Abstract
Cardiogenic shock (CS) caused by acute myocardial infarction (AMI) accounts for most deaths in the population with AMI and continues to be associated with high short-term mortality. Several temporary mechanical circulatory support (MCS) devices have been developed to treat CS and studied in randomized controlled trials (RCTs) of patients with AMI-CS. Unfortunately, none of these RCTs has demonstrated an improvement in survival with temporary MCS in AMI-CS. Potential reasons for these negative results in RCTs are numerous and reflect the challenges of enrolling critically ill patients with CS. Researchers have used observational study designs to provide insights about outcomes associated with the use of temporary MCS in AMI-CS. These observational studies have yielded conflicting results, in some cases contrary to the results of RCTs. Several limitations pertinent to both RCTs and observational analyses, mostly relating to selection bias and failure to consider unmeasured confounding variables and population heterogeneity, preclude drawing strong inferences regarding the effects of temporary MCS on survival in populations with AMI-CS. Understanding these limitations is essential to correctly interpreting the literature regarding temporary MCS to treat AMI-CS and is necessary to inform the design of future studies that will potentially provide stronger evidence. Optimally matching temporary MCS devices to the needs of individual patients with AMI-CS will presumably be more successful than indiscriminate application in unselected patients. In this review, we discuss the existing literature on temporary MCS to treat AMI-CS and describe the specific challenges that must be overcome to develop an improved evidence base for guiding clinical practice.
Keywords: acute myocardial infarction, cardiogenic shock, extracorporeal membrane oxygenator, intra-aortic balloon pump, mechanical circulatory support, ventricular assist device
Central Illustration
Highlights
-
•
Mortality due to acute myocardial infarction-cardiogenic shock is high.
-
•
Temporary mechanical circulatory support has not improved outcomes in acute myocardial infarction-cardiogenic shock.
-
•
Interpretation of randomized trials and observation studies remains difficult.
-
•
Matching temporary mechanical circulatory support to the patients most likely to benefit is challenging.
-
•
Without better studies, the effects of temporary mechanical circulatory support in acute myocardial infarction-cardiogenic shock will remain uncertain.
Introduction
Cardiogenic shock (CS) affects 5%-10% of patients with acute myocardial infarction (AMI), accounting for most in-hospital deaths in this population.1, 2, 3 Despite advances in percutaneous coronary intervention (PCI) and systems of care in AMI, the prevalence of CS caused by AMI (AMI-CS) has not declined and may in fact be rising.4, 5, 6 The short-term mortality in patients with AMI-CS remains high (approximately 30%-50% at 30 days) despite early coronary artery reperfusion and use of increasingly sophisticated temporary mechanical circulatory support (MCS) devices.5,6 Few randomized controlled trials (RCTs) have adequately tested interventions in AMI-CS, and most have shown negative results.7,8 Even the groundbreaking SHOCK trial failed to show a difference in the primary outcome of 30-day survival, although survival at 6 months improved with early revascularization.9 Nonetheless, mortality remains high even after successful reperfusion therapy, so enhanced therapies and treatment strategies are urgently needed to improve the dismal outcomes for patients with AMI-CS.
During the past decade, there has been expanding utilization of MCS in AMI-CS.10, 11, 12 However, although MCS devices can improve forward cardiac output and coronary perfusion and reduce pulmonary congestion, these beneficial effects may be offset by bleeding and vascular complications.11 Unfortunately, developing high-quality evidence to support the safety and efficacy of temporary MCS devices in AMI-CS has been challenging. This report aimed to summarize the results from previous studies (observational and randomized) examining the utility of MCS in AMI-CS, describe the limitations of these studies, and review the ongoing trials with the greatest potential to provide class I evidence to inform future clinical decision making in this high-risk population.
Rationale and pitfalls of temporary MCS in AMI-CS
CS is defined by end-organ hypoperfusion resulting from ineffective cardiac output, typically with associated systemic arterial hypotension.13,14 Treatment strategies for CS emphasize hemodynamic stabilization to restore systemic perfusion.2,3,13 Vasopressors and inotropes can be used for this purpose but, often, provide inadequate support and can produce cardiac and noncardiac toxicity and complications.13 An expanding array of temporary MCS devices has been developed with the goal of supporting the circulation and improving perfusion in patients with CS while minimizing the need for inotropes or vasopressors to foster myocardial recovery.11,12 The intra-aortic balloon pump (IABP) was first introduced more than 50 years ago and remains the most commonly used temporary MCS device in the United States in the contemporary era despite a decrease in its use over time.4,6,10,15, 16, 17, 18 Multiple percutaneous ventricular assist devices (pVADs) have been introduced, the most widely used being the Impella family of devices (Abiomed), with the TandemHeart family of devices (LivaNova) used less frequently.12 Venoarterial extracorporeal membrane oxygenation (VA-ECMO) support has also been available for many years, with recently increasing use for treatment of CS because of acute and chronic cardiac disease.6,10,15,18,19
Temporary MCS devices can increase cardiac output and arterial pressure to restore tissue perfusion, facilitate vasopressor and inotrope weaning, and unload the left ventricle.11 It is logical that these favorable hemodynamic effects would translate directly into improved patient outcomes for patients with AMI-CS, presuming that they are not outweighed by device-related complications. Short-term mortality has been the focus of most RCTs for determining the efficacy of temporary MCS devices in AMI-CS, whereas long-term survival has been examined less frequently in this context.20 Other relevant nonfatal patient-centered end points such as heart, kidney and other organ failure, vascular complications, stroke, and bleeding may be assessed. However, clinical improvements with MCS devices have not been demonstrated in any published RCT to date, with possible explanations ranging from trial design issues (small sample size or enrollment of patients not likely to benefit) to true lack of efficacy or even harm.7,8,20, 21, 22 As reviewed further, neither have the results of observational studies been conclusive.
Substantial heterogeneity exists within the population with AMI-CS, the presence of which in previous studies may have contributed to the failure to demonstrate treatment efficacy of temporary MCS devices. The hemodynamic patterns, shock severity, presence of established organ failure, and complicating factors can differ markedly among patients with AMI-CS due to left ventricular (LV) failure.23 CS is characterized by a downward spiral starting with initial LV dysfunction leading to systemic hypoperfusion and end-organ ischemia.13 This can culminate in the development of a “hemometabolic” shock phenotype that may not respond to hemodynamic support alone, resulting in a dissociation between the acute hemodynamic efficacy of temporary MCS and its ability to improve outcomes.24 The term hemometabolic shock, also called cardiometabolic shock, has been used to describe an end stage of CS where accumulated metabolic abnormalities (eg, lactic acidosis) from multiorgan failure create a self-perpetuating shock state.24,25 Although there is no universal definition of hemometabolic shock, one proposed definition involves the combination of Society for Cardiovascular Angiography & Interventions (SCAI) SHOCK stage D/E CS with severe metabolic (lactic) acidosis and multiorgan failure.25 Using machine learning clustering based on laboratory variables, a hemometabolic shock phenotype was identified within the population with CS that demonstrated severe shock, right-sided congestion, marked lactic acidosis, transaminitis, and acute kidney injury.26,27 Thus, delayed deployment of temporary MCS after established end-organ failure (ie, hemometabolic shock) may fail to improve survival despite successful hemodynamic stabilization.
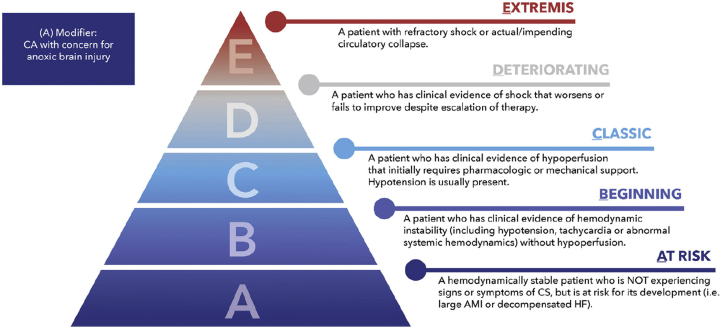
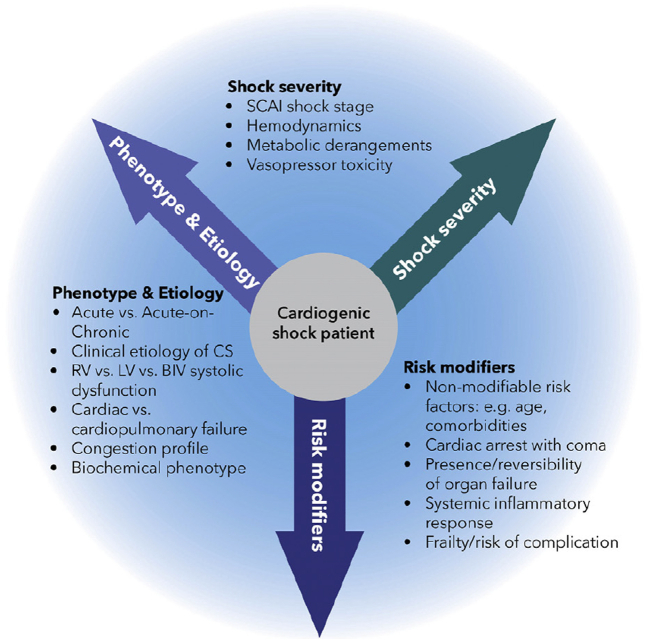
Temporary MCS devices are likely to exhibit different risk-benefit profiles across hemodynamic phenotypes and shock severity, and it would be ideal to match the severity of CS to the type and magnitude of support. In some patients, right ventricular (RV) failure is the predominant cause of AMI-CS and determinant of its prognosis.28,29 Isolated LV temporary MCS in a patient with biventricular or RV-predominant CS may provide inadequate hemodynamic support.23,30 The SCAI SHOCK classification (Figure 1) offers a standardized approach to define shock severity that has proven to be clinically relevant and facile.14,23,31 Patients with CS will show several clinical or demographic variables that will influence their response to treatment, likelihood for recovery, and survival.23 The prognostic factors that influence decision making can be conceptualized using a 3-axis model of CS (Figure 2), as proposed by the SCAI SHOCK classification working group.14,32 For example, cardiac arrest (CA) occurs in approximately half of patients with AMI-CS and is consistently associated with greater shock severity, advanced organ failure, and worse outcomes often driven by the presence of anoxic brain injury, which may not be modifiable even if temporary MCS provides myocardial recovery.7,32, 33, 34, 35 Hence, there is no reason to expect that universal application of a single type of temporary MCS device across a heterogeneous population with AMI-CS would improve outcomes, even if temporary MCS may be beneficial in certain subgroups.
Figure 1.
Updated SCAI SHOCK classification schema. Reproduced with permission from Naidu et al.14 AMI, acute myocardial infarction; CS, cardiogenic shock; HF, heart failure; SCAI, Society for Cardiovascular Angiography & Interventions.
Figure 2.
SCAI SHOCK Classification 3-axis model for conceptualization of patients with CS. Reproduced with permission from Naidu et al.14 CS, cardiogenic shock; LV, left ventricle; RV, right ventricle; SCAI, Society for Cardiovascular Angiography & Interventions.
Randomized trials of temporary MCS in CS
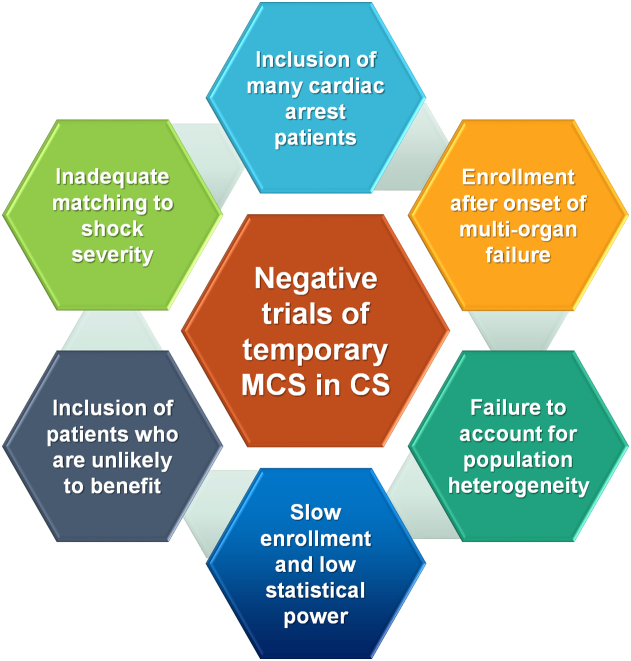
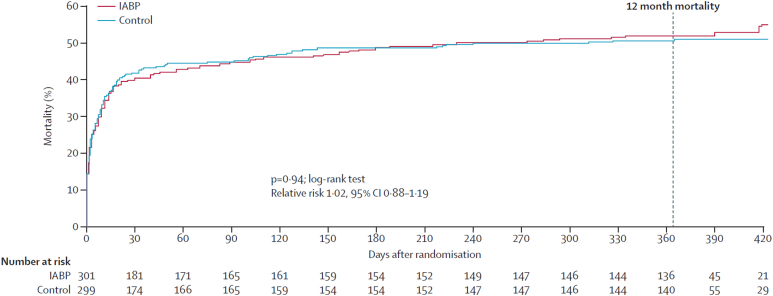
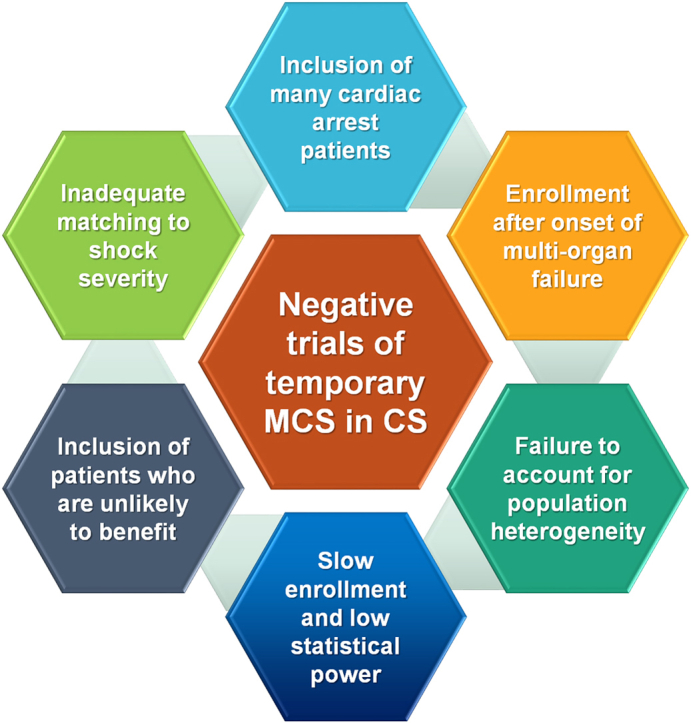
The few RCTs that have been performed examining the use of temporary MCS in AMI-CS (Table 1) have failed to demonstrate improvement in outcomes owing to several potential reasons (Central Illustration).7,20, 21, 22,36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46 The IABP-SHOCK trial in 40 patients with AMI-CS observed minimal improvement in hemodynamic end points, organ dysfunction, or severity of illness with IABP compared with that with vasopressors and inotropes alone.38,47 The largest and highest-quality RCT of temporary MCS in AMI-CS published to date is the IABP-SHOCK-II trial, in which 600 patients with AMI-CS who underwent early revascularization were randomly assigned to routine IABP use and medical therapy alone.21 Mortality at 30 days was similar with IABP and control therapy (relative risk [RR], 0.96; 95% CI, 0.79-1.17), although complications did not differ between the groups; however, survival between the groups remained similar during a long-term follow-up (Figure 3).21,48,49 Of note, the IABP was placed after revascularization in 86.6% of patients, although mortality was independent of the timing of IABP insertion. A Cochrane meta-analysis of all RCTs did not demonstrate a decrease in mortality with IABP in AMI-CS.22 The lack of efficacy and potential for an increased risk of stroke with routine IABP use in AMI-CS led to a class III recommendation in recent guidelines.3
Table 1.
Published randomized trials of temporary MCS in AMI-CS.
| Study | Year of publication | N | Mortalitya with intervention, % | Mortalitya with comparator, % | RR (95% CI)b | Additional findings |
|---|---|---|---|---|---|---|
| IABP vs medical therapy | ||||||
| Arias et al36 | 2005 | 40 | 32.3 | 55.6 | 0.58 (0.27-1.26) | – |
| TACTICS37 | 2005 | 57 | 30.0 | 33.3 | 0.90 (0.42-1.93) | No difference in complications. Terminated early owing to slow enrollment (planned n = 538) |
| IABP-SHOCK38 | 2010 | 40 | 36.8 | 28.6 | 1.28 (0.45-3.72) | No improvement in organ failure or hemodynamics with IABP |
| IABP-SHOCK-II21 | 2012 | 598 | 39.7 | 41.3 | 0.96 (0.79-1.17) | No difference in complications |
| Pooled | – | 735 | 38.2 | 40.3 | 0.95 (0.79-1.13) | – |
| TandemHeart vs IABP | ||||||
| Thiele et al39 | 2005 | 41 | 42.9 | 45.0 | 0.95 (0.48-1.90) | Better hemodynamics and more complications with pVAD |
| Burkhoff et al40 | 2006 | 33 | 47.4 | 35.7 | 1.33 (0.57-3.10) | Better hemodynamics with pVAD. Terminated early owing to slow enrollment (planned n = 90) |
| Pooled | – | 74 | 45.0 | 41.2 | 1.09 (0.64-1.85) | – |
| Impella vs IABP | ||||||
| ISAR-SHOCK41 | 2008 | 26 | 46.2 | 46.2 | 1.00 (0.44-2.29) | Better hemodynamics with pVAD |
| IMPRESS42 | 2017 | 48 | 45.8 | 50.0 | 0.92 (0.51-1.66) | More bleeding with pVAD |
| IMPELLA-STIC43 | 2020 | 12 | 33.3 | 0.0 | 5.00 (0.29-84.44) | More bleeding with pVAD. Terminated early owing to slow enrollment (planned n = 60) |
| Pooled | – | 86 | 44.2 | 41.9 | 1.06 (0.65-1.72) | – |
| VA-ECMO vs no MCS ± rescue ECMO | ||||||
| ECLS-Shock44 | 2019 | 42 | 19 | 33 | 0.57 (0.19-1.66) | No difference in major complications |
| ECMO-CS45 | 2022 | 117 | 50.0 | 47.5 | 1.11 (0.66-1.87) | Similar risk of serious complications |
| Pooled | – | 159 | 45.6 | 48.8 | 0.93 (0.67-1.30) | – |
CS, cardiogenic shock; ECLS, extracorporeal life support; ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; LV, left ventricular; MCS, mechanical circulatory support; PCI, percutaneous coronary intervention; pVAD, percutaneous ventricular assist device; VA-ECMO, venoarterial extracorporeal membrane oxygenation.
Either in-hospital or 30-day mortality.
Central Illustration.
Potential reasons why published RCTs of temporary MCS in AMI-CS have not demonstrated significant differences in mortality. AMI-CS, acute myocardial infarction related cardiogenic shock; MCS, mechanical circulatory support; RCT, randomized controlled trial.
Figure 3.
Kaplan-Meier curves demonstrating long-term mortality in patients with AMI-CS who were randomized to IABP vs medical therapy in the IABP-SHOCK-II trial. Reproduced with permission from Thiele et al.48 AMI, acute myocardial infarction; CS, cardiogenic shock; IABP, intra-aortic balloon pump.
Early RCTs showed greater hemodynamic improvements with pVAD compared with IABP in AMI-CS.20,39, 40, 41,43 Unfortunately, an adequately powered RCT of pVAD and IABP (or medical therapy only) comparison to assess clinical outcomes has not been completed. A meta-analysis including 148 patients from 4 RCTs did not find a difference in survival between pVAD and IABP groups (pooled RR, 1.01; 95% CI, 0.70-1.44), and complications increased with pVAD use.20,42 Unfortunately, most of these trials were stopped prematurely because of slow enrollment. In addition to being underpowered, these studies were limited by patient selection criteria.33 For example, the largest RCT to date (IMPRESS, n = 48) enrolled patietns with high-risk AMI-CS requiring mechanical ventilation, nearly all of whom had experienced CA; in-hospital and 6-month mortality were similar with IABP and pVAD treatments, and the cause of death was anoxic brain injury in approximately half of the patients who died.42
The ECLS-Shock pilot study compared 30-day and 1-year mortality in 42 patients with AMI-CS who were randomly assigned to VA-ECMO vs no MCS, finding no differences in mortality at either time point, major complications, or neurologic outcomes between the groups.44,46 The recently-published multicenter ECMO-CS trial is the largest reported RCT of advanced MCS devices in CS, comparing a strategy of early VA-ECMO vs rescue VA-ECMO in 122 patients with CS of SCAI SHOCK stages D or E of various etiologies (two-thirds due to AMI); patients who were comatose after CA were excluded.45 Delayed (rescue) VA-ECMO was used in 39% of the rescue control group. Unfortunately, the ECMO-CS trial did not demonstrate a significant difference between the groups in the 30-day primary end point of death, resuscitated CA, or escalation of MCS (63.8% vs 71.2% respectively; hazard ratio, 0.72; 95% CI, 0.46-1.12),45 nor did the 30-day mortality differ (50.0% vs 47.5% respectively). Serious adverse events were frequent in both groups (60.3% vs 61.0%).45 Ongoing RCTs examining temporary MCS use in AMI-CS are summarized in Table 2, several of which are adequately powered for mortality assessment.50,51
Table 2.
Ongoing randomized trials of temporary MCS in AMI-CS.
| Name | NCT No. | Started recruiting | Projected N | Intervention |
|---|---|---|---|---|
| Populations with AMI-CS | ||||
| ECLS-SHOCK | NCT03637205 | Yes | 420 | VA-ECMO |
| EUROSHOCKa | NCT03813134 | Yes | 428 | VA-ECMO |
| ANCHOR | NCT04184635 | Yes | 400 | VA-ECMO |
| DanGer Shock | NCT01633502 | Yes | 360 | Impella CP |
| ULYSS | NCT05366452 | No | 204 | Impella CP |
| RECOVER-IV | NCT05506449 | No | 560 | Impella CP |
| Patients with CS receiving ECMO | ||||
| REVERSE | NCT03431467 | Yes | 96 | Impella CP |
| UNLOAD ECMO | NCT05577195 | No | 198 | Impella CP |
| ECMOsorb | NCT05027529 | Yes | 54 | Cytosorb |
| AMI + preshock | ||||
| SCAI-B | NCT04989777 | No | 512 | IABP |
AMI-CS, acute myocardial infarction-cardiogenic shock; CS, cardiogenic shock; ECLS, extracorporeal life support; ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; MCS, mechanical circulatory support; VA-ECMO, venoarterial extracorporeal membrane oxygenation.
From clinicaltrials.gov search on October 31, 2022.
This study was terminated early owing to slow recruitment and other factors.
Observational studies of temporary MCS devices in CS
The association between IABP use and outcomes in patients with AMI-CS has been examined in numerous studies, culminating in several meta-analyses with conflicting results.52 Some, but not all, recent observational analyses using propensity adjustment methods have shown a potential association between IABP use and lower mortality in various CS cohorts.4,53, 54, 55, 56 One study using inverse probability of treatment weighting suggested a benefit of IABP in a mixed CS cohort, particularly in the lower SCAI SHOCK stages.53 However, other recent studies have failed to demonstrate an improvement in outcomes and have reported a greater risk of complications with IABP use in AMI-CS, consistent with the results of most meta-analyses.4,55, 56, 57 The observed association between IABP use and outcomes in AMI-CS may vary based on the use of PCI vs thrombolytic therapy and perhaps the timing of IABP use in relation to PCI (pre-PCI preferred in most studies).56,58, 59, 60 Overall, these conflicting observational studies do not provide strong support for the routine use of IABP in AMI-CS.
More controversial is the interpretation of observational trials of pVAD use in AMI-CS. Recent retrospective and prospective comparative observational studies of pVAD and VA-ECMO use in AMI-CS are summarized in Table 3.61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72 Retrospective observational studies examining outcomes with Impella vs IABP in AMI-CS from administrative or insurance claim databases have often used propensity matching to try to adjust for differences in baseline covariates between the groups. One cohort analysis matched patients who received an Impella in a registry to patients from the IABP-SHOCK-II study, finding no benefit and a greater risk of complications with Impella.61 Other propensity-adjusted studies have demonstrated worse outcomes with Impella in comparison with IABP, including higher mortality and more complications.62, 63, 64, 65,73 Recent observational studies examining the use of VA-ECMO in AMI-CS have shown similar survival rates during short-term and long-term follow-ups when compared with patients receiving Impella, and the largest study showed more complications with VA-ECMO after propensity adjustment.67, 68, 69, 70 Several studies have focused on the question of whether using an IABP or Impella to unload the LV during VA-ECMO support is beneficial, with most studies suggesting better survival when either device is used for this purpose despite a higher risk of vascular complications and bleeding.71,74, 75, 76, 77
Table 3.
Observational comparative studies comparing short-term mortality in patients with AMI-CS treated with advanced temporary MCS devices published in the past 5 years.
| Study | Year | Design | N | Mortality with MCS, % | Mortality with comparator, % | OR (95% CI)a | Complications |
|---|---|---|---|---|---|---|---|
| pVAD vs IABP | |||||||
| Schrage et al61 | 2019 | Retrospective, propensity adjusted | 230 | 45.2 | 46.1 | 0.97 (0.57-1.62) | More complications with pVAD |
| Helgestad et al18 | 2020 | Retrospective, propensity adjusted | 80 | 40.0 | 77.5 | 0.19 (0.07-0.52) | No difference in complications |
| Dhruva et al62 | 2020 | Retrospective, propensity adjusted | 3360 | 45.0 | 34.1 | 1.58 (1.38-1.82) | More complications with pVAD |
| Vallabhajosyula et al63 | 2020 | Retrospective, propensity adjusted | 2838 | 28.4 | 26.7 | 1.09 (0.92-1.28) | More complications with pVAD |
| Desai et al64 | 2021 | Retrospective, propensity adjusted | 886 | 40.5 | 36.8 | 1.17 (0.89-1.54) | More complications with pVAD |
| Jin et al65 | 2022 | Retrospective, propensity adjusted | 10,230 | 49.6 | 29.0 | 1.72 (1.25-2.38) | More complications with pVAD |
| Miller et al66 | 2022 | Retrospective, propensity adjusted | 1634 | 36.2 | 25.8 | 1.63 (1.32-2.02) | More complications with pVAD |
| ECMO vs pVAD | |||||||
| Garan et al67 | 2019 | Prospective | 51 | 45.0 | 45.2 | 0.99 (0.32-3.08) | No difference in complications |
| Schiller et al68 | 2019 | Retrospective | 94 | 34.7 | 37.5 | 0.89 (0.38-2.06) | Complications not reported |
| Karami et al69 | 2020 | Retrospective | 128 | 52.7 | 49.0 | 1.22 (0.57-2.56) | More complications with ECMO |
| Karatolios et al70 | 2021 | Retrospective, propensity adjusted | 183 | 61.4 | 49.4 | 1.63 (0.88-3.03) | More complications with ECMO |
AMI-CS, acute myocardial infarction-cardiogenic shock; CS, cardiogenic shock; ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; MCS, mechanical circulatory support; pVAD, percutaneous ventricular assist device.
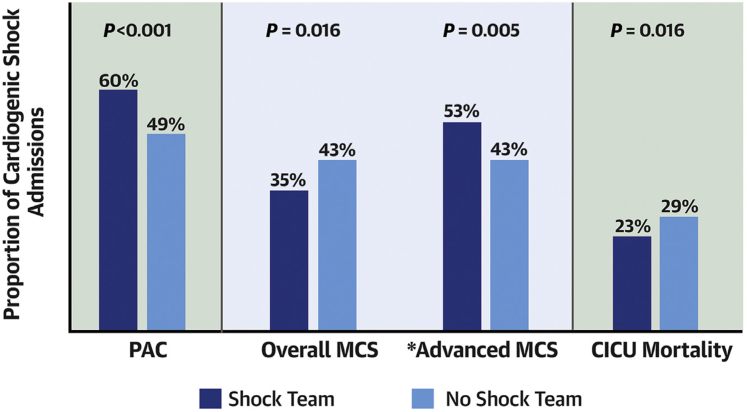
By comparison, the prospective National Cardiogenic Shock Initiative (NCSI) protocol incorporating early Impella placement before PCI in AMI-CS showed improved outcomes compared with historical controls, and a meta-analysis of observational studies suggested that Impella placement before PCI may be beneficial in AMI-CS.72,78, 79, 80 The single-arm NCSI results suggest that a structured protocol for AMI-CS care incorporating up-front Impella placement and other best practices may be associated with improved survival and that bleeding and vascular complications may be reduced with meticulous attention to large-bore vascular access and closure.72,79 The implementation of a shock team can streamline the utilization of temporary MCS to provide individualized care with the potential to improve outcomes in patients with CS.81,82 In the Critical Care Cardiology Trials Network (CCCTN) registry (Figure 4), centers with an institutional shock team used less temporary MCS overall but were more likely to use advanced MCS; patients with CS at centers with a shock team showed lower mortality, suggesting that judicious use of temporary MCS might be beneficial.81
Figure 4.
Utilization of temporary MCS and mortality for patients with CS in centers participating in the Critical Care Cardiology Trails Network (CCCTN) based on the presence of an institutional shock team. ∗Advanced MCS includes percutaneous LVADs and ECMO. Reproduced with permission from Papolos et al.81 CICU, critical intensive care unit; CS, cardiogenic shock; MCS, mechanical circulatory support; PAC, pulmonary artery catheter.
Collectively, these conflicting retrospective observational studies have not provided compelling evidence that routine use of advanced temporary MCS devices improves clinical outcomes in unselected patients with AMI-CS, and the relative risks for serious complications are uncertain. As discussed further, these studies seem to be affected by confounding by indication because sicker patients receive escalating therapies (ie, pVAD or ECMO), and traditional adjustment models are incapable of fully measuring and adjusting for this source of bias.
Strengths and limitations of observational studies and randomized trials
The well-known limitations of both RCTs and observational studies are magnified in AMI-CS (Table 4). Only RCTs can establish a causal relationship between a therapy and benefit or harm. However, RCTs on AMI-CS typically have enrolled highly selected patients and have been performed at experienced tertiary-care referral centers with high resource availability and substantial local expertise, representing a best-case scenario for complex device usage. It has been estimated that only one-third of patients with CS in contemporary practice would have qualified for entry into major RCTs, and substantial differences between trial-eligible and trial-ineligible patients have been observed suggesting that RCT results may not generalize to the broader population with AMI-CS represented in observational studies.83 Indeed, patients with AMI-CS enrolled in RCTs generally differ from those in registries, with a lower overall risk profile and more aggressive care.84 Moreover, observational studies experience a selection bias in the choice of device for individual patients, and the composition of the AMI-CS population in a given study can strongly affect the findings.33,83,84 Prospective single-arm studies, including the NCSI and PROTECT-III studies, can provide important information about the outcomes observed using temporary MCS devices in experienced hands and selected patients, but the lack of a control group is a substantial limitation.72,79,80,85
Table 4.
Comparative strengths and weaknesses of observational studies and randomized trials in AMI-CS.
| Randomized controlled trials | Observational analyses |
|---|---|
| Strengths | |
|
|
| Weaknesses | |
|
|
Confounding and suboptimal data quality of observational studies in AMI-CS
Confounding by indication is highly problematic in observational analyses of AMI-CS treatments, and patients who receive temporary MCS typically dramatically differ from patients who are not chosen to receive this therapy. It is challenging to identify the reasons that one MCS device was chosen rather than another in studies from administrative databases, with the use of advanced MCS devices typically reflecting the need for more potent support in sicker patients with a greater hemodynamic compromise. Multivariable analysis and propensity methods are thus frequently used to adjust for measured differences in patient characteristics that drive treatment decisions but have limitations.86 Examples have been published in which propensity-adjusted observational analyses of noncritical cardiovascular interventions replicated the findings of RCTs; however, the feasibility and reliability of determining propensity in the setting of critical illness may differ from the outpatient setting.87,88 Traditional patient-level clinical factors such as sex and comorbidities that are used to estimate the propensity for elective or nonurgent cardiovascular treatments are not the crucial prognostic variables that drive decision making in AMI-CS treatment.87,88 As emphasized by the SCAI SHOCK classification 3-axis model, the salient variables for decision making regarding temporary MCS use in AMI-CS treatment encompass measures of shock severity, clinical or hemodynamic phenotype, and nonmodifiable risk modifiers such as anoxic brain injury from CA that cannot often be gleaned from observational data sets.12,14,23 Numerous important risk factors for mortality in patients with CS cannot be reliably identified or quantified from administrative databases, including success of revascularization, SCAI SHOCK stage, ventricular function, hemodynamics, clinical phenotype, vasopressor requirements, circumstances of CA, magnitude of lactic acidosis, degree of end-organ failure, baseline functional status, frailty, code status, and goals of care.14,23,25, 26, 27, 28, 29,32,35 Therefore, even well-conducted propensity-adjusted observational studies drawn from administrative databases generally offer a low level of evidence because they cannot incorporate the most crucial variables and should be considered at best hypothesis generating.73
Most importantly, even the most sophisticated statistical adjustments cannot account for unmeasured confounders in observational studies. Anticipated prognosis, resuscitation status, goals of care, and patient preferences also influence these decisions yet are not captured in administrative or claims databases. Escalation from one temporary MCS device to another can be challenging to categorize in observational analyses; such patients are a particularly high-risk group that should be recognized as failure of the original device rather than ascribed to the “higher-level” device, a common error.19,29,67 A related challenge relates to the availability and quality of relevant data, particularly from administrative data based on billing/claims for which the inaccuracy of discharge diagnoses may influence the findings.89 This highlights the importance of using a standardized shock severity assessment such as the SCAI SHOCK classification, but such categorization is not typically captured in administrative databases, which can likewise not reliably differentiate the hemodynamic phenotypes of AMI-CS or quantify the severity of complications such as organ failure.14 Prospective observational studies with dedicated case report forms collecting the essential variables (such as NCSI and PROTECT-III72,79,80,85) can overcome some but not all these limitations. Therefore, propensity-adjusted analyses are not a valid surrogate for RCTs on AMI-CS.
Logistic challenges of randomized trials in AMI-CS
Randomized trials offer substantial benefits compared with observational data, most importantly balancing the rates of unmeasured confounders between the study groups. However, conducting RCTs in patients with AMI-CS introduces numerous hurdles that must be overcome, such as the ability to rapidly obtain informed consent from critically ill patients (including those who may be unresponsive) or from their legal representatives (who may be distraught or not physically present). In addition, many physicians have an implicit bias in believing that MCS devices are either mandatory for patient survival or, conversely, are harmful without proven evidence of benefit. Because they perceive lack of equipoise, both these physician groups have declined participation in previous AMI-CS RCTs. Hence, published AMI-CS RCTs have been limited by small sample sizes leading to inconclusive evidence from underpowered analyses, with most being terminated before their planned recruitment was achieved.
Even completed adequately powered RCTs may not be large enough to evaluate important subgroups, and negative findings from a large subgroup may mask a positive treatment effect in other patients. For example, in the SHOCK trial (in which the primary end point of 30-day mortality was not reduced by early revascularization), only one-quarter of patients (n = 73) were randomly assigned within 6 hours of symptom onset.9 This subgroup showed a significant reduction in 30-day mortality, whereas no difference in survival was seen in those patients randomized beyond 6 hours; this plausible interaction would ideally be examined in larger RCTs but has not been.9 Whether there is an important interaction between the time from symptom onset to AMI-CS and the use of MCS is unknown, but equally critical to establish. In a second example, nearly half of the patients in the IABP-SHOCK-II trial experienced CA; there is no sound reason to expect that an IABP would ameliorate death from anoxic brain injury.21 Conversely, to date, most RCTs in AMI-CS have included predominantly patients with SCAI SHOCK stage C and D, which leaves uncertainties about interventions in patients with greater or lesser shock severity.7 Despite these challenges, RCTs are considered the highest level of evidence and the gold standard for determining safety and efficacy. Observational analyses, which are often drawn from much larger datasets, may provide important complementary exploratory evidence about low frequency adverse events and usage patterns of drugs and interventions in real-world practice.
Where does this leave the practicing physician?
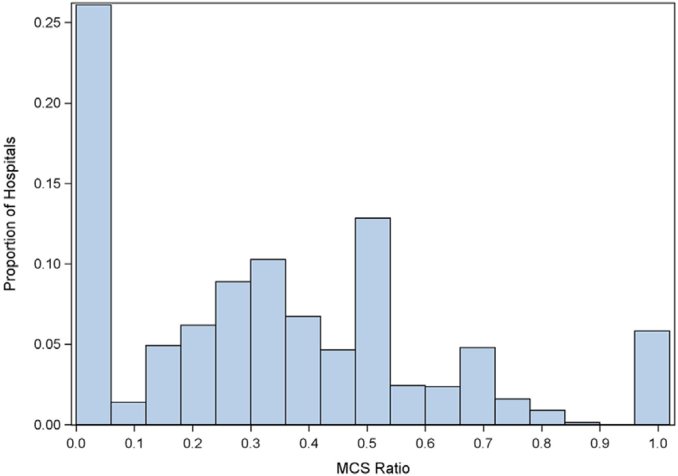
The conflicting findings between studies regarding potential benefits and harms of temporary MCS in AMI-CS and the lack of adequately powered RCTs have left providers with uncertainty regarding whether and in whom to use these devices. In addition, the high cost of some temporary MCS devices raises important issues regarding cost-effectiveness.64 Moreover, not all operators are facile at large bore femoral or alternative vascular access and closure (as is necessary for pVADs and VA-ECMO), resulting in hesitancy by some physicians to use these devices without irrefutable evidence of their benefit. Differences in baseline care (such as the success of revascularization) and variable timing of device implantation add additional layers of complexity.58, 59, 60 Local standards of care and treatment protocols may vary with provider experience (volume-outcome relationships), availability of cardiac intensivists, and presence of shock teams (including the distinction between hubs and spoke centers).80,81 Not surprisingly, there is marked variability in temporary MCS device utilization for CS across centers (Figure 5) and between countries, an undesirable situation that can only be rectified by a new generation of high-quality evidence resulting in strong uniform guideline recommendations.10,15, 16, 17
Figure 5.
Variability in the use of temporary MCS devices for AMI-CS in the United States Nationwide Inpatient Sample in 2014. MCS ratio denotes proportion of AMI-CS hospitalizations using temporary MCS. Reproduced with permission from Strom et al.16 AMI, acute myocardial infarction; CS, cardiogenic shock; MCS, mechanical circulatory support.
Suggestions for future research
Large-scale multi-national RCTs with adequate statistical power and appropriate enrollment of a population that not only mirrors the typical AMI-CS population encountered in clinical practice but excludes patients not likely to benefit are essential to establish standards of care.50,51 Such studies may be sponsored by the investigators (often with governmental funding support) or industries. Emergency exception from informed consent has been successfully used to enroll critically ill patients in the setting of CA is being used to facilitate enrollment in future AMI-CS trials.90,91 Pragmatic RCTs embedded into usual clinical practice could evaluate different strategies that are within current standards of care, either comparing different temporary MCS devices or care protocols incorporating these devices. Trial efficiency can be improved with factorial designs testing multiple interventions in combination or using an adaptive design.92,93 Finally, cluster randomization at the hospital level may obviate the need for individual patient informed consent but is less robust than multicenter individual patient RCTs owing to potential bias resulting from differences in patient profiles, operator skill and systems of care. Observational studies continue to have value in AMI-CS outcomes research. However, we must move away from retrospective analyses of administrative databases to prospective enrollment of patients in registries using dedicated case report forms that collect reasons for MCS device usage, shock stage and other critical prognostic factors, and adjudicate outcomes. Identifying which patients are likely to benefit, experience a neutral effect, or can be harmed by temporary MCS device usage is essential to optimize outcomes in this extremely high-risk patient cohort.
Conclusions
The great variability in studies published to date regarding the safety and efficacy of temporary MCS devices in AMI-CS poses a challenge for operators and health care systems to select the appropriate patients in whom these devices should be used. Implementation of a multidisciplinary shock team can facilitate matching the right device to the right patient at the right time, and emerging observational data suggests that this process of care is associated with survival.81 Quality improvement protocols should enable institutions to assess their own temporary MCS practices to learn from their successes and failures, applying rigorous methodology that can allow these findings to translate to the research arena. As with all technology, it is likely that selective rather than indiscriminate use of temporary MCS devices will optimize outcomes and cost-effectiveness. Although developing the next generation of temporary MCS devices that provide even more potent hemodynamic support with a lower rate of complications will be helpful, learning how best to apply these devices in the right situations (ie, survivable patients) is even more essential. Moving the field forward will require a deeper understanding of the core pathophysiologic mechanisms that drive heterogeneity and outcomes in AMI-CS. The strengths and limitations of existing and ongoing observational studies and RCTs need to be appreciated to properly interpret the present evidence base. Most importantly, acknowledging the lack of high-quality evidence regarding MCS device use in AMI-CS and the marked variability between centers in the adoption of these devices is the definition of equipoise and should compel widespread and enthusiastic investigator participation into ongoing and future RCTs to generate the highest level of evidence as soon as possible.
Acknowledgments
Declaration of competing interest
Deepak L. Bhatt discloses the following relationships—Advisory Board: AngioWave, Bayer, Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, High Enroll, Janssen, Level Ex, McKinsey, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, and Stasys; Board of Directors: AngioWave (stock options), Boston VA Research Institute, Bristol Myers Squibb (stock), DRS.LINQ (stock options), High Enroll (stock), Society of Cardiovascular Patient Care, TobeSoft; Chair: Inaugural Chair, and American Heart Association Quality Oversight Committee; Consultant: Broadview Ventures; Data Monitoring Committees: Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo; for the ABILITY-DM trial, funded by Concept Medical), Novartis, Population Health Research Institute; Rutgers University (for the NIH-funded MINT Trial); Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor-in-Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Oakstone CME (Course Director, Comprehensive Review of Interventional Cardiology), Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees), Wiley (steering committee); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), and VA CART Research and Publications Committee (Chair); Patent: Sotagliflozin (named on a patent for sotagliflozin assigned to Brigham and Women’s Hospital who assigned to Lexicon, with no financial benefits for both); Research Funding: Abbott, Acesion Pharma, Afimmune, Aker Biomarine, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CinCor, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, Youngene, and 89Bio; Royalties: Elsevier (Editor, Braunwald’s Heart Disease); Site Co-Investigator: Abbott, Biotronik, Boston Scientific, CSI, Endotronix, St. Jude Medical (now Abbott), Philips, SpectraWAVE, Svelte, and Vascular Solutions; Trustee: American College of Cardiology; Unfunded Research: FlowCo and Takeda. Gregg W. Stone has received speaker honoraria from Medtronic, Pulnovo, Infraredx, Abiomed, and Abbott; has served as a consultant to Valfix, TherOx, Robocath, HeartFlow, Ablative Solutions, Vectorious, Miracor, Neovasc, Ancora, Elucid Bio, Occlutech, CorFlow, Apollo Therapeutics, Impulse Dynamics, Cardiomech, Gore, Amgen, Adona Medical, and Millennia Biopharma; and has equity/options from Ancora, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, Valfix, and Xenter. Dr. Stone’s daughter is an employee at IQVIA. Institutional disclosure: Dr. Stone’s employer, Mount Sinai Hospital, receives research support from Abbott, Abiomed, Bioventrix, Cardiovascular Systems Inc, Phillips, Biosense-Webster, Shockwave, Vascular Dynamics, Pulnovo, and V-wave. Jacob C. Jentzer and Srihari S. Naidu reported no financial interests.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics statement and patient consent
This was a review article and therefore no patient contact or patient-level data was involved. The authors adhered to relevant ethical guidelines.
References
- 1.Omer M.A., Tyler J.M., Henry T.D., et al. Clinical characteristics and outcomes of STEMI patients with cardiogenic shock and cardiac arrest. JACC Cardiovasc Interv. 2020;13(10):1211–1219. doi: 10.1016/j.jcin.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Chioncel O., Parissis J., Mebazaa A., et al. Epidemiology, pathophysiology and contemporary management of cardiogenic shock—a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2020;22(10):1315–1341. doi: 10.1002/ejhf.1922. [DOI] [PubMed] [Google Scholar]
- 3.Zeymer U., Bueno H., Granger C.B., et al. Acute Cardiovascular Care Association position statement for the diagnosis and treatment of patients with acute myocardial infarction complicated by cardiogenic shock: a document of the Acute Cardiovascular Care Association of the European Society of Cardiology. Eur Heart J Acute Cardiovasc Care. 2020;9(2):183–197. doi: 10.1177/2048872619894254. [DOI] [PubMed] [Google Scholar]
- 4.Rathod K.S., Koganti S., Iqbal M.B., et al. Contemporary trends in cardiogenic shock: Incidence, intra-aortic balloon pump utilisation and outcomes from the London Heart Attack Group. Eur Heart J Acute Cardiovasc Care. 2018;7(1):16–27. doi: 10.1177/2048872617741735. [DOI] [PubMed] [Google Scholar]
- 5.Osman M., Syed M., Patibandla S., et al. Fifteen-year trends in incidence of cardiogenic shock hospitalization and in-hospital mortality in the United States. J Am Heart Assoc. 2021;10(15) doi: 10.1161/JAHA.121.021061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schrage B., Becher P.M., Gossling A., et al. Temporal trends in incidence, causes, use of mechanical circulatory support and mortality in cardiogenic shock. ESC Heart Fail. 2021;8(2):1295–1303. doi: 10.1002/ehf2.13202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tyler J.M., Brown C., Jentzer J.C., et al. Variability in reporting of key outcome predictors in acute myocardial infarction cardiogenic shock trials. Catheter Cardiovasc Interv. 2022;99(1):19–26. doi: 10.1002/ccd.29710. [DOI] [PubMed] [Google Scholar]
- 8.Thiele H., Ohman E.M., de Waha-Thiele S., Zeymer U., Desch S. Management of cardiogenic shock complicating myocardial infarction: an update 2019. Eur Heart J. 2019;40(32):2671–2683. doi: 10.1093/eurheartj/ehz363. [DOI] [PubMed] [Google Scholar]
- 9.Hochman J.S., Sleeper L.A., Webb J.G., et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med. 1999;341(9):625–634. doi: 10.1056/NEJM199908263410901. [DOI] [PubMed] [Google Scholar]
- 10.Shah M., Patnaik S., Patel B., et al. Trends in mechanical circulatory support use and hospital mortality among patients with acute myocardial infarction and non-infarction related cardiogenic shock in the United States. Clin Res Cardiol. 2018;107(4):287–303. doi: 10.1007/s00392-017-1182-2. [DOI] [PubMed] [Google Scholar]
- 11.Rihal C.S., Naidu S.S., Givertz M.M., et al. Society for Cardiovascular Angiography and Interventions (SCAI); Heart Failure Society of America (HFSA); Society for Thoracic Surgeons (STS); American Heart Association (AHA); American College of Cardiology (ACC). 2015 SCAI/ACC/HFSA/STS clinical expert consensus statement on the use of percutaneous mechanical circulatory support devices in cardiovascular care: endorsed by the American Heart Association, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencionista; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d'intervention. J Am Coll Cardiol. 2015;65(7):2140–2141. doi: 10.1016/j.jacc.2015.02.043. [DOI] [PubMed] [Google Scholar]
- 12.Henry T.D., Tomey M.I., Tamis-Holland J.E., et al. American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Arteriosclerosis, Thrombosis and Vascular Biology; and Council on Cardiovascular and Stroke Nursing. Invasive management of acute myocardial infarction complicated by cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2021;143(15):e815–e829. doi: 10.1161/CIR.0000000000000959. [DOI] [PubMed] [Google Scholar]
- 13.van Diepen S., Katz J.N., Albert N.M., et al. American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Mission: Lifeline. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136(16):e232–e268. doi: 10.1161/CIR.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 14.Naidu S.S., Baran D.A., Jentzer J.C., et al. SCAI SHOCK stage classification expert consensus update: a review and incorporation of validation studies. J Soc Cardiovasc Angrogr Interv. 2022;1(1):100008. [Google Scholar]
- 15.Strom J.B., Zhao Y., Shen C., et al. National trends, predictors of use, and in-hospital outcomes in mechanical circulatory support for cardiogenic shock. EuroIntervention. 2018;13(18):e2152–e2159. doi: 10.4244/EIJ-D-17-00947. [DOI] [PubMed] [Google Scholar]
- 16.Strom J.B., Zhao Y., Shen C., et al. Hospital variation in the utilization of short-term nondurable mechanical circulatory support in myocardial infarction complicated by cardiogenic shock. Circ Cardiovasc Interv. 2019;12(1) doi: 10.1161/CIRCINTERVENTIONS.118.007270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berg D.D., Barnett C.F., Kenigsberg B.B., et al. Clinical practice patterns in temporary mechanical circulatory support for shock in the Critical Care Cardiology Trials Network (CCCTN) registry. Circ Heart Fail. 2019;12(11) doi: 10.1161/CIRCHEARTFAILURE.119.006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helgestad O.K.L., Josiassen J., Hassager C., et al. Contemporary trends in use of mechanical circulatory support in patients with acute MI and cardiogenic shock. Open Heart. 2020;7(1) doi: 10.1136/openhrt-2019-001214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jentzer J.C., Baran D.A., Bohman J.K., et al. Cardiogenic shock severity and mortality in patients receiving venoarterial extracorporeal membrane oxygenator support. Eur Heart J Acute Cardiovasc Care. 2022;11(12):891–903. doi: 10.1093/ehjacc/zuac119. [DOI] [PubMed] [Google Scholar]
- 20.Thiele H., Jobs A., Ouweneel D.M., et al. Percutaneous short-term active mechanical support devices in cardiogenic shock: a systematic review and collaborative meta-analysis of randomized trials. Eur Heart J. 2017;38(47):3523–3531. doi: 10.1093/eurheartj/ehx363. [DOI] [PubMed] [Google Scholar]
- 21.Thiele H., Zeymer U., Neumann F.J., et al. IABP-SHOCKII Trial Investigators. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367(14):1287–1296. doi: 10.1056/NEJMoa1208410. [DOI] [PubMed] [Google Scholar]
- 22.Unverzagt S., Buerke M., de Waha A., et al. Intra-aortic balloon pump counterpulsation (IABP) for myocardial infarction complicated by cardiogenic shock. Cochrane Database Syst Rev. 2015;2015(3):CD007398. doi: 10.1002/14651858.CD007398.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jentzer J.C., Rayfield C., Soussi S., et al. Advances in the staging and phenotyping of cardiogenic shock part 1: clinical context. JACC Adv. 2022;1(4):1–14. [Google Scholar]
- 24.Esposito M.L., Kapur N.K. Acute mechanical circulatory support for cardiogenic shock: the "door to support" time. F1000Res. 2017;6:737. doi: 10.12688/f1000research.11150.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jentzer J.C., Kashani K.B., Wiley B.M., et al. Laboratory markers of acidosis and mortality in cardiogenic shock: developing a definition of hemometabolic shock. Shock. 2022;57(1):31–40. doi: 10.1097/SHK.0000000000001812. [DOI] [PubMed] [Google Scholar]
- 26.Jentzer J.C., Soussi S., Lawler P.R., Kennedy J.N., Kashani K.B. Validation of cardiogenic shock phenotypes in a mixed cardiac intensive care unit population. Catheter Cardiovasc Interv. 2022;99(4):1006–1014. doi: 10.1002/ccd.30103. [DOI] [PubMed] [Google Scholar]
- 27.Zweck E., Thayer K.L., Helgestad O.K.L., et al. Phenotyping cardiogenic shock. J Am Heart Assoc. 2021;10(14) doi: 10.1161/JAHA.120.020085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burstein B., van Diepen S., Wiley B.M., Anavekar N.S., Jentzer J.C. Biventricular function and shock severity predict mortality in cardiac ICU patients. Chest. 2022;161(3):697–709. doi: 10.1016/j.chest.2021.09.032. [DOI] [PubMed] [Google Scholar]
- 29.Thayer K.L., Zweck E., Ayouty M., et al. Invasive hemodynamic assessment and classification of in-hospital mortality risk among patients with cardiogenic shock. Circ Heart Fail. 2020;13(9) doi: 10.1161/CIRCHEARTFAILURE.120.007099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiley B.M., Eckman P.M., Jentzer J.C. Vasopressor load: sounding the alarm in management of cardiogenic shock associated with acute myocardial infarction. Crit Care Med. 2021;49(5):865–869. doi: 10.1097/CCM.0000000000004906. [DOI] [PubMed] [Google Scholar]
- 31.Jentzer J.C. Understanding cardiogenic shock severity and mortality risk assessment. Circ Heart Fail. 2020;13(9) doi: 10.1161/CIRCHEARTFAILURE.120.007568. [DOI] [PubMed] [Google Scholar]
- 32.Jentzer J.C., Henry T.D., Barsness G.W., Menon V., Baran D.A., Van Diepen S. Influence of cardiac arrest and SCAI shock stage on cardiac intensive care unit mortality. Catheter Cardiovasc Interv. 2020;96(7):1350–1359. doi: 10.1002/ccd.28854. [DOI] [PubMed] [Google Scholar]
- 33.Jentzer J.C., van Diepen S., Henry T.D. Understanding how cardiac arrest complicates the analysis of clinical trials of cardiogenic shock. Circ Cardiovasc Qual Outcomes. 2020;13(9) doi: 10.1161/CIRCOUTCOMES.120.006692. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed A.M., Tabi M., Wiley B.M., et al. Outcomes associated with cardiac arrest in patients in the cardiac intensive care unit with cardiogenic shock. Am J Cardiol. 2022;169:1–9. doi: 10.1016/j.amjcard.2021.12.041. [DOI] [PubMed] [Google Scholar]
- 35.Jentzer J.C., van Diepen S., Barsness G.W., et al. Cardiogenic shock classification to predict mortality in the cardiac intensive care unit. J Am Coll Cardiol. 2019;74(17):2117–2128. doi: 10.1016/j.jacc.2019.07.077. [DOI] [PubMed] [Google Scholar]
- 36.Arias E.A., Gonzalez-Chon O., Garcia-Lopez S.M., et al. [Impact of the intra-aortic balloon pump in the mortality due to cardiogenic shock secondary to acute myocardial infarction] Arch Cardiol Mex. 2005;75(3):260–266. [PubMed] [Google Scholar]
- 37.Ohman E.M., Nanas J., Stomel R.J., et al. Thrombolysis and counterpulsation to improve survival in myocardial infarction complicated by hypotension and suspected cardiogenic shock or heart failure: results of the TACTICS trial. J Thromb Thrombolysis. 2005;19(1):33–39. doi: 10.1007/s11239-005-0938-0. [DOI] [PubMed] [Google Scholar]
- 38.Prondzinsky R., Lemm H., Swyter M., et al. Intra-aortic balloon counterpulsation in patients with acute myocardial infarction complicated by cardiogenic shock: the prospective, randomized IABP SHOCK Trial for attenuation of multiorgan dysfunction syndrome. Crit Care Med. 2010;38(1):152–160. doi: 10.1097/CCM.0b013e3181b78671. [DOI] [PubMed] [Google Scholar]
- 39.Thiele H., Sick P., Boudriot E., et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2005;26(13):1276–1283. doi: 10.1093/eurheartj/ehi161. [DOI] [PubMed] [Google Scholar]
- 40.Burkhoff D., Cohen H., Brunckhorst C., O'Neill W.W., TandemHeart Investigators Group A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am Heart J. 2006;152(3):469.e1–469.e8. doi: 10.1016/j.ahj.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 41.Seyfarth M., Sibbing D., Bauer I., et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol. 2008;52(19):1584–1588. doi: 10.1016/j.jacc.2008.05.065. [DOI] [PubMed] [Google Scholar]
- 42.Ouweneel D.M., Eriksen E., Sjauw K.D., et al. Percutaneous mechanical circulatory support versus intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2017;69(3):278–287. doi: 10.1016/j.jacc.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 43.Bochaton T., Huot L., Elbaz M., et al. Mechanical circulatory support with the Impella(R) LP5.0 pump and an intra-aortic balloon pump for cardiogenic shock in acute myocardial infarction: the IMPELLA-STIC randomized study. Arch Cardiovasc Dis. 2020;113(4):237–243. doi: 10.1016/j.acvd.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Brunner S., Guenther S.P.W., Lackermair K., et al. Extracorporeal life support in cardiogenic shock complicating acute myocardial infarction. J Am Coll Cardiol. 2019;73(18):2355–2357. doi: 10.1016/j.jacc.2019.02.044. [DOI] [PubMed] [Google Scholar]
- 45.Ostadal P., Rokyta R., Karasek J., et al. Extracorporeal membrane oxygenation in the therapy of cardiogenic shock: results of the ECMO-CS randomized clinical trial. Circulation. 2023;147:454–464. doi: 10.1161/CIRCULATIONAHA.122.062949. [DOI] [PubMed] [Google Scholar]
- 46.Lackermair K., Brunner S., Orban M., et al. Outcome of patients treated with extracorporeal life support in cardiogenic shock complicating acute myocardial infarction: 1-year result from the ECLS-Shock study. Clin Res Cardiol. 2021;110(9):1412–1420. doi: 10.1007/s00392-020-01778-8. [DOI] [PubMed] [Google Scholar]
- 47.Prondzinsky R., Unverzagt S., Russ M., et al. Hemodynamic effects of intra-aortic balloon counterpulsation in patients with acute myocardial infarction complicated by cardiogenic shock: the prospective, randomized IABP shock trial. Shock. 2012;37(4):378–384. doi: 10.1097/SHK.0b013e31824a67af. [DOI] [PubMed] [Google Scholar]
- 48.Thiele H., Zeymer U., Neumann F.J., et al. Intraaortic Balloon Pump in Cardiogenic Shock II (IABP-SHOCK II) Trial Investigators. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet. 2013;382(9905):1638–1645. doi: 10.1016/S0140-6736(13)61783-3. [DOI] [PubMed] [Google Scholar]
- 49.Thiele H., Zeymer U., Thelemann N., et al. IABP-SHOCK II Trial (Intraaortic Balloon Pump in Cardiogenic Shock II) Investigators; IABP-SHOCK II Investigators. Intraaortic balloon pump in cardiogenic shock complicating acute myocardial infarction: long-term 6-year outcome of the randomized IABP-SHOCK II trial. Circulation. 2019;139(3):395–403. doi: 10.1161/CIRCULATIONAHA.118.038201. [DOI] [PubMed] [Google Scholar]
- 50.Udesen N.J., Moller J.E., Lindholm M.G., et al. DanGer Shock investigators. Rationale and design of DanGer shock: Danish-German cardiogenic shock trial. Am Heart J. 2019;214:60–68. doi: 10.1016/j.ahj.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 51.Banning A.S., Adriaenssens T., Berry C., et al. Veno-arterial extracorporeal membrane oxygenation (ECMO) in patients with cardiogenic shock: rationale and design of the randomised, multicentre, open-label EURO SHOCK trial. EuroIntervention. 2021;16(15):e1227–e1236. doi: 10.4244/EIJ-D-20-01076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Acconcia M.C., Caretta Q., Romeo F., et al. Meta-analyses on intra-aortic balloon pump in cardiogenic shock complicating acute myocardial infarction may provide biased results. Eur Rev Med Pharmacol Sci. 2018;22(8):2405–2414. doi: 10.26355/eurrev_201804_14833. [DOI] [PubMed] [Google Scholar]
- 53.Jentzer J.C., van Diepen S., Henry T.D., Baran D.A., Barsness G.W., Holmes D.R., Jr. Influence of intra-aortic balloon pump on mortality as a function of cardiogenic shock severity. Catheter Cardiovasc Interv. 2022;99(2):293–304. doi: 10.1002/ccd.29800. [DOI] [PubMed] [Google Scholar]
- 54.Yuan S., He J., Cai Z., et al. Intra-aortic balloon pump in cardiogenic shock: a propensity score matching analysis. Catheter Cardiovasc Interv. 2022;99(suppl 1):1456–1464. doi: 10.1002/ccd.30102. [DOI] [PubMed] [Google Scholar]
- 55.Wang W., Yang F., Lin X., et al. The preference, effect, and prognosis of intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock patients: a retrospective cohort study. Biomed Res Int. 2021;2021 doi: 10.1155/2021/6656926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chu S., Sun P., Zhang Y., et al. Intra-aortic balloon pump on in-hospital outcomes of cardiogenic shock: findings from a nationwide registry, China. ESC Heart Fail. 2021;8(4):3286–3294. doi: 10.1002/ehf2.13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Timoteo A.T., Nogueira M.A., Rosa S.A., Belo A., Ferreira R.C., Pro A.C.S.I. Role of intra-aortic balloon pump counterpulsation in the treatment of acute myocardial infarction complicated by cardiogenic shock: Evidence from the Portuguese nationwide registry. Eur Heart J Acute Cardiovasc Care. 2016;5(7):23–31. doi: 10.1177/2048872615606600. [DOI] [PubMed] [Google Scholar]
- 58.Hawranek M., Gierlotka M., Pres D., Zembala M., Gasior M. Nonroutine use of intra-aortic balloon pump in cardiogenic shock complicating myocardial infarction with successful and unsuccessful primary percutaneous coronary intervention. JACC Cardiovasc Interv. 2018;11(18):1885–1893. doi: 10.1016/j.jcin.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 59.Romeo F., Acconcia M.C., Sergi D., et al. The outcome of intra-aortic balloon pump support in acute myocardial infarction complicated by cardiogenic shock according to the type of revascularization: a comprehensive meta-analysis. Am Heart J. 2013;165(5):679–692. doi: 10.1016/j.ahj.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 60.Cui K., Lyu S., Liu H., et al. Timing of initiation of intra-aortic balloon pump in patients with acute myocardial infarction complicated by cardiogenic shock: a meta-analysis. Clin Cardiol. 2019;42(11):1126–1134. doi: 10.1002/clc.23264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schrage B., Ibrahim K., Loehn T., et al. Impella support for acute myocardial infarction complicated by cardiogenic shock. Circulation. 2019;139(10):1249–1258. doi: 10.1161/CIRCULATIONAHA.118.036614. [DOI] [PubMed] [Google Scholar]
- 62.Dhruva S.S., Ross J.S., Mortazavi B.J., et al. Association of use of an intravascular microaxial left ventricular assist device vs intra-aortic balloon pump with in-hospital mortality and major bleeding among patients with acute myocardial infarction complicated by cardiogenic shock. JAMA. 2020;323(8):734–745. doi: 10.1001/jama.2020.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vallabhajosyula S., Subramaniam A.V., Murphree D.H., Jr., et al. Complications from percutaneous-left ventricular assist devices versus intra-aortic balloon pump in acute myocardial infarction-cardiogenic shock. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0238046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Desai R., Hanna B., Singh S., et al. Percutaneous ventricular assist device vs. intra-aortic balloon pump for hemodynamic support in acute myocardial infarction-related cardiogenic shock and coexistent atrial fibrillation: a nationwide propensity-matched analysis. Am J Med Sci. 2021;361(1):55–62. doi: 10.1016/j.amjms.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 65.Jin C., Yandrapalli S., Yang Y., Liu B., Aronow W.S., Naidu S.S. A comparison of in-hospital outcomes between the use of Impella and IABP in acute myocardial infarction cardiogenic shock undergoing percutaneous coronary intervention. J Invasive Cardiol. 2022;34(2):E98–E103. doi: 10.25270/jic/21.00096. [DOI] [PubMed] [Google Scholar]
- 66.Miller P.E., Bromfield S.G., Ma Q., et al. Clinical outcomes and cost associated with an intravascular microaxial left ventricular assist device vs intra-aortic balloon pump in patients presenting with acute myocardial infarction complicated by cardiogenic shock. JAMA Intern Med. 2022;182(9):926–933. doi: 10.1001/jamainternmed.2022.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garan A.R., Takeda K., Salna M., et al. Prospective comparison of a percutaneous ventricular assist device and venoarterial extracorporeal membrane oxygenation for patients with cardiogenic shock following acute myocardial infarction. J Am Heart Assoc. 2019;8(9) doi: 10.1161/JAHA.119.012171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schiller P., Hellgren L., Vikholm P. Survival after refractory cardiogenic shock is comparable in patients with Impella and veno-arterial extracorporeal membrane oxygenation when adjusted for SAVE score. Eur Heart J Acute Cardiovasc Care. 2019;8(8):329–337. doi: 10.1177/2048872618799745. [DOI] [PubMed] [Google Scholar]
- 69.Karami M., den Uil C.A., Ouweneel D.M., et al. Mechanical circulatory support in cardiogenic shock from acute myocardial infarction: Impella CP/5.0 versus ECMO. Eur Heart J Acute Cardiovasc Care. 2020;9(2):164–172. doi: 10.1177/2048872619865891. [DOI] [PubMed] [Google Scholar]
- 70.Karatolios K., Chatzis G., Markus B., et al. Comparison of mechanical circulatory support with venoarterial extracorporeal membrane oxygenation or Impella for patients with cardiogenic shock: a propensity-matched analysis. Clin Res Cardiol. 2021;110(9):1404–1411. doi: 10.1007/s00392-020-01777-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grandin E.W., Nunez J.I., Willar B., et al. Mechanical left ventricular unloading in patients undergoing venoarterial extracorporeal membrane oxygenation. J Am Coll Cardiol. 2022;79(13):1239–1250. doi: 10.1016/j.jacc.2022.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Basir M.B., Kapur N.K., Patel K., et al. National Cardiogenic Shock Initiative Initiative Investigators. Improved outcomes associated with the use of shock protocols: updates from the National Cardiogenic Shock Initiative. Catheter Cardiovasc Interv. 2019;93(7):1173–1183. doi: 10.1002/ccd.28307. [DOI] [PubMed] [Google Scholar]
- 73.Moustafa A., Khan M.S., Saad M., Siddiqui S., Eltahawy E. Impella support versus intra-aortic balloon pump in acute myocardial infarction complicated by cardiogenic shock: a meta-analysis. Cardiovasc Revasc Med. 2022;34:25–31. doi: 10.1016/j.carrev.2021.01.028. [DOI] [PubMed] [Google Scholar]
- 74.Nishi T., Ishii M., Tsujita K., et al. Outcomes of venoarterial extracorporeal membrane oxygenation plus intra-aortic balloon pumping for treatment of acute myocardial infarction complicated by cardiogenic shock. J Am Heart Assoc. 2022;11(7) doi: 10.1161/JAHA.121.023713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vallabhajosyula S., O'Horo J.C., Antharam P., et al. Concomitant intra-aortic balloon pump use in cardiogenic shock requiring veno-arterial extracorporeal membrane oxygenation. Circ Cardiovasc Interv. 2018;11(9) doi: 10.1161/CIRCINTERVENTIONS.118.006930. [DOI] [PubMed] [Google Scholar]
- 76.Schrage B., Becher P.M., Bernhardt A., et al. Left ventricular unloading is associated with lower mortality in patients with cardiogenic shock treated with venoarterial extracorporeal membrane oxygenation: results from an international, multicenter cohort study. Circulation. 2020;142(22):2095–2106. doi: 10.1161/CIRCULATIONAHA.120.048792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Russo J.J., Aleksova N., Pitcher I., et al. Left ventricular unloading during extracorporeal membrane oxygenation in patients with cardiogenic shock. J Am Coll Cardiol. 2019;73(6):654–662. doi: 10.1016/j.jacc.2018.10.085. [DOI] [PubMed] [Google Scholar]
- 78.Iannaccone M., Franchin L., Hanson I.D., et al. Timing of Impella placement in PCI for acute myocardial infarction complicated by cardiogenic shock: an updated meta-analysis. Int J Cardiol. 2022;362:47–54. doi: 10.1016/j.ijcard.2022.05.011. [DOI] [PubMed] [Google Scholar]
- 79.Basir M.B., Schreiber T., Dixon S., et al. Feasibility of early mechanical circulatory support in acute myocardial infarction complicated by cardiogenic shock: the Detroit cardiogenic shock initiative. Catheter Cardiovasc Interv. 2018;91(3):454–461. doi: 10.1002/ccd.27427. [DOI] [PubMed] [Google Scholar]
- 80.O'Neill W.W., Grines C., Schreiber T., et al. Analysis of outcomes for 15,259 US patients with acute myocardial infarction cardiogenic shock (AMICS) supported with the Impella device. Am Heart J. 2018;202:33–38. doi: 10.1016/j.ahj.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 81.Papolos A.I., Kenigsberg B.B., Berg D.D., et al. Critical Care Cardiology Trials Network I. Management and outcomes of cardiogenic shock in cardiac ICUs with versus without shock teams. J Am Coll Cardiol. 2021;78(13):1309–1317. doi: 10.1016/j.jacc.2021.07.044. [DOI] [PubMed] [Google Scholar]
- 82.Tehrani B.N., Truesdell A.G., Sherwood M.W., et al. Standardized team-based care for cardiogenic shock. J Am Coll Cardiol. 2019;73(13):1659–1669. doi: 10.1016/j.jacc.2018.12.084. [DOI] [PubMed] [Google Scholar]
- 83.Schrage B., Beer B.N., Savarese G., et al. Eligibility for mechanical circulatory support devices based on current and past randomised cardiogenic shock trials. Eur J Heart Fail. 2021;23(11):1942–1951. doi: 10.1002/ejhf.2274. [DOI] [PubMed] [Google Scholar]
- 84.Megaly M., Buda K., Alaswad K., et al. Comparative analysis of patient characteristics in cardiogenic shock studies: differences between trials and registries. JACC Cardiovasc Interv. 2022;15(3):297–304. doi: 10.1016/j.jcin.2021.11.036. [DOI] [PubMed] [Google Scholar]
- 85.O'Neill W.W., Anderson M., Burkhoff D., et al. Improved outcomes in patients with severely depressed LVEF undergoing percutaneous coronary intervention with contemporary practices. Am Heart J. 2022;248:139–149. doi: 10.1016/j.ahj.2022.02.006. [DOI] [PubMed] [Google Scholar]
- 86.Elze M.C., Gregson J., Baber U., et al. Comparison of propensity score methods and covariate adjustment: evaluation in 4 cardiovascular studies. J Am Coll Cardiol. 2017;69(3):345–357. doi: 10.1016/j.jacc.2016.10.060. [DOI] [PubMed] [Google Scholar]
- 87.Noseworthy P.A., Gersh B.J., Kent D.M., et al. Atrial fibrillation ablation in practice: assessing CABANA generalizability. Eur Heart J. 2019;40(16):1257–1264. doi: 10.1093/eurheartj/ehz085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yao X., Gersh B.J., Holmes D.R., Jr., et al. Association of surgical left atrial appendage occlusion with subsequent stroke and mortality among patients undergoing cardiac surgery. JAMA. 2018;319(20):2116–2126. doi: 10.1001/jama.2018.6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jentzer J.C., Ahmed A.M., Vallabhajosyula S., et al. Shock in the cardiac intensive care unit: Changes in epidemiology and prognosis over time. Am Heart J. 2021;232:94–104. doi: 10.1016/j.ahj.2020.10.054. [DOI] [PubMed] [Google Scholar]
- 90.Kern K.B., Radsel P., Jentzer J.C., et al. Randomized pilot clinical trial of early coronary angiography versus no early coronary angiography after cardiac arrest without ST-segment elevation: the PEARL Study. Circulation. 2020;142(21):2002–2012. doi: 10.1161/CIRCULATIONAHA.120.049569. [DOI] [PubMed] [Google Scholar]
- 91.Yannopoulos D., Bartos J., Raveendran G., et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open-label, randomised controlled trial. Lancet. 2020;396(10265):1807–1816. doi: 10.1016/S0140-6736(20)32338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lawler P.R., Hochman J.S., Zarychanski R. What are adaptive platform clinical trials and what role may they have in cardiovascular medicine? Circulation. 2022;145(9):629–632. doi: 10.1161/CIRCULATIONAHA.121.058113. [DOI] [PubMed] [Google Scholar]
- 93.Bhatt D.L., Mehta C. Adaptive designs for clinical trials. N Engl J Med. 2016;375(1):65–74. doi: 10.1056/NEJMra1510061. [DOI] [PubMed] [Google Scholar]