Abstract
Background and Aims
While most Helicobacter pylori-infected individuals remain asymptomatic throughout their lifetime, in a significant proportion, the resulting severe chronic gastritis drives the development of gastric cancer. In this study, we examine a new therapeutic target, a host potassium channel regulatory subunit, SUR2 (encoded by ABCC9), with potential to protect against H pylori-associated diseases.
Methods
SUR2 gene (ABCC9) expression in human gastric biopsies was analyzed by quantitative polymerase chain reactions. Helicobacter-infected mice were administered the SUR2-channel agonists, pinacidil and nicorandil, then gastric tissues analyzed by histology, immunohistochemistry and quantitative polymerase chain reaction, and splenic tissues by enzyme-linked immunosorbent assays. In vitro studies were performed on human and mouse macrophages, human gastric epithelial cells and mouse splenocytes.
Results
ABCC9 expression in human and mouse stomachs is downregulated with H pylori infection. Treatment of Helicobacter-infected mice with SUR2 channel modulators, pinacidil or nicorandil, significantly reduced gastritis severity. In gastric epithelial cells, nicorandil-induced opening of the SUR2 channel increased intracellular K+ and prevented H pylori-mediated Ca2+ influx and downstream pro-inflammatory signaling.
Conclusion
SUR2 is a novel host factor that regulates Helicobacter pathogenesis. Pharmacological targeting of SUR2 provides a potential approach for reducing the severity of H pylori-associated gastritis, without eradicating infection.
Keywords: SUR2, Gastritis, Helicobacter pylori, Therapeutic, Potassium channel
Introduction
While most individuals infected with Helicobacter pylori remain asymptomatic throughout their lifetime, in some, the resulting chronic inflammation (gastritis) over decades drives development of peptic ulcers, gastric MALT (mucosa-associated lymphoid tissue) lymphoma, or gastric adenocarcinoma (gastric cancer).1 Gastric cancer is the most serious consequence of H pylori infection, being the fifth most prevalent cancer and fourth leading cause of cancer-related deaths globally.2 Progression to malignancy is a multistep process1,3 and largely preventable, provided H pylori is eradicated early enough, thereby removing the inflammatory drive toward cancer.
Despite increasing antimicrobial resistance, therapies based on antibiotics currently remain the frontline method for treating H pylori infection.4 However, an expert World Health Organization panel recently listed H pylori among the top 10 pathogenic bacteria for which new therapies are urgently needed.5 There is therefore a high priority to identify novel antibiotics, or alternative treatments, for either eradicating H pylori infection or preventing associated diseases.5
Elements that modify the severity of H pylori-induced gastritis, including environmental, bacterial virulence and host factors, influence the susceptibility of a host to associated diseases. Potassium channels comprise a heterogenous family of host factors for which little is known regarding H pylori pathogenesis. These transmembrane proteins, by the selective transportation of potassium ions (K+) across cell membranes, are involved in an array of biological processes in diverse cell types. They consist of several subsets, including voltage-gated potassium channels which open and close in response to transmembrane electrical potential, and inwardly rectifying potassium channels (Kir) that transfer positive charge into a cell via the inward transportation of ions, in particular K+. While not well studied, some of these channels are emerging as pro-malignant factors that contribute to gastric cancer progression.6,7
ATP-sensitive Kir (KATP) channels typically comprise a core of 4 pore-forming Kir subunits, surrounded by 4 sulphonylurea receptors (SURs), forming a functional hetero-octomeric complex.8 SUR subunits serve as regulatory clamps around the channel pore, controlling the flow of K+ ions into the cell. KATP channels in the heart, brain, pancreas, and vascular smooth muscles are well studied, as their aberrant expression is associated with pathologies including coronary artery disease, neurological diseases, hypertension, cardiac ischemia, and diabetes mellitus.9 Moreover, several KATP channel modulating drugs, including pinacidil and nicorandil, have been approved for clinical practice and used in the treatment of these conditions. However, there are only sparse reports of KATP channels in the stomach, which are neither investigated in detail nor in the context of Helicobacter-induced gastritis and associated diseases.
This study demonstrates that KATP channels containing SUR2 (encoded by the ABCC9 gene) play an important role in H pylori-induced gastritis. Notably, treatment with either of 2 KATP channel agonists, pinacidil and nicorandil, ameliorated gastritis in a murine model of Helicobacter infection.
Materials and Methods
Human gastric biopsies
Gastric mucosal tissues were obtained from H pylori positive individuals with clinically significant chronic atrophic gastritis and disease-free controls as previously described (approval number 097.1998, Melbourne Health Research Directorate Human Ethics Committee).10
Bacterial culture and infection of mice
H pylori Sydney strain 1 (SS1),11 H pylori clinical isolate 251,12 and H felis (ATCC 49179, CS1)13 were cultured as previously described.14 Mice were infected with a single orogastric dose of 107 H pylori SS1 or H felis CS1 in 100 μL brain heart infusion broth (Oxoid).
Mouse infection studies
All animal procedures were approved by the Animal Ethics Committee, University of Melbourne (AEC1714170 and AEC1814406) and carried out in adherence to the Animal research: reporting of in vivo experiments (ARRIVE) guidelines. Adult female C57BL/6 mice (6–7 weeks old; Walter and Eliza Hall Institute, Kew, Australia) were housed in filter-top cages (5 mice per cage) with ad libitum access to feed and sterile water. Randomization was carried out as follows: upon arrival, mice were arbitrarily assigned to cages. After one week of acclimatization, mice were ear-clipped and assigned a number within the cage. Cages were then randomly taken from the rack and assigned a group number (2 cages or 10 mice per group). Sample size calculation was performed a priori (based on our previous experience with the Helicobacter infection models) with the power of the experiment set to 80% and a 0.05 significance threshold. Each mouse (or tissues collected from each mouse, such as stomach or spleen, as indicated in Results and graphs) is considered an experimental unit for the studies reported here.
For the drug treatment study (N = 60), there were 3 control groups (no treatment, pinacidil treatment, nicorandil treatment) and 3 H felis infection groups (no treatment, pinacidil treatment, nicorandil treatment). For the treatment groups, mice were maintained on drinking water containing nicorandil or pinacidil (Sigma-Aldrich; 1mg/kg/day). Mice were infected as described above (uninfected mice received media only) and drug treatments (via drinking water) were started at after 4 weeks of infection. At study conclusion (twelve weeks post infection), the following parameters were measured: stomach histology for grading of atrophy and immune cell infiltration, food and water consumption, qPCR analysis for Helicobacter colonization, gastric cytokines and parietal cell marker expression (qPCR), spleen cytokine levels (enzyme-linked immunosorbent assay [ELISA]), and SUR2 expression (immunohistochemistry). No exclusion criteria were set, and all experimental units were included in analysis.
Quantification of gene expression by qPCR
Primers used in this study are listed in Table. RNA extracted from stomach tissues using TRIzol reagent (Invitrogen) was converted to cDNA with GoScript Reverse Transcription System (Promega). For qPCR, duplicate reactions of 15μL containing 7.5μL QuantiTect SYBR Green PCR Master Mix (Qiagen), 300 nM primers (Table) and 2μL of cDNA (1:10 dilution), were performed in an Mx3000P cycler (Agilent Technologies). Primer efficiencies were calculated with LinRegPCR13 and gene expression quantified relative to Rpl32.
Table.
Primers Used in This Study
| Primer name | Primer sequence (5′->3′) |
|---|---|
| Human qPCR primers | |
| ABCC9 all transcripts F | ATGGTGTACTACAAAATTCCTGC |
| ABCC9 all transcripts R | TCACAGACATGCACAAACAGG |
| SUR2A F | TTCTATTATGGATGCAGGCC |
| SUR2B R | ACCAAAGTGGAAAAGAGGCC |
| SUR2B F | GTTATTGTGATGAAGCGAGG |
| SUR2B R | TTACAGAGGTCAAGCTGATG |
| RPL32 F | CATCTCCTTCTCGGCATCA |
| RPL32 R | ACCCTGTTGTCAATGCCTC |
| Mouse qPCR primers | |
| Abcc9 all transcripts F | CTTTGTGGACGCACTCAACC |
| Abcc9 all transcripts R | TGTGTCCGGGAAAATGAAGC |
| Rpl32 F | GAGGTGCTGCTGATGTGC |
| Rpl32 R | GGCGTTGGGATTGGTGACT |
| Sur2A F | GTAACCATAGCTCACCGTGTCTC |
| Sur2A R | CATTCTTGTGCTGGAGCAGG |
| Sur2B F | CCATAGCTCATCGGGTTCACAC |
| Sur2B R | ACACTCCATCTTCCTGGGCC |
| Ifn-g F | CAGCAACAGCAAGGCGAAA |
| Ifn-g R | CTGGACCTGTGGGTTGTTGAC |
| Il-6 F | GAAAATTTCCTCTGGTCTTCTGG |
| Il-6 R | TGGAAATTGGGGTAGGAAGG |
| Il-17a F | tccagaaggccctcagacta |
| Il-17a R | agcatcttctcgaccctgaa |
| Mip-2 F | AGTGAACTGCGCTGTCAATG |
| Mip-2 R | TTCAGGGTCAAGGCAAACTT |
| Genomic DNA qPCR primers | |
| Mouse Gapdh F | TGCACCACCAACTGCTTAG |
| Mouse Gapdh R | GGATGCAGGGATGATGTTC |
| H felis flaB F | TTCGATTGGTCCTACAGGCTCAGA |
| H felis flaB R | TTCTTGTTGATGACATTGACCAACGCA |
F, forward primer; R, reverse primer.
To quantify bacteria, longitudinally halved mouse stomachs were homogenized in 1mL of TRIzol reagent and genomic DNA extracted according to the manufacturer’s protocol and used to quantify H felis genome copies (with primers targeting flaB) relative to mouse Gapdh copies by qPCR as above.
Assessment of gastritis
Longitudinally halved stomachs were fixed in 10% neutral buffered formalin, processed, embedded in paraffin and 5μm sections stained with hematoxylin and eosin. Stained sections were blinded and assessed histologically for infiltrating inflammatory immune cells and atrophy as previously described.15
Mammalian cell culture and stimulation
RAW264.7 (mouse macrophage, ATCC TIB71) and AGS (human gastric epithelial, ATCC CRL-1739) cell lines,16 red-cell depleted primary splenocytes and intraperitoneal macrophages from naïve C57BL/6 mice, were cultured in Roswell Park Memorial Institute medium 1640 supplemented with 10% heat-inactivated fetal calf serum (Gibco). Cells (2x105 per ml) were stimulated with live H pylori SS1 or 251 (multiplicity of infection = 10), or SS1 lysate (10μg/ml) with or without nicorandil (100μM). Supernatants were collected 24 hours poststimulation and cytokines analyzed by ELISA. SUR2/Kir6.1 expression in AGS cells was visualized by immunofluorescence assay.
Immunofluorescence assay with AGS cells
AGS cells grown on Nunc Lab-Tek 8-chamber slides were fixed with 4% paraformaldehyde for 20 minutes at ambient temperature. Cells were then blocked with 2% goat (SUR2) or donkey (Kir6.1) serum in 0.3M glycine-phosphate buffered saline (PBS) buffer, followed by overnight incubation at 4 °C with mouse antihuman SUR2 (1:300; Abcam #ab174631) or rabbit anti-human Kir6.1 (1:100; Abcam #ab251809) primary antibodies. Cells were then incubated with goat anti-mouse IgG Alexa Fluor 488 (SUR2; Thermo Fisher Scientific #A11001) or donkey anti-rabbit Alexa Fluor 488 (Kir6.1; Thermo Fisher Scientific #A21206) diluted to 1:500. Actin cytoskeletons were stained with phalloidin-iFluor 647 (Abcam #176759) and cells mounted in ProLong Gold Antifade mountant with 4′,6-diamidino-2-phenylindole counterstain (Invitrogen). Fluorescence images were captured using a Zeiss LSM 780 laser scanning confocal microscope and processed using ZEN software (Zeiss).
Quantification of cytokines by ELISA
Cell culture supernatants and spleen homogenates (in PBS) were centrifuged to remove cell/tissue debris prior to quantification of cytokines by ELISA as previously described (2). DuoSet ELISA kits (R&D Systems) were used to quantify mouse interleukins IL-6, IL-17A, macrophage inflammatory protein 2 (MIP-2), interferon gamma (IFN-γ), and human IL-8 (using the manufacturer’s protocol). Plates were read at 450nm in a Multiskan GO microplate reader (Thermo Fisher Scientific) and sample concentration of each cytokine was determined against a standard curve of relevant recombinant cytokine.
Immunohistochemistry with mouse stomach tissues
Immunohistochemistry was performed on formalin-fixed paraffin embedded stomach cross-sections (5μm thick). Tissue sections were de-waxed and rehydrated followed by antigen retrieval in boiling sodium citrate buffer (10mM sodium citrate, 0.05% Tween 20, pH 6.0) for 40 minutes. Primary antibodies used were as follows: mouse antihuman SUR2 (Abcam #ab174631, in combination with Vector M.O.M immunodetection kit) and mouse IgG1 isotype control (Abcam), each diluted 1:200 and rabbit anti-mouse CK19 (ab15463, Abcam #ab15463) diluted 1:50. Immunofluorescent detection of bound primary antibodies was achieved using the following secondary antibody conjugates: donkey anti-rabbit IgG-Alexa Fluor 594 and goat anti-mouse IgG Alexa Fluor 488 (Thermo Fisher Scientific). Samples were then mounted in Pro-Long Gold medium containing 4′,6-diamidino-2-phenylindole counterstain (Invitrogen). Fluorescence images were captured using a Zeiss LSM 780 laser scanning confocal microscope and processed using ZEN Blue software (Zeiss).
Ca2+/K+ ion indicator dye assay
Black, optical-bottom, 96-well plates (Thermo Scientific) seeded with AGS cells (2x105 cells/ml) in serum-free Roswell Park Memorial Institute 1640 media were loaded with 2μM of either intracellular Ca2+ indicator Cal-520 AM or K+ indicator ION Potassium Green-2 AM (Abcam) fluorescent dyes in 0.02% Pluronic F-127 and 0.04% dimethyl sulfoxide and incubated in the dark for 90 minutes. Cells were washed twice with PBS and serum-free media (no pH indicator) added to the wells. Reads were taken in an FLUOstar OPTIMA (BMG Labtech) fluorescent microplate reader (excitation/emission: Ca2+ 485/520, K+ 520-10/560-10) at baseline (F0) and at indicated time-points after addition of H pylori (MOI = 10) and/or nicorandil (100μM). Intracellular Ca2+ and K+ levels are expressed as percentage baseline corrected fluorescence intensities (F/F0∗100).
Statistical analyses
Data were analyzed using GraphPad Prism version 7.0 (GraphPad Software Inc; San Diego, USA) with the Mann-Whitney U test, two-way ANOVA or Wilcoxon matched-pairs test, as specified. Area under curve analysis was performed for mice water intake and body weight measurements. P-values <.05 were considered statistically significant.
Results
Reduced ABCC9 expression in human and mouse stomachs upon H pylori infection
To examine the potential role of SUR2 channels in H pylori-associated diseases, we first assessed the effect of H pylori on ABCC9 gene expression in infected gastric tissues. This identified ABCC9 expression to be significantly lower in individuals with H pylori infection/chronic atrophic gastritis, as compared to uninfected controls (Figure 1A). A similar observation was made in mice, where gastric Abcc9 expression was reduced in C57BL/6 mice infected with H pylori for 2, 4, and 12 months (Figure 1B).
Figure 1.
Reduced ABCC9 expression in stomachs infected with H pylori SS1. H pylori infection significantly reduced overall ABCC9 gene expression (all transcripts) in (A) human gastric mucosa (normal = 16, H pylori = 15) and (B) mouse stomachs at 2-, 4-, and 12-month postinfection (mpi) as determined by quantitative polymerase chain reaction (Mann-Whitney). SUR2B splice variant expression was significantly higher than SUR2A in both (C) humans and (D) mice at 4 mpi (Wilcoxon matched-pairs test).
The ABCC9 gene undergoes alternative splicing, generating variants with tissue-specific expression; for example, cardiac and skeletal muscles predominantly express SUR2A, while SUR2B is more common in vascular smooth muscles. Analysis of these variants identified SUR2B as the predominant isoform in both human (Figure 1C) and mouse gastric tissues (Figure 1D), irrespective of H pylori infection status.
Nicorandil suppresses the pro-inflammatory response to H pylori by epithelial cells
In vitro stimulation assays were performed to determine if SUR2 can modify the inflammatory response of gastric epithelial and immune cells to H pylori. RAW264.7 murine macrophage and human AGS gastric epithelial cell lines were selected as a prescreen demonstrated their concomitant expression of both the Abcc9/SUR2 and Kcnj8/KIR6.1 subunits that are required for a functional KATP channel (Figure 2A and B). These cells were then stimulated with H pylori, with or without addition of the SUR2 channel agonist, nicorandil. Nicorandil treatment had no effect on the pro-inflammatory IL-6 and MIP-2 response of RAW264.7 macrophages to H pylori stimulation (Figure 2C and D). A similar lack of effect was also observed using non-induced mouse primary splenocytes and intraperitoneal macrophages. AGS cells were then stimulated with lysed or live H pylori and culture supernatants examined for IL-8 secretion. IL-8 is known to be the most up-regulated cytokine in H pylori exposed gastric epithelial cells.17 In contrast to the immune cells, nicorandil treatment significantly reduced the IL-8 response of AGS cells to stimulation with either lysed or live H pylori SS1 (Figure 2E and F).
Figure 2.
Nicorandil inhibits the inflammatory effect of H pylori on epithelial cells. A) KATP channel subunit transcript expression in human AGS gastric epithelial and murine RAW 264.7 macrophage cell lines. B) Immunofluorescence images showing expression of SUR2 and Kir6.1 proteins in AGS cells. Blue, 4′,6-diamidino-2-phenylindole; red, actin; green, SUR2/Kir6.1 as labeled. C, D) Nicorandil (100 μM) had no effect on (C) IL-6 and (D) MIP-2 secretion by RAW264.7 macrophages, primary mouse intraperitoneal (IP) macrophages or splenocytes (as annotated) stimulated for 24 hours with H pylori SS1 lysate. E–G) Nicorandil treatment (100 μM) significantly reduced the IL-8 response of AGS cells to 24 hours stimulation with (E) H pylori SS1 lysate, (F) live H pylori SS1 and (G) live Cag-positive H pylori 251(MOI = 10). Graphs show group medians (horizontal bar), interquartile range (box), 10th and 90th percentiles (bars). P values were calculated using Mann-Whitney tests (ns, P > .05).
As H pylori SS1 has a dysfunctional cag pathogenicity island (cagPAI),12 we further evaluated H pylori strain 251 which possesses a functional cagPAI and is therefore considered more pathogenic. Nicorandil significantly reduced the IL-8 response of AGS cells to H pylori 251 (Figure 2G), demonstrating that SUR2 activation also exerted an anti-inflammatory response on epithelial cells exposed to cagPAI positive H pylori.
Nicorandil-induced increases in intracellular K+ levels counteract intracellular Ca2+ influx in response to pro-inflammatory stimuli
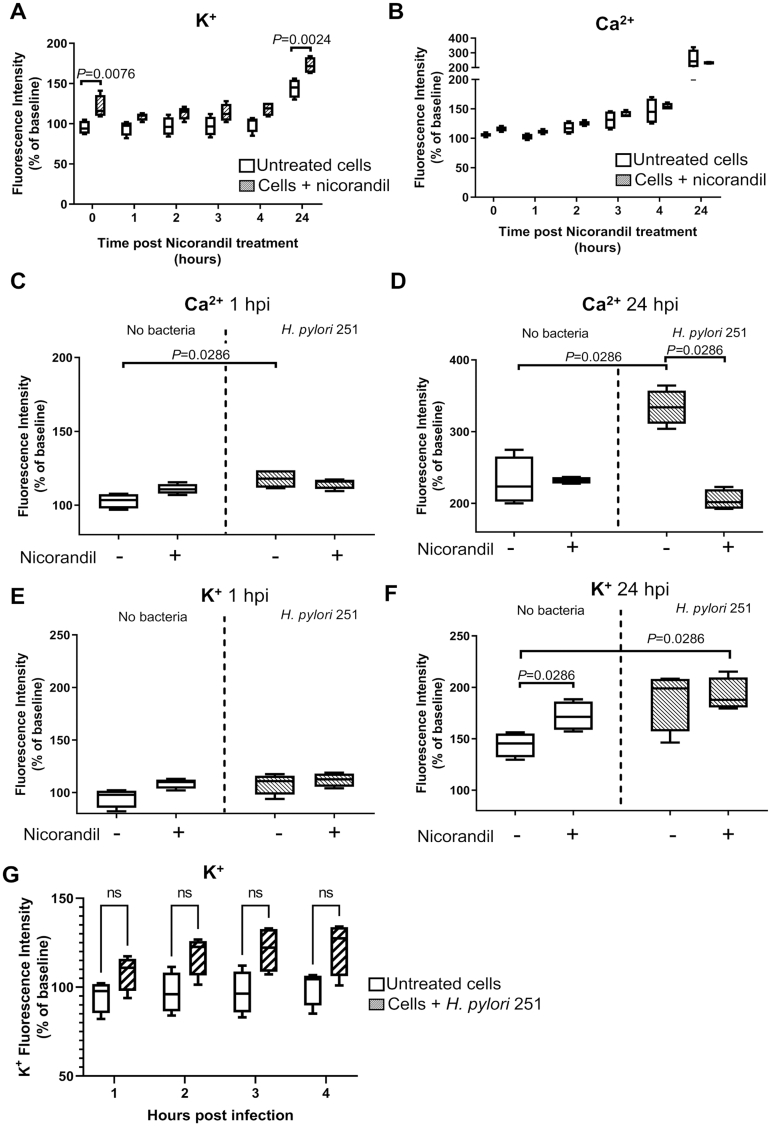
The effect of KATP channel activators including pinacidil and nicorandil on smooth muscle tissues is well studied, revealing these compounds cause muscle relaxation via membrane hyperpolarization and subsequent closure of voltage-gated calcium channels.18,19 The effect of these activators on other cell types is less well-known. Nicorandil treatment reduced IL-8 secretion by H pylori-stimulated AGS cells (Figure 2E–G), which has been shown to be regulated by Ca2+ signaling.20,21 We therefore hypothesized that the inhibitory effects of nicorandil treatment on cytokine secretion could be due to SUR2-mediated regulatory effects on intracellular Ca2+. Nicorandil treatment of unstimulated AGS cells preloaded with fluorogenic Ca2+- or K+-sensitive dyes produced an immediate increase in intracellular K+, which was sustained for at least 24 hours (Figure 3A), and a modest early increase in intracellular Ca2+ which gradually normalized relative to untreated cells (Figure 3B). Intracellular Ca2+ levels were, as expected, increased by H pylori stimulation from one to 24 hours postinfection (Figure 3C and D). This increase was completely prevented by nicorandil treatment thus demonstrating the direct effect of this channel modulating drug on intracellular Ca2+.
Figure 3.
Nicorandil treatment prevents H pylori-induced Ca2+ influx in AGS cells. Intracellular potassium K+ (A) and Ca2+ (B) in untreated (clear box) or nicorandil-treated (100 μM; shaded box) AGS cells, expressed as a percentage of initial baseline reads from each well. Intracellular K+ spiked immediately after nicorandil addition and stayed elevated at 24 hours post-treatment. Ca2+ increased slightly but this effect was not significant. C) Intracellular Ca2+ levels increased upon H pylori 251 stimulation (MOI = 10) at 1 hour post infection (hpi) and D) 24 hpi. Nicorandil treatment significantly reduced H pylori induced Ca2+ influx, comparable to uninfected cells. Intracellular K+ at 1 hpi (E) and 24 hpi (F). P values were calculated by Mann-Whitney tests. G) Intracellular K+ in uninfected and H pylori-stimulated cells (without nicorandil treatment). All differences among infected cells and uninfected controls were nonsignificant (ns, P > .05) as calculated with two-way ANOVA followed by Sidak’s multiple comparisons test.
Intracellular K+ levels spiked significantly upon nicorandil treatment in uninfected cells at 24 hours postdrug treatment (Figure 3F) but were comparable between untreated, infected cells and uninfected controls (Figure 3G) thus showing that K+ level within the cell was not greatly affected by H pylori stimulation. Given the previously demonstrated role of Ca2+-signaling on regulating cytokine secretion by gastric epithelial cells,21 the nicorandil-mediated inhibition of pro-inflammatory IL-8 secretion by H pylori-stimulated AGS cells is therefore potentially explained by the suppression of Ca2+-dependent pro-inflammatory signaling (Figure 3F).
Treatment with SUR2 channel agonists reduce gastritis severity in Helicobacter-infected mice
To examine the effect of modulating SUR2 function on Helicobacter-induced gastritis, groups of mice were infected with H felis for 4 weeks, then treated with pinacidil or nicorandil for an additional 8 weeks, before assessment of gastritis severity at 12 weeks postinfection (Figure 4A). These drugs were selected to provide an analysis of the effect of differential agonism of SUR2 channels: pinacidil activates both SUR2A/B channels while nicorandil has a greater specificity for SUR2B.21
Figure 4.
SUR2-KATP channel agonists do not impact H felis CS1 colonization but counteract infection-related SUR2 downregulation in a mouse gastritis model. A) Mice were infected with H felis CS1 for 4 weeks, then treated for another 8 weeks with either pinacidil or nicorandil (via drinking water). Age-matched uninfected controls (with or without drug treatment) were analyzed in parallel. Drug treatment had no effect on (B) mouse body weights, or (C) daily water intake as measured by ‘area under curve’ analysis. D) Gastric colonization with H felis, measured by qPCR, was not affected by drug treatment. E) Sur2B expression in stomach halves, measured by qPCR. F) SUR2 expression in the stomach mucosa as detected by immunofluorescence staining in a 5μm cross-section through the corpus epithelium; SUR2 (green), cytokeratin 19 (CK19, an epithelial cell marker; red) and cell nuclei (4′,6-diamidino-2-phenylindole, blue). G) SUR2 expression was significantly reduced upon H felis infection (3 mpi) and restored upon pinacidil and nicorandil treatment. Infection groups as labeled. Scale bars, 100μm. qPCR data analyzed by Mann-Whitney tests: ns, not-significant P > .05; other P values as annotated.
Long-term administration of both pinacidil and nicorandil was well tolerated with no impact on water intake or body weights, either with or without H felis infection (Figure 4B and C) and no effect on H felis colonization (Figure 4D). Gene expression studies on stomach halves showed that SUR2B expression was downregulated upon infection (Figure 4E). Nicorandil, but not pinacidil, treatment was successful in increasing SUR2B expression in infected stomach tissues. Immunohistochemical staining showed gastric SUR2 expression to be predominantly located in the crypts of the corpus epithelium (Figure 4F). The expected reduction in SUR2 tissue expression resulting from H felis infection was ameliorated by treatment with either pinacidil or nicorandil (Figure 4G).
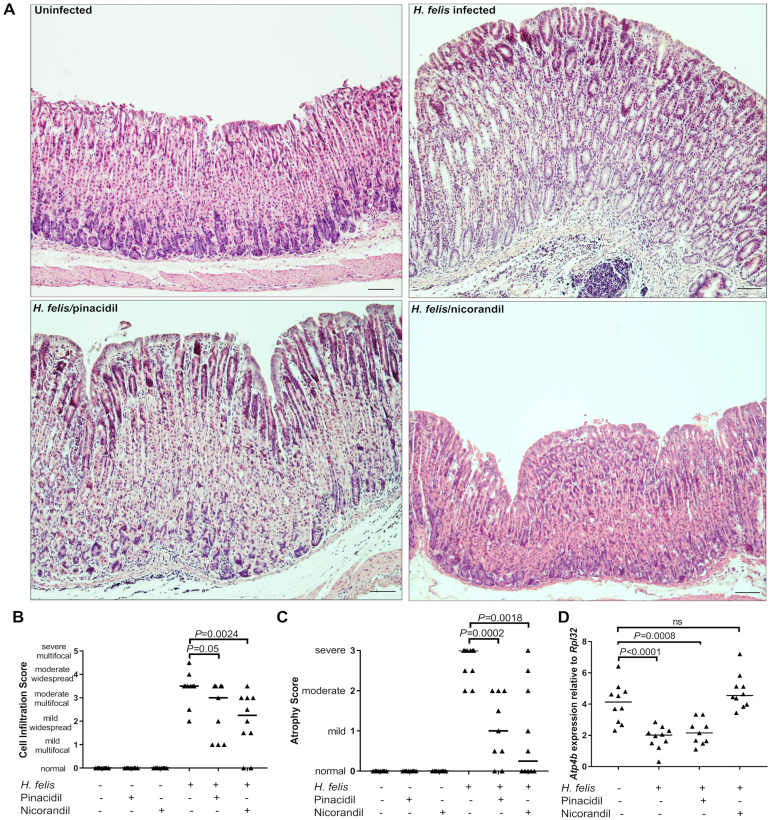
To further characterize the anti-inflammatory effects of pinacidil and nicorandil in H felis-infected mouse stomach tissues, the expression of key pro-inflammatory cytokines was analyzed. While gastric expression of Il-6, Mip-2, Il-17A, and Ifnγ genes were all significantly increased upon H felis infection, both pinacidil and nicorandil treatments reduced expression of all 4 cytokines in infected mice, in some cases to baseline levels (Figure 5A–D). This appeared to be predominantly a localized effect, as cytokine levels in splenic tissues from the same mice showed drug-induced reduction of only IFN-γ (Figure 5E–H). Histological analysis (Figure 6A) showed that while infected control mice typically presented with severe atrophic gastritis characterized by moderate immune cell infiltration and severe atrophy with associated parietal cell loss, groups treated with either pinacidil or nicorandil had significantly reduced levels of atrophy and immune cell infiltrates (Figure 6B and C). There was a trend toward greater effectiveness with nicorandil treatment, which reduced the atrophy score to zero (similar to normal uninfected controls) in 50% (5/10) of infected mice (Figure 6C). This was supported by qPCR analysis of the parietal cell marker Atp4b, which encodes the β subunit of H+/K+ ATPase; parietal cell loss (marked by a reduction in Atp4b levels) in infected mice was restored to baseline by nicorandil, but not pinacidil treatment (Figure 6D).
Figure 5.
Anti-inflammatory effects of pinacidil and nicorandil are associated with a reduced local gastric cytokine response. (A–D) mRNA levels of cytokines in stomach halves from H felis CS1 infected, drug-treated mice (as described in Figure 4A) were quantified by qPCR. (E–H) Cytokines in splenic homogenates were quantified by enzyme-linked immunosorbent assay. Graphs show each mouse as an individual marker and group medians are depicted by horizontal lines. P values were calculated using Mann-Whitney tests.
Figure 6.
Histopathological analysis of mouse stomachs. A) Representative gastric histopathology (H&E-stained sections) from H felis CS1 infected mice and uninfected control at 12 weeks postinfection (as annotated). B–D) Analysis of mouse gastric sections at 12 weeks postinfection showed that both pinacidil and nicorandil significantly reduced immune cell infiltration (B) and atrophy (C) compared to untreated/infected controls. D) Loss of Atp4b parietal cell marker expression in H felis infected gastric tissues was restored by nicorandil treatment. Data points on each graph show individual mice with group medians depicted by horizontal lines. P values were calculated using Mann-Whitney tests.
Discussion
This study identifies SUR2 as a new host factor of importance in H pylori pathogenesis that can be pharmacologically targeted to protect against the pathological consequences of this infection. Most diseases associated with chronic H pylori infection, including gastric cancer and peptic ulcer disease, arise from a prolonged and exaggerated gastritis. It is now well recognized that this continuous H pylori-induced inflammatory assault on the gastric tissue is the key driver for metaplastic changes and the development of gastric cancer. Factors that modulate the severity of H pylori-induced gastritis therefore influence the susceptibility or resistance of an individual regarding progression to malignancy. Despite their considerable potential to modify inflammation, potassium channels have been poorly studied in the context of H pylori infection and pathogenesis. There are more than 75 different potassium channels, some of which have been described as gastric oncogenic factors and may serve as potential prognostic biomarkers.6,7 However, their functions in the stomach have not been well investigated apart from a role for a few potassium channels in assisting acid secretion.23,24 This study of SUR2 provides the first direct evidence that a potassium channel might play an important role in Helicobacter pathogenesis.
The important functionality of SUR2 was demonstrated by the ability of 2 KATP channel modulators, pinacidil and nicorandil, to reduce the severity of gastritis caused by Helicobacter infection in mice. Notably, half of the infected nicorandil-treated mice presented with zero gastric atrophy, in comparison with infected controls, all of which had moderate to severe atrophic gastritis. This was associated with a reduction in pro-inflammatory cytokines in the stomach, consistent with a marked reduction in gastritis. To our knowledge, this is the first report of a drug treatment targeting a host factor and producing a therapeutic reduction of Helicobacter-induced gastritis, without impacting bacterial colonization levels thereby demonstrating a direct effect on inflammation. Given the different selectiveness of these 2 drugs, the observation that nicorandil had better efficacy than pinacidil indicates these drug-mediated reductions in Helicobacter-induced gastritis involved the targeting of SUR2B rather than SUR2A.
The distribution of SUR2B expression suggested these drug-mediated effects would likely act via immune and/or epithelial cells. Studying murine macrophages and splenocytes revealed no obvious effect of nicorandil treatment on H pylori-induced secretion of pro-inflammatory cytokines by immune cells. In contrast, nicorandil treatment of human gastric epithelial cells significantly reduced H pylori-induced production of IL-8, an important chemokine in H pylori pathogenesis involved in the recruitment of inflammatory immune cells. In fact, IL-8 has been shown to be the most upregulated gene in genome-wide profiling of gastric epithelial cells exposed to H pylori.16 Hence, these data support the anti-gastritis effects of nicorandil being due to its action on gastric epithelial cells. Importantly, this anti-inflammatory effect was also induced in response to the more pathogenic cagPAI positive strain of H pylori.
A key part of the H pylori-epithelial cell interaction involves the activation of host cell pattern recognition receptors, leading to PI3K activation,25 then an increase in intracellular Ca2+21 which promotes activation of a mitogen-activated protein kinase/nuclear factor kappa B driven pro-inflammatory responses.26 Here, analyses showed that opening the SUR2 potassium channel leads to K+ influx in gastric epithelial cells, which prevents the rise in intracellular Ca2+ triggered by H pylori. Previous studies in pancreatic islet cells and cardiomyocytes have also shown that opening KATP channels can attenuate Ca2+ accumulation by preventing membrane depolarization and the closing of voltage-gated calcium channels.18,19,27 Data presented in this study therefore suggest that pinacidil and nicorandil suppressed gastritis in infected mice via KATP channel mediated attenuation of Helicobacter-induced Ca2+ signaling in gastric epithelial cells.
The identification of new drug treatments for H pylori infection to prevent associated diseases has been identified by a World Health Organization expert panel as highly important due to the level of antimicrobial resistance of H pylori to current treatment regimens.5 This study raises the possibility of a different approach than treating the infection, instead by pharmacologically targeting SUR2 to treat the disease-causing gastritis. Such an approach could potentially confer an advantage over conventional eradication therapies, as modifying a host factor to protect against gastritis will not apply selective pressure to the bacteria, and so is unlikely to generate the drug resistance that occurs with antibiotics. Moreover, there is evidence that H pylori infection might provide some protection against other disease states including asthma and inflammatory bowel disease.28,29 Hence, targeting SUR2 to control gastritis might retain any beneficial effects associated with H pylori colonization.
In this study, pinacidil and nicorandil were used as model drugs to demonstrate the effect of modifying SUR2 activation. However, while these drugs have been used clinically, their effects on the cardiovascular system and pancreatic cells could be contraindicative for the treatment of H pylori gastritis in some individuals. Pinacidil can cause fluid retention,30 and nicorandil has been associated with an increased risk for gastrointestinal ulceration, although this has not been measured in the context of H pylori infection31 and has been shown to be protective in chemically induced gastric ulcer animal models.32 Hence translating these findings to a treatment for H pylori-induced gastritis might require the development of a more specific pharmacotherapy approach for targeting gastric SUR2B.
Conclusion
In conclusion, we have identified a novel host factor, SUR2, which is of significance in H pylori infection. This is the first potassium channel identified to play an important role in H pylori pathogenesis, influencing the severity of Helicobacter-induced gastritis. Importantly, SUR2 is shown to be a druggable target, allowing for the treatment of gastritis without eradicating Helicobacter infection. By using 2 different model drugs, we have shown that SUR2B is the primary gastric SUR2 subunit that can be targeted to modify gastritis, and the anti-inflammatory effects of the SUR2-channel agonists are most likely mediated via gastric epithelial cells where drug-induced K+ influx prevents the rise in intracellular Ca2+ that can otherwise trigger downstream pro-inflammatory signaling. Overall, targeting SUR2 offers a promising avenue for treating H pylori-induced gastritis and peptic ulcer disease as well as preventing the development of gastric cancer, while avoiding issues relating to antimicrobial resistance.
Acknowledgments
Authors' Contributions:
Sohinee Sarkar performed the experiments, analyzed the data, compiled the figures, and wrote the manuscript. Ghazal Alipour Talesh performed immune analysis experiments. Trevelyan R. Menheniott acquired human patient tissues and obtained funding. Philip Sutton conceived the study, performed data analysis, participated in manuscript writing, obtained funding, and supervised the research.
Footnotes
Conflicts of Interest: The authors disclose no conflicts.
Funding: This study was supported by Project Grant GNT1123357 from the National Health and Medical Research Council of Australia, and the Victorian Government's Operational Infrastructure Support Program. PS was supported by a fellowship from the DHB Foundation.
Ethical Statement: The corresponding author, on behalf of all authors, jointly and severally, certifies that their institution has approved the protocol for any investigation involving humans or animals and that all experimentation was conducted in conformity with ethical and humane principles of research.
Data Transparency Statement: All data generated or analyzed during this study are included in this published article (or available from the corresponding author upon reasonable request).
Reporting Guidelines: ARRIVE/Care and Use of Laboratory Animals.
References
- 1.Correa P., Piazuelo M.B. The gastric precancerous cascade. J Dig Dis. 2012;13(1):2–9. doi: 10.1111/j.1751-2980.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Tan P., Yeoh K.G. Genetics and molecular pathogenesis of gastric adenocarcinoma. Gastroenterology. 2015;149(5):1153–1162.e3. doi: 10.1053/j.gastro.2015.05.059. [DOI] [PubMed] [Google Scholar]
- 4.Malfertheiner P., Megraud F., Rokkas T., et al. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022; 71:1724-1762 [Google Scholar]
- 5.Savoldi A., Carrara E., Graham D.Y., et al. Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta-analysis in World Health Organization regions. Gastroenterology. 2018;155(5):1372–1382.e17. doi: 10.1053/j.gastro.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ji C.D., Wang Y.X., Xiang D.F., et al. Kir2.1 interaction with Stk38 promotes invasion and metastasis of human gastric cancer by enhancing MEKK2-MEK1/2-ERK1/2 signaling. Cancer Res. 2018;78(11):3041–3053. doi: 10.1158/0008-5472.CAN-17-3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X., Cai H., Zheng W., et al. An individualized prognostic signature for gastric cancer patients treated with 5-Fluorouracil-based chemotherapy and distinct multi-omics characteristics of prognostic groups. Oncotarget. 2016;7(8):8743–8755. doi: 10.18632/oncotarget.7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aguilar-Bryan L., Clement J.P., 4th, Gonzalez G., et al. Toward understanding the assembly and structure of KATP channels. Physiol Rev. 1998;78(1):227–245. doi: 10.1152/physrev.1998.78.1.227. [DOI] [PubMed] [Google Scholar]
- 9.Tian C., Zhu R., Zhu L., et al. Potassium channels: structures, diseases, and modulators. Chem Biol Drug Des. 2014;83(1):1–26. doi: 10.1111/cbdd.12237. [DOI] [PubMed] [Google Scholar]
- 10.Jackson C.B., Judd L.M., Menheniott T.R., et al. Augmented gp130-mediated cytokine signalling accompanies human gastric cancer progression. J Pathol. 2007;213(2):140–151. doi: 10.1002/path.2218. [DOI] [PubMed] [Google Scholar]
- 11.Lee A., O'Rourke J., De Ungria M.C., et al. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology. 1997;112(4):1386–1397. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 12.Philpott D.J., Belaid D., Troubadour P., et al. Reduced activation of inflammatory responses in host cells by mouse-adapted Helicobacter pylory isolates. Cell Microbiol. 2002;4(5):285–296. doi: 10.1046/j.1462-5822.2002.00189.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee A., Hazell S.L., O'Rourke J., et al. Isolation of a spiral-shaped bacterium from the cat stomach. Infect Immun. 1988;56(11):2843–2850. doi: 10.1128/iai.56.11.2843-2850.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGuckin M.A., Every A.L., Skene C.D., et al. Muc1 mucin limits both Helicobacter pylori colonization of the murine gastric mucosa and associated gastritis. Gastroenterology. 2007;133(4):1210–1218. doi: 10.1053/j.gastro.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Sutton P., Danon S.J., Walker M., et al. Post-immunisation gastritis and Helicobacter infection in the mouse: a long term study. Gut. 2001;49(4):467–473. doi: 10.1136/gut.49.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barranco S.C., Townsend C.M., Jr., Casartelli C., et al. Establishment and characterization of an in vitro model system for human adenocarcinoma of the stomach. Cancer Res. 1983;43(4):1703–1709. [PubMed] [Google Scholar]
- 17.Eftang L.L., Esbensen Y., Tannæs T.M., et al. Interleukin-8 is the single most up-regulated gene in whole genome profiling of H. pylori exposed gastric epithelial cells. BMC Microbiol. 2012;12:9. doi: 10.1186/1471-2180-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kukovetz W.R., Holzmann S., Pöch G. Molecular mechanism of action of nicorandil. J Cardiovasc Pharmacol. 1992;20(Suppl 3):S1–S7. doi: 10.1097/00005344-199206203-00002. [DOI] [PubMed] [Google Scholar]
- 19.Jovanovic S., Jovanovic A. Pinacidil prevents membrane depolarisation and intracellular Ca2+ loading in single cardiomyocytes exposed to severe metabolic stress. Int J Mol Med. 2001;7(6):639–643. doi: 10.3892/ijmm.7.6.639. [DOI] [PubMed] [Google Scholar]
- 20.Nozawa Y., Nishihara K., Peek R.M., et al. Identification of a signaling cascade for interleukin-8 production by Helicobacter pylori in human gastric epithelial cells. Biochem Pharmacol. 2002;64(1):21–30. doi: 10.1016/s0006-2952(02)01030-4. [DOI] [PubMed] [Google Scholar]
- 21.Marlink K.L., Bacon K.D., Sheppard B.C., et al. Effects of Helicobacter pylori on intracellular Ca2+ signaling in normal human gastric mucous epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2003;285(1):G163–G176. doi: 10.1152/ajpgi.00257.2002. [DOI] [PubMed] [Google Scholar]
- 22.Shindo T., Yamada M., Isomoto S., et al. SUR2 subtype (A and B)-dependent differential activation of the cloned ATP-sensitive K+ channels by pinacidil and nicorandil. Br J Pharmacol. 1998;124(5):985–991. doi: 10.1038/sj.bjp.0701927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan J., Liu W., Karvar S., et al. Potassium channel KCNJ15 is required for histamine-stimulated gastric acid secretion. Am J Physiol Cell Physiol. 2015;309(4):C264–C270. doi: 10.1152/ajpcell.00012.2015. [DOI] [PubMed] [Google Scholar]
- 24.Lambrecht N.W., Yakubov I., Scott D., et al. Identification of the K efflux channel coupled to the gastric H-K-ATPase during acid secretion. Physiol Genomics. 2005;21(1):81–91. doi: 10.1152/physiolgenomics.00212.2004. [DOI] [PubMed] [Google Scholar]
- 25.Nagy T.A., Frey M.R., Yan F., et al. Helicobacter pylori regulates cellular migration and apoptosis by activation of phosphatidylinositol 3-kinase signaling. J Infect Dis. 2009;199(5):641–651. doi: 10.1086/596660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brandt S., Kwok T., Hartig R., et al. NF-kappaB activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc Natl Acad Sci U S A. 2005;102(26):9300–9305. doi: 10.1073/pnas.0409873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raphemot R., Swale D.R., Dadi P.K., et al. Direct activation of β-cell KATP channels with a novel xanthine derivative. Mol Pharmacol. 2014;85(6):858–865. doi: 10.1124/mol.114.091884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castaño-Rodríguez N., Kaakoush N.O., Lee W.S., et al. Dual role of Helicobacter and Campylobacter species in IBD: a systematic review and meta-analysis. Gut. 2017;66(2):235–249. doi: 10.1136/gutjnl-2015-310545. [DOI] [PubMed] [Google Scholar]
- 29.Blaser M.J., Chen Y., Reibman J. Does Helicobacter pylori protect against asthma and allergy? Gut. 2008;57(5):561–567. doi: 10.1136/gut.2007.133462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlsen J.E., Kardel T., Jensen H.A., et al. Trap-Jensen J. Pinacidil, a new vasodilator: pharmacokinetics and pharmacodynamics of a new retarded release tablet in essential hypertension. Eur J Clin Pharmacol. 1983;25(4):557–561. doi: 10.1007/BF00542128. [DOI] [PubMed] [Google Scholar]
- 31.Lee C.C., Chang S.S., Lee S.H., et al. Use of nicorandil is associated with increased risk for gastrointestinal ulceration and perforation- A nationally representative population-based study. Sci Rep. 2015;5:11495. doi: 10.1038/srep11495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva R.O., dos Santos G.M., Nicolau L.A., et al. Sulfated-polysaccharide fraction from red algae Gracilaria caudata protects mice gut against ethanol-induced damage. Mar Drugs. 2011;9(11):2188–2200. doi: 10.3390/md9112188. [DOI] [PMC free article] [PubMed] [Google Scholar]