Abstract
Background
The FlexNav delivery system (DS) features a hydrophilic coating, stability layer, and integrated sheath to facilitate valve deployment in vessel diameters ≥5.0 mm.
Methods
Data were pooled from 2 concurrent prospective, multicenter, premarket studies (PORTICO IDE [n = 147] and FlexNav EU CE Mark [n = 46]) to evaluate the safety and efficacy of the FlexNav DS to deliver the Portico valve in the Global FlexNav study. The primary end point was Valve Academic Research Consortium (VARC)-2 major vascular complication rate at 30 days. These outcomes were compared with those of the commercially available valve arm from the PORTICO IDE study.
Results
The Global FlexNav study enrolled 193 high- or extreme-risk subjects for sugery. The mean age was 84.8 years, and 59.6% were women, with a mean Society of Thoracic Surgeons score of 5.2%. At 1 year, the rate of all-cause mortality was 5.2%, disabling stroke 2.1%, and mild or less paravalvular leak 99.4%. The mean aortic gradient was maintained at 7.4 ± 4.3 mm Hg through 1 year. At 1 year, 96.8% of subjects were classified as New York Heart Association class I or II. A pacemaker was implanted in 15.4% of subjects at 30 days and 18.4% at 1 year. The results of the Portico valve in the Global FlexNav study are comparable with the results from the commercially available valve arm in the PORTICO IDE study.
Conclusion
The FlexNav DS was shown to be safe for the delivery of the Portico valve, which demonstrated sustained treatment benefits at 1 year with low rates of all-cause mortality or disabling stroke, improved heart failure symptoms, and excellent valve performance.
Keywords: aortic insufficiency, aortic regurgitation, transcatheter aortic valve replacement
Central Illustration
Highlights
-
•
The Portico valve with FlexNav DS was used in high and extreme risk subjects.
-
•
The devices provided excellent safety and valve performance from 30 days to 1 year.
-
•
They demonstrated low vascular complications or life-threatening bleeding.
-
•
They provided low mortality, disabling strokes, and excellent functional outcomes.
-
•
They sustained large aortic valve area and low PVL and mean aortic gradients.
Introduction
Transcatheter aortic valve replacement (TAVR) is the standard of care for the treatment of severe symptomatic aortic stenosis (AS) of trileaflet aortic valve, irrespective of surgical risk,1, 2, 3, 4, 5, 6, 7 and is a reliable alternative for subjects in whom open heart surgery may not be feasible.7,8 The balloon-expandable intra-annular Sapien 3 valve (Edwards Lifesciences), the self-expanding supra-annular Evolut valve (Medtronic), and the self-expanding intra-annular Portico THV (THV) (Abbott Structural Heart) are approved by the US FDA for the treatment of severe AS in high- and extreme-risk subjects.9
The Portico resheathable transcatheter aortic valve system is a self-expanding THV with large, open cells and bovine intra-annular leaflets uniquely designed to provide excellent hemodynamics. The approval of the Portico valve was based on the clinical outcomes of the PORTICO IDE randomized clinical trial.9,10 In this trial, the rates of death or disabling stroke, cardiac symptom reduction, and functional status were not significantly different between the Portico valve and commercial valves at 1 year and 2 years. The first-generation Portico valve delivery system (DS) used in the PORTICO IDE study required the use of a separate 18F or 19F arterial introducer sheath, requiring a minimum vessel diameter of 6.0 mm. The next-generation FlexNav DS was designed to address the limitations of the first-generation DS. Unlike the first-generation DS, the FlexNav DS has a hydrophilic-coated integrated sheath to reduce the delivery profile diameter to 14F or 15F equivalent to minimize vessel trauma at the access site and features a stability layer to minimize system manipulations and support precise valve deployment.
Methods
Study design
Two prospective, multicenter, nonrandomized, studies enrolled concurrently under identical eligibility criteria and were designed to allow data to be pooled between the 2 studies. The Global FlexNav DS analysis reported in this study includes 180 subjects previously reported and an additional 13 subjects from the PORTICO IDE continued access arm, thereby 193 subjects enrolled at 28 sites in Europe, Australia, and the United States between October 2018 and February 2020. Of the total 193 subjects, 147 subjects were enrolled in the PORTICO IDE study (NCT02000115) at 20 US sites and 3 Australian sites, and 46 subjects were enrolled in the FlexNav EU CE Mark study (NCT03724812) at 5 European sites. Both studies received regulatory approval and were approved by an ethics committee/institutional review board at each investigational site and ethical oversight per local and national regulations. All subjects provided written informed consent per the national and local requirements. Clinical events were adjudicated by a common independent clinical events committee per the Valve Academic Research Consortium (VARC)-2 definitions.11 Echocardiograms were analyzed by an independent echocardiography core laboratory (MedStar Health Research Institute). Subjects were evaluated at baseline, discharge, and 30-day, 6-month, and 1-year visits. For a list of investigational sites, refer to Supplemental Table S1.
Enrollment
All enrolled subjects were evaluated by the local site heart team and were screened per eligibility criteria. Subjects must have presented with New York Heart Association (NYHA) ≥II and severe native AS, defined as aortic valve area ≤1.0 cm2 (or indexed aortic valve area ≤0.6 cm2/m2), with a mean pressure gradient of >40 mm Hg, jet velocity >4.0 m/s, or Doppler velocity index <0.25. High-risk subjects were defined as having a Society of Thoracic Surgeons (STS) predicted mortality risk score of ≥8% or comorbidities that predicted an operative mortality risk at 30 days of ≥15% (as determined by cardiologist and ≥1 surgeon per respective protocols). Extreme-risk subjects were defined as having a probability of death or serious morbidity at 30 days of >50%. For a full list of eligibility criteria, study definitions, and subject selection committee criteria (confirming eligibility, STS risk, and procedural access suitability), refer to Supplemental Table S2.10
Study devices
Portico THV
Comprehensive descriptions of the Portico valve have been reported previously.9,12,13 Key design features include a radio-opaque, nonflared, self-expanding nitinol frame with intra-annular leaflet placement and large, open cells to minimize coronary obstruction. Portico valves are available in the sizes of 23.0, 25.0, 27.0, and 29.0 mm sizes and can be fully resheathed and repositioned before full release (80% deployment).
FlexNav delivery system
The FlexNav DS key features include an integrated sheath that reduces the distal profile to 14F-15F equivalent and allows the catheter system to directly access vessels ≥5.0 mm, a hydrophilic coating that enhances deliverability, and a stability layer that facilitates precise valve placement to minimize manipulations and malposition. In addition, an ergonomically designed proximal handle offers a deployment wheel allowing for adjustments, a micro-adjustment wheel to align the valve and radio-opaque tip, and a deployment indicator. These improvements were designed to minimize vascular complications, improve deliverability with precise deployment, and overcome transfemoral vascular access limiting factors (eg, narrowing, tortuosity, and calcification).
Procedure
Study subjects received general or local anesthesia per site discretion. Investigators were permitted to use a separate arterial introducer sheath (18F with 23.0 or 25.0 mm Portico valves and 19F with 27.0 or 29.0 mm Portico valves) or use the FlexNav DS integrated sheath alone for arterial access. Predilation of the native aortic valve with balloon valvuloplasty was recommended. The Portico valve could be resheathed and repositioned before full deployment for ideal valve positioning. If valve underexpansion or mild aortic insufficiency was observed, postdilation was recommended to increase the paravalvular seal with the native valve. The investigational site standard of care was followed for antithrombotic and antiplatelet regimens.
End points
The primary safety end point was VARC-2–defined major vascular complications at 30 days. A nonhierarchical descriptive safety end point at 30 days evaluated the composite rate of all-cause mortality, disabling stroke, life-threatening bleeding requiring transfusion, acute kidney injury requiring dialysis, or major vascular complications. Adverse events were defined using the VARC-2 standardized criteria, and major vascular complications were adjudicated as access site or nonaccess site related. Additional 30-day descriptive end points included technical device success (defined as successful vascular access, delivery and deployment of the Portico valve, retrieval with FlexNav DS, and a single valve correctly positioned), procedural outcomes, valve performance (effective orifice area, mean aortic transvalvular gradient, paravalvular leak [PVL], and prosthesis-patient mismatch), functional status (6-minute walk and NYHA class), and quality of life (Kansas City Cardiomyopathy Questionnaire). One-year descriptive end points included a composite of all-cause mortality or disabling stroke, all-cause mortality, disabling stroke, nondisabling stroke, and valve performance (effective orifice area, mean aortic transvalvular gradient, and PVL).
Statistical analysis
Baseline characteristics, procedural outcomes, and study end points were summarized using descriptive statistics. Paired t tests (echocardiographic data and Kansas City Cardiomyopathy Questionnaire score) or the Wilcoxon signed-rank test (NYHA class) were used to compare descriptive outcomes from baseline (or discharge) to 1 year. To assure complete ascertainment, sites were asked to complete a survival status check (telephone call) to confirm the subject’s survival status at 365 days from the date of the index procedure. To compare the performance of the Portico valve with the FlexNav DS with commercially available valves, subjects from the Global FlexNav study (n = 193) were analyzed using a propensity score methodology to subjects from the commercially available valve (CAV) arm of the PORTICO IDE study (n = 362). This methodology reduced confounding in the comparison of outcomes by considering differences in baseline subject characteristics. First, a logistic regression model was used on the key demographic and baseline characteristics to calculate the propensity score for each subject. Then, the combined subject cohort (N = 555) was divided into 5 quintiles based on their propensity scores. Differences in baseline characteristics between the 2 groups within each quintile (n = 111 per quintile) were compared. The propensity quintile was judged to be adequate if group comparison P values for those baseline characteristics were >0.05 after adjusting for the propensity score quintiles. An independent statistician with no knowledge of the clinical outcome data performed the propensity score analysis. The analysis of individual components and composite end point of mortality and disabling stroke were based on Kaplan-Meier event rate difference between groups within each quintile, and moderate or severe paravalvular leak at 1 year were based on proportion difference between the groups. The analysis included all subjects from the Global FlexNav study and the CAV arm of the PORTICO IDE study who underwent the TAVR procedure. Moreover, the analysis was conducted using both average treatment effect (ATE) weight and average treatment effect on the treated (ATT) weight approaches.14, 15, 16 The 95% CIs were calculated for the overall differences between the 2 groups for both ATE and ATT approaches. SAS version 9.4 (SAS Institute) was used for all statistical analyses.
Study oversight
Clinical data were monitored and verified by source documentation at the clinical site. Study data were entered into each respective study database on the case report forms and signed by the site principal investigator. All data discrepancies were resolved before locking the database and pooling the data. Abbott managed the Oracle software (Oracle Corporation) database (21 CFR part 11 compliant) and overall study oversight.
Results
Baseline demographics
Baseline demographics for subjects in the Global FlexNav cohort and the CAV arm of the PORTICO IDE study (CAV group) are presented in Table 1. In the FlexNav cohort, the mean age was 84.8 ± 5.7 years, 59.6% (115/193) were women, the mean STS score was 5.22% (calculated using the later version of the STS short-term risk calculator released on November 15, 2018), and 21.2% (41/193) of subjects were deemed extreme risk. In the CAV group, the mean age was 83.6 ± 7.0 years, 53.3% (193/362) were women, the mean STS score was 6.55% (calculated using an older version of the STS short-term risk calculator before November 15, 2018), and 16.9% (61/362) of subjects were deemed extreme risk. Both groups represent a high and extreme surgical risk population.
Table 1.
Baseline demographics.
| Global FlexNav DS cohort |
CAV group |
|
|---|---|---|
| (n = 193) | (n = 362) | |
| Age at consent, y | 84.8 ± 5.7 (193) | 83.6 ± 7.0 (362) |
| Female sex | 59.6 (115/193) | 53.3 (193/362) |
| NYHA class | ||
| Class II | 39.9 (77/193) | 27.1 (98/362) |
| Class III | 56.5 (109/193) | 63.5 (230/362) |
| Class IV | 3.6 (7/193) | 9.4 (34/362) |
| STS predicted risk of mortality, % | 5.20 ± 2.84 (193) | 6.55 ± 3.37 (362) |
| STS predicted risk of morbidity and mortality, % | 20.35 ± 8.58 (193) | 27.39 ± 8.16 (362) |
| EuroSCORE II, % | 4.64 ± 3.47 (193) | 6.68 ± 5.88 (362) |
| Aortic valve repair | 0.0 (0/193) | 0.0 (0/362) |
| Aortic valve replacement | 0.0 (0/147) | 0.0 (0/362) |
| Balloon valvuloplasty | 3.1 (6/193) | 6.1 (22/362) |
| Cerebral vascular accident | 7.8 (15/193) | 13.5 (49/362) |
| Cerebrovascular disease | 9.8 (19/193) | 16.6 (60/362) |
| Chronic lung disease | 26.9 (52/193) | 39.8 (144/362) |
| Coronary artery bypass graft | 14.0 (27/193) | 20.7 (75/362) |
| Coronary artery disease | 59.1 (114/193) | 69.1 (250/362) |
| Diabetes | 32.1 (62/193) | 39.2 (142/362) |
| Oral control | 62.9 (39/62) | 50.0 (71/142) |
| Hostile mediastinum/prohibitive chest deformity | 3.1 (6/193) | 5.2 (19/362) |
| Hypertension | 87.6 (169/193) | 89.5 (324/362) |
| Infectious endocarditis | 0.0 (0/193) | 0.0 (0/362) |
| Kidney disease | 19.2 (37/193) | 25.7 (93/362) |
| PTCA with stent | 22.8 (44/193) | 28.5 (103/362) |
| Peripheral vascular disease | 12.4 (24/193) | 18.0 (65/362) |
| Porcelain aorta | 0.5 (1/193) | 2.8 (10/362) |
| Previous myocardial infarction | 10.9 (21/193) | 11.0 (40/362) |
| Previous permanent pacemaker | 9.3 (18/193) | 16.6 (60/362) |
| PTCA without stent | 1.6 (3/193) | 3.3 (12/362) |
| Pulmonary hypertension | 31.1 (60/193) | 34.3 (124/362) |
| Severe liver disease | 0.0 (0/193) | 0.8 (3/362) |
| Transient ischemic attack | 5.7 (11/193) | 6.9 (25/362) |
| Frailty index: total frailty score (of 4) | 1.7 ± 0.7 (193) | 1.9 ± 0.8 (362) |
| Katz index of activities of daily living (≤4) | 5.2 (10/193) | 11.3 (41/362) |
| Grip strength (<body mass index and sex-based cutoff) | 86.5 (167/193) | 81.8 (296/362) |
| 15-Foot walk test (≥height and sex-based cutoff) | 67.0 (122/182) | 74.5 (263/353) |
| Albumin (<3.5 g/dL) | 17.6 (34/193) | 23.0 (86/374) |
| SSC approved risk classification | ||
| Extreme risk | 21.2 (41/193) | 16.9 (61/362) |
| High risk | 78.8 (152/193) | 83.1 (301/362) |
CAV, commercially available valve; DS, delivery system; PTCA, percutaneous transluminal coronary angioplasty; SSC, Subject Selection Committee; STS, Society of Thoracic Surgeons.
Continuous parameters: values are represented by as mean ± SD (n). Categorical parameters: rate per subject (%) is calculated n/N, where n is the number of subjects with an event and N is the total number of subjects at risk.
Procedural characteristics
Procedural characteristics are presented in Table 2. In the Global FlexNav cohort, conscious sedation was used in 59.1% (114/193) of subjects, 100% (193/193) of subjects were treated through transfemoral access, total fluoroscopy time was 21.2 ± 8.18 minutes, and previous balloon aortic valvuloplasty was performed in 94.8% (183/193) of subjects. In the CAV group, conscious sedation was used in 32.0% (116/362) of subjects, 95.0% (343/361) were treated through transfemoral access, the total fluoroscopy time was 19.5 ± 63.91 minutes, and previous balloon aortic valvuloplasty was performed in 55.4% (200/361) of subjects. Postimplant balloon valvuloplasty was performed in 29.5% (57/193) of the Global FlexNav cohort compared with 20.4% (76/361) of the CAV group. Postimplant depth was similar in both groups, with an implant depth of 4.21 ± 2.75 mm in the Global FlexNav cohort and 4.48 ± 2.34 mm in the CAV group. Procedure success was 96.9% (187/193) in the Global FlexNav cohort, with 3.1% (6/193) of subjects requiring a second TAVR valve, and 98.3% (356/362) in the CAV group, with 0.3% (1/362) of subjects converting to surgery and 1.4% (5/362) of subjects requiring a second TAVR valve. The length of hospital stay was similar in both groups (3.0 ± 2.3 days for the Global FlexNav cohort and 2.9 ± 2.2 for the CAV group).
Table 2.
Procedural characteristics.
| Global FlexNav DS cohort (n = 193) | CAV group (n = 362) | |
|---|---|---|
| Anesthesia: conscious sedation | 59.1 (114/193) | 32.0 (116/362) |
| Transfemoral access | 100.0 (193/193) | 95.0 (343/361) |
| Total fluoroscopy time, min | 21.21 ± 8.18 (191) | 19.51 ± 63.91 (359) |
| Pre-BAV performed | 94.8 (183/193) | 55.4 (200/361) |
| Final deployed stent depth, mm | 4.21 ± 2.75 (176) | 4.48 ± 2.34 (266) |
| Postimplant balloon valvuloplasty | 29.5 (57/193) | 20.4 (74/362) |
| Resheathing attempted | 43.0 (83/193) | NA |
| Implanted valve sizea, mm | ||
| 20.0 | NA | 1.9 (7/361) |
| 23.0 | 2.6 (5/191) | 26.9 (97/361) |
| 25.0 | 28.8 (55/191) | NA |
| 26.0 | NA | 41.6 (150/361) |
| 27.0 | 32.5 (62/191) | NA |
| 29.0 | 36.1 (69/191) | 24.1 (87/361) |
| 31.0 | NA | 1.9 (7/361) |
| 34.0 | NA | 3.3 (12/361) |
| Length of hospital stay, d | 3.0 ± 2.3 (193) | 2.9 ± 2.2 (362) |
| Procedural successb | 96.9 (187/193) | 98.3 (356/362) |
| Procedural mortalityc | 0.0 (0/193) | 0.0 (0/362) |
| Conversion to SAVR | 0.0 (0/193) | 0.3 (1/362) |
| Need for a second TAVR valve | 3.1 (6/193) | 1.4 (5/362) |
| No valve implanted | 0.0 (0/193) | 0.0 (0/362) |
| Successful vascular access | 100.0 (193/193) | 100.0 (362/362) |
BAV, balloon aortic valvuloplasty; CAV, commercially available valve; TAVR, transcatheter aortic valve replacement; SAVR, surgical aortic valve replacement.
Continuous parameters: values are represented as mean ± SD (n). Categorical parameters: rate per subject (%) is calculated n/N, where n is the number of subjects with an event and N is the total number of subjects at risk. NA refers to data not available.
Device size based on the final valve implanted. Two subjects who required a second valve were implanted with a non–Portico valve and not included in this summary.
Procedural success is defined as the absence of procedural mortality and correct positioning of a single transcatheter prosthetic heart valve in the proper anatomical location.
Procedural mortality is defined as deaths that occurred during the index procedure.
Clinical outcomes
Clinical outcomes for the Global FlexNav cohort and the CAV group are presented in Table 3. At 30 days, the rate of major vascular complications in the Global FlexNav cohort was 5.7% (11/193). All major vascular complications occurred on the same day as the index procedure, and most (5.2%, 10/193) were adjudicated as access site related. The nonhierarchical composite safety end point rate at 30 days was 9.8% (19/193) and was primarily driven by major vascular complications (5.7%, 11/193) and life-threatening bleeding requiring transfusion (4.1%, 8/193). At 30 days, all-cause mortality was 1.0% (2/193) and disabling stroke was 2.1% (4/193). There were no events of acute kidney injury (AKI) requiring dialysis through 30 days. In the CAV group, the rate of all-cause mortality at 30 days was 1.4% (5/362), disabling stroke was 0.8% (3/362), major vascular complications was 6.6% (24/362), and life-threatening bleeding was 3.6% (13/362). The rate of new permanent pacemaker implantation at 30 days was 15.4% (27/175) in the Global FlexNav cohort compared with 11.3% (35/302) in the CAV group.
Table 3.
Key clinical outcomes at 30 d and 1 y.
| Global FlexNav DS cohort (N = 193) | CAV group (N = 362) | |||
|---|---|---|---|---|
| Event | 30 d | 1 y | 30 d | 1 y |
| All-cause mortality | 1.0 | 5.2 | 1.4 | 11.8 |
| Cardiovascular | 1.0 | 2.1 | NA | NA |
| Disabling stroke | 2.1 | 2.1 | 0.8 | 2.6 |
| Life-threatening bleeding requiring transfusion | 4.1 | NA | 3.6 | NA |
| Acute kidney injury requiring dialysis | 0.0 | NA | 0.8 | NA |
| Major vascular complications | 5.7 | NA | 6.6 | NA |
| New pacemaker implantationa | 15.4 | 18.4 | 11.3 | NA |
Rate per subject (%) is calculated n/N, where n is the number of subjects with an event and N is the total number of subjects at risk. NA indicates data not available.
Total number of subjects without a permanent pacemaker at baseline is used as the denominator for calculating the new pacemaker implantation rate.
At 1 year, the all-cause mortality and disabling stroke rates were 5.2% (10/193) (including 1 COVID-19 death) and 2.1% (4/193) in the Global FlexNav cohort and 11.8% (42/362) and 2.6% (9/362) in the CAV group.
The propensity quintile analysis comparing the FlexNav cohort with the CAV group, presented in Table 4, resulted in a propensity quintile adjusted difference for the composite of all-cause mortality or disabling stroke at 1 year of −8.9% for ATE and −9.1% for ATT with 95% CIs not including zero, indicating that the FlexNav cohort is no worse than the CAV group. Similarly, the results for all-cause mortality at 1 year showed the adjusted difference was −9.4% for ATE and −9.3% for ATT with 95% CIs not including zero, indicating that the FlexNav cohort is no worse than the CAV group. The propensity analysis for disabling stroke at 1 year had an adjusted difference of 0.1% for ATE and −0.3% for ATT, with 95% CIs crossing zero, suggesting no difference between the cohorts.
Table 4.
A propensity score analysis for mortality and disabling stroke at 1 y.
| Event | Global FlexNav (n = 193): PS-SM ATE ratea | CAV (n = 362): PS-SM ATE ratea | ATE adjusted differencea (95% CI) | Global FlexNav (n = 193): PS-SM ATT rateb | CAV (n = 362): PS-SM ATT rateb | ATT adjusted differenceb (95% CI) |
|---|---|---|---|---|---|---|
| Composite end point of mortality or disabling stroke | 5.0 | 13.9 | −8.9 (−14 to −3.7) | 6.2 | 15.3 | −9.1 (−15.4 to −2.7) |
| Mortality | 3.3 | 12.7 | −9.4 (−13.7 to −5.1) | 5.2 | 14.5 | −9.3 (−15.5 to −3.1) |
| Disabling stroke | 2.6 | 2.5 | 0.1 (−3.4 to 3.6) | 2.1 | 2.4 | −0.3 (−3.5 to 2.8) |
ATE, average treatment effect; PS-SM ATE, propensity score stratified mean average treatment effect
Values given are percentages.
Event rates are expressed as PS-SM ATE rates across quintiles and ATE-adjusted difference.
Event rates are expressed as propensity score stratified mean ATE on the treated (PS-SM ATT) rates across quintiles and ATE on the treated (ATT)-adjusted difference.
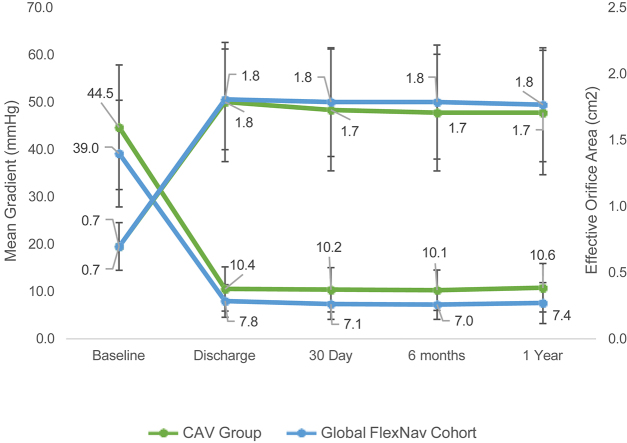
Valve performance
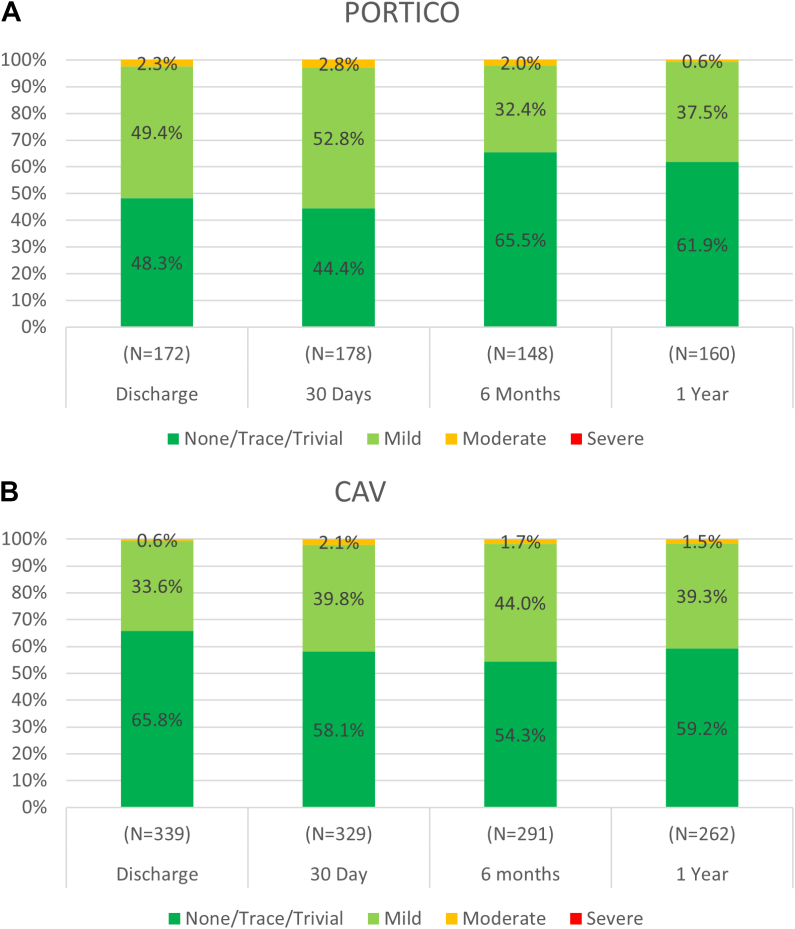
Valve hemodynamics for the Global FlexNav cohort and the CAV group are presented in the Central Illustration. The mean aortic gradients were numerically lower in the Global FlexNav cohort than those in the CAV group at 30 days (7.1 ± 3.2 mm Hg vs 10.2 ± 4.7 mm Hg) and at 1 year (7.4 ± 4.3 mm Hg vs 10.6 ± 5.1 mm Hg). The mean effective orifice area was similar in the Global FlexNav cohort and the CAV group at 30 days (1.8 ± 0.4 cm2 vs 1.8 ± 0.5 cm2) and at 1 year (1.8 ± 0.4 cm2 vs 1.7 ± 0.5 cm2). In the Global FlexNav cohort, the proportion of subjects with mild or less PVL at 30 days was 97.2% (173/178) and at 1 year was 99.4% (159/160); there were no cases of severe PVL (Figure 1). In the CAV group, the proportion of subjects with mild or less PVL at 30 days was 97.9% (322/329) and at 1 year was 98.5% (258/262); there were no cases of severe PVL. When comparing PVL performance of the Portico valve to CAV, the results of the propensity score analysis for moderate or severe PVL at 1 year demonstrated an adjusted difference of −0.9% for ATE and −0.8% for ATT, with 95% CIs crossing zero, suggesting no difference between the cohorts (Table 5).
Central Illustration.
The hemodynamic outcomes of mean gradient and effective orifice area at the baseline, discharge, 30-day, 6-month, and 1-year visit for the Global FlexNav (blue) and CAV (green) groups. CAV, commercially available valve.
Figure 1.
Paravalvular leak through 1 year. The distribution of paravalvular leak at 30 days, 6 months, and 1 year for the Global FlexNav (A) and CAV (B) groups. CAV, commercially available valve.
Table 5.
A propensity score analysis for moderate or severe PVL at 1 y.
| Event | Global FlexNav (n = 193): PS-SM ATE ratea | CAV (n = 362): PS-SM ATE ratea | ATE adjusted differencea (95% CI) | Global FlexNav (n = 193): PS-SM ATT rateb | CAV (n = 362): PS-SM ATT rateb | ATT adjusted differenceb (95% CI) |
|---|---|---|---|---|---|---|
| Moderate or severe PVL | 1.0 | 1.9 | −0.9 (−3.6 to 1.8) | 0.5 | 1.3 | −0.8 (−2.7 to 1.2) |
ATE, average treatment effect; ATT, average treatment effect on the treated; CAV, commercially available valve; PS-SM ATE, propensity score stratified mean average treatment effect
PVL, paravalvular leak.
Event rates are expressed as propensity score stratified mean average treatment effect (PS-SM ATE) rates across quintiles and average treatment effect (ATE)-adjusted difference.
Event rates are expressed as propensity score stratified mean ATE on the treated (PS-SM ATT) rates across quintiles and ATT-adjusted difference.
Functional status
Functional status through 1 year for the Global FlexNav cohort and the CAV group are presented in Figure 2. At 30 days, 95.7% (179/187) of the Global FlexNav cohort subjects were in NYHA functional class I or II, with 81.2% (152/187) improving by at least 1 NYHA classification. At 1 year, 96.8% (152/157) of subjects were NYHA class I or II. In the CAV group, 91.8% (256/279) of subjects were NYHA class I or II at 1 year.
Figure 2.
The NYHA functional classification from baseline through 1 year. The NYHA functional class of subjects at baseline, 30-day and 1 year for the Global FlexNav (A) and CAV (B) groups. CAV, commercially available valve.
Discussion
The principal findings of Global FlexNav study are that the Portico THV with the FlexNav DS for the treatment of severe symptomatic AS in high- or extreme surgical-risk subjects was associated with (1) high procedural success with no procedural deaths; (2) major vascular complication rate of 5.7% (11/193), comparable with the rates observed with the other contemporary commercially available THVs; (3) excellent 30-day and 1-year clinical outcomes, with 1.0% (2/193) all-cause mortality and 2.1% (4/193) disabling stroke at 30 days and 5.2% (10/193) all-cause mortality and 2.1% (4/193) disabling stroke at 1 year; (4) significant and sustained improvement in hemodynamics through 1 year; (5) none to mild paravalvular regurgitation at 1 year in 99.4% (159/160) of subjects; and (6) significant and sustained improvement in functional status through 1 year. The key safety and effectiveness results of the Portico valve in the Global FlexNav study are in line with the results of contemporary valves from the CAV group on the PORTICO IDE study.
When comparing the performance of the Portico valve with the FlexNav DS with that of CAVs, the quintiles propensity score methodology demonstrated that both mortality and the composite of mortality and disabling stroke were not worse than the CAV cohort. Although not directly studied in a head-to-head manner, the Global FlexNav and PORTICO IDE inclusion and exclusion criteria and subject selection and approval process were almost identical, and both studies enrolled high and extreme surgical-risk subjects with severe symptomatic AS.
The outcomes of TAVR with the Portico valve in the Global FlexNav DS cohort were improved compared with those of the Portico valve using the first-generation Portico delivery system in the PORTICO IDE study, in a similar surgical-risk subject population with severe AS. The same Portico valve was used in both the Global FlexNav study and the PORTICO IDE study; however, the next-generation FlexNav delivery system with an integrated sheath was incorporated in the Global FlexNav study. The mean STS score in the PORTICO IDE study was 6.4%, whereas the mean STS score in the Global FlexNav study was 5.2% (the PORTICO IDE study STS scores were calculated using the older version of the STS risk calculator, whereas Global FlexNav cohort STS scores were calculated using the later version of the STS short-term risk calculator released on November 15, 2018). The 30-day all-cause mortality of 1.0% observed in the Global FlexNav study was lower than the expected mortality based on the STS score. The procedural mortality (0.0% vs 0.5%), 30-day all-cause mortality (1.0% vs 4.5%), major vascular complication rates (5.7% vs 9.6%), and pacemaker rates (15.4% vs 27.7%) were lower in the Global FlexNav study than those in the PORTICO IDE trial. Despite the use of the same Portico valve in the 2 studies, the rates of significant paravalvular regurgitation were lower in the Global FlexNav study than in the PORTICO IDE study at both 30 days (2.8% vs 6.1%) and 1 year (0.6% vs 7.6%), which may likely be attributed to the precise placement afforded by the FlexNav DS (4.2 mm vs 6.4 mm).9
The Portico valve is the third TAVR valve type commercially available in the United States for the treatment of AS. The Portico valve design offers a few advantages over the commercially available balloon-expandable Sapien 3 valves or the self-expanding Evolut valves. The valve hemodynamics (mean aortic gradients and effective orifice areas) associated with the self-expanding Portico valves were superior to the balloon-expandable Sapien 3 valve and similar to self-expanding Evolut valves.9 Coronary access is more favorable with Portico valve with the large open cells and intra-annular location of the valve leaflets to facilitate easier coronary access than that with the Evolut valve. For the same reason, the Portico valve is likely to be a better landing zone for TAVR-in-TAVR compared with the Evolut valves.
The pacemaker rates continue to be higher with the self-expanding Evolut or Portico valves, compared with that of the Sapien valve. The cusp-overlap technique of implant has been associated with a reduction in pacemaker rates for the Evolut valve, with the OPTIMIZE PRO study reporting a 30-day pacemaker rate of 9.8%.17 A similar technique has been implemented for Abbott TAVR after the Global FlexNav study was complete. The new technique provides procedural steps to help achieve a higher implant depth, with a target implant depth of 3 mm, and is intended to result in a lower risk of conduction system impairment after TAVR. For the Global FlexNav study, the recommended target implant depth was 3.0-5.0 mm. The number of subjects with a final deployed stent depth in the recommended range of 3.0-5.0 mm was 42.0%, with 30.1% less than the 3.0-mm depth and 27.8% greater than the 5.0-mm depth. Although implant depth did not correlate with pacemaker rates in the Global FlexNav study, the CONFIDENCE registry with 1001 Portico subjects reported that higher implantation depth led to lower pacemaker implantation rates, as low as 12.6% at 30-days when implanted at 2.0-4.0 mm.18
The incorporation of a polyethylene terephthalate skirt to the Sapien 3 valve and a porcine pericardial tissue wrap to the Evolut valve were associated with a reduction in paravalvular regurgitation with the Sapien 3 and Evolut valves. The rate of mild or less paravalvular leak in this study with the Portico valve, without a sealing skirt technology, was 97.2% at 30 days and 99.4% at 1 year. Despite the use of the same valve that was used in the PORTICO IDE study, there was a remarkably low rate of moderate or greater paravalvular regurgitation in the Global FlexNav study than that in the PORTICO IDE study, which was likely owing to achieving a better implant depth. Abbott incorporated the paravalvular regurgitation sealing NaviSeal Cuff in the next-generation Navitor valve, which has demonstrated an improvement in paravalvular leak with mild or less PVL in 100% of subjects at 30 days.19
Study limitations were previously reported in the publication reporting the 30-day outcomes of a similar cohort.10 This was a single-arm study, and there was no concurrent control group; thus, comparisons with other studies and valve types are only descriptive.
Conclusions
The use of the FlexNav DS for delivery of Portico valves demonstrated safety at 30 days and sustained treatment benefits at 1 year, with low rates of all-cause mortality or disabling stroke, improved heart failure symptoms, and enhanced valve performance.
Acknowledgments
The authors thank Nadine Ottoson (Abbott) for assisting with the writing of the manuscript and preparation of tables and figures and Hongfei Guo, PhD (Abbott), for providing statistical support.
Declaration of competing interest
Gregory P. Fontana is a consultant for and has received proctoring fees from Abbott, Medtronic, and LivaNova; is a member of the structural heart advisory board for Abbott; and is a principal investigator for Abbott and Medtronic clinical studies. Francesco Bedogni has served as a consultant for Boston Scientific, Medtronic, and Abbott. Mark Groh is a member of the structural heart safety advisory board for Abbott. David Smith has received speaking fees and proctoring fees from Abbott. Bassem M. Chehab has received grant and research support from Abbott and received speaking and proctoring fees from Abbott, Edwards Lifesciences, Medtronic, and Boston Scientific. Gerald Yong is a physician proctor for Abbott Vascular, Medtronic, and Edwards Lifesciences. Stephen Worthley has received research grants from Abbott and Biotronikand and is a principal investigator for Abbott clinical studies. Ganesh Manoharan has served as a proctor for Boston Scientific, Medtronic, and Abbott.
Ron Waksman is an advisory board member for Amgen, Boston Scientific, Cardioset, Cardiovascular Systems Inc., Medtronic, Philips, and Pi-Cardia; is a consultant for Amgen, Biotronik, Boston Scientific, Cardioset, Cardiovascular Systems Inc., Medtronic, Philips, and Picardia; has received grant support from AstraZeneca, Biotronik, Boston Scientific, and Chiesi; is a Speakers Bureau member for AstraZeneca and Chiesi; and is an investor in MedAlliance. Ravi K. Ramana has received speaking, consulting, and proctoring fees from Edwards Lifesciences; has received consulting fees from Abbott and Medtronic; and has received research support from Boston Scientific. Federico M. Asch is the director of an academic echocardiography core laboratory that has institutional research contracts with Abbott, Edwards Lifesciences, Boston Scientific, Medtronic, LivaNova, Biotronik, and Vascular Innovations. Tarun Chakravarty is a consultant for Edwards Lifesciences, Medtronic, Abbott, and Boston Scientific. Raj R. Makkar has received research grants and speaking and proctoring fees from Edwards Lifesciences, Abbott, Medtronic, and Boston Scientific and is a principal investigator for Abbott clinical studies. H. Edward Garrett has reported no financial interests.
Funding sources
This research was funded by Abbott.
Ethics statement and patient consent
The research reported has adhered to the relevant ethical guidelines and patient consent has been obtained.
Footnotes
To access the supplementary material accompanying this article, visit the online version of the Journal of the Society for Cardiovascular Angiography & Interventions at https://doi.org/10.1016/j.jscai.2022.100562
Supplementary material
References
- 1.Leon M.B., Smith C.R., Mack M., et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 2.Smith C.R., Leon M.B., Mack M.J., et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 3.Popma J.J., Adams D.H., Reardon M.J., et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. 2014;63(19):1972–1981. doi: 10.1016/j.jacc.2014.02.556. [DOI] [PubMed] [Google Scholar]
- 4.Adams D.H., Popma J.J., Reardon M.J. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;371(19):967–968. doi: 10.1056/NEJMc1408396. [DOI] [PubMed] [Google Scholar]
- 5.Leon M.B., Smith C.R. Transcatheter Aortic-Valve Replacement. N Engl J Med. 2016;375(7):700–701. doi: 10.1056/NEJMc1606814. [DOI] [PubMed] [Google Scholar]
- 6.Reardon M.J., Van Mieghem N.M., Popma J.J., et al. SURTAVI Investigators. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376(14):1321–1331. doi: 10.1056/NEJMoa1700456. [DOI] [PubMed] [Google Scholar]
- 7.Mack M.J., Leon M.B., Thourani V.H., et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380(18):1695–1705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 8.Popma J.J., Deeb G.M., Yakubov S.J., et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380(18):1706–1715. doi: 10.1056/NEJMoa1816885. [DOI] [PubMed] [Google Scholar]
- 9.Makkar R.R., Cheng W., Waksman R., et al. Self-expanding intra-annular versus commercially available transcatheter heart valves in high and extreme risk patients with severe aortic stenosis (PORTICO IDE): a randomised, controlled, non-inferiority trial. Lancet. 2020;396(10252):669–683. doi: 10.1016/S0140-6736(20)31358-1. [DOI] [PubMed] [Google Scholar]
- 10.Fontana G.P., Bedogni F., Groh M., et al. Safety profile of an intra-annular self-expanding transcatheter aortic valve and next-generation low-profile delivery system. JACC Cardiovasc Interv. 2020;13(21):2467–2478. doi: 10.1016/j.jcin.2020.06.041. [DOI] [PubMed] [Google Scholar]
- 11.Kappetein A.P., Head S.J., Généreux P., et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document (VARC-2) Eur J Cardiothorac Surg. 2012;42(5):S45–S60. doi: 10.1093/ejcts/ezs533. [DOI] [PubMed] [Google Scholar]
- 12.Möllmann H., Linke A., Holzhey D.M., et al. Implantation and 30-day follow-up on all 4 valve sizes within the portico transcatheter aortic bioprosthetic family. JACC Cardiovasc Interv. 2017;10(15):1538–1547. doi: 10.1016/j.jcin.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 13.Tzikas A., Amrane H., Bedogni F., et al. Transcatheter aortic valve replacement using the portico system: 10 things to remember. J Interv Cardiol. 2016;29(5):523–529. doi: 10.1111/joic.12322. [DOI] [PubMed] [Google Scholar]
- 14.Austin P.C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenbaum P.R., Rubin D.B. Reducing bias in observational studies using sub-classification on the propensity score. J Am Stat Assoc. 1984;79(387):516–524. [Google Scholar]
- 16.Lu N., Xu Y., Yue L.Q. Some considerations on design and analysis plan on a nonrandomized comparative study using propensity score methodology for medical device premarket evaluation. Stat Biopharm Res. 2020;12(2):155–163. [Google Scholar]
- 17.Grugg KJ. Impact of a standardized TAVI technique and care pathway in the Optimize PRO study. Paper presented at EuroPCR 2022 Slide Presentations Conference; May 17-20, 2022; Paris, France.
- 18.Mollmann H. CONFIDENCE registry: valve hemodynamics and 1-year survival following implantation of the PorticoTM valve. Paper presented at TCT 2022; September 16-19, 2022; Boston, MA.
- 19.Søndergaard L. Paper presented at EuroPCR conference; 2021. 30-Day outcomes from a next generation TAVI device with an active sealing cuff. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.