Introduction
Pulse oximetry is a mainstay technology used to manage children with cyanotic congenital heart disease (CCHD). Using light absorption, the percentage of oxyhemoglobin (SpO2) is calculated as a noninvasive estimate of arterial oxygen saturation (SaO2). While managing children with CCHD, providers must ensure that the patient is within the acceptable range of hypoxemia because unexpectedly low SaO2 indicates the need for intervention. In children with structurally normal hearts, the SpO2 accuracy declines at lower SaO2. The fact that pulse oximetry overestimates SaO2 during hypoxia in patients with darker skin pigmentation has also been documented.1 However, the degree of errors due to race and/or single-ventricle physiology in patients with CCHD has not been fully elucidated.2, 3, 4, 5 In this research letter, we used time-matched SpO2-SaO2 pairs collected during cardiac catheterization to investigate SpO2 error as a function of SaO2, self-reported race, and single-ventricle palliation.
Methods
We conducted a single-center, retrospective review of children with single-ventricle palliation undergoing cardiac catheterization. SpO2 was measured using the IntelliVue X3 system (Philips), and SaO2 was measured from arterial samples using the GEM Premier 4000 system (Werfen). Time-matched data pairs (recorded at a maximum of 60-second intervals) were extracted from the procedure log and race identifiers from the Society of Thoracic Surgeons and IMproving Pediatric and Adult Congenital Treatments databases. Self-identified African Americans (AAs) were compared with those who did not identify as AA. Patients were dichotomized to those with (Blalock-Thomas-Taussig shunt], patent ductus arteriosus stent, central shunt, and hybrid) and without diastolic runoff lesions.
Linear mixed-effect modeling was used to describe the relationship among the AA race, SaO2, and SpO2. The model included a random effect for the subjects and an interaction term to determine whether the AA race affects the relationship between SaO2 and SpO2. In the models, the SaO2 was converted to a percentage <100% such that the intercept represented the expected SpO2 when the SaO2 was 100%. This study was approved by the Office of Research Integrity and Compliance.
Results
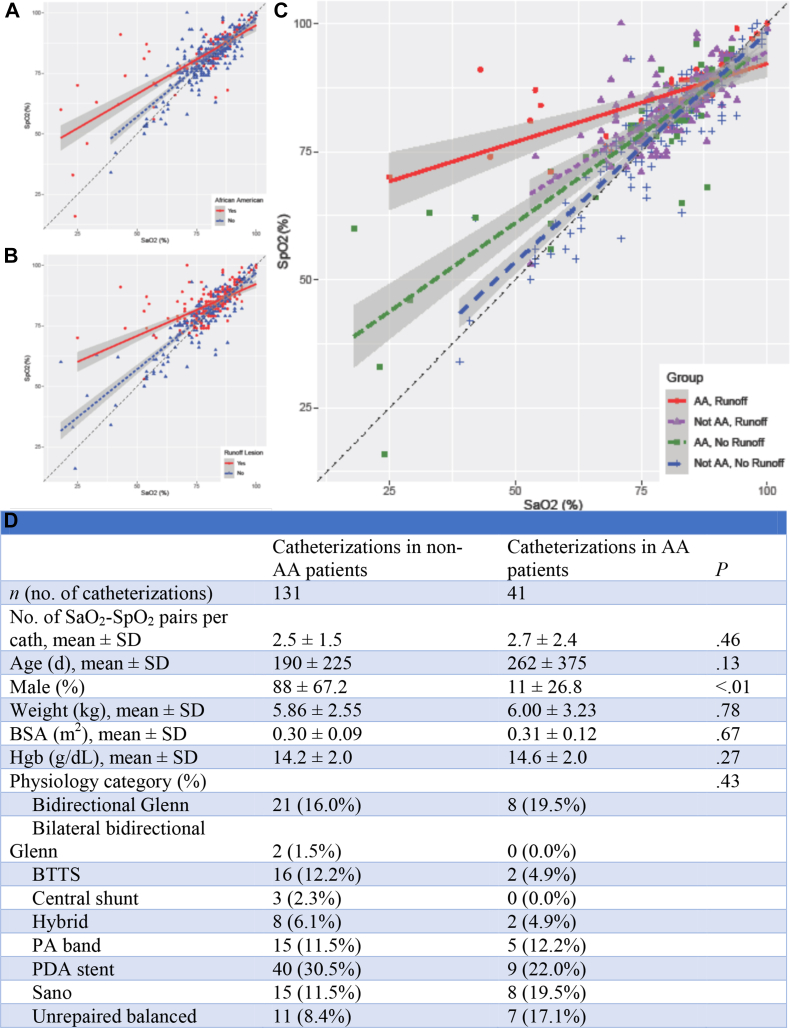
Four hundred thirty-nine SpO2-SaO2 pairs were extracted from 172 catheterizations performed on 130 patients (Figure 1D). Thirty patients (23%) representing 112 SpO2-SaO2 pairs (26%) were AAs. The median difference between SpO2 and SaO2 was +1% (IQR, −1% to +4%). The difference between SpO2 and SaO2 increased at lower SaO2 (B = −0.38; P < .001). A significant interaction existed between the AA race and SaO2 in the model of SpO2 (P < .001), indicating that the relationship between SaO2 and SpO2 varied with the presence of the AA race. A significant interaction with SaO2 also existed for age, sex, weight, body surface area, cardiac index, arterial blood pressure, pulse pressure, PCO2, bicarbonate, the stage of palliation, and the presence of diastolic runoff, meaning that each of these factors affects the accuracy of SpO2. The interaction between the AA race and SaO2 in determining SpO2 persisted even after controlling for these variables (P < .001). In the final model, which compared AA with non-AA subjects, the intercept was lower (91% vs 96%, respectively; P < .001) and the slope was less negative (−0.46 vs −0.75, respectively; P < .001), indicating that in AA patients, SpO2 increasingly overestimated SaO2 at lower SaO2 (Figure 1A). For example, at an SaO2 of 80%, the expected SpO2 was 82% for AA patients vs 81% for non-AA patients, whereas at an SaO2 of 60%, the expected SpO2 was 73% for AA patients vs 66% for non-AA patients. Diastolic runoff was also correlated with SpO2 values, which overestimated SaO2 (Figure 1B), with the AA race and diastolic runoff representing additive risk factors (Figure 1C).
Figure. 1.
(A) Percentage of oxyhemoglobin (SpO2) as a function of arterial oxygen saturation (SaO2) and race. (B) SpO2 as a function of SaO2 and diastolic runoff lesions. (C) SpO2 as a function of SaO2, race, and diastolic runoff lesions. (D) Patient demographics. AA, African American; BSA, body surface area; BTTS, Blalock-Thomas-Taussig shunt; cath, catheterization; Hgb, hemoglobin; PA, pulmonary artery; PDA, patent ductus arteriosus; SaO2, arterial oxygen saturation; SpO2, percentage of oxyhemoglobin.
Discussion
Pulse oximetry significantly underestimates critical hypoxemia in self-identified AA patients and in the presence of runoff lesions, with these errors being additive. Falsely elevated SpO2 readings may cause clinicians to underdiagnose critical hypoxemia in AA patients with single-ventricle palliation and diastolic runoff, a population known to be at a high risk of mortality in the interstage period.
Without access to the manufacturer’s proprietary algorithm for SpO2, we can only speculate the reason for increased errors in the setting of runoff lesions. In principle, all pulse oximeters measure the ratio of pulsatile-to-nonpulsatile absorbance. These data were collected from healthy volunteers, and an empiric algorithm was generated. We posit that in the setting of runoff lesions, the pulsatile component is significantly increased, thereby rendering the empiric formula less accurate. A next-generation device capable of correcting for the presence of widened pulse pressures could be invaluable.
The primary limitation of this study is that the correlation between skin tone and SpO2 accuracy was not directly investigated. Rather, self-identified race was used as a proxy for skin tone. A single brand of pulse oximeter at a single institution was studied, and additional data are required to validate these results more broadly. Finally, potential factors, such as methemoglobinemia or carboxyhemoglobin, were not directly measured in this study but are known to affect the accuracy of pulse oximetry.
Conclusion
SpO2 accuracy is negatively impacted by worsening hypoxemia, the AA race, and the presence of diastolic runoff, a novel finding of this study. The wide pulse pressure associated with runoff lesions was outside of the standard physiology based on which pulse oximetry was designed and validated and, thus, warrants further study. Ultimately, these data demonstrate that pulse oximetry can be falsely reassuring in our highest-risk patients with CCHD and present concerns that warrant further attention from device manufacturers and clinicians alike.
Acknowledgments
Declaration of competing interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics statement
This study was approved by the Office of Research Integrity and Compliance (IRB 2021-4435).
References
- 1.Bickler P.E., Feiner J.R., Severinghaus J.W. Effects of skin pigmentation on pulse oximeter accuracy at low saturation. Anesthesiology. 2005;102(4):715–719. doi: 10.1097/00000542-200504000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Harris B.U., Stewart S., Verma A., et al. Accuracy of a portable pulse oximeter in monitoring hypoxemic infants with cyanotic heart disease. Cardiol Young. 2019;29(8):1025–1029. doi: 10.1017/s1047951119001355. [DOI] [PubMed] [Google Scholar]
- 3.Kim E.H., Lee J.H., Song I.K., et al. Accuracy of pulse oximeters at low oxygen saturations in children with congenital cyanotic heart disease: an observational study. Paediatr Anaesth. 2019;29(6):597–603. doi: 10.1111/pan.13642. [DOI] [PubMed] [Google Scholar]
- 4.Ross P.A., Newth C.J., Khemani R.G. Accuracy of pulse oximetry in children. Pediatrics. 2014;133(1):22–29. doi: 10.1542/peds.2013-1760. [DOI] [PubMed] [Google Scholar]
- 5.Sjoding M.W., Dickson R.P., Iwashyna T.J., Gay S.E., Valley T.S. Racial bias in pulse oximetry measurement. N Engl J Med. 2020;383(25):2477–2478. doi: 10.1056/NEJMc2029240. [DOI] [PMC free article] [PubMed] [Google Scholar]