Abstract
Interleukin 8 (IL-8) is a member of the rapidly growing superfamily of those cytokines which are thought to be involved in the regulation of inflammatory processes and cell proliferation. In neutrophils, IL-8 triggers a variety of cellular responses by interacting with specific cell-surface receptors. To examine whether IL-8 receptors are coupled to activation of guanine-nucleotide-binding proteins (G-proteins), we have investigated the influence of IL-8 on GTP hydrolysis by and guanosine 5'-[gamma-[35S]thio]triphosphate (GTP[35S]) binding to purified human neutrophil plasma membranes. IL-8 stimulated high-affinity GTPase about 2-fold at 100 nM, and half-maximal stimulation was observed at 1 nM. The peptide-stimulated GTPase was confined to plasma membranes upon subcellular fractionation, and was due to an increase in Vmax. rather than a decrease in Km. High-affinity binding of GTP[35S] to neutrophil plasma membranes was stimulated half-maximally and maximally (up to 5-fold) by IL-8 at about 10 nM and 100 nM respectively. GTP[35S] binding to the membranes was also stimulated by two IL-8-related cytokines, neutrophil-activating peptide 2 (NAP-2) and melanoma growth-stimulatory activity (gro/MGSA). Taken together, these results demonstrate that receptors for IL-8 and related cytokines are coupled to and activate G-proteins in neutrophil plasma membranes, indicating that G-protein activation is an important intermediate step in the induction of neutrophil functions by IL-8 and its congeners.
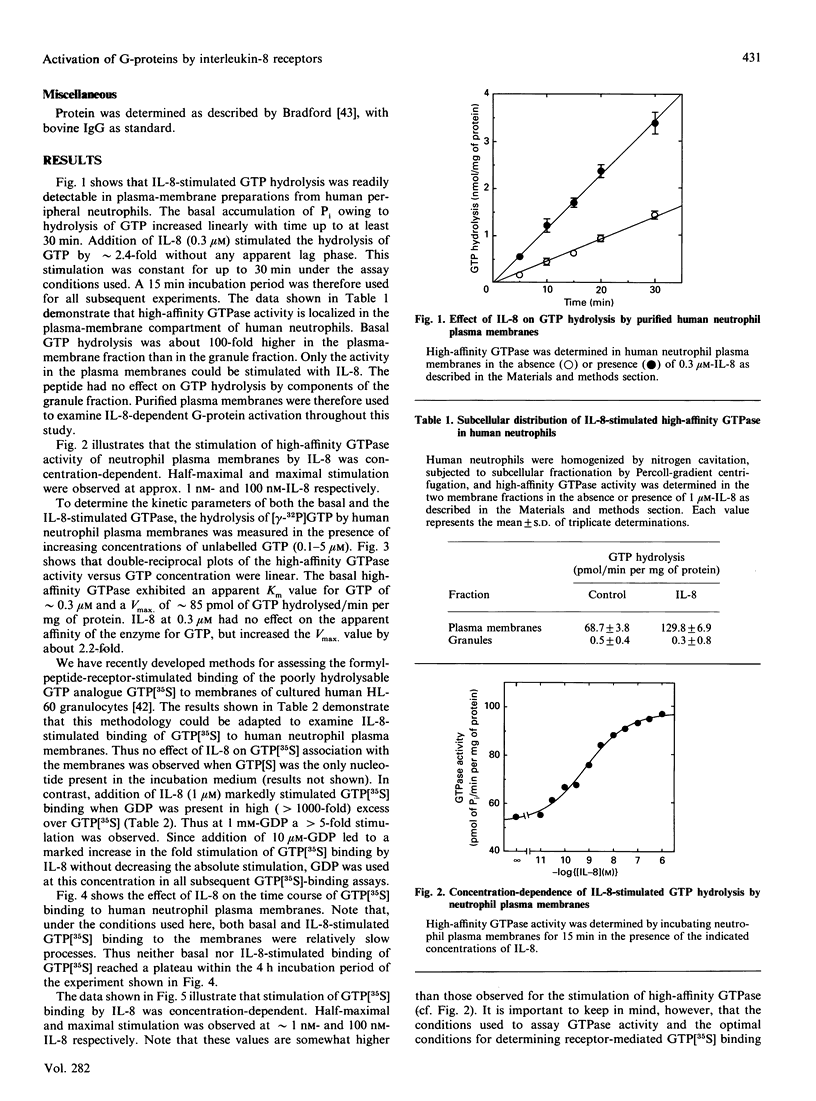
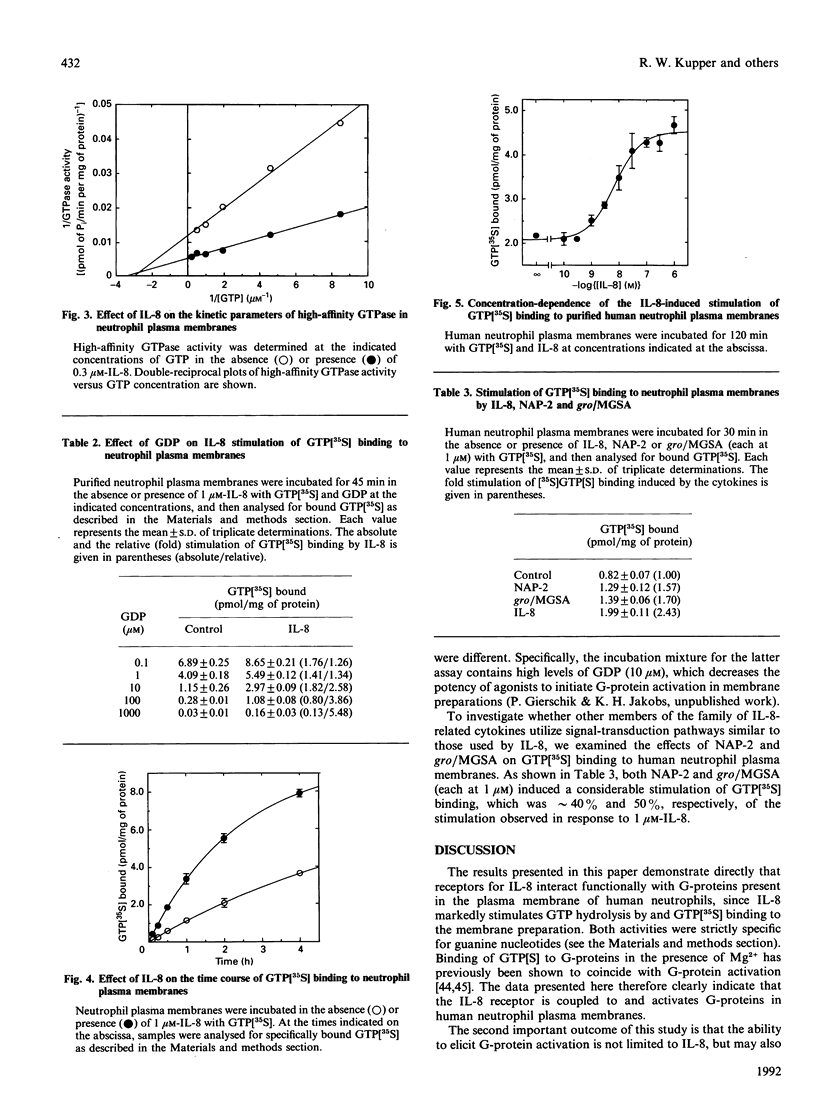
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anisowicz A., Bardwell L., Sager R. Constitutive overexpression of a growth-regulated gene in transformed Chinese hamster and human cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7188–7192. doi: 10.1073/pnas.84.20.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggiolini M., Walz A., Kunkel S. L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989 Oct;84(4):1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balentien E., Han J. H., Thomas H. G., Wen D. Z., Samantha A. K., Zachariae C. O., Griffin P. R., Brachmann R., Wong W. L., Matsushima K. Recombinant expression, biochemical characterization, and biological activities of the human MGSA/gro protein. Biochemistry. 1990 Nov 6;29(44):10225–10233. doi: 10.1021/bi00496a011. [DOI] [PubMed] [Google Scholar]
- Banga H. S., Walker R. K., Winberry L. K., Rittenhouse S. E. Pertussis toxin can activate human platelets. Comparative effects of holotoxin and its ADP-ribosylating S1 subunit. J Biol Chem. 1987 Nov 5;262(31):14871–14874. [PubMed] [Google Scholar]
- Becker E. L., Kermode J. C., Naccache P. H., Yassin R., Munoz J. J., Marsh M. L., Huang C. K., Sha'afi R. I. Pertussis toxin as a probe of neutrophil activation. Fed Proc. 1986 Jun;45(7):2151–2155. [PubMed] [Google Scholar]
- Besemer J., Hujber A., Kuhn B. Specific binding, internalization, and degradation of human neutrophil activating factor by human polymorphonuclear leukocytes. J Biol Chem. 1989 Oct 15;264(29):17409–17415. [PubMed] [Google Scholar]
- Borregaard N., Heiple J. M., Simons E. R., Clark R. A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983 Jul;97(1):52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Clark-Lewis I., Moser B., Walz A., Baggiolini M., Scott G. J., Aebersold R. Chemical synthesis, purification, and characterization of two inflammatory proteins, neutrophil activating peptide 1 (interleukin-8) and neutrophil activating peptide. Biochemistry. 1991 Mar 26;30(12):3128–3135. doi: 10.1021/bi00226a021. [DOI] [PubMed] [Google Scholar]
- Davatelis G., Tekamp-Olson P., Wolpe S. D., Hermsen K., Luedke C., Gallegos C., Coit D., Merryweather J., Cerami A. Cloning and characterization of a cDNA for murine macrophage inflammatory protein (MIP), a novel monokine with inflammatory and chemokinetic properties. J Exp Med. 1988 Jun 1;167(6):1939–1944. doi: 10.1084/jem.167.6.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewald B., Baggiolini M. Methods for assessing exocytosis by neutrophil leukocytes. Methods Enzymol. 1986;132:267–277. doi: 10.1016/s0076-6879(86)32014-7. [DOI] [PubMed] [Google Scholar]
- Doi T., Greenberg S. M., Rosenberg R. D. Structure of the rat platelet factor 4 gene: a marker for megakaryocyte differentiation. Mol Cell Biol. 1987 Feb;7(2):898–904. doi: 10.1128/mcb.7.2.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta R., Yamagishi J., Kotani H., Sakamoto F., Fukui T., Matsui Y., Sohmura Y., Yamada M., Yoshimura T., Larsen C. G. Production and characterization of recombinant human neutrophil chemotactic factor. J Biochem. 1989 Sep;106(3):436–441. doi: 10.1093/oxfordjournals.jbchem.a122870. [DOI] [PubMed] [Google Scholar]
- Furutani Y., Nomura H., Notake M., Oyamada Y., Fukui T., Yamada M., Larsen C. G., Oppenheim J. J., Matsushima K. Cloning and sequencing of the cDNA for human monocyte chemotactic and activating factor (MCAF). Biochem Biophys Res Commun. 1989 Feb 28;159(1):249–255. doi: 10.1016/0006-291x(89)92430-3. [DOI] [PubMed] [Google Scholar]
- Gierschik P., Falloon J., Milligan G., Pines M., Gallin J. I., Spiegel A. Immunochemical evidence for a novel pertussis toxin substrate in human neutrophils. J Biol Chem. 1986 Jun 15;261(17):8058–8062. [PubMed] [Google Scholar]
- Gierschik P., Moghtader R., Straub C., Dieterich K., Jakobs K. H. Signal amplification in HL-60 granulocytes. Evidence that the chemotactic peptide receptor catalytically activates guanine-nucleotide-binding regulatory proteins in native plasma membranes. Eur J Biochem. 1991 May 8;197(3):725–732. doi: 10.1111/j.1432-1033.1991.tb15964.x. [DOI] [PubMed] [Google Scholar]
- Gierschik P., Sidiropoulos D., Jakobs K. H. Two distinct Gi-proteins mediate formyl peptide receptor signal transduction in human leukemia (HL-60) cells. J Biol Chem. 1989 Dec 25;264(36):21470–21473. [PubMed] [Google Scholar]
- Gierschik P., Sidiropoulos D., Spiegel A., Jakobs K. H. Purification and immunochemical characterization of the major pertussis-toxin-sensitive guanine-nucleotide-binding protein of bovine-neutrophil membranes. Eur J Biochem. 1987 May 15;165(1):185–194. doi: 10.1111/j.1432-1033.1987.tb11210.x. [DOI] [PubMed] [Google Scholar]
- Gierschik P., Sidiropoulos D., Steisslinger M., Jakobs K. H. Na+ regulation of formyl peptide receptor-mediated signal transduction in HL 60 cells. Evidence that the cation prevents activation of the G-protein by unoccupied receptors. Eur J Pharmacol. 1989 Dec 5;172(6):481–492. doi: 10.1016/0922-4106(89)90031-x. [DOI] [PubMed] [Google Scholar]
- Goldsmith P., Gierschik P., Milligan G., Unson C. G., Vinitsky R., Malech H. L., Spiegel A. M. Antibodies directed against synthetic peptides distinguish between GTP-binding proteins in neutrophil and brain. J Biol Chem. 1987 Oct 25;262(30):14683–14688. [PubMed] [Google Scholar]
- Goldsmith P., Rossiter K., Carter A., Simonds W., Unson C. G., Vinitsky R., Spiegel A. M. Identification of the GTP-binding protein encoded by Gi3 complementary DNA. J Biol Chem. 1988 May 15;263(14):6476–6479. [PubMed] [Google Scholar]
- Gray L. S., Huber K. S., Gray M. C., Hewlett E. L., Engelhard V. H. Pertussis toxin effects on T lymphocytes are mediated through CD3 and not by pertussis toxin catalyzed modification of a G protein. J Immunol. 1989 Mar 1;142(5):1631–1638. [PubMed] [Google Scholar]
- Grob P. M., David E., Warren T. C., DeLeon R. P., Farina P. R., Homon C. A. Characterization of a receptor for human monocyte-derived neutrophil chemotactic factor/interleukin-8. J Biol Chem. 1990 May 15;265(14):8311–8316. [PubMed] [Google Scholar]
- Higashijima T., Ferguson K. M., Sternweis P. C., Ross E. M., Smigel M. D., Gilman A. G. The effect of activating ligands on the intrinsic fluorescence of guanine nucleotide-binding regulatory proteins. J Biol Chem. 1987 Jan 15;262(2):752–756. [PubMed] [Google Scholar]
- Leonard E. J., Yoshimura T. Human monocyte chemoattractant protein-1 (MCP-1). Immunol Today. 1990 Mar;11(3):97–101. doi: 10.1016/0167-5699(90)90035-8. [DOI] [PubMed] [Google Scholar]
- Lindley I., Aschauer H., Seifert J. M., Lam C., Brunowsky W., Kownatzki E., Thelen M., Peveri P., Dewald B., von Tscharner V. Synthesis and expression in Escherichia coli of the gene encoding monocyte-derived neutrophil-activating factor: biological equivalence between natural and recombinant neutrophil-activating factor. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9199–9203. doi: 10.1073/pnas.85.23.9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster A. D., Unkeless J. C., Ravetch J. V. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985 Jun 20;315(6021):672–676. doi: 10.1038/315672a0. [DOI] [PubMed] [Google Scholar]
- Matsushima K., Morishita K., Yoshimura T., Lavu S., Kobayashi Y., Lew W., Appella E., Kung H. F., Leonard E. J., Oppenheim J. J. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988 Jun 1;167(6):1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeish K. R., Gierschik P., Schepers T., Sidiropoulos D., Jakobs K. H. Evidence that activation of a common G-protein by receptors for leukotriene B4 and N-formylmethionyl-leucyl-phenylalanine in HL-60 cells occurs by different mechanisms. Biochem J. 1989 Jun 1;260(2):427–434. doi: 10.1042/bj2600427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser B., Clark-Lewis I., Zwahlen R., Baggiolini M. Neutrophil-activating properties of the melanoma growth-stimulatory activity. J Exp Med. 1990 May 1;171(5):1797–1802. doi: 10.1084/jem.171.5.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser B., Schumacher C., von Tscharner V., Clark-Lewis I., Baggiolini M. Neutrophil-activating peptide 2 and gro/melanoma growth-stimulatory activity interact with neutrophil-activating peptide 1/interleukin 8 receptors on human neutrophils. J Biol Chem. 1991 Jun 5;266(16):10666–10671. [PubMed] [Google Scholar]
- Nogimori K., Ito K., Tamura M., Satoh S., Ishii S., Ui M. Chemical modification of islet-activating protein, pertussis toxin. Essential role of free amino groups in its lymphocytosis-promoting activity. Biochim Biophys Acta. 1984 Sep 28;801(2):220–231. doi: 10.1016/0304-4165(84)90071-0. [DOI] [PubMed] [Google Scholar]
- Nogimori K., Tamura M., Yajima M., Ito K., Nakamura T., Kajikawa N., Maruyama Y., Ui M. Dual mechanisms involved in development of diverse biological activities of islet-activating protein, pertussis toxin, as revealed by chemical modification of lysine residues in the toxin molecule. Biochim Biophys Acta. 1984 Sep 28;801(2):232–243. doi: 10.1016/0304-4165(84)90072-2. [DOI] [PubMed] [Google Scholar]
- Northup J. K., Smigel M. D., Gilman A. G. The guanine nucleotide activating site of the regulatory component of adenylate cyclase. Identification by ligand binding. J Biol Chem. 1982 Oct 10;257(19):11416–11423. [PubMed] [Google Scholar]
- Poncz M., Surrey S., LaRocco P., Weiss M. J., Rappaport E. F., Conway T. M., Schwartz E. Cloning and characterization of platelet factor 4 cDNA derived from a human erythroleukemic cell line. Blood. 1987 Jan;69(1):219–223. [PubMed] [Google Scholar]
- Richmond A., Balentien E., Thomas H. G., Flaggs G., Barton D. E., Spiess J., Bordoni R., Francke U., Derynck R. Molecular characterization and chromosomal mapping of melanoma growth stimulatory activity, a growth factor structurally related to beta-thromboglobulin. EMBO J. 1988 Jul;7(7):2025–2033. doi: 10.1002/j.1460-2075.1988.tb03042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson E. A., Yoshimura T., Leonard E. J., Tanaka S., Griffin P. R., Shabanowitz J., Hunt D. F., Appella E. Complete amino acid sequence of a human monocyte chemoattractant, a putative mediator of cellular immune reactions. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1850–1854. doi: 10.1073/pnas.86.6.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta A. K., Oppenheim J. J., Matsushima K. Identification and characterization of specific receptors for monocyte-derived neutrophil chemotactic factor (MDNCF) on human neutrophils. J Exp Med. 1989 Mar 1;169(3):1185–1189. doi: 10.1084/jem.169.3.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta A. K., Oppenheim J. J., Matsushima K. Interleukin 8 (monocyte-derived neutrophil chemotactic factor) dynamically regulates its own receptor expression on human neutrophils. J Biol Chem. 1990 Jan 5;265(1):183–189. [PubMed] [Google Scholar]
- Schall T. J., Bacon K., Toy K. J., Goeddel D. V. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990 Oct 18;347(6294):669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- Sherry B., Tekamp-Olson P., Gallegos C., Bauer D., Davatelis G., Wolpe S. D., Masiarz F., Coit D., Cerami A. Resolution of the two components of macrophage inflammatory protein 1, and cloning and characterization of one of those components, macrophage inflammatory protein 1 beta. J Exp Med. 1988 Dec 1;168(6):2251–2259. doi: 10.1084/jem.168.6.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strnad C. F., Carchman R. A. Human T lymphocyte mitogenesis in response to the B oligomer of pertussis toxin is associated with an early elevation in cytosolic calcium concentrations. FEBS Lett. 1987 Dec 10;225(1-2):16–20. doi: 10.1016/0014-5793(87)81123-7. [DOI] [PubMed] [Google Scholar]
- Tamura M., Nogimori K., Yajima M., Ase K., Ui M. A role of the B-oligomer moiety of islet-activating protein, pertussis toxin, in development of the biological effects on intact cells. J Biol Chem. 1983 Jun 10;258(11):6756–6761. [PubMed] [Google Scholar]
- Tanaka S., Robinson E. A., Yoshimura T., Matsushima K., Leonard E. J., Appella E. Synthesis and biological characterization of monocyte-derived neutrophil chemotactic factor. FEBS Lett. 1988 Aug 29;236(2):467–470. doi: 10.1016/0014-5793(88)80078-4. [DOI] [PubMed] [Google Scholar]
- Tekamp-Olson P., Gallegos C., Bauer D., McClain J., Sherry B., Fabre M., van Deventer S., Cerami A. Cloning and characterization of cDNAs for murine macrophage inflammatory protein 2 and its human homologues. J Exp Med. 1990 Sep 1;172(3):911–919. doi: 10.1084/jem.172.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen M., Peveri P., Kernen P., von Tscharner V., Walz A., Baggiolini M. Mechanism of neutrophil activation by NAF, a novel monocyte-derived peptide agonist. FASEB J. 1988 Aug;2(11):2702–2706. [PubMed] [Google Scholar]
- Walz A., Baggiolini M. A novel cleavage product of beta-thromboglobulin formed in cultures of stimulated mononuclear cells activates human neutrophils. Biochem Biophys Res Commun. 1989 Mar 31;159(3):969–975. doi: 10.1016/0006-291x(89)92203-1. [DOI] [PubMed] [Google Scholar]
- Walz A., Baggiolini M. Generation of the neutrophil-activating peptide NAP-2 from platelet basic protein or connective tissue-activating peptide III through monocyte proteases. J Exp Med. 1990 Feb 1;171(2):449–454. doi: 10.1084/jem.171.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz A., Peveri P., Aschauer H., Baggiolini M. Purification and amino acid sequencing of NAF, a novel neutrophil-activating factor produced by monocytes. Biochem Biophys Res Commun. 1987 Dec 16;149(2):755–761. doi: 10.1016/0006-291x(87)90432-3. [DOI] [PubMed] [Google Scholar]
- Westwick J., Li S. W., Camp R. D. Novel neutrophil-stimulating peptides. Immunol Today. 1989 May;10(5):146–147. doi: 10.1016/0167-5699(89)90164-3. [DOI] [PubMed] [Google Scholar]
- Wolpe S. D., Cerami A. Macrophage inflammatory proteins 1 and 2: members of a novel superfamily of cytokines. FASEB J. 1989 Dec;3(14):2565–2573. doi: 10.1096/fasebj.3.14.2687068. [DOI] [PubMed] [Google Scholar]
- Wolpe S. D., Sherry B., Juers D., Davatelis G., Yurt R. W., Cerami A. Identification and characterization of macrophage inflammatory protein 2. Proc Natl Acad Sci U S A. 1989 Jan;86(2):612–616. doi: 10.1073/pnas.86.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T., Matsushima K., Tanaka S., Robinson E. A., Appella E., Oppenheim J. J., Leonard E. J. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9233–9237. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]


