral, such that the tension required and tolerated on the VCD anchoring sutures or footplates is less (with excessive tension causing a “pull-through” phenomenon where the anchoring mechanism tears through the arterial wall), while there is a greater tendency to “pinch” the artery with successful closure device placement.
For transaxillary devices whose dwell time is limited, a planned “pre-close technique” using a suture-mediated VCD may suffice. The Impella CP (Abiomed) now has a “wire re-access port” which can accommodate a stiff 0.035ʺ guidewire. While there are theoretical risks for infection, no clear evidence precludes VCD placement with delayed closure, though many prefer open repair or suture-mediated VCDs over a collagen-plug VCD in such cases.
Manual compression of the axillary artery has been demonstrated to be a viable alternative to VCDs, particularly for sheath sizes <9F in patients who are not on anticoagulation (Figure 5).11 Compression over or near the second rib may be the most effective location for hemostasis and avoidance of pseudoaneurysm formation. However, a developing hematoma in this location may be hard to appreciate given the anatomy and therefore upstream balloon tamponade during manual compression should be considered as a precaution.
Figure 5.

Manual pressure of the axillary artery (arrow) around the second rib with nonocclusive hemostasis confirmed by angiography.
Complications and management
Familiarity with potential axillary artery complications and their management is essential for patient safety. The axillary artery is more fragile than the femoral artery making it theoretically more prone to complications during instrumentation, though comparative data is limited.2 The Axillary Access Registry to Monitor Safety (ARMS) Registry (which included 102 consecutive patients who underwent transaxillary access for mechanical hemodynamic support across 10 U.S. sites) reported a 15.7% (16/102) rate of procedural complications, 10% of which were due to minor access site bleeding and hematoma.3 There were no instances of major bleeding. Other in-hospital complications (occurring >6 hours after the procedure up until discharge) included access site bleeding requiring transfusion (6/102), access site hematoma >4 cm (3/102), neurologic complaints (3/102), pseudoaneurysm formation (1/102), and hand ischemia (1/102).
Bleeding
Bleeding is the most common complication from transaxillary artery access. The risk of bleeding increases with larger-bore sheaths, patient age, longer sheath dwell time, tortuosity, urgency of the procedure, and anticoagulation.2 Hemostasis failure can lead to bleeding into the axillary space with possible compression of the adjacent brachial plexus and permanent nerve injury. As the axillary artery is outside the thoracic cavity, a hemothorax should not occur if vessel puncture was accurate.
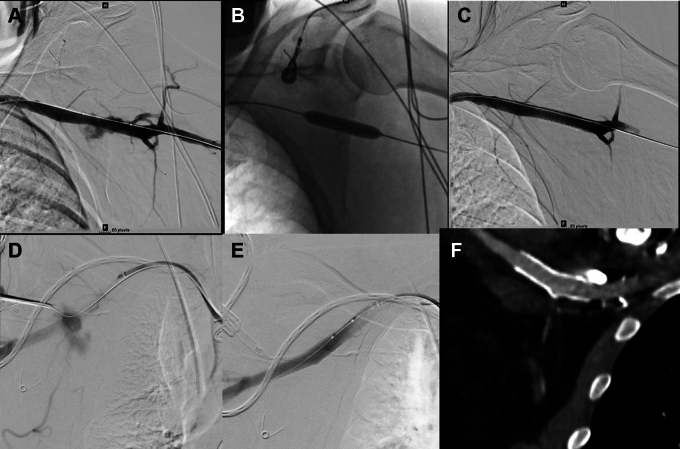
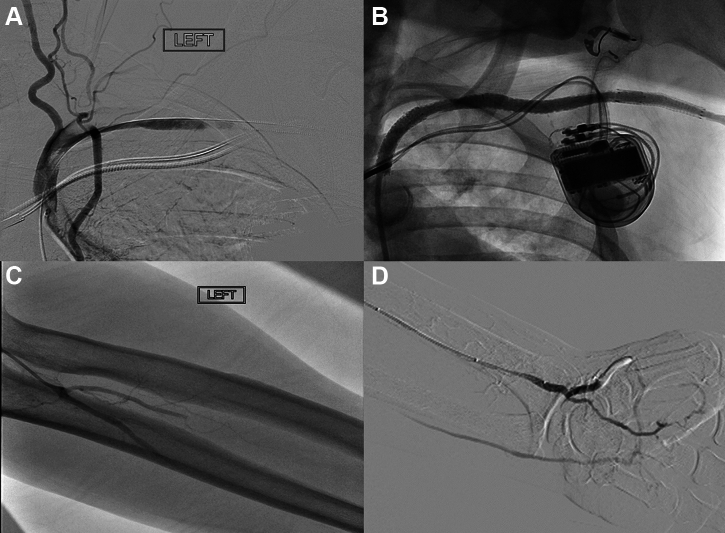
Failure of the initial closure strategy (especially balloon tamponade) (Figures 6 and 7) may be managed with a stent graft to cover the arteriotomy and achieve hemostasis.2,3 Access options for stent graft delivery include the femoral artery, ipsilateral brachial artery, or ipsilateral radial artery (if the stent graft can be deployed through a smaller access). The self-expanding Viabahn covered stent endoprosthesis (W.L. Gore) is preferred over balloon-expandable stent grafts for superior apposition and crush resistance. Details of sheath and wire compatibility of the various Viabahn stent sizes are listed in Table 1. Proximity to relevant side branches including the internal mammary and vertebral arteries should be considered before covered stent deployment. A short stent graft may not adequately seal a large arteriotomy while a longer one may cover important branches.
Figure 6.
Covered stent use for bleeding. A) Bleeding at axillary puncture site B) balloon inflation across the puncture site C) Stent graft bailout. D) Extravasation at axillary artery puncture site after Perclose closure failure. Angiogram performed while testing closure. Note maintenance of wire access at axillary puncture site and safety wire across axillary artery E) Hemostasis after deployment of self-expanding Viabahn (W.L. Gore) covered stent F) CTA at 1-month follow-up showing patent axillary artery covered stent.
Figure 7.
Dissection. A) Angiography showing extensive dissection and thrombosis of the left subclavian artery. B) After extensive thrombectomy, angioplasty and stenting of the left subclavian and axillary artery. Note thrombus shift into the vertebral and left internal mammary artery which was asymptomatic C) Distal embolization to the left radial, ulnar and interosseous arteries D) Restoration of flow to all 3 forearm arteries after aspiration thrombectomy and low-pressure balloon angioplasty.
Table 1.
Equipment and supplies recommended for axillary artery access
| Key attributes | Example | |
|---|---|---|
| Ultrasound | Small profile probe Vascular linear array transducer Minimum 6-cm depth |
L25x 6-13 MHz Transducer for Edge (Sonosite) |
| Micropuncture kit | 21-gauge, echogenic needle 0.018ʺ guidewire |
Micropuncture Access Kit (Cook) |
| Sheath for secondary access (femoral) | 7F-8F 90-110 cm length preferred for dry closure Side port for angiography Braided construction Hydrophilic coating |
Flexor Shuttle Sheath (Cook) Pinnacle Destination Sheath (Terumo) |
| Vascular closure device | Suture-mediated closure Pre-close capability, maintain access |
Perclose Proglide (Abbott) |
| Dry closure balloon | Compliant balloon 6-12 mm diameter sized 1.0-1.1:1 to vessel 20-40 mm length Long shaft 120 cm 0.018ʺ or 0.035ʺ wire compatible 7F sheath compatible |
Multiple products suitable |
| Covered stents for bailout | Self-expanding covered stents preferred Oversize by 10-20% relative to vessel 120 cm catheter length 2.5, 5, 7.5 cm lengths |
Viabahn Endoprosthesis (W.L. Gore) 5 mm and 6 mm diameter (6F sheath, 0.018ʺ guidewire) 7 mm and 8 mm diameter (7F sheath, 0.018ʺ guidewire) 5 mm and 6 mm diameter (7F sheath, 0.035ʺ guidewire) 7 mm to 10 mm diameter (8F sheath, 0.035ʺ guidewire) |
Direct surgical repair of the artery may be preferable in some cases to definitively manage bleeding and avoid the risks of stent grafts including side branch occlusion, restenosis, thrombosis, infection and reduced mobility. In the majority of such cases, the arteriotomy can be repaired primarily, unless there is significant atherosclerotic disease in the access vessel or the vessel wall has been significantly disrupted, in which case a patch angioplasty or a short interposition graft may be required.
Pseudoaneurysm
Bleeding from an axillary arteriotomy may not be immediately apparent and may result in pseudoaneurysm (PSA) formation or an access site hematoma, which together occurred in 4% of patients in the ARMS registry.4 Access site hematomas may be managed conservatively with observation. However, pseudoaneurysms, especially those that are larger than 2 cm, or in patients who are chronically anticoagulated, are unlikely to resolve spontaneously. Treatment of axillary artery PSA depends on the size and symptoms. Ultrasound-guided thrombin injection, with or without a temporary occluding balloon to mitigate the risk of distal embolization is often a feasible option with a high success rate.12 Coverage of the arteriotomy with a stent graft or open surgical repair are effective though more invasive options.13
Dissection
Axillary artery dissection is a rare complication with a rate of 4% in patients undergoing large-bore vascular access.14 If the dissection is non-flow limiting and clinically asymptomatic, conservative management may be sufficient. However, if the dissection is associated with ischemic hand symptoms, stenting with either bare-metal or covered stents may be necessary (Figure 7). Hand ischemia from a dissection after percutaneous transaxillary access is possible and operators must be poised to address this as well as any distal embolization that may occur.
Upper extremity ischemia
While the highly collateralized nature of the shoulder is generally protective, ischemia of the upper extremity may result from an occlusive sheath, hematoma formation, or vessel dissection or thrombosis. If the ischemia persists or develops after a sheath is removed, immediate angiography and/or CTA would be warranted. Management includes balloon angioplasty if there is a focal stenosis or occlusion, open thrombectomy and arterial repair, or percutaneous thromboembolectomy.14,15
Prolonged mechanical circulatory support with a pVAD can cause ischemia. “Peeling away” the Impella 14F introducer sheath leaving the smaller 9F repositioning sheath may be sufficient to restore limb perfusion. Antegrade distal perfusion can also be achieved by connecting the side arm of the large bore sheath to the side arm of a radial or brachial sheath using a male-to-male connector and arterial tubing.15
Thrombosis
The rate of vascular complications increases with prolonged dwell times for transaxillary devices. In-situ thrombosis associated with the device or sheath can occur over time despite anticoagulation. In one study of the Impella pVAD, while no patients with implant duration of 0 or 1 day had a thrombus, the rate of thrombus formation increased to 67% for implant durations of 4-7 days and 75% for implant durations of >7 days (P = 0.03 for trend).16 Most patients with nonocclusive thrombus were managed conservatively with heparin for 72 hours following explant and arteriotomy hemostasis, but 4 patients (14%) required balloon angioplasty or rheolytic thrombectomy at the time of explant for occlusive clot displaced into the brachial artery. In contrast, for the lower profile IABP, a large study of 195 patients with a mean duration of support of 19 days had relatively low risks of hematoma (4.6%), bacteremia (9.2%), and arm ischemia (3.5%).17
Infection
Indwelling sheaths theoretically pose a risk of infection at the axillary access site, though almost no such infections have been reported in the literature. Prophylactic antibiotics should be administered when the device is not promptly removed.
Neurological complications
Although brachial plexus injury has long been considered a significant risk associated with axillary access, the reported incidence in the literature is low. The few published case series to date have not reported any severe brachial plexus injuries.8,14 One series noted a single case of transient ipsilateral thumb numbness. Tingling in the C8 distribution was reported in 3% of patients in the ARMS registry.3 Nevertheless, all patients should be evaluated for ipsilateral pain, paresthesias, and weakness during or following transaxillary procedures, particularly when bleeding has occurred.
Stroke
Stroke can occur with any arterial instrumentation or device, or spontaneously due to patient factors. The exact risk from axillary access is unclear and may vary by device, indication, and duration, with reported rates of 1% for short-term use of a pVAD, 2.6% for the IABP and 6.5% for TAVR.3,7,17 There are no data to suggest that laterality of access can modify the risk of stroke.
Best practices for axillary access
Multidisciplinary team
Transaxillary large-bore procedures are advanced procedures performed on patients with significant comorbidities. Facilities starting a transaxillary program should have interventionalists experienced with large-bore access, vascular or cardiothoracic surgery services, cardiothoracic radiology, and critical care nursing capable of detecting and managing complications. Heart failure or transplant specialists would be necessary for pre-procedure planning as well as management of prolonged mechanical circulatory support. If local expertise in transaxillary access is not available, then transfer to a center with such a program would be appropriate (Table 2).
Table 2.
Applications of transaxillary access
| Indication | Example devices | Sheath sizes | Duration | Insertion recommendations | Removal and closure recommendations | Comments/areas of uncertainty |
|---|---|---|---|---|---|---|
| Fenestrated, branched, or chimney endovascular aneurysm repair (EVAR) or transcatheter aortic valve replacement (TAVR) |
Complex EVAR: iCast (Getinge) VBX (W.L. Gore) Viabahn (W.L. Gore) TAVR: Sapien 3 (Edwards) Evolut R (Medtronic) Portico (Abbott) |
Complex EVAR: 6F-16F TAVR: 14F-19F |
Hours, in procedure room only |
|
|
|
| Short-term mechanical circulatory support | Impella (Abiomed) | 13F-14F | Hours to days |
|
|
|
| Extended mechanical support with intra-aortic balloon counterpulsation | MEGA/Sensation (Getinge) Ultraflex (Arrow Teleflex) |
7.5F-8F | Days to weeks |
|
|
|
| Peripheral endovascular angiography and intervention | Any traditional coronary sheath or peripheral (eg, Destination, Terumo) sheath | 4F-8F | Hours |
|
|
|
Imaging
Multiple modalities for imaging (including fluoroscopy and ultrasound) should be available to ensure access to the appropriate segment of artery and assess vessel size prior to placement of larger catheters. Pre-procedural CT scans can be particularly helpful to exclude tortuosity and atherosclerosis but not always possible especially in patients who have acute or chronic kidney injury, or who are unstable and require percutaneous mechanical circulatory support.
Real-time ultrasound guidance has been demonstrated across multiple access venous and arterial sites to improve first-pass success rates and reduce vascular complications. Familiarity with the technique will likely facilitate accurate placement of transaxillary devices. As the utility of ultrasound has not been confirmed for the axillary artery in randomized trials, however, alternative techniques of access including placement of a guidewire in the axillary artery to serve as a fluoroscopic target for needle puncture are reasonable.
Vessel location
Though some experienced groups have suggested other locations, given the anatomical considerations previously discussed, this panel recommends the second segment of the axillary artery as the preferred site for puncture, regardless of left or right sided access. Some patients have a pacemaker/defibrillator implanted in the left anterior chest wall and this may require a more lateral puncture location.
Exit strategy
Just as with femoral large bore access, it is important to consider how the device will be removed, prior to placement. Considerations regarding removal depend on many factors including estimated dwell time, size of vascular catheter, stability of the patient, and the need for anticoagulation following device removal.
Concentration of expertise
All vascular access techniques have a learning curve, and there are unique challenges to the transaxillary route. If a center anticipates low volume, the number of operators should be limited to concentrate the expertise initially and if the practice expands, these individuals can facilitate peer training of other personnel.
Quality assurance
As with any procedure, including usual catheterization laboratory or operating room cases, outcomes and complications should be tracked to optimize outcomes.
Gaps to address in the future
Ideal approach to removal of transaxillary devices
There are many approaches described to remove transaxillary devices, and no clear evidence which approach may be superior. Important aspects to consider are the availability of suture-mediated closure device placement at the time of insertion, length of time the device has been deployed in the patient's body, level of anticoagulation the patient will require following removal, and the size of the arteriotomy, as well as the circumstances of placement. Open surgical removal and direct repair of the arteriotomy may be preferable to endovascular closure for devices with prolonged dwell times.
Securing devices for longer-term use (outside the cath lab environment)
Regardless of the device placed, if the patient is ambulatory, there needs to be careful consideration to securing the device to prevent migration. In the case of the IABP, current catheters are longer than needed for transaxillary use, leading to questions about how to secure the excess length. Involving colleagues from nursing can ensure that everyone on the team is comfortable with the access site management including avoiding dressings that might lead to a breach in the sterile sleeve when changed. Avoid securing catheters to the upper arm, as movement will lead to catheter migration which can be challenging to correct.
Comparative efficacy and safety of percutaneous transaxillary access
While there are no randomized trials comparing percutaneous axillary access with surgical axillary or other arterial access sites, the ongoing ARMS registry and the SUPER AXA registry (NCT04589962) comparing surgical and percutaneous axillary access will continue to provide data on the safety and efficacy of percutaneous axillary access.
Training and privileging
As percutaneous axillary access is a relatively new technique, training criteria and requirements should be developed that are applicable broadly to all interventionalists/specialties utilizing this approach. To maximize procedural safety, it would be best for providers to have foundational knowledge of axillary anatomy plus any device-specific knowledge prior to placing transaxillary devices. Graduate medical education programs should develop training curricula in percutaneous axillary artery access. For those in practice, participation in a formal training program designed for percutaneous transaxillary procedures is recommended. These should include a focus on the following domains:
-
•
Knowledge of the anatomy of the upper chest wall and extremity including arterial, venous, and brachial plexus anatomy and common anomalies
-
•
Knowledge of a variety of imaging techniques relative to pre-procedural planning, especially computed tomography and ultrasound for anatomic assessment and access site selection for transaxillary access
-
•
Experience with devices from the femoral approach including management of large-bore arterial access and device removal
-
•
Appropriate selection of which axillary artery to use as well as contraindications to axillary access or specific device use
-
•
Didactic training in imaging modalities (fluoroscopy, angiography and ultrasound techniques) as applied to the axillary artery
-
•
Formal training for large bore access, closure devices, and other techniques for hemostasis
-
•
For devices of 10F or larger: Appropriate training in techniques for endovascular “dry” hemostasis and use of covered stents for access site hemostasis
-
•
Interactive education with hands-on or online simulation-based training
Privileging is ultimately decided by individual hospitals. Proper training is important to ensure excellent outcomes and enhance patient safety. There are no specific number of procedures that define a competent operator. The requirements for hospital privileges should ideally be the same for each specialty involved in percutaneous transaxillary procedures. In addition, requirements should be the same for operators in practice and those recently completing training.
Privileging considerations include the following:
-
•
Evidence of participation/completion of a formal didactic and hands-on training program as outlined
-
•
Outline the potential need or role for remote or on-site proctoring
-
•
Successful completion of industry required device specific training programs where applicable (IABP, TAVR, MCS, TEVAR, etc.)
-
•
Ongoing formal monitoring program to evaluate outcomes by operator using local or registry based outcomes data
-
•
Determination of interval of ongoing CME training that may be determined by frequency and/or complexity of procedures performed by operators.
Certificates of training may eventually become available from professional societies, industry, or medical education providers.
Summary
Axillary artery access has become increasingly widespread as an alternative to the femoral route for large-bore TAVR, EVAR, and MCS procedures. Best practices for transaxillary procedures include involvement of a multidisciplinary team, multimodality imaging including CT and ultrasound, access at the second axillary segment, and expertise in large-bore dry-closure and hemostasis. While the technique is demonstrably safe and effective at experienced centers, education and training on its unique anatomic and clinical challenges will ensure safe dissemination across the growing number of facilities performing such procedures.
Peer review statement
Given her role as Deputy Editor, Suzanne Baron had no involvement in the peer review of this article and has no access to information regarding its peer review.
Footnotes
To access the supplementary material accompanying this article, visit the online version of the Journal of the Society for Cardiovascular Angiography & Interventions at 10.1016/j.jscai.2022.100041.
Supplementary material
References
- 1.Szerlip M., Feldman D.N., Aronow H.D., et al. SCAI publications committee manual of standard operating procedures. Catheter Cardiovasc Interv. 2020;96(1):145–155. doi: 10.1002/ccd.28754. [DOI] [PubMed] [Google Scholar]
- 2.Schäfer U., Ho Y., Frerker C., et al. Direct percutaneous access technique for transaxillary transcatheter aortic valve implantation: “the Hamburg Sankt Georg approach.”. JACC Cardiovasc Interv. 2012;5(5):477–486. doi: 10.1016/j.jcin.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 3.McCabe J.M., Kaki A.A., Pinto D.S., et al. Percutaneous axillary access for placement of microaxial ventricular support devices: the Axillary Access Registry to Monitor Safety (ARMS) Circ Cardiovasc Interv. 2021;14(1) doi: 10.1161/CIRCINTERVENTIONS.120.009657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tayal R., Hirst C.S., Garg A., Kapur N.K. Deployment of acute mechanical circulatory support devices via the axillary artery. Expert Rev Cardiovasc Ther. 2019;17(5):353–360. doi: 10.1080/14779072.2019.1606712. [DOI] [PubMed] [Google Scholar]
- 5.Dawson K., Jones T.L., Kearney K.E., McCabe J.M. Emerging role of large-bore percutaneous axillary vascular access: a step-by-step guide. Interv Cardiol. 2020;15:e07. doi: 10.15420/icr.2019.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenbaum A.N., Jain C.C., Shadrin I.Y., El Hajj S.C., El Sabbagh A., Behfar A. Percutaneous axillary intra-aortic balloon pump insertion technique as bridge to advanced heart failure therapy. ASAIO J. 2021;67(4):e81–e85. doi: 10.1097/MAT.0000000000001259. [DOI] [PubMed] [Google Scholar]
- 7.Dahle T.G., Kaneko T., McCabe J.M. Outcomes following subclavian and axillary artery access for transcatheter aortic valve replacement: Society of the Thoracic Surgeons/American College of Cardiology TVT Registry Report. JACC Cardiovasc Interv. 2019;12(7):662–669. doi: 10.1016/j.jcin.2019.01.219. [DOI] [PubMed] [Google Scholar]
- 8.Harris E., Warner C.J., Hnath J.C., Sternbach Y., Darling R.C., III Percutaneous axillary artery access for endovascular interventions. J Vasc Surg. 2018;68(2):555–559. doi: 10.1016/j.jvs.2017.11.066. [DOI] [PubMed] [Google Scholar]
- 9.Arnett D.M., Lee J.C., Harms M.A., et al. Caliber and fitness of the axillary artery as a conduit for large-bore cardiovascular procedures. Catheter Cardiovasc Interv. 2018;91(1):150–156. doi: 10.1002/ccd.27416. [DOI] [PubMed] [Google Scholar]
- 10.Thawabi M., Tayal R., Khakwani Z., Sinclair M., Cohen M., Wasty N. Suggested bony landmarks for safe axillary artery access. J Invasive Cardiol. 2018;30(3):115–118. [PubMed] [Google Scholar]
- 11.Tayal R., DiVita M., Sossou C.W., et al. Efficacy of manual hemostasis for percutaneous axillary artery intra-aortic balloon pump removal. J Interv Cardiol. 2020;2020 doi: 10.1155/2020/8375878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown A.D., Hirpara D.H., Jaberi A., Oreopoulos G.D., Simons M.E. Successful balloon assisted percutaneous thrombin injection of right subclavian artery pseudoaneurysm. J Vasc Access. 2017;18(5):e62–e65. doi: 10.5301/jva.5000694. [DOI] [PubMed] [Google Scholar]
- 13.Chen L., Peng F., Wang T., Chen D., Yang J. Traumatic pseudoaneurysm of axillary artery combined with brachial plexus injury. PLoS One. 2014;9(11) doi: 10.1371/journal.pone.0113099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meertens M., Laturnus J., Ling A., Atkinson N., Mees B., Wagner T. Percutaneous axillary artery access in complex endovascular aortic repair. J Vasc Interv Radiol. 2019;30(6):830–835. doi: 10.1016/j.jvir.2018.12.735. [DOI] [PubMed] [Google Scholar]
- 15.Kaki A., Alraies M.C., Kajy M., Blank N., Glazier J.J., Mohamad T., Elder M., Schreiber T. Large bore occlusive sheath management. Catheter Cardiovasc Interv. 2019;93(4):678–684. doi: 10.1002/ccd.28101. [DOI] [PubMed] [Google Scholar]
- 16.Jones T.L., Kearney K.E., McCabe J.M. Prevalence and predictors of vascular thrombus formation after percutaneous axillary artery impella insertion. Circ Cardiovasc Interv. 2019;12(8) doi: 10.1161/CIRCINTERVENTIONS.119.008046. [DOI] [PubMed] [Google Scholar]
- 17.Bhimaraj A., Agrawal T., Duran A., et al. Percutaneous left axillary artery placement of intra-aortic balloon pump in advanced heart failure patients. JACC Heart Fail. 2020;8(4):313–323. doi: 10.1016/j.jchf.2020.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.