Abstract

The increasing occurrence of infectious diseases caused by antimicrobial resistance organisms urged the necessity to develop more potent, selective, and safe antimicrobial agents. The unique magnetic and tunable properties of iron oxide nanoparticles (IONPs) make them a promising candidate for different theragnostic applications, including antimicrobial agents. Though IONPs act as a nonspecific antimicrobial agent, their antimicrobial activities are directly or indirectly linked with their synthesis methods, synthesizing precursors, size, shapes, concentration, and surface modifications. Alteration of these parameters could accelerate or decelerate the production of reactive oxygen species (ROS). An increase in ROS role production disrupts bacterial cell walls, cell membranes, alters major biomolecules (e.g., lipids, proteins, nucleic acids), and affects metabolic processes (e.g., Krebs cycle, fatty acid synthesis, ATP synthesis, glycolysis, and mitophagy). In this review, we will investigate the antibacterial activity of bare and surface-modified IONPs and the influence of physiochemical parameters on their antibacterial activity. Additionally, we will report the potential mechanism of IONPs’ action in driving this antimicrobial activity.
1. Introduction
Antimicrobial resistance (AMR) is a natural phenomenon in which pathogenic microorganisms develop mechanisms to resist antimicrobial drugs.1 Due to the severity of AMR, it has become a worldwide concern owing to its correlation with escalated morbidity and mortality. According to the World Health Organization (WHO), AMR has been identified as a global threat to public health, and this era has been reported as the “postantibiotic” era, in which minor infections will be the primary cause of death.2 According to a study published in 2019, approximately 7.7 million fatalities were ascribed to bacterial infections caused explicitly by antibiotic-resistant bacterial strains.3 Similarly, a report released in 2022 highlighted that AMR was the primary cause of 1.27 million fatalities and had an indirect role in 4.95 million deaths.4 Moreover, bacteria develop AMR mechanisms through unsupervised exposure and the wide availability of antibiotics.5,6 Considering the limited therapeutic options and the severity of AMR, researchers are developing magnetic nanoparticles (MNPs)-based alternative antibacterial agents.7−9
Recently, MNPs have received attention in various fields due to their unique magnetic properties, including paramagnetism, tunable magnetism, biocompatibility, drug delivery, and antimicrobial and surface modification properties.10−16 Like other MNPs, iron oxide nanoparticles (IONPs) are used as magnetic resonance imaging (MRI) contrast dye, biosensors, diagnosing diseases, drug delivery vehicles, pollutant removal, biomedical machinery, and antimicrobial agents.17−22 Though divalent metals like iron ions are essential for the growth of microbes, IONPs display nonspecific antibacterial activity by generating electrostatic attraction or repulsion force and reactive oxygen species (ROS).23−26 Moreover, other parameters could influence the antimicrobial activity of IONPs, including synthesis methods, precursors, size, and concentration.27 Furthermore, depending on the characteristics of the materials, the physicochemical, electrical, optical, and biological properties of IONPs can be influenced via surface modification. Notably, the coated nanocomplexes exhibit more potent antimicrobial activity than bare IONPs.28
Gudkov et al.29 reported that the antibacterial activity of IONPs could be influenced by altering their synthesis methods and size and discussed several bacteriostatic mechanisms including ROS generation, electrostatic interaction, disruption of the cell membrane, and fragmentation of DNA and protein molecules by inducing free radicals. Moreover, Arias et al.30 described numerous clinical applications of IONPs (e.g., antimicrobial, anticancer, drug delivery, anticonvulsants, and immunosuppressive agents), toxic effects, and the extent of biocompatibility between IONPs and eukaryotic cells or tissues. Another review addressed the biocompatibility and cytotoxicity of IONPs and how their antibacterial activity was evaluated on a various model organisms using multiple physical and chemical criteria.31 Alprol et al.32 highlighted that IONPs synthesized through the green methods are able to exhibit potent antibacterial activity against Gram-positive and moderate inhibition against Gram-negative bacteria by inducing oxidative stress by generating ROS. Furthermore, these authors summarized a few influential parameters associated with the antibacterial efficacy of the IONPs and highlighted their mechanisms against various types of bacteria. Recently, Tasnim et al.33 discussed the antimicrobial mechanisms and activity of several metal-doped IONPs against a range of bacteria and the potential drawbacks of metal-doped IONPs. Nevertheless, the aforementioned reviews were unable to report the influence of diverse coating materials on the physiochemical properties of IONPs, a comparative analysis of the antibacterial activities of the uncoated and surface-coated IONPs-complexes, their combined mechanistic understanding, and the impact of several parameters like shape, concentration, and precursors on the antibacterial activities of IONPs. Therefore, enlightening the influential parameters of the antibacterial activities of the coated and uncoated IONPs should be weighed up for further research purposes, molecular analysis, and drug development.
In this review, we intend to scrutinize the antimicrobial activities of the uncoated and coated IONPs associated with their physicochemical parameters and their potential mechanisms of action(s). Additionally, we will discuss the antimicrobial efficacy of IONPs concerning their precursors, manufacturing techniques, size, shape, concentration, and surface modification materials.
2. Pathogenic Bacteria
Pathogenic bacteria are microorganisms that induce infectious diseases within the host organism.34 They exhibit a diverse array of virulence determinants encompassing adherence factors, invasion factors, toxins, capsules, and siderophores, thereby eliciting pathogenicity and instigating disease through host cell invasion and evasion of host defense mechanisms.35,36
According to the structural composition of the bacterial cell wall, bacteria can be classified into two distinct categories: Gram-positive and Gram-negative.37 Several studies have shown that a significant proportion of pathogenic bacteria exhibit Gram-negative characteristics, and due to their distinctive cellular structure, they are more susceptible to antibiotic resistance.38 Gram-negative bacteria have a complex cellular structure consisting of a peptidoglycan cell wall surrounded by the lipopolysaccharide (LPS) layer. The LPS comprises several virulence factors, including Lipid-A, also known as endotoxin, which is the main virulence factor that causes disease.39 Gram-negative bacteria release their virulence factor to cause disease during active cellular growth, and after the cell has been lysed, endotoxin is released.40 Upon releasing these toxins, a robust inflammatory response is initiated within the host organism, potentially resulting in severe and life-threatening symptoms.41,42 Multiple toxins can potentially inflict diverse harm on the host cell. For instance, producing Shiga toxin by E. coli and releasing cholera toxin (CT) by V. cholerae can lead to severe watery diarrhea.43 The prevailing ailments resulting from pathogenic bacteria include tuberculosis caused by Mycobacterium tuberculosis,44 stomach infections caused by H. pylori,45 and typhoid fever caused by Salmonella typhi.(46) More importantly, pathogenic bacteria are able to infiltrate the host’s tissue and employ various strategies to exploit the host, potentially resulting in adverse health outcomes and a diverse array of illnesses.47 Pathogen invasion necessitates a designated point of entry originating from a contaminated source, such as consumable items or inhaling noxious substances. Moreover, pathogens can be transmitted through direct and indirect contact with an infected individual or in poorly ventilated environments.48
Pathogenic bacteria pose a significant challenge with the emergence of antibiotic-resistant strains. The overutilization of antibiotics in bacterial populations leads to genetic mutations or the expression of resistance genes, resulting in antibiotic-resistant bacteria development.49 Resistant genes can be transferred to other bacteria through gene transfer, which may occur through many processes, including conjugation, transmission, and transduction. When pathogenic bacteria develop antibiotic resistance, they become significantly more challenging to treat and present an increased risk to the host organism.50
3. Iron Oxide Nanoparticles (IONPs)
Ferromagnetic IONPs find extensive application due to their intricate structure. IONPs exhibit either spinel or inverse spinel crystalline structures, which are determined by the specific type of IONPs and their underlying condition of synthesis.51 Iron cations and oxide ions are arranged in tetrahedral and octahedral sites.52,53 However, the positioning of the iron ions is influenced by their oxidation state and structure.54 Fe3+ ions prefer occupying octahedral and tetrahedral sites while maintaining their high spin complex, which arises from their ability to polarize water molecules, leading to the facile cleavage of OH bonds. This phenomenon gives rise to a complex with a reduced charge density and increased stability, accompanied by a smaller ionic radius. In contrast, it was observed that Fe2+ exhibits a preference for occupying exclusively octahedral positions within a complex. Moreover, the preferred attributes of the lower charge density and wider ionic radius of Fe2+ enable them to maintain a high spin configuration.55
IONPs have 14 different types of structures, and 10 of them are found in the Earth’s crust due to the abundant existence of oxygen, hydrogen, and iron. Among them, the predominant rock-forming minerals are hematite, magnetite, and goethite. Maghemite, ferrihydrite, and lepidocrocite have a moderate abundance, whereas wustite, bernalite, and feroxyhyte are the least abundant. Hematite (Fe2O3) and magnetite (Fe3O4) have been extensively studied and applied in different clinical and biomedical domains due to their distinctive attributes such as ferromagnetism, biocompatibility, adjustable magnetic properties, coercivity, high magnetic saturation, magneto-optic performance, anisotropy, magneto-crystalline anisotropy, surface modification properties, and photothermal properties.56−61Figure 1 shows the schematical representation of the most abundant IONPs and oxyhydroxides.
Figure 1.
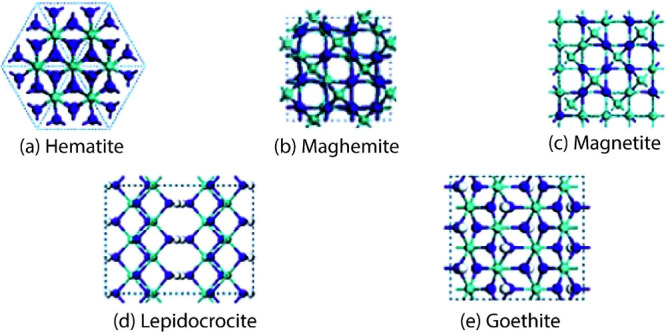
Schematic representation of (a) hematite, (b) maghemite, (c) magnetite, (d) lepidocrocite, and (e) goethite structures. These structures are viewed from the ⟨001⟩ or ⟨0001⟩ directions. The idea of the diagram is taken from Guo and Barnard.82
There are multiple methodologies for synthesizing IONPs, which can broadly be categorized into two fundamental approaches. The top-down approach involves producing NPs from larger particles.62 A different approach involves using the bottom-up method, where NPs are created by utilizing individual molecules and gradually assembling them into the desired nanostructure.63−65 The methodologies employed in this study encompass coprecipitation (COP), sol–gel (SG), hydrothermal (HDT), one-pot (OP), green synthesis (GRS), sonochemical (SNC), laser pyrolysis (LP), solid-state pyrolytic (SSP), polyol synthesis (PYS), and the microemulsion method (MEL).17,66−75 Utilizing these synthesis methods significantly influences various characteristics of the IONPs, including their size, cation distribution, magnetic properties,10 and antimicrobial properties.76 Moreover, the versatile synthesis process can significantly influence the physicochemical properties of IONPs, which have found widespread applications, including contrast dyes for MRI, specific drug delivery agents, gene carriers for gene therapy, potential antimicrobial agents, potential cancer therapy agents, and biosensors.77−79 Besides drug delivery, IONPs could enhance the efficacy of Food and Drug Administration (FDA)-approved drugs.80,81
4. IONPs as an Antimicrobial Agent
IONPs possess unique antibacterial properties due to their distinctive physical and chemical attributes, such as their surface charge and ability to generate ROS.83 The features of IONPs depend on various aspects, such as their synthesis process, precursors, size, shape, and concentration.9,84
IONPs could be synthesized using various techniques, including COP, SG, HDT, GRS, LP, MEL, OP, SNC, and PSY methods.85 Chemically synthesized methods necessitate the use of intense radiation and result in the production of very hazardous residues. The GRS method requires minimal precursors and energy for initiation, with plant extracts as the reducing agent. Interestingly, the plant extracts possess inherent therapeutic characteristics that contribute to their notable antibacterial efficacy.85,86 Moreover, various iron precursors utilized in the synthesis procedures may influence the properties of IONPs (Table 1). Hence, precursors and synthesis methods are considered crucial parameters that influence the antibacterial activity of IONPs.
Table 1. Antimicrobial activity and potential mechanism of chemically synthesized IONPsa.
| NPs | Particle size (nm) | Shape | Precursor | Synthesis method | Coating Material | Con. (μg/mL) | Organism | ZOI (mm) | MIC (μg/mL) | MBC (μg/mL) | IC50 (μg/mL) | Mechanism | ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe3O4 | ∼254 | Spherical | FeCl3·6H2O, FeCl2·4H2O, NaOH, DW & HCl | COP | RHL | NR | Escherichia coli (O157: H7) | ∼9 | 64 | 128 | NR | The RHL coated-Fe3O4@PVA@p-CoA/GA biosurfactant mediated the structural and confirmational changes on the cell surface. Moreover, the coating material intercalated with the DNA, inhibited the replication, transcription and translation process by generating ROS. The ROS induced oxidative stress that led to apoptosis of bacterial cells. | (87) |
| Escherichia coli (O26: H11) | ∼10 | 4 | 8 | ||||||||||
| Escherichia coli (O78: H10) | ∼12 | 32 | 64 | ||||||||||
| Staphylococcus aureus (MSSA) | ∼12 | 16 | 32 | ||||||||||
| Staphylococcus aureus (MRSA) | ∼10 | 32 | 64 | ||||||||||
| Staphylococcus aureus (VRSA) | ∼8 | 64 | 128 | ||||||||||
| RHL, PVA, GA, p-CoA | NR | Escherichia coli (O157: H7) | 35 | 32 | 64 | ||||||||
| Escherichia coli (O26: H11) | 45 | 1 | 2 | ||||||||||
| Escherichia coli (O78: H10) | 40 | 16 | 32 | ||||||||||
| MSSA | 40 | 4 | 8 | ||||||||||
| MRSA | 30 | 16 | 32 | ||||||||||
| VRSA | 25 | 32 | 64 | ||||||||||
| Fe2O3 | 36.1 | Spherical | FeCl3·6H2O, TiCl4·2H2O, CTAB & NaOH | COP | PR | 5000 | Bacillus subtilis | 13 | NR | NR | NR | The Fe2O3 and TiO2 polyester nanocomposite enhanced the interaction with bacterial cell membranes. It created an ionic imbalance in the bacterial cell, resulting in cell apoptosis. | (88) |
| Staphylococcus aureus | 18 | ||||||||||||
| Escherichia coli | 13 | ||||||||||||
| Pseudomonas aeruginosa | 12 | ||||||||||||
| Candida albicans | 29 | ||||||||||||
| Aspergillus niger | 26 | ||||||||||||
| Fe3O4 | 48 | Spherical | FeSO4, FeCl3, DW, NH4OH & HNO3, | COP | RHL | NR | Pseudomonas aeruginosa | NR | 1000 | NR | NR | Fe3O4 induced oxidative stress in the bacterial cell by producing ROS which caused cell death. Again, the Rhamnolipid-coated Fe3O4 interacted with extracellular polymeric substances (EPS), which could affect the hydrophobicity of bacterial cell surface and hinder biofilm generation. | (89) |
| Staphylococcus aureus | NR | 1000 | NR | ||||||||||
| IO QDNPs | <80 | Spherical | Fe (NO3)3.9H2O, argon gas, DW, ethanol & NaOH | COP and HDT. | NR | NR | Escherichia coli PTCC 1330 | NR | 1 | NR | NR | The IO-QDNPs changed membrane potential and damaged the biological mechanism by interacting with the cell membranes and subunit of the ribosome. Again, ROS-mediated structural and confirmational changes in DNA were responsible for bacterial cell death. | (90) |
| Staphylococcus aureus PTCC 1053 | NR | 0.5 | |||||||||||
| Serratia marcescens PTCC1621 | NR | 0.5 | |||||||||||
| Pseudomonas aeruginosa PTCC1074 | NR | 0.5 | |||||||||||
| Staphylococcus aureus PTCC. 1112 | NR | 1 | |||||||||||
| Micrococcus luteus PTCC. 1110 | NR | 4 | |||||||||||
| Bacillus subtilis PTCC. 1023 | NR | 0.5 | |||||||||||
| Staphylococcus epidermidis PTCC 1114 | NR | 0.5 | |||||||||||
| Fe3O4 | ∼39.56 | Spherical | FeCl2·4H2O, FeCl3·6H2O & NH4OH | COP | TiO2 | NR | Escherichia coli | NR | 150 | NR | NR | TiO2-coated IONPs had potent toxicity against bacterial cells, so they exhibited antibacterial activity when they interacted with the cell membranes. | (91) |
| Klebsiella pneumonia | NR | 150 | |||||||||||
| Bacillus subtilis | NR | 150 | |||||||||||
| Staphylococcus aureus | NR | 150 | |||||||||||
| Escherichia coli | NR | 150 | |||||||||||
| Klebsiella pneumonia | NR | 150 | |||||||||||
| Bacillus subtilis | NR | 150 | |||||||||||
| Staphylococcus aureus | NR | 150 | |||||||||||
| SPION | 23.32 ± 1.17 | NR | Iron salt and dimercaptosuccinic acid | Two-step COP process. | NR | NR | MARS | NR | 530.796 ± 15.241 | NR | NR | SPION mediated the conformational and structural changes on the proteins of the cell surface and disrupted the cell membrane. | (92) |
| 495.993 ± 3.909 | NR | ||||||||||||
| 581.353 ± 10.26 | NR | ||||||||||||
| 457.871 ± 21.064 | NR | ||||||||||||
| Fe3O4 | 31.09 | Spherical | FeCl2·2H2O, FeCl3·6H2O, N2, DW & NH4OH. | COP | NR | NR | Staphylococcus epidermidis | 17.33 ± 0.57 | 25 | 50 | NR | Generally, photolytic generation of ROS induces oxidative stress in the cell. Also, due to the vibration of magnetic fields, IONPs lead bacterial cells to death. | (93) |
| Proteus mirabilis | 17.51 ± 0.57 | 50 | 100 | ||||||||||
| Acinetobacter baumannii | 17.66 ± 0.57 | 50 | 100 | ||||||||||
| PEG | NR | Staphylococcus epidermidis | 19.66 ± 0.57 | 25 | 50 | ||||||||
| Proteus mirabilis | 21.66 ± 0.57 | 50 | 100 | ||||||||||
| Acinetobacter baumannii | 22.00 ± 0.46 | 50 | 100 | ||||||||||
| GEN | NR | Staphylococcus epidermidis | 21.33 ± 1.15 | 50 | 100 | ||||||||
| Proteus mirabilis | 23.66 ± 0.57 | 25 | 50 | ||||||||||
| Acinetobacter baumannii | 23.66 ± 0.57 | 50 | 100 | ||||||||||
| PEG+ GEN | NR | Staphylococcus epidermidis | 23.66 ± 0.057 | 50 | 100 | ||||||||
| Proteus mirabilis | 25.66 ± 0.57 | 25 | 50 | ||||||||||
| Acinetobacter baumannii | 26.33 ± 0.57 | 50 | 100 | ||||||||||
| Fe2O3 | 14.2 ± 0.5 | Spherical | FeCl2·4H2O, FeCl3·6H2O, DW, NH4OH, HNO3, HCl & Fe (NO3)3·9H2O | COP | Teicoplanin | NR | Bacillus subtilis (ATCC 6633) | NR | 2 | >128 | NR | Fe2O3 NPs induced ROS, which induced oxidative stress and led to programmed cell death as well as autophagic activity. | (94) |
| Staphylococcus aureus (ATCC 6538Pb (MSSA)) | NR | 2 | 128 | ||||||||||
| Staphylococcus aureus (ATCC 43300 (MRSA) | NR | 2 | >128 | ||||||||||
| Enterococcus. faecalis (ATCC 29212) | NR | 1 | 32 | ||||||||||
| Enterococcus. faecalis (ATCC 51299 (VanB) | NR | 2 | >128 | ||||||||||
| Enterococcus. faecalis (9160188401-EF-34 (VanA) | NR | >128 | >128 | ||||||||||
| Escherichia coli (ATCC 35218) | NR | >128 | >128 | ||||||||||
| Fe2O3 | 25.34 | Hexagonal | FeSO4·7H2O, Co (NO3)2·6H2O, NaOH & DW | COP | NR | 400 | Bacillus subtilis | 11 | Escherichia coli | Fe2O3 NPs induced ROS, which triggered oxidative stress and led to cell death. | (59) | ||
| Staphylococcus aureus | 12 | 900 | NR | ||||||||||
| Escherichia coli | 19 | ||||||||||||
| Salmonella typhi | 12 | ||||||||||||
| 600 | Bacillus subtilis | 15 | |||||||||||
| Staphylococcus aureus | 13 | ||||||||||||
| Escherichia coli | 20 | ||||||||||||
| Salmonella typhi | 13 | ||||||||||||
| 800 | Bacillus subtilis | 17 | |||||||||||
| Staphylococcus aureus | 15 | ||||||||||||
| Escherichia coli | 21 | ||||||||||||
| Salmonella typhi | 16 | ||||||||||||
| Fe3O4 | 10.64 ± 4.73 | Spherical | FeSO4·7H2O, FeCl3·6 H2O, DW & NaOH | COP | Oleic acid | NR | Escherichia coli | NR | NR | NR | NR | The antibacterial activity of Fe3O4 NPs was mediated by electrostatic interaction between NPs and the cell membranes. Also, the ROS played a vital role in cell apoptosis. | (95) |
| Enterococcus hirae | NR | ||||||||||||
| Fe3O4 | 10–30 | Spherical | NR | COP | NR | 12.5 | Escherichia coli | 7 | NR | NR | NR | Fe3O4 mediated antibacterial activity by causing damaged to the proteins and DNA in the bacterial cell by generating ROS. | (96) |
| Proteus mirabilis | 7 | ||||||||||||
| Bacillus subtills | 7 | ||||||||||||
| 25 | Escherichia coli | 7 | |||||||||||
| Proteus mirabilis | 7 | ||||||||||||
| Bacillus subtills | 7 | ||||||||||||
| 50 | Escherichia coli | 8 | |||||||||||
| Proteus mirabilis | 8 | ||||||||||||
| Bacillus subtills | 8 | ||||||||||||
| Fe3O4 + Plant extract | |||||||||||||
| 12.5 | Escherichia coli | 11 | NR | NR | NR | ||||||||
| Proteus mirabilis | 16 | ||||||||||||
| Bacillus subtills | 7 | ||||||||||||
| 25 | Escherichia coli | 12 | |||||||||||
| Proteus mirabilis | 17 | ||||||||||||
| Bacillus subtills | 8 | ||||||||||||
| 50 | Escherichia coli | 13 | |||||||||||
| Proteus mirabilis | 18 | ||||||||||||
| Bacillus subtills | 10 | ||||||||||||
| Fe3O4 | 6–9 | Spherical | FeCl3, FeSO4, DW, NH4OH, PEG & ethyl alcohol | COP | NR | NR | Serratia marcescens | NR | 32 | NR | NR | ROS produced by IONPs moderated physical damage or chemical damage by interacting with the cell membranes, leading to bacterial cell death. | (97) |
| Escherichia coli | NR | 64 | |||||||||||
| Pseudomonas aeruginosa | NR | 128 | |||||||||||
| Listeria monocytogenes | NR | 32 | |||||||||||
| Fe3O4 | 24 | NR | FeCl3, FeCl2, DW, Argon gas & NaOH | COP | NR | 40 | Bacillus cereus | 13 | 5 | 80 | NR | IONPs could interact with DNA and proteins and mediate their conformational changes. | (66) |
| Klebsiella pneumoniae | 15 | ||||||||||||
| 80 | Bacillus cereus | 22 | |||||||||||
| Klebsiella pneumoniae | 26 | ||||||||||||
| Fe3O4 | 10–14 | Spherical | FeCl3, FeCl2, DW, & NaOH | COP | NR | 500 | Staphylococcus aureus | <10 | Staphylococcus aureus | NR | The IONPs damaged the cell membrane by inducing ROS. When IONPs are conjugated with gentamicin, bacterial growth is constrained by both mechanisms. By inhibiting the protein synthesis and damaging the cell membrane. | (98) | |
| Escherichia coli | <10 | 25,000 | 30,000 | ||||||||||
| Bacillus subtills | <10 | ||||||||||||
| Pseudomonas aeruginosa | <10 | ||||||||||||
| 1000 | Staphylococcus aureus | <10 | Escherichia coli | NR | |||||||||
| Escherichia coli | <10 | 28,000 | 35,000 | ||||||||||
| Bacillus subtills | <10 | ||||||||||||
| Pseudomonas aeruginosa | <10 | ||||||||||||
| 3000 | Staphylococcus aureus | <10 | Bacillus subtills | NR | |||||||||
| Escherichia coli | <10 | 20,000 | 25,000 | ||||||||||
| Bacillus subtills | <10 | ||||||||||||
| Pseudomonas aeruginosa | <10 | ||||||||||||
| 5000 | Staphylococcus aureus | <10 | Pseudomonas aeruginosa | NR | |||||||||
| Escherichia coli | <10 | 30,000 | 35,000 | ||||||||||
| Bacillus subtills | <10 | ||||||||||||
| Pseudomonas aeruginosa | <10 | ||||||||||||
| GEN | 500 | Staphylococcus aureus | <10 | Staphylococcus aureus | NR | ||||||||
| Escherichia coli | <10 | 2000 | 3000 | ||||||||||
| Bacillus subtills | 12.5 | ||||||||||||
| Pseudomonas aeruginosa | <10 | ||||||||||||
| 1000 | Staphylococcus aureus | 10 | Escherichia coli | NR | |||||||||
| Escherichia coli | <10 | 2500 | 3000 | ||||||||||
| Bacillus subtills | 13 | ||||||||||||
| Pseudomonas aeruginosa | <10 | ||||||||||||
| 3000 | Staphylococcus aureus | 13 | Bacillus subtills | NR | |||||||||
| Escherichia coli | <10 | 1500 | 2500 | ||||||||||
| Bacillus subtills | 25 | ||||||||||||
| Pseudomonas aeruginosa | <10 | ||||||||||||
| 5000 | Staphylococcus aureus | 16 | Pseudomonas aeruginosa | NR | |||||||||
| Escherichia coli | 11 | 3000 | 4000 | ||||||||||
| Bacillus subtills | 33 | ||||||||||||
| Pseudomonas aeruginosa | <10 | ||||||||||||
| Fe3O4 | ∼100 | Spherical | Oleic amine, ethylene glycol, FeCl3·6H2O, & sodium acetate | HDT | NR | 200 | Klebsiella pneumoniae | 22 ± 1.0 | Klebsiella pneumoniae | NR | IONPs generate ROS molecules that interact with the ion transportation channel and the DNA and induce oxidative stress to eliminate bacterial cells. | (68) | |
| Staphylococcus aureus | 28 ± 0.5 | 50 | 100 | ||||||||||
| Enterococcus faecalis | 25 ± 0.0 | ||||||||||||
| Pseudomonas aureginosa | 18 ± 1.0 | ||||||||||||
| 100 | Klebsiella pneumoniae | 20. ± 0.0 | Staphylococcus aureus | ||||||||||
| Staphylococcus aureus | 25 ± 0.0 | 6.25 | 12.5 | ||||||||||
| Enterococcus faecalis | 20 ± 1.0 | ||||||||||||
| Pseudomonas aureginosa | 16 ± 0.0 | ||||||||||||
| 50 | Klebsiella pneumoniae | 18 ± 1.0 | Enterococcus faecalis | ||||||||||
| Staphylococcus aureus | 22 ± 2.0 | 6.25 | 12.5 | ||||||||||
| Enterococcus faecalis | 17 ± 0.5 | ||||||||||||
| Pseudomonas aureginosa | 15 ± 0.5 | ||||||||||||
| 25 | Klebsiella pneumoniae | 15 ± 0 | Pseudomonas aureginosa | ||||||||||
| Staphylococcus aureus | 20 ± 1.0 | 50 | 50 | ||||||||||
| Enterococcus faecalis | 11 ± 0.0 | ||||||||||||
| Pseudomonas aureginosa | 12 ± 0.0 | ||||||||||||
| Fe3O4 | 35 | Spherical | Fe (NO3)3·9H2O, DW, & NH3 | CC | NR | 25 | Escherichia coli | 15 | NR | NR | NR | Fe3O4 generated ROS that disrupted membrane protein and penetrated the bacterial membrane. It induced oxidative stress, which led to cell death. | (99) |
| Proteus vulgaris | 11 | ||||||||||||
| Staphylococcus aureus | 9 | ||||||||||||
| Xanthomonas | 10 | ||||||||||||
| 30 | Escherichia coli | 16 | |||||||||||
| Proteus vulgaris | 12 | ||||||||||||
| Staphylococcus aureus | 12 | ||||||||||||
| Xanthomonas | 12 | ||||||||||||
| 40 | Escherichia coli | 17 | |||||||||||
| Proteus vulgaris | 15 | ||||||||||||
| Staphylococcus aureus | 14 | ||||||||||||
| Xanthomonas | 14 | ||||||||||||
| 50 | Escherichia coli | 17 | |||||||||||
| Proteus vulgaris | 20 | ||||||||||||
| Staphylococcus aureus | 15 | ||||||||||||
| Xanthomonas | 15 | ||||||||||||
| 60 | Escherichia coli | 15 | |||||||||||
| Proteus vulgaris | 20 | ||||||||||||
| Staphylococcus aureus | 11 | ||||||||||||
| Xanthomonas | 8 | ||||||||||||
| 80 | Escherichia coli | 15 | |||||||||||
| Proteus vulgaris | 12 | ||||||||||||
| Staphylococcus aureus | 12 | ||||||||||||
| Xanthomonas | 14 | ||||||||||||
| 100 | Escherichia coli | 21 | |||||||||||
| Proteus vulgaris | 21 | ||||||||||||
| Staphylococcus aureus | 15 | ||||||||||||
| Xanthomonas | 15 | ||||||||||||
| IO | 14–23 | Hexagonal | FeSO4·7H2O, β-Cyclodextrin, N2 & sodium borohydride | CS | Beta-cyclodextrin | NR | Staphylococcus aureus | 43.83 ± 0.75 | 100 | NR | NR | IONPs interacted with the cell wall and facilitated cell permeability, hindering cellular activity and resulting in the disruption of bacterial cells. | (100) |
| Klebsiella pneumonia | 39.83 ± 1.33 | 300 | |||||||||||
| Salmonella typhi | 20 ± 0.63 | 200 | |||||||||||
| Fe3O4 | ∼25 | NR | Fe (NO3)3, DW, Ethylene glycol. | SG | NR | 5 | Bacillus spp. | 23 | NR | NR | NR | The addition of citric acid increased the ROS generation and the electro-hole pair generation, leading to the decay of the bacterial surface. | (101) |
| Escherichia coli | 19 | ||||||||||||
| 10 | Bacillus spp. | 25 | |||||||||||
| Escherichia coli | 20 | ||||||||||||
| 15 | Bacillus spp. | 26 | |||||||||||
| Escherichia coli | 21 | ||||||||||||
| 20 | Bacillus spp. | 27 | |||||||||||
| Escherichia coli | 22 | ||||||||||||
| Citric acid | 5 | Bacillus spp. | 32 | ||||||||||
| Escherichia coli | 29 | ||||||||||||
| 10 | Bacillus spp. | 31 | |||||||||||
| Escherichia coli | 22 | ||||||||||||
| 15 | Bacillus spp. | 33 | |||||||||||
| Escherichia coli | 23 | ||||||||||||
| 20 | Bacillus spp. | 36 | |||||||||||
| Escherichia coli | 26 | ||||||||||||
| Fe3O4 | NR | NR | Fe (NO3)3·9H2O, ethanol | SG | NR | 50 | Enterobacter aerogenes | NR | NR | NR | NR | Fe3+ ions interact with the negatively charged membrane, decreasing the membrane integrity. By entering the cell, IONPs produce ROS, which disrupts the cell organelles and eradicates the cell. | (102) |
| 100 | |||||||||||||
| 150 | Staphylococcus aureus | ||||||||||||
| 200 | |||||||||||||
| Fe2O3 | 50–110 | Spherical | SDS, DMF & iron pressed pellet. | PLA | NR | Fe2O3 + DMF | The electrons in the photocatalytic process were absorbed by free radicals and produced OH– radicals. These radicals inhibited the growth of bacteria by induced oxidative stress generated by ROS. | (103) | |||||
| 4250 (20 mJ) | Escherichia coli | 19 | NR | NR | NR | ||||||||
| Pseudomonas aeruginosa | 17 | ||||||||||||
| Serritia marcescens | 20 | ||||||||||||
| Staphylococcus aureus | 21 | ||||||||||||
| 4250 (40 mJ) | Escherichia coli | 22 | |||||||||||
| Pseudomonas aeruginosa | 16 | ||||||||||||
| Serritia marcescens | 24 | ||||||||||||
| Staphylococcus aureus | 18 | ||||||||||||
| 4250 (60 mJ) | Escherichia coli | 18 | |||||||||||
| Pseudomonas aeruginosa | 27 | ||||||||||||
| Serritia marcescens | 17 | ||||||||||||
| Staphylococcus aureus | 22 | ||||||||||||
| 4250 (80 mJ) | Escherichia coli | 17 | |||||||||||
| Pseudomonas aeruginosa | 27 | ||||||||||||
| Serritia marcescens | 20 | ||||||||||||
| Staphylococcus aureus | 23 | ||||||||||||
| Fe2O3 + SDS | |||||||||||||
| 4250 (20 mJ) | Escherichia coli | 25 | |||||||||||
| Pseudomonas aeruginosa | 13 | ||||||||||||
| Serritia marcescens | 27 | ||||||||||||
| Staphylococcus aureus | 30 | ||||||||||||
| 4250 (40 mJ) | Escherichia coli | 27 | |||||||||||
| Pseudomonas aeruginosa | 27 | ||||||||||||
| Serritia marcescens | 30 | ||||||||||||
| Staphylococcus aureus | 35 | ||||||||||||
| 4250 (60 mJ) | Escherichia coli | 23 | |||||||||||
| Pseudomonas aeruginosa | 22 | ||||||||||||
| Serritia marcescens | 15 | ||||||||||||
| Staphylococcus aureus | 28 | ||||||||||||
| 4250 (80 mJ) | Escherichia coli | 20 | |||||||||||
| Pseudomonas aeruginosa | 19 | ||||||||||||
| Serritia marcescens | 18 | ||||||||||||
| Staphylococcus aureus | 30 |
NPs: Nanoparticles; Con.: Concentration; ZOI: zone of inhibition; ref: References; ROS: Reactive oxygen species; NR: Not reported; MIC: Minimum inhibitory concentration; MBC: Minimum bactericidal concentration; IC50: Half maximal inhibitory concentration; COP: Coprecipitation; HDT: Hydrothermal; CS: Chemical synthesis; SG: Sol–gel; TDP: Thermal decomposition; PLA: Pulsed laser ablation; FeCl3·6H2O: Ferric chloride hexahydrate; FeCl2·4H2O: Ferrous chloride tetrahydrate; FeSO4: Ferrous sulfate; TiCl4·2H2O: Titanium tetrachloride; FeSO4·7H2O: Ferrous sulfate; Fe (NO3)3·9H2O: Ferric nitrate nonahydrate; DW: Deionized water; CTAB: Trimethylammonium bromide; PR: polyester resin; RHL: Rhamnolipid; DMF: Dimethylformamide; GEN: Gentamicin; kan: Kanamycin; CC: Chemical combustion; PEG: Poly(ethylene glycol); IO: Iron oxide; QNDPs: Quantum dots nanoparticles; SPION: Superparamagnetic Iron oxide nanoparticles; PVA: Poly(vinyl alcohol) polymer; GA: Gallic acid; p-CoA: p-Coumaric acid; RHL coated-Fe3O4@PVA@p-CoA/GA: Rhamnolipid coated Iron oxide with Poly(vinyl alcohol) polymer; Gallic acid & p-Coumaric acid; MSSA: Methicillin-sensitive Staphylococcus aureus; MRSA: Methicillin-resistant Staphylococcus aureus; VRSA: Vancomycin-resistant Staphylococcus aureus.
The antimicrobial efficacy of IONPs also varies by size, which influences the surface area-to-volume ratio. The small size of the IONPs indicates a larger surface area to volume ratio; consequently, the increased surface area would allow IONPs to cover a more significant portion of the bacteria, resulting in more frequent interactions with the bacterial cell membrane and causing damage through electrostatic attraction and repulsion force.104−106 The antibacterial effectiveness of the IONPs also depends on the bacterial strains due to their differences in cell wall structure. The Gram-positive bacteria have a thick peptidoglycan layer consisting of a net negative charge due to teichoic acid.107 On the contrary, Gram-negative bacteria consist of a thin peptidoglycan layer and an overlaid lipopolysaccharide layer, which has an elevated net negative charge. IONPs interact with the negatively charged bacterial cell membrane through an electrostatic force that induces depolarization and disrupts the integrity of the cell membrane.103,108,109 Hence, due to the structural difference in the cellular composition, Gram-positive bacteria are more susceptible to IONPs than Gram-negative bacteria. Moreover, IONPs implicate nonspecific mechanistic behavior to eradicate pathogenic bacteria, such as inducing oxidative stress by producing ROS.110−112 These have the ability to inhibit the function of DNA, proteins, and enzymes, leading to bacterial cell death.76,113 IONPs do not adhere to a single strategy for inhibiting bacteria. As a result, bacteria struggle to develop resistance mechanisms against them.114
5. Parameters Influencing Antimicrobial Activity
5.1. Synthesis
5.1.1. COP Method
COP is the most common, economical, and versatile method for generating IONPs. This method generates IONPs under alkaline conditions through precipitation in a nonoxidizing environment where Fe2+/Fe3+ salt precursors are mixed in a 1:2 ratio.96,115−117 Moreover, the COP technique can effectively influence the size and morphology of the IONPs by altering some variables during synthesis, including the ionic strength, pH, temperature, concentration, and ratio of the precursors.118
IONPs synthesized via the COP method exhibited notable antibacterial activity against bacteria; for instance, the bare Fe3O4 exhibited moderate antibacterial activity against E. coli, P. mirabilis, and B. subtilis.(96) However, the antibacterial activity can be increased through surface modification of IONPs. Recently, Sharaf et al.87 reported that IONPs generated and modified using COP procedures showed notable antibacterial efficacy against several E. coli and S. aureus. An inhibitory zone ranging from 9 to 12 mm was detected against both bacteria when the Fe3O4 was coated with RHL. Interestingly, surface modification of the IONPs using RHL, PVA, GA, and p-CoA caused a 4-fold increase in zone of inhibition (ZOI). Conversely, the Fe2O3 generated by COP without other substances demonstrated an enhanced antibacterial effect compared to magnetite. It displayed a ZOI ranging from 11 to 21 mm against B. cereus, S. aureus, E. coli, and S. typhi at greater concentrations.59 Compared to bare Fe3O4 NPs, the Fe2O3 NPs showed pronounced bacteriostatic action against E. coli and S. aureus,59,98 while a study by Abdulsada et al.93 displayed that IONPs synthesized and modified with poly(ethylene glycol) (PEG) and gentamicin (GEN) through COP had an elevated antibacterial activity compared to the bare IONPs. Despite having the same synthesis method, IONPs exhibit a varying response against identical bacteria, and several factors may contribute to this variation, including precursors, shape, size, and coating material. Thus, IONPs synthesized by the COP method exhibited remarkable antimicrobial properties.99
5.1.2. SG Method
The SG method is a bottom-up synthesis method where irreversible chemical reactions occur. Certain chemical interactions, including hydrolysis and condensation, form a sol and a three-dimensional polymeric structure termed a gel. The polycondensation reaction generates a polymeric network that IONPs can retain. In most cases, nitrate precursors are utilized in SG methods for several key benefits, including uniformity in size and high levels of purity and quantity for IONPs.119 For these reasons, IONPs produced through the SG method exhibit noteworthy antibacterial activity against various bacteria at varying concentrations. The SG method is widely utilized to synthesize doped metal IONPs; however, some research indicates the synthesis of IONPs through the SG method and observes their antibacterial activity against some Gram-negative and positive bacteria. For instance, Fe3O4 was generated through the SG procedure, and its antibacterial activity was observed at different concentrations ranging from 5 to 20 μg/mL on different Gram-negative and positive bacteria. IONPs at different concentrations exhibited 19 to 36 mm ZOI for E. coli and B. cereus(101) (Table 2). Additionally, Brahmbhatt et al.102 conducted an antibiogram with Fe3O4 produced by SG and gave a visual aid for ZOI; however, the experiment lacked statistical analysis regarding the antibacterial activity of IONPs.
Table 2. Antimicrobial activity and potential mechanism of Green synthesized IONPsa.
| NPs | Particle size (nm) | Shape | Precursor | Synthesis method | Coating Material | Con. (μg/mL) | Organism | ZOI (mm) | MIC (μg/mL) | MBC (μg/mL) | IC50 (μg/mL) | Mechanism | ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α-Fe2O3 | 28.17 | Spherical | FeCl3·6H2O, & Capparis zeyl anica leaf extract | GRS | NR | 25 | Staphylococcus aureus | 23 ± 2.23 | NR | NR | 15.6 | Fe2O3 eliminates bacterial cells by inducing oxidative stress by generating ROS molecules. | (140) |
| Streptococcus pyogenes | 25 ± 2.56 | ||||||||||||
| Escherichia coli | 23 ± 1.34 | ||||||||||||
| Pseudomonas aeruginosa | 22 ± 1.23 | ||||||||||||
| Candida albicans | 19 ± 0.98 | ||||||||||||
| Aspergillus niger | 17 ± 1.62 | ||||||||||||
| Fe2O3 | 13.13–24.93 | Hexagonal | Purpureocillium lilacinum,& FeSO4 | GRS | NR | NR | Staphylococcus aureus | 26.5 | NR | NR | NR | Fe2O3 mediated structural or conformational change of DNA, which disrupted enzymatic activity by generating hydroxyl free radicals. Additionally, Fe2O3 induced oxidative stress, leading to apoptosis of bacterial cells. | (131) |
| Bacillus subtilis | 24.8 | ||||||||||||
| Escherichia coli | 19.5 | ||||||||||||
| Pseudomonas aeruginosa | 17 | ||||||||||||
| IO | 3.31–10.69 | Spherical | FeCl3& Penicillium spp. | GRS | NR | 250 | Staphylococcus aureus | 12 ± 0.6 | NR | NR | NR | IONPs diffuse into the intercellular membrane and damage DNA and protein and decompose the lipopolysaccharide layer by generating ROS, which induces oxidative stress. Hence, bacterial growth was inhibited. | (139) |
| Escherichia coli | 11.3 ± 1.2 | ||||||||||||
| Klebsiella pneumoniae | 11.3 ± 0.6 | ||||||||||||
| Shigella sonnie | 11.3 ± 0.6 | ||||||||||||
| Pseudomonas aureginosa | 11.3 ± 0.6 | ||||||||||||
| Fe2O3 | 40 | Spherical | Citrus leaf extract, DW, & FeCl3. | GRS | NR | 100 | Bacillus subtilis | 26.1 ± 0.24 | NR | NR | NR | Fe2O3 disrupted the enzymatic activity by interacting with cell surface proteins and the biological metabolites, resulting in bacterial cell apoptosis. | (69) |
| Klebsiella pneumoniae | 21.5 ± 0.36 | ||||||||||||
| 50 | Bacillus subtilis | 24.1 ± 0.37 | |||||||||||
| Klebsiella pneumoniae | 18.3 ± 0.49 | ||||||||||||
| 25 | Bacillus subtilis | 12.8 ± 0.69 | |||||||||||
| Klebsiella pneumoniae | 11.7 ± 0.53 | ||||||||||||
| Fe2O3 | 5–100 | Spherical | FeCl3, Tamarix Aphylla extract& NaOH | ′GRS | NR | 1000 | Escherichia coli | 11 | 30 ± 0.5 | NR | NR | Fe2O3 diffused into the cytoplasmic space, disrupted the enzymatic activity, damaged the DNA and led to death. | (141) |
| Bacillus subtilis | 12 | 30 ± 0.5 | |||||||||||
| FeO | 93.9 | Spherical | FeSO4·7H2O Thymbra spicata leaf extract& ethyl alcohol. | GRS | NR | Bacillus cereus | NR | 200 | 200 | NR | The free radical generated by FeO NPs interacted with the cellular membrane, which induced oxidative stress and caused cell apoptosis. | (142) | |
| Staphylococcus aureus | >200 | >200 | |||||||||||
| Escherichia coli | >200 | >200 | |||||||||||
| Salmonella typhimurium | 100 | 200 | |||||||||||
| NR | Bacillus cereus | NR | 200 | >200 | NR | ||||||||
| Staphylococcus aureus | >200 | >200 | |||||||||||
| Escherichia coli | >200 | >200 | |||||||||||
| Salmonella typhimurium | 200 | >200 | |||||||||||
| IONPs | 150–200 | Spherical | FeSO4·7H2O, Lawsonia inermis extract, NaOH, DW & methanol. | GRS | l-tyr | 40 | Staphylococcus aureus | 16 | NR | NR | NR | l-tyrosine-coated IONPs interacted with the lipid bilayer, disrupting the bacterial cell membrane and leading to cell apoptosis. | (143) |
| Staphylococcus tryphimurium | 15 | ||||||||||||
| FeO | 82 ± 7.0 | Spherical | FeCl3, PMC, & DW | GRS | NR | 10 | Escherichia coli | 20 | NR | NR | NR | FeO-NPs interacted with the bacteria’s thiol group, which induced oxidative stress and caused cell death. | (144) |
| Salmonella typhi | 24 | ||||||||||||
| Staphylococcus aureus | 26 | ||||||||||||
| Shigella | 24 | ||||||||||||
| Pasteurella multocida | 18 | ||||||||||||
| Pseudomonas aeruginosa | 16 | ||||||||||||
| FeO | 10–25 | Spherical | FeCl3 & GMAM | GRS | NR | NR | Escherichia coli | NR | NR | 200 | NR | FeO-NPs interacted with bacterial cell membranes and induced oxidative stress by producing ROS, leading to cell death. | (145) |
| Pseudomonas aeruginosa | NR | NR | 200 | ||||||||||
| Staphylococcus aureus | NR | NR | 200 | ||||||||||
| Fe3O4 | 84.81 | NR | FeCl2, FeCl3, DW, MH, CCP, & NaOH | GRS | Kan | 25 μg (NPs) + 5 μg (antibiotic) | Bacillus cereus (ATCC 13601) | 10.35 ± 0.17 | Antibiotics or anticandidal-coated Fe3O4 adheres to the cell surface via electromagnetic attractions; they interact with the thiol group which induces oxidative stress and leads to microbial cell apoptosis. | (123) | |||
| Escherichia coli (ATCC 43890) | 18.44 ± 0.41 | ||||||||||||
| Listeria monocytogenes (ATCC 19115) | 16.00 ± 0.86 | ||||||||||||
| Staphylococcus aureus (ATCC 49444) | 13.09 ± 0.30 | ||||||||||||
| Salmonella typhimurium (ATCC 43174) | 13.17 ± 0.26 | ||||||||||||
| Rif | 25 μg (NPs) + 5 μg (antibiotic) | Bacillus cereus (ATCC 13601) | 9.36 ± 0.28 | ||||||||||
| Escherichia coli (ATCC 43890) | 9.62 ± 0.51 | ||||||||||||
| Listeria monocytogenes (ATCC 19115) | 0 | ||||||||||||
| Staphylococcus aureus (ATCC 49444) | 21.99 ± 1.32 | ||||||||||||
| Salmonella typhimurium (ATCC 43174) | 0 | ||||||||||||
| Amp | 25 μg (NPs) + 5 μg (antibiotic) | Candida albicans (KACC 30003) | 10.63 ± 0.55 | ||||||||||
| Candida albicans (KACC 30062) | 17.68 ± 0.52 | ||||||||||||
| Candida glabrata (KBNO6P00368) | 10.11 ± 0.20 | ||||||||||||
| Candida glochares (KACC 30061) | 17.35 ± 0.35 | ||||||||||||
| Candida saitoana (KACC 41238) | 12.30 ± 0.54 | ||||||||||||
| 48.91 | GRS | Kan | 25 μg (NPs) + 5 μg (antibiotic) | Bacillus cereus (ATCC 13601) | 10.04 ± 0.41 | ||||||||
| Escherichia coli (ATCC 43890) | 19.65 ± 0.60 | ||||||||||||
| Listeria monocytogenes (ATCC 19115) | 13.10 ± 0.34 | ||||||||||||
| Staphylococcus aureus (ATCC 49444) | 12.28 ± 0.15 | ||||||||||||
| Salmonella typhimurium (ATCC 43174) | 12.42 ± 0.28 | ||||||||||||
| Rif | 25 μg (NPs) + 5 μg (antibiotic) | Bacillus cereus (ATCC 13601) | 10.66 ± 0.16 | ||||||||||
| Escherichia coli (ATCC 43890) | 9.29 ± 0.57 | ||||||||||||
| Listeria monocytogenes (ATCC 19115) | 0 | ||||||||||||
| Staphylococcus aureus (ATCC 49444) | 24.42 ± 0.26 | ||||||||||||
| Salmonella typhimurium (ATCC 43174) | 0 | ||||||||||||
| Amp | 25 μg (NPs) + 5 μg (antibiotic) | Candida albicans (KACC 30003) | 10.33 ± 0.46 | ||||||||||
| Candida albicans (KACC 30062) | 16.15 ± 0.23 | ||||||||||||
| Candida glabrata (KBNO6P00368) | 9.81 ± 0.57 | ||||||||||||
| Candida glochares (KACC 30061) | 16.44 ± 0.21 | ||||||||||||
| Candida saitoana (KACC 41238) | 10.01 ± 0.16 | ||||||||||||
| α-Fe2O3 | 34 | Quasi-spherical | FeCl3, P. guajava leaf extracted, DW & acetone | GRS | NR | 20 | Escherichia coli | 29 | NR | NR | NR | Fe2O3 produced ROS, which broke the DNA strands, inactivated enzymes and led to lipid peroxidation. This activity triggered mechanisms such as penetration of cell membrane and induced oxidative stress which mediated cell death. | (122) |
| Staphylococcus aureus | 39 | ||||||||||||
| 50 | Escherichia coli | 10 | |||||||||||
| Staphylococcus aureus | 13 | ||||||||||||
| 100 | Escherichia coli | 12 | |||||||||||
| Staphylococcus aureus | 15 | ||||||||||||
| Fe3O4 | 37.86 | Spherical | FeCl3, FeCl2, Zea mays L., & ear leaf extract. | GRS | Fe3O4 (25 μ) + kan (5 μ) | Bacillus cereus ATCC 13061 | 9.8 ± 0.34 | NR | NR | NR | The ROS mediated by Fe3O4 NPs damaged DNA, protein and cellular membranes. Thus, resulted in bacterial cell death | (146) | |
| Escherichia coli ATCC 43890 | 18.86 ± 0.82 | ||||||||||||
| Listeria monocytogenes ATCC 19115 | 13.54 ± 0.30 | ||||||||||||
| Staphylococcus aureus ATCC 49444 | 13.09 ± 0.15 | ||||||||||||
| Salmonella typhimurium ATCC 43174 | 13.3 ± 0.47 | ||||||||||||
| Fe3O4 (25 μ) + Rif (5 μ) | Bacillus cereus ATCC 13061 | 0 | |||||||||||
| Escherichia coli ATCC 43890 | 0 | ||||||||||||
| Listeria monocytogenes ATCC 19115 | 0 | ||||||||||||
| Staphylococcus aureus ATCC 49444 | 20.90 ± 0.50 | ||||||||||||
| Salmonella typhimurium ATCC 43174 | ±0.34 | ||||||||||||
| α- Fe2O3 | 20 | Spherical | Fe (NO3)3·9H2O, DW, & S. cordifolia extract. | GRS | NR | 50 | Escherichia coli | 11.33 ± 0.58 | NR | NR | NR | The ROS-mediated free radicals could disrupt the cell membrane via different interactions such as electrostatic, dipole, hydrogen, etc. | (121) |
| Bacillus subtills | 16 ± 1 | ||||||||||||
| Staphylococcus aureus | 13.67 ± 0.58 | ||||||||||||
| Klebsiella pneumoniae | 12 ± 1 |
NPs: Nanoparticles; Con.: Concentration; ZOI: zone of inhibition; ref: References; ROS: Reactive oxygen species; NR: Not reported; MIC: Minimum inhibitory concentration; MBC: Minimum bactericidal concentration; IC50: Half maximal inhibitory concentration; FeCl3.6H2O: Ferric chloride hexahydrate; Fe (NO3)3·9H2O: Ferric nitrate nonahydrate; DW: Deionized water; PEG: Poly(ethylene glycol); Kan: Kanamycin; Rif: Rifampicin; GRS: Green synthesis; GMAM: gray mangrove Avicennia marina; Amp: amphotericin; LPE: linear polyester; MH: Maize silky hairs; CCP: Leaves of Chinese cabbage; PMC: Psidium guajava-moringa composite; l-tyr: l-tyrosine; S. cordifolia: Sida cordifolia.
5.1.3. Other Chemical Synthesis Methods
IONPs can be synthesized using other chemically induced methods, including chemical precipitation, modified coprecipitation,120 wet chemical, matrix-mediated method, hydrothermal, and laser ablation methods which are commercially available from several companies. In the modified coprecipitation method, cationic and anionic solutions are mixed for nucleation, and the aggregation of NPs is controlled by the pH and temperature.120
The HDT produces IONPs with excellent crystalline structure at high temperature and pressure in an aqueous solution. The method also enhanced magnetism, uniform size distribution, and reduced aggregation. As a result, the IONPs produced through thermal decomposition would exhibit exceptional stability, while their compact size would significantly boost their antimicrobial effectiveness. For example, when Fe3O4 NPs are synthesized using HDT, they show more substantial antibacterial effects on Gram-positive than Gram-negative bacteria. IONPs displayed a ZOI range of 12–28 mm for Gram-positive and 12–22 mm for Gram-negative bacteria.68 Therefore, IONPs synthesized through the HDT method demonstrated satisfactory antibacterial activity.
The liquid solution is subjected to pulsed laser beams to conduct the laser ablation synthesis procedure for synthesizing IONPs.121 The method consists of a high-power laser submerged in an aqueous solution, targeting the precursors. In this method, IONPs can be obtained using a single-step procedure. The IONPs produced using these methods are entirely surfactant-free, resulting in exceptional stability and purity. Thus, IONPs produced through the PLS method displayed moderate antibacterial activity against Gram-positive and Gram-negative bacteria.103
5.1.4. GRS Method
GRS refers to a method where the biology-mediated synthesis of NPs is conducted. A range of biological elements, including microorganisms and leaf extracts like Chinese cabbage, mare silk, Psidium guajava,(122)Punica granatum, Zea mays,(123) and Sida cordifolia(91) could complete the synthesis procedure. This synthesis procedure is slightly modified,121 where the extraction of biological components is used as a reducing or stabilizing agent that is considered less toxic, convenient, economical, simple, eco-friendly, and biocompatible.30,71,99,124,125 IONPs synthesized through GRS acquire significant antibacterial activity against pathogenic bacteria compared to other chemical-induced synthesis methods123,125 due to the inherent therapeutic characteristics of the plant extracts (Table 2).85,126−128
According to a study by Singh et al.85 the antimicrobial effectiveness of the MNPs depends on two parameters: (i) the precursors used to synthesize the nanomaterials and (ii) the size of the particles. The plant extracts utilized in synthesizing IONPs for antibacterial purposes consist of phytochemicals that possess preexisting therapeutic or medicinal capabilities. As a result, IONPs produced by GRS using the green precursors induce a combined antimicrobial action, which exhibits a comparatively heightened antibacterial activity than other chemically induced methods.129,130Purpureocillium lilacinum was used to synthesize Fe2O3 and showed antibacterial activity against Gram-positive and negative bacteria, especially Gram-positive bacteria.131 This fungus is a well-known biocontrol agent used to produce leucinostatin antibiotics, which could be used against bacteria and fungus.132 Again, penicillium spp.,(133)Citrus leaf extract,134Tamarix aphylla extract,135Thymbra spicata leaf extract,136Lawsone inermis extract,137 and P. guajava leaf extract138 possess prior antimicrobial activity. Hammad et al.131 reported that Fe2O3 NPs generated by Capparis zeylanica leaf extract showed approximately 23 and 22 mm ZOI for S. aureus and P. aeruginosa, respectively. Again, IONPs were synthesized using Penicillium spp and displayed an approximate ZOI of 12 and 11 mm for similar organisms.139 In both cases, the IONPs generated by Capparis zeylanica showed a more pronounced antibacterial effect.
IONPs synthesized using Citrus leaf extracts inhibit the growth of B. cereus, K. pneumoniae, and B. subtilis. Similarly, Fe2O3 NPs produced from Tamarix aphylla and Thymbra spicata extracts are able to eradicate E. coli and S. aureus bacteria in a dose-dependent manner. Additionally, modifying the surface of IONPs with different types of antimicrobial agents, including kanamycin (Kan), rifampicin (Rif), and amphotericin (Amp), has proven to be more effective against microbes than the bare particles synthesized by GRS.69,141,142 Moreover, IONPs synthesized via the GRS method have demonstrated notable antibacterial efficacy in compared to chemically synthesized methods (Tables 1, 2).
5.2. Size
IONPs have distinctive antimicrobial properties due to their physical and chemical characteristics, including the large surface area-to-volume ratio, superparamagnetic properties, and self-assembly.147,148 These attributes are relevant to various factors, including their size, which influences their antimicrobial properties. Different synthesis procedures can influence the range of effective sizes of the IONPs.149 Moreover, small IONPs exhibited enhanced stability and a higher surface-area-to-volume ratio, increasing free energy content, surface charge, and reactivity. The surface reacts with the microbes to achieve content stabilization, effectively diffusing into the cytoplasmic region and permeating the bacterial cell wall or membrane.9,95,150 The heightened reactivity exhibited by NPs of smaller dimensions leads to an augmented generation of ROS, thereby promoting electron transfer due to the quantum confinement effect.151 Furthermore, to optimally utilize drug delivery and antibacterial activity, the NPs’ size should be less than 100 nm, commonly referred to as superparamagnetic iron oxide nanoparticles (SPIONs).152−157
Generally, smaller IONPs show enhanced antimicrobial activity compared to larger IONPs. IONPs with a size of around 10–100 nm display moderate antibacterial activity against a range of bacteria. For instance, hematite with a size ranging from 13.13 to 24.93 nm showed excellent antibacterial activity, where the recorded ZOI for E. coli was 23 mm.131 Again, hematite with a slightly large size like 28.17 nm showed decreased bacteriostatic activity with a 19.5 mm ZOI.140 The size difference between the two hematites was approximately 3–5 nm, influencing the antibacterial efficacy. Moreover, magnetite also showed significant antibacterial activity against numerous bacteria in sizes ranging from 8 to 260 nm. Moreover, where the size of IONPs is less than 10 nm and higher than 100 nm, the antibacterial activity of the IONPs decreases, such as IONPs ranging from 3.31 to 10 nm shows a ZOI of 11 ± 0.6 mm and 40 nm sized IONPs showed 21.5 ± 0.36 mm for K. pneumoniae.(69,139) Nevertheless, the antibacterial efficacy of this diverse range of IONPs lack to consistently correlate with their size due to other intrinsic parameters like precursors, synthesis methods, and coating materials (Figure 2). For instance, the effectiveness of antibacterial agents can vary depending on their dimensions and other inherent factors, like the materials used for their coating. In the case of both coated and uncoated IONPs, it was observed that their sizes varied within a range of approximately 6–254 nm and 3–200 nm, respectively, when employing the COP and GRS methods. However, it is often observed that smaller-sized IONPs tend to interact more frequently with pathogenic bacteria, resulting in dose reduction.158−161 Consequently, smaller IONPs exhibit a greater level of antibacterial activity.162−164
Figure 2.
An illustration of the numerous IONPs parameters that influenced the antibacterial activity. Here, AA: antimicrobial activity, AN: agar nutrient, AMH: agar Mueller-Hinton solution, LB: lysogenic broth, Amx: amoxicillin, TB: tryptic-soy broth, APD: agar potato dextrose solution, BN: broth of nutrient, BS: bacteriostatic effect, BC: bactericidal effect, and BG: bacterial growth.
5.3. Shape
The shape of the IONPs is crucial for their antibacterial activity. Numerous papers have documented different shapes of IONPs, such as hexagonal, quasi-hexagonal, and spherical. Interestingly, the majority of the research papers focused on the prevalence of spherical-shaped IONPs. These morphological differences are achieved by modifying the precursor molecules and temperature.165 Diverse-shaped IONPs utilize different approaches to interact with bacteria; hexagonal IONPs are larger compared to spherical ones. As a result, the hexagonal IONPs interact with the bacterial cell wall due to their sharp edges, which results in cell wall distortion.166,167 However, the spherical-shaped IONPs could diffuse into the intercellular space. Additionally, there is empirical evidence that displays this particular variant is not effective against all types of bacteria.168−170 A study revealed that nanoparticles with a spherical shape exhibit the highest level of effectiveness in combating bacterial infections.171 Moreover, it is evident that spherical IONPs exhibit the most potent antibacterial activity. For example, when studying hexagonal IONPs using the COP method, the findings showed an average ZOI between 11 and 12 mm (Tables 1 & 2).59 Conversely, IONPs with a spherical shape showed a 17 mm inhibition.93 A larger ZOI suggests that spherical IONPs have superior antibacterial activity than their hexagonal counterparts.
5.4. Concentration
The concentration of IONPs significantly affects their antimicrobial action, depending on various aspects such as dose, saturation effect, and cellular absorption. The antimicrobial activity of IONPs frequently depends on their dose.172,173 With the increased concentration, the bacterial cell surface may become saturated with IONPs, thereby increasing the probability of cellular uptake and consequently enhancing antimicrobial effects.174,175 Nevertheless, saturation may impose a constraint on IONPs, thereby diminishing their antibacterial effectiveness.176−179
The antimicrobial efficacy of the IONPs against pathogenic microorganisms is contingent upon their concentration. Due to the increase in concentration, a corresponding escalation in bacteriostatic activity was observed. Numerous studies have displayed the dose-dependent effect of IONPs against pathogenic microorganisms. For instance, Mehrabi et al.68 have examined the dose-dependent effect of IONPs on several pathogenic bacteria, where the antimicrobial activity of IONPs was observed at different concentrations, at the lowest concentration of 25 μg/mL, approximately 20 mm and 15 mm, and at the highest concentration of 200 μg/mL, 28 mm and 22 mm ZOI for S. aureus and K. pneumonia. The elevated concentration would indicate a higher possibility of endocytosis and enhanced generation of ROS. The increased production of ROS would improve oxidative stress and quickly eliminate microbial cells. Another study displayed a concentration-dependent approach of Fe2O3 NPs against the bacteria B. subtilis, S. aureus, E. coli, and S. typhi (Table 1). At a 400 μg/mL concentration, the observed zone diameters were 11, 12, and 19 mm. However, at a higher concentration of 800 μg/mL, the observed zone diameters were 15, 16, 17, and 21 mm, respectively.122 The remarked zone diameter values exhibited a proportional relationship with the varying concentrations, whereby lower concentrations corresponded to smaller ZOI. In comparison, higher concentrations were associated with augmented zones of inhibition. Sharaf et al.87 stated that the concentration of RHL-coated IONPs determines whether various bacterial strains exhibit bactericidal or bacteriostatic activity. The MIC and MBC concentrations of IONPs exhibited variation against distinct isolates of S. aureus, including MRSA, VRSA, and MSSA, which were 16, 32, and 64 μg/mL, respectively. Different isolates of E. coli bacteria also displayed multiple concentrations of MIC and MBC; hence, the increased concentration would elevate the antibacterial activity of the IONPs.
5.5. Precursors
IONPs can be synthesized using different iron salt precursors, e.g., chloride, sulfate, perchlorates, and nitrate salt precursors.180 Moreover, the antimicrobial activity of IONPs can be significantly influenced by the utilization of distinct iron salt precursors during their synthesis. It is worth noting that the resulting IONPs exhibit varying levels of antimicrobial activity depending on the specific salts used during synthesis. The Fe3O4 sample, synthesized through the utilization of FeCl3·6H2O and FeCl2·4H2O, demonstrated a notable ZOI of 20 mm68 while IONPs synthesized by Fe(NO3)3·9H2O showed a 9 mm ZOI99 for S. aureus. Furthermore, the iron precursor employed in the green synthesis process may also impact the bacteriostatic activity of the synthesized compounds. Fe2O3 NPs were synthesized utilizing various iron precursors via a sustainable and environmentally friendly approach known as green synthesis. Hematite was synthesized individually, employing FeCl3·6H2O and FeSO4 as precursors, respectively. IONPs that were synthesized using FeCl3·6H2O exhibited a zone diameter of 21 and 23 mm140 while IONPs synthesized by FeSO4 exhibited a ZOI of 26, 19.5, and 17 mm for S. aureus, E. coli, and P. aeruginosa, respectively.131 The provided data (Table 1) indicated that IONPs produced using chloride salts exhibited greater efficacy compared to those synthesized using sulfate salt when tested against E. coli and P. aeruginosa. However, the IONPs synthesized using sulfate salt demonstrated superior effectiveness against S. aureus. Additionally, IONPs synthesized utilizing a chloride precursor exhibited a zone diameter measuring 26 and 21.5 mm, respectively.69 On the contrary, Fe(NO3)3 precursor-derived IONPs showed 12 and 16 mm, ZOI for K. pneumoniae and B. subtilis, respectively.121 In this case, the chloride precursor was highly influential in inhibiting the microorganisms compared to the IONPs produced by nitrate salt. Hence, the inspection suggested that different precursors used to synthesize IONPs would influence their antimicrobial properties.181−183
5.6. Surface Modification to Enhance Antibacterial Activity of IONPs
The surface modification of IONPs could influence their physical, chemical, and biological characteristics. There are various methods to modify the surface of IONPs; however, using coating techniques to modify the surface of IONPs is highly effective. Moreover, the application areas would vary according to the coating materials utilized on IONPs because the properties of the coating materials alter the activity of the IONPs. Several studies have been conducted which offer valuable insights into surface modification that increases the antibacterial activity of IONPs.184−187 Hence, IONPs coated with antimicrobial agents such as gentamicin,98 Kan, Rif, Amp,188 poly(vinyl alcohol), teicoplanin,94 dimethylformamide, sodium dodecyl sulfate,189 and gallic acid (GA) form a nanocomplex, then these complexes tend to exhibit a combined effect against pathogenic bacteria (Tables 1 & 2). For instance, the bare IONPs exhibited ZOI, and the diameters were 17.33 ± 0.57 mm, 17.51 ± 0.57 mm, and 17.66 ± 0.57 mm while the IONPs conjugated with PEG+GEN demonstrated enhanced antimicrobial activity, with zone diameters of 23.66 ± 0.57 mm, 25.66 ± 0.57 mm, and 26.33 ± 0.57 mm against S. epidermidis, P. mirabilis, and A. baumannii, respectively. Hence, an increase in the diameter of the inhibitory zone by 7 mm was observed, which is attributed to the bacteriostatic properties exhibited by the coating material.93 Additionally, similar results were observed as antibiotics, beta-cyclodextrin,100 and l-tyrosine were coated on IONPs.143
The coating material used to improve the antibacterial activity must have inherent bacteriostatic qualities; hence, utilizing various coating materials would influence the antibacterial activity of IONPs. For instance, Kan-coated IONPs showed an inhibition zone diameter of 10.35 ± 0.17 mm, 18.44 ± 0.41 mm, 16 ± 0.86 mm, and 13.17 ± 0.26 mm while Rif-coated IONPs exhibited 9.36 ± 0.28 mm, 9.62 ± 0.28 mm, and 21.99 ± 1.32 mm against B. cereus, E. coli, L. monocytogenes, S. aureus, and S. typhimurium, respectively.123 Therefore, the different coating materials exhibited different ZOI. Additionally, utilizing multiple coating materials would display an increase in ZOI. For instance, the MICs of RHL-coated IONPs against various strains were found to be 4 μg/mL, 16 μg/mL, 32 μg/mL, 32 μg/mL, 64 μg/mL, and 64 μg/mL, while the MICs of RHL, PVA, GA, and p-CoA-coated IONPs against the same strains were determined to be 1 μg/mL, 4 μg/mL, 16 μg/mL, 16 μg/mL, 32 μg/mL, and 32 μg/mL E. coli (O26:H11), MSSA, E. coli (O78:H10), MRSA, E. coli (O157:H7), and VRSA, respectively. Hence, coating IONPs with multiple materials reduced the MIC dose to almost half against pathogenic bacteria compared to the MIC of single-coated IONPs. The coating materials RHL, PVA, GA, and p-CoA have significant antimicrobial properties as well as specific mechanisms to inhibit microbial cells.87 As a result, after coating them with IONPs, the individual materials started exhibiting their bacteriostatic mechanisms and the antimicrobial activity of the whole nanocomplex increased. As these coated IONPs complexes have displayed enhanced antimicrobial activity compared to bare IONPs, it is conclusive to state that the surface modifications of the IONPs would enhance their antimicrobial activity.190,191
Another approach could be doping impurities. It refers to the intentional introduction of specific foreign material into the lattice structure of the mother sample. Doping at various ratios to the other sample Fe3O4 could increase the antimicrobial activity. Since the dopant atom would influence the physicochemical properties of the IONPs, it would increase the interaction with the microorganism.192 Additionally, IONPs can achieve magnetic characteristics from dopant atoms, which could create severe vibration and damage the elasticity of the cell membrane and rupture the cells.189,191,192 Therefore, doping would increase the antibacterial activity of IONPs.
6. Antimicrobial Mechanisms of IONPs
Studies have shown that IONPs have a moderate antimicrobial effect on various bacteria that are susceptible to it. In-depth research is being carried out to gain insights into the antibacterial mechanisms of IONPs. Until this point, these mechanisms involved the generation of ROS through the Fenton reaction leading to lipid peroxidation, DNA damage, and protein damage. Additionally, the presence of free radicals causes lipid peroxidation, which in turn initiates ferroptosis, resulting in oxidative stress and, ultimately, cell death.193,194 Additionally, IONPs exhibit electrostatic interaction with bacteria and hinder cellular integrity. Thus far, these mentioned mechanistic understandings of IONPs have been theorized.
6.1. Cell Membrane Disruption
The cellular membrane of bacteria is composed of a bilayer of phospholipids interspersed with specialized proteins embedded within it. The semipermeable membrane exhibits hydrophilic polar heads and covalently linked hydrophobic nonpolar tails with ion channels.49 Bacterial cell membranes are negatively charged due to highly electronegative groups on their constituent phospholipids and lipopolysaccharides.195 IONPs can have either a positive or negative charge, leading to electrostatic interactions with the bacterial cell membrane. The antibacterial activity resulting from electrostatic interaction can differ depending on the surface charge of IONPs and the specific bacterial species. The surface charge of the IONPs interacts with the bacterial cell membrane through electrostatic attraction or repulsion and disrupts their integrity by increasing cell permeability, which allows frequent ion exchange into the cell and leads to cell death.196 Additionally, IONPs adhere to the bacterial cell membrane, and due to their nanosized nature and high surface-to-volume ratio, they can cause physical disruption to the cell membrane.30,149
Generally, synthesized IONPs consist of an overall negative charge due to the inherited hydroxyl group. However, the surface charge of the IONPs is dependent on the pH, being positive at low pH and negative at high pH. In both states, IONPs exhibit antibacterial activity through electrostatic attraction and repulsion. Positively charged iron ions present in IONPs have the potential to exert an influence on the integrity of cell membranes. An interruption occurs via electrostatic forces, upon the interaction of the iron ion with the cell wall. The metal ion then either attaches itself to the bacterial cell’s membranes or uses protein channels to enter the intracellular space.197
6.2. ROS Production
One of the most auspicious antibacterial mechanisms exhibited by IONPs involves the generation of ROS through photocatalysis, cellular aerobic metabolism, and the Fenton reaction. ROS can be generated by reducing O2 and the oxidation of H2O.198 In photocatalysis, UV light is required to generate ROS such as hydrogen peroxide (H2O2), hydroxy radical (−OH), and superoxide (·O2–).199,200 However, ROS generated through the Fenton reaction do not require any solar light. The Fe3+ is reduced to Fe2+ by accepting an electron in the Fenton reaction. As Fe2+ is unstable in the presence of O2, it is rapidly oxidized and produces ·O2–, reduces the Fe3+ to Fe2+, and their dismutation continuously produces H2O2. The reduced Fe2+ increases and reacts with H2O2, generating free radicals such as −OH.201−204
The following reaction displays a generalized Fenton reaction for generating ROS.
These ROS inhibited bacterial growth in various ways, including peroxidation of lipids in the cell membrane, damaging DNA, inhibiting replication, transcription, and translation.196,205−211 Additionally, free radicals can reduce the antioxidant system, including enzymes and natural elements, e.g., catalase, peroxidase, ascorbic acid, and carotenes, which could deactivate enzymes and inhibit bacterial growth. Hence, ROS were held responsible for a cascade of malfunctions that resulted in bacterial mortality by inducing oxidative stress in the cell (Figure 3).
Figure 3.
A diagrammatic illustration of IONPs antibacterial and antibiofilm mechanisms. IONPs trigger oxidative stress and cell lysis by generating ROS, altering membrane depolarization, damaging protein or nucleic acid, inactivating enzymes, damaging mitochondria or ribosome, impairing efflux pump, disrupting cytoplasm and cell membrane. ROS: Reactive oxygen species; IONPs: Iron oxide nanoparticles.
6.3. Damage to Cellular Content
The cellular composition of a bacterial cell is primarily composed of DNA, proteins, and lipids. Once the IONPs have successfully traversed the cellular membrane, they possess the capability to engage with various constituents within the cytoplasmic milieu. Damage to DNA and proteins induced by reactive oxygen species (ROS) is commonly investigated in the field. However, it is worth noting that certain studies have suggested alternative outcomes based on the specific characteristics of ROS, such as their size, shape, and concentration.212 Although IONP’s toxicity is suggested, it also exhibits apparent genotoxic properties. The IONPs demonstrated that they directly intercalate with DNA, breaking DNA strands.213,214 Additionally, the IONPs may interact with the proteins and enzymes necessary for ATP synthesis.212 These ions might obstruct ribosomal activities and stop them from synthesizing proteins. Gradually, interacting with the cytoplasmic content, replication, transcription, and translation might be inhibited by IONPs, which also have antibacterial properties.23,196,215
6.4. Enzyme Inactivation
IONPs could inhibit bacterial growth by interfering with the enzymatic activities of the microbial cells. IONPs hinder the enzymatic pathways and inhibit bacterial growth by binding with the enzyme, denaturing the enzyme, interfering with the cofactors, and disrupting energy production.216,217 IONPs can interact with either the surface or the active site of enzymes. Due to their nonspecific binding mechanism exhibited by IONPs, they possess the potential to function as irreversible enzyme inhibitors. IONPs exhibit diminutive dimensions, rendering them at the nanoscale level. Additionally, a molecular entity exhibits the inherent capability to act as an enzymatic inhibitor because it can imitate the native substrate and form a binding interaction with the enzyme’s catalytic site.218
6.5. Efflux Pump
IONPs could inhibit bacterial growth by interacting with efflux pumps. The efflux pump is a transport system localized in the cytoplasmic membrane to expel harmful substances from the cell. So, bacteriostatic activity could be achieved by preventing the release of the efflux pump. There are possible mechanisms by which an efflux pump could be inhibited. The first possible mechanism to inhibit the efflux pump is the direct binding of IONPs to the efflux pump. The disruption of efflux kinetics could be another mechanism that inhibits microbial cell growth.219 To comprehensively grasp the impact of the efflux pump on drug resistance, it is imperative to ascertain the kinetic constants. Hence, perturbing the efflux kinetics would exert an impact on the MDR efflux pump.219 The initial mechanism elucidated the noteworthy potential of direct binding between IONPs and an efflux pump to impede the proliferation of microbial cells. Nevertheless, the interaction between IONPs and the efflux pump may give rise to certain challenges. One of the prevailing concerns pertains to the dimensions of the efflux pump and its inert response in the face of the IONPs.220 Therefore, IONPs might interact with the other membrane proteins to become associated with a nearby efflux pump.221
7. Toxicity of IONPs
The utilization of IONPs as a therapeutic agent has become increasingly frequent in the clinical field. Therefore, conducting a cytotoxicity assay to assess the biocompatibility of IONPs both in vitro and in vivo is crucial. Frequently used techniques for investigating the toxicity and assessing the biocompatibility of IONPs in vitro include cell viability assay, LDH assay to examine cell membrane integrity, MTT assay to evaluate mitochondrial function, immunohistochemistry to detect markers of apoptosis, hemolysis assay, microscopic analysis of intracellular localization, and genotoxicity assessment for specific cell expression.222−228 The toxicity of IONPs is influenced by various parameters, including their dimensions, morphology, surface coating, method of administration, attached peptide, medication, and targeting agents.229−231 Smaller IONPs with a size lower than 10 nm are generally more hazardous than larger ones due to their higher surface area, which creates a higher possibility of penetrating the cells.232 However, in the case of an in vivo system, smaller IONPs are rapidly eliminated through extravasation and renal clearance, whereas the spleen captures the larger ones through mechanical filtration.233−235 Moreover, the surface charge can significantly influence the cytotoxicity of IONPs. The membrane potential of a cell is usually negative, and the surface charge of the IONPs is negative and sometimes positive; their interaction can be repulsive or attractive. The smaller NPs with a high surface area would have a higher change in ratio, increasing the electrostatic interaction between the cell and IONPs and damaging them. Furthermore, several coating materials are able to influence the toxicity of IONPs. For instance, longer exposure to IONPs could induce cytotoxicity due to the production of free radicals. However, toxic effects may be neutralized through PEGylation.236 PEG forms a hydrophilic barrier around IONPs, reducing the interaction between IONPs and cell membranes. More importantly, PEG coating enhances their antimicrobial activity and biocompatibility. Additionally, IONPs are less likely to cause significant health problems since Fe3+ is widely found in the human body. Hence, the release of the iron would not cause any major side effects in the in vivo system.237−239
8. Limitations and Future Directions
IONPs have moderate antibacterial activity due to several factors, including their interaction mechanisms, nutrient deprivation, varying susceptibility, dose determination, and cytotoxicity.240−242 IONPs interact with microbes through different mechanisms; however, these mechanisms depend on their size. Smaller IONPs have a larger surface area and can interact physically and more frequently with the bacterial cell membrane.243 On the contrary, the larger particles are not able to interact much frequently. Due to having a smaller surface area to volume ratio, their determined dose to eradicate bacteria increases.244,245 Moreover, dose determination becomes extremely difficult when the pathogens have variable susceptibility. Furthermore, IONPs are able to interfere with microbial nutrient uptake, as most pathogenic bacteria require iron for their growth and survival.246,247 Additionally, a few of the IONPs interact with cells, producing ROS that can be toxic for cells and induce pro-inflammatory mediators.248−251
Though IONPs have several limitations as therapeutic agents, these limitations can be minimized using biocompatible precursors and surface modification. For instance, in the GRS synthesis method, bioreductants, which consist of phytochemicals with prior antimicrobial activity, are used instead of chemicals.252,253 Therefore, a combined mechanism is initiated against eliminating bacteria; hence, the potency of IONPs increases.254 Additionally, surface modification approaches can help address IONPs’ main drawbacks, such as stability issues, cytotoxicity, biocompatibility, and moderate antibacterial activity.149,255 Biopolymers such as PEG and chitosan are used as coating material to modify the surface of IONPs.256,257 These biopolymers consist of antibacterial and biocompatible properties.258 Hence, surface-modified IONPs would be more potent as an antimicrobial agent because these biopolymers would compress the size of the particles and increase the surface area to volume ratio, resulting in a combined mechanism against pathogenic bacteria and acquiring increased bioavailability while keeping the cytotoxicity to a minimum by reducing the interaction between IONPs and tissues.259−261
IONPs exhibit moderate antibacterial activity as a therapeutic agent against pathogenic bacteria since the dose of IONPs would vary depending on the bacterial species, size of IONPs, and ROS production capabilities. For better and more precise outcomes, the GRS synthesis method and surface modification can be utilized to produce stable IONPs with enhanced biocompatibility and antibacterial activity. Furthermore, IONPs can be used in different clinical sectors, including cancer detection and treatment, magnetic hyperthermia, multimodal imaging, soil and water purifications, and biosensors.42,196,262,263
9. Conclusion
The antibacterial activity of the bare and coated IONPs depends on the synthesis methods, size, concentration, precursors, surface charge, and the materials used for coating. Among several synthesis methods, IONPs synthesized through GRS exhibited notable antibacterial efficacy. The phytochemicals with prior antimicrobial properties used in producing IONPs displayed a combined mechanism against bacteria. Additionally, IONPs expressed moderate antibacterial activity within the size range of approximately 10–100 nm. They exhibited higher cellular uptake and efficient antibacterial activity within this range, while IONPs with sizes smaller than 10 nm were less effective against bacteria. Furthermore, their properties to inhibit bacteria depend on their diminutive dimensions, and spherical-shaped IONPs show more significant potential for inhibiting bacteria than other shapes. Moreover, depending on the utilization of different iron salt precursors (e.g., sulfate, chloride, and nitrate) the antibacterial properties of IONPs vary. Among all the iron salt precursors, chloride salt has been proven to be the most effective precursor utilized to synthesize IONPs as an antibacterial agent. Although IONPs are used as therapeutic agents, they show moderate antibacterial activity. The moderate activities are increased by using different biopolymers (e.g., PEG, chitosan, L-try, antibiotics) as coating material. Surface modification via biocompatible coating material or drug candidates would increase stability, enhance biocompatibility, and simultaneously augment their antibacterial efficacy to address the pressing issue of infectious diseases caused by AMR organisms.
Acknowledgments
The authors are also thankful to the School of Data and Sciences Course Waiver for Research, APC Funding, and Q1 Publication Award Policy of Brac University, Bangladesh.
Author Contributions
Conceptualization, M.S.S.; writing original draft preparation, K.N.S., S.U., M.S.S., figure drawing S.U., M.H.R.; review and editing, S.U, M.H.R., and M.S.S.; project administration, M.S.S., M.H.R., and S.U.
The authors declare no competing financial interest.
References
- Abushaheen M. A.; Fatani A. J.; Alosaimi M.; Mansy W.; George M.; Acharya S.; Rathod S.; Divakar D. D.; Jhugroo C.; Vellappally S.; et al. Antimicrobial resistance, mechanisms and its clinical significance. Disease-a-Month 2020, 66 (6), 100971 10.1016/j.disamonth.2020.100971. [DOI] [PubMed] [Google Scholar]
- Llor C.; Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Therapeutic advances in drug safety 2014, 5 (6), 229–241. 10.1177/2042098614554919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salam M. A.; Al-Amin M. Y.; Salam M. T.; Pawar J. S.; Akhter N.; Rabaan A. A.; Alqumber M. A. In Antimicrobial resistance: a growing serious threat for global public health; Healthcare, MDPI, 2023; p 1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang K. W. K.; Millar B. C.; Moore J. E. Antimicrobial resistance (AMR). British Journal of Biomedical Science 2023, 80, 11387 10.3389/bjbs.2023.11387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srakocic S.; Biggers A.. How Do Bacteria Become Resistant to Antibiotics? In Healthline, 2022.https://www.healthline.com/health/antibiotics/how-do-bacteria-become-resistant-to-antibiotics#resistance
- Struelens M. J. The epidemiology of antimicrobial resistance in hospital acquired infections: problems and possible solutions. BMJ: British Medical Journal 1998, 317 (7159), 652–652. 10.1136/bmj.317.7159.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Materón E. M.; Miyazaki C. M.; Carr O.; Joshi N.; Picciani P. H.; Dalmaschio C. J.; Davis F.; Shimizu F. M. Magnetic nanoparticles in biomedical applications: A review. Applied Surface Science Advances 2021, 6, 100163 10.1016/j.apsadv.2021.100163. [DOI] [Google Scholar]
- Shekoufeh B.; Lotfipour F.; Azhar L. Magnetic nanoparticles for antimicrobial drug delivery. Die Pharmazie-An International Journal of Pharmaceutical Sciences 2012, 67 (10), 817–821. 10.1691/ph.2012.1163. [DOI] [PubMed] [Google Scholar]
- Allafchian A.; Hosseini S. S. Antibacterial magnetic nanoparticles for therapeutics: a review. IET nanobiotechnology 2019, 13 (8), 786–799. 10.1049/iet-nbt.2019.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cursaru L. M.; Piticescu R. M.; Dragut D. V.; Tudor I. A.; Kuncser V.; Iacob N.; Stoiciu F. The Influence of Synthesis Parameters on Structural and Magnetic Properties of Iron Oxide Nanomaterials. Nanomaterials 2020, 10 (1), 85. 10.3390/nano10010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ummah A. M.; Peng Y.-H.; Ho C.-H. Structure, property and magneto-optical interaction of wide-band-gap layered magnetism near the Néel temperature with antiferromagnetic to paramagnetic transition. FlatChem. 2023, 41, 100536 10.1016/j.flatc.2023.100536. [DOI] [Google Scholar]
- Thakare N. R.; Singh R.; Talukdar H.; Yadav D.; Hazarika S.; Ingole P. G.; Ahn Y.-H., Functionalization of biogenic and biomimetic magnetic nanosystems for biomedical applications. In Functionalized Magnetic Nanosystems for Diagnostic Tools and Devices; Elsevier, 2024; pp 229–255. [Google Scholar]
- Gupta I.; Sirohi S.; Roy K. Strategies for functionalization of magnetic nanoparticles for biomedical applications. Materials Today: Proceedings 2023, 72, 2757–2767. 10.1016/j.matpr.2022.10.065. [DOI] [Google Scholar]
- Ahmadpour F.; Ganjali F.; Radinekiyan F.; Eivazzadeh-Keihan R.; Salimibani M.; Bahreinizad H.; Mahdavi M.; Maleki A. Fabrication and characterization of a novel magnetic nanostructure based on pectin–cellulose hydrogel for in vitro hyperthermia during cancer therapy. RSC Adv. 2024, 14 (19), 13676–13684. 10.1039/D3RA08067F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salam M.; Zheng H.; Liu Y.; Zaib A.; Rehman S. A. U.; Riaz N.; Eliw M.; Hayat F.; Li H.; Wang F. Effects of micro (nano) plastics on soil nutrient cycling: state of the knowledge. Journal of Environmental Management 2023, 344, 118437 10.1016/j.jenvman.2023.118437. [DOI] [PubMed] [Google Scholar]
- Gupta N.; Parsai T.; Kulkarni H. V. A review on the fate of micro and nano plastics (MNPs) and their implication in regulating nutrient cycling in constructed wetland systems. Journal of Environmental Management 2024, 350, 119559 10.1016/j.jenvman.2023.119559. [DOI] [PubMed] [Google Scholar]
- Negrescu A. M.; Killian M. S.; Raghu S. N. V.; Schmuki P.; Mazare A.; Cimpean A. Metal Oxide Nanoparticles: Review of Synthesis, Characterization and Biological Effects. J. Funct. Biomater. 2022, 13 (4), 274 10.3390/jfb13040274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez L.; Cima-Cabal M. D.; Duarte A. C.; Rodriguez A.; Garcia P.; Garcia-Suarez M. D. M. Developing Diagnostic and Therapeutic Approaches to Bacterial Infections for a New Era: Implications of Globalization. Antibiotics (Basel) 2020, 9 (12), 916. 10.3390/antibiotics9120916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadfar S. M.; Roemhild K.; Drude N. I.; von Stillfried S.; Knüchel R.; Kiessling F.; Lammers T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Delivery Rev. 2019, 138, 302–325. 10.1016/j.addr.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Łojewska E.; Sakowicz T. An Alternative to Antibiotics: Selected Methods to Combat Zoonotic Foodborne Bacterial Infections. Curr. Microbiol. 2021, 78 (12), 4037–4037. 10.1007/s00284-021-02665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-López E.; Gomes D.; Esteruelas G.; Bonilla L.; Lopez-Machado A. L.; Galindo R.; Cano A.; Espina M.; Ettcheto M.; Camins A.; Silva A. M.; Durazzo A.; Santini A.; Garcia M. L.; Souto E. B. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10 (2), 292–292. 10.3390/nano10020292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakil M. S.; Bhuiya M. S.; Morshed M. R.; Babu G.; Niloy M. S.; Hossen M. S.; Islam M. A. Cobalt Ferrite Nanoparticle’s Safety in Biomedical and Agricultural Applications: A Review of Recent Progress. Curr. Med. Chem. 2023, 30 (15), 1756–1775. 10.2174/0929867329666221007113951. [DOI] [PubMed] [Google Scholar]
- Karnwal A.; Kumar G.; Pant G.; Hossain K.; Ahmad A.; Alshammari M. B. Perspectives on Usage of Functional Nanomaterials in Antimicrobial Therapy for Antibiotic-Resistant Bacterial Infections. ACS Omega 2023, 8 (15), 13492–13508. 10.1021/acsomega.3c00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makabenta J. M. V.; Nabawy A.; Li C.-H.; Schmidt-Malan S.; Patel R.; Rotello V. M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nature Reviews Microbiology 2021, 19 (1), 23–36. 10.1038/s41579-020-0420-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochvaldová L.; Večeřová R.; Kolář M.; Prucek R.; Kvítek L.; Lapčík L.; Panáček A. Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis. Nanotechnol. Rev. 2022, 11 (1), 1115–1142. 10.1515/ntrev-2022-0059. [DOI] [Google Scholar]
- Slavin Y. N.; Asnis J.; Häfeli U. O.; Bach H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. Journal of Nanobiotechnology 2017, 15, 65 10.1186/s12951-017-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assa F.; Jafarizadeh-Malmiri H.; Ajamein H.; Anarjan N.; Vaghari H.; Sayyar Z.; Berenjian A., A biotechnological perspective on the application of iron oxide nanoparticles. In Nano Research; Tsinghua University Press, 2016; Vol. 9, pp 2203–2225. [Google Scholar]
- Lee N. Y.; Ko W. C.; Hsueh P. R. Nanoparticles in the Treatment of Infections Caused by Multidrug-Resistant Organisms. Front Pharmacol 2019, 10, 1153. 10.3389/fphar.2019.01153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudkov S. V.; Burmistrov D. E.; Serov D. A.; Rebezov M. B.; Semenova A. A.; Lisitsyn A. B. Do Iron Oxide Nanoparticles Have Significant Antibacterial Properties?. Antibiotics 2021, 10 (7), 884. 10.3390/antibiotics10070884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias L. S.; Pessan J. P.; Vieira A. P. M.; De Lima T. M. T.; Delbem A. C. B.; Monteiro D. R. Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis, Drugs, Antimicrobial Activity, and Toxicity. Antibiotics 2018, 7 (2), 46. 10.3390/antibiotics7020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezealigo U. S.; Ezealigo B. N.; Aisida S. O.; Ezema F. I. Iron oxide nanoparticles in biological systems: Antibacterial and toxicology perspective. JCIS Open 2021, 4, 100027 10.1016/j.jciso.2021.100027. [DOI] [Google Scholar]
- Alprol A. E.; Mansour A. T.; Abdelwahab A. M.; Ashour M. Advances in Green Synthesis of Metal Oxide Nanoparticles by Marine Algae for Wastewater Treatment by Adsorption and Photocatalysis Techniques. Catalysts 2023, 13 (5), 888. 10.3390/catal13050888. [DOI] [Google Scholar]
- Tasnim N. T.; Ferdous N.; Rumon M. M. H.; Shakil M. S. The Promise of Metal-Doped Iron Oxide Nanoparticles as Antimicrobial Agent. ACS Omega 2024, 9, 16. 10.1021/acsomega.3c06323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglangit F.; Yu Y.; Deng H. Bacterial pathogens: threat or treat (a review on bioactive natural products from bacterial pathogens). Natural Product Reports 2021, 38 (4), 782–821. 10.1039/D0NP00061B. [DOI] [PubMed] [Google Scholar]
- Janeway C.; Travers P.; Walport M.; Shlomchik M. J.. Immunobiology: the immune system in health and disease; Garland Pub: New York, 2001; Vol. 2. [Google Scholar]
- Peterson J. W.Bacterial pathogenesis. Medical Microbiology, 4th ed.; University of Texas Medical Branch at Galveston, 1996. [PubMed] [Google Scholar]
- Silhavy T. J.; Kahne D.; Walker S. The Bacterial Cell Envelope. Cold Spring Harbor Perspectives in Biology 2010, 2 (5), a000414 10.1101/cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breijyeh Z.; Jubeh B.; Karaman R. Resistance of gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules 2020, 25 (6), 1340. 10.3390/molecules25061340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.; Niesel D. W.; Peterson J. W.; Klimpel G. R. Lipoprotein Release by Bacteria: Potential Factor in Bacterial Pathogenesis. Infect. Immun. 1998, 66 (11), 5196–5196. 10.1128/IAI.66.11.5196-5201.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertics P.; Gavala M.; Denlinger L.. Endotoxins. In Encyclopedia of Respiratory Medicine; ScienceDirect, 2006; pp 80–85. [Google Scholar]
- Zhang W.; Jiang H.; Wu G.; Huang P.; Wang H.; An H.; Liu S.; Zhang W. The pathogenesis and potential therapeutic targets in sepsis. MedComm 2023, 4 (6), e418 10.1002/mco2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A.; Waris A.; Khan M. A.; Asim M.; Khan A. U.; Khan S.; Zeb J. Recent advancement, immune responses, and mechanism of action of various vaccines against intracellular bacterial infections. Life Sciences 2023, 314, 121332 10.1016/j.lfs.2022.121332. [DOI] [PubMed] [Google Scholar]
- Jurėnas D.; Fraikin N.; Goormaghtigh F.; Van Melderen L. Biology and evolution of bacterial toxin–antitoxin systems. Nature Reviews Microbiology 2022 20:6 2022, 20 (6), 335–350. 10.1038/s41579-021-00661-1. [DOI] [PubMed] [Google Scholar]
- Smith I. Mycobacterium tuberculosis Pathogenesis and Molecular Determinants of Virulence. Clin. Microbiol. Rev. 2003, 16 (3), 463–463. 10.1128/CMR.16.3.463-496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollaard A. M.; Verspaget H. W.; Ali S.; Visser L. G.; Veenendaal R. A.; Van Asten H. A. G. H.; Widjaja S.; Surjadi C.; Van Dissel J. T. Helicobacter pylori infection and typhoid fever in Jakarta, Indonesia. Epidemiology and Infection 2006, 134 (1), 163–163. 10.1017/S0950268805004875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A. K.; Dhasmana N.; Dubey N.; Kumar N.; Gangwal A.; Gupta M.; Singh Y. Bacterial Virulence Factors: Secreted for Survival. Indian Journal of Microbiology 2017, 57 (1), 1–1. 10.1007/s12088-016-0625-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y.; Hand T. W. Role of the Microbiota in Immunity and inflammation. Cell 2014, 157 (1), 121–121. 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba C. P. Environmentally Transmitted Pathogens. Environmental Microbiology 2009, 445–445. 10.1016/B978-0-12-370519-8.00022-5. [DOI] [Google Scholar]
- C Reygaert W. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS microbiology 2018, 4 (3), 482. 10.3934/microbiol.2018.3.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munita J. M.; Arias C. A., Mechanisms of Antibiotic Resistance. Microbiology spectrum 2016, 4 ( (2), ). 10.1128/microbiolspec.VMBF-0016-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-Alvarez G., Synthesis, characterisation and applications of iron oxide nanoparticles, PhD Thesis, KTH: Sweden, 2004. [Google Scholar]
- Bock D. C.; Tallman K. R.; Guo H.; Quilty C.; Yan S.; Smith P. F.; Zhang B.; Lutz D. M.; McCarthy A. H.; Huie M. M.; Burnett V.; Bruck A. M.; Marschilok A. C.; Takeuchi E. S.; Liu P.; Takeuchi K. J. De)lithiation of spinel ferrites Fe3O4, MgFe2O4, and ZnFe2O4: A combined spectroscopic, diffraction and theory study. Phys. Chem. Chem. Phys. 2020, 22 (45), 26200–26215. 10.1039/D0CP02322A. [DOI] [PubMed] [Google Scholar]
- Jian W.; Jia R.; Wang J.; Zhang H. X.; Bai F. Q. Iron oxides with a reverse spinel structure: Impact of active sites on molecule adsorption. Inorganic Chemistry Frontiers 2019, 6 (10), 2810–2816. 10.1039/C9QI00790C. [DOI] [Google Scholar]
- Sanchez-Lievanos K. R.; Stair J. L.; Knowles K. E. Cation Distribution in Spinel Ferrite Nanocrystals: Characterization, Impact on their Physical Properties, and Opportunities for Synthetic Control. Inorg. Chem. 2021, 60 (7), 4291–4305. 10.1021/acs.inorgchem.1c00040. [DOI] [PubMed] [Google Scholar]
- Nagashima M.; Ishida T.; Akasaka M. Distribution of Fe among octahedral sites and its effect on the crystal structure of pumpellyite. Physics and Chemistry of Minerals 2006, 33 (3), 178–191. 10.1007/s00269-006-0066-1. [DOI] [Google Scholar]
- Wu W.; He Q.; Jiang C. Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Functionalization Strategies. Nanoscale Research Letters 2008, 3 (11), 397–415. 10.1007/s11671-008-9174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonvin D.; Arakcheeva A.; Millan A.; Pinol R.; Hofmann H.; Mionic Ebersold M. Controlling structural and magnetic properties of IONPs by aqueous synthesis for improved hyperthermia. RSC Adv. 2017, 7 (22), 13159–13170. 10.1039/C7RA00687J. [DOI] [Google Scholar]
- Wu W.; Wu Z.; Yu T.; Jiang C.; Kim W.-S. Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16 (2), 023501 10.1088/1468-6996/16/2/023501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan M.; Kumar Y.; Periyasamy L.; Viswanath A. K. Antibacterial applications of α-Fe2O3/Co3O4 nanocomposites and study of their structural, optical, magnetic and cytotoxic characteristics. Applied Nanoscience (Switzerland) 2018, 8 (1–2), 137–153. 10.1007/s13204-018-0656-5. [DOI] [Google Scholar]
- Clarke D. M.; Marquina C.; Lloyd D. C.; Vallejo-Fernandez G. Effect of the distribution of anisotropy constants on the magnetic properties of iron oxide nanoparticles. J. Magn. Magn. Mater. 2022, 559, 169543–169543. 10.1016/j.jmmm.2022.169543. [DOI] [Google Scholar]
- Estelrich J.; Busquets M. A. Iron Oxide Nanoparticles in Photothermal Therapy. Molecules: A Journal of Synthetic Chemistry and Natural Product Chemistry 2018, 23 (7), 1567. 10.3390/molecules23071567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amendola V.; Meneghetti M.; Granozzi G.; Agnoli S.; Polizzi S.; Riello P.; Boscaini A.; Anselmi C.; Fracasso G.; Colombatti M.; Innocenti C.; Gatteschi D.; Sangregorio C. Top-down synthesis of multifunctional iron oxide nanoparticles for macrophage labelling and manipulation. J. Mater. Chem. 2011, 21 (11), 3803–3813. 10.1039/c0jm03863f. [DOI] [Google Scholar]
- Abid N.; Khan A. M.; Shujait S.; Chaudhary K.; Ikram M.; Imran M.; Haider J.; Khan M.; Khan Q.; Maqbool M. Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: A review. Adv. Colloid Interface Sci. 2022, 300, 102597–102597. 10.1016/j.cis.2021.102597. [DOI] [PubMed] [Google Scholar]
- Mahmud N.; Anik M. I.; Hossain M. K.; Khan M. I.; Uddin S.; Ashrafuzzaman M.; Rahaman M. M. Advances in nanomaterial-based platforms to combat COVID-19: Diagnostics, preventions, therapeutics, and vaccine developments. ACS Applied Bio Materials 2022, 5 (6), 2431–2460. 10.1021/acsabm.2c00123. [DOI] [PubMed] [Google Scholar]
- Uddin S.; Islam M. R.; Chowdhury M. R.; Wakabayashi R.; Kamiya N.; Moniruzzaman M.; Goto M. Lipid-based ionic-liquid-mediated nanodispersions as biocompatible carriers for the enhanced transdermal delivery of a peptide drug. ACS applied bio materials 2021, 4 (8), 6256–6267. 10.1021/acsabm.1c00563. [DOI] [PubMed] [Google Scholar]
- Ansari S. A.; Oves M.; Satar R.; Khan A.; Ahmad S. I.; Jafri M. A.; Zaidi S. K.; Alqahtani M. H. Antibacterial activity of iron oxide nanoparticles synthesized by co-precipitation technology against Bacillus cereus and Klebsiella pneumoniae. Polish Journal of Chemical Technology 2017, 19 (4), 110–115. 10.1515/pjct-2017-0076. [DOI] [Google Scholar]
- Fexy A. Structural Study on Iron Oxide Nanoparticles Prepared by Sol-Gel Method. Int. J. Scientific Eng. Res. 2018, 9 (7), 324–329. [Google Scholar]
- Mehrabi M.; Ghasemi M. F.; Rasti B.; Falahati M.; Mirzaie A.; Hasan A. Nanoporous iron oxide nanoparticle: hydrothermal fabrication, human serum albumin interaction and potential antibacterial effects. J. Biomol. Struct. Dyn. 2021, 39 (7), 2595–2606. 10.1080/07391102.2020.1751296. [DOI] [PubMed] [Google Scholar]
- Hooda R.; Sharma M. Green Synthesis, Characterization and Antibacterial Activity of Iron Oxide Nanoparticles. Plant Archives 2020, 20 (1), 1196–1200. [Google Scholar]
- Yadav V. K.; Ali D.; Khan S. H.; Gnanamoorthy G.; Choudhary N.; Yadav K. K.; Thai V. N.; Hussain S. A.; Manhrdas S. Synthesis and Characterization of Amorphous Iron Oxide Nanoparticles by the Sonochemical Method and Their Application for the Remediation of Heavy Metals from Wastewater. Nanomaterials 2020, 10 (8), 1551. 10.3390/nano10081551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko A. I.; Sobol E. N.; Simakin A. V.; Serkov A. A.; Sukhov I. A.; Shafeev G. A. Biofunctional magnetic ‘core–shell’ nanoparticles generated by laser ablation of iron in liquid. Laser Physics 2015, 25 (2), 025607–025607. 10.1088/1054-660X/25/2/025607. [DOI] [Google Scholar]
- Hachani R.; Lowdell M.; Birchall M.; Hervault A.; Mertz D.; Begin-Colin S.; Thanh N. T. B. D. K. Polyol synthesis, functionalisation, and biocompatibility studies of superparamagnetic iron oxide nanoparticles as potential MRI contrast agents. Nanoscale 2016, 8 (6), 3278–3287. 10.1039/C5NR03867G. [DOI] [PubMed] [Google Scholar]
- Kekalo K.; Koo K.; Zeitchick E.; Baker I. Microemulsion Synthesis of Iron Core/Iron Oxide Shell Magnetic Nanoparticles and Their Physicochemical Properties. Materials Research Society symposia proceedings. Materials Research Society 2012, 1416, 61–66. 10.1557/opl.2012.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M. R.; Uddin S.; Chowdhury M. R.; Wakabayashi R.; Moniruzzaman M.; Goto M. Insulin transdermal delivery system for diabetes treatment using a biocompatible ionic liquid-based microemulsion. ACS Appl. Mater. Interfaces 2021, 13 (36), 42461–42472. 10.1021/acsami.1c11533. [DOI] [PubMed] [Google Scholar]
- Uddin S.; Islam M. R.; Moshikur R. M.; Wakabayashi R.; Moniruzzaman M.; Goto M. Modification with Conventional Surfactants to Improve a Lipid-Based Ionic-Liquid-Associated Transcutaneous Anticancer Vaccine. Molecules 2023, 28 (7), 2969. 10.3390/molecules28072969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghunath A.; Perumal E. Metal oxide nanoparticles as antimicrobial agents: a promise for the future. International journal of antimicrobial agents 2017, 49 (2), 137–152. 10.1016/j.ijantimicag.2016.11.011. [DOI] [PubMed] [Google Scholar]
- Ali A.; Zafar H.; Zia M.; ul Haq I.; Phull A. R.; Ali J. S.; Hussain A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnology, science and applications 2016, 9, 49–67. 10.2147/NSA.S99986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.; Zhang T.; Gao J. Biocompatible iron oxide nanoparticles for targeted cancer gene therapy: A review. Nanomaterials 2022, 12 (19), 3323. 10.3390/nano12193323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin S.; Chowdhury M. R.; Wakabayashi R.; Kamiya N.; Moniruzzaman M.; Goto M. Lipid based biocompatible ionic liquids: synthesis, characterization and biocompatibility evaluation. Chem. Commun. 2020, 56 (89), 13756–13759. 10.1039/D0CC04491A. [DOI] [PubMed] [Google Scholar]
- Patil S.; Mishra V. S.; Reddy P. C.; Lochab B. Multifunctional Multicomponent Highly Biocompatible pH-Responsive Iron-Oxide Embedded Nanodiamond Cargo for Artesunate to Inhibit Cancer Growth. ACS Materials Letters 2023, 5 (2), 336–340. 10.1021/acsmaterialslett.2c01027. [DOI] [Google Scholar]
- Kannan D.; Yadav N.; Ahmad S.; Namdev P.; Bhattacharjee S.; Lochab B.; Singh S. Pre-clinical study of iron oxide nanoparticles fortified artesunate for efficient targeting of malarial parasite. EBioMedicine 2019, 45, 261–277. 10.1016/j.ebiom.2019.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H.; Barnard A. S. Naturally occurring iron oxide nanoparticles: morphology, surface chemistry and environmental stability. Journal of Materials Chemistry A 2013, 1 (1), 27–42. 10.1039/C2TA00523A. [DOI] [Google Scholar]
- Nasiri K.; Masoumi S. M.; Amini S.; Goudarzi M.; Tafreshi S. M.; Bagheri A.; Yasamineh S.; Alwan M.; Arellano M. T. C.; Gholizadeh O. Recent advances in metal nanoparticles to treat periodontitis. J. Nanobiotechnol. 2023, 21 (1), 283. 10.1186/s12951-023-02042-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajinkya N.; Yu X.; Kaithal P.; Luo H.; Somani P.; Ramakrishna S. Magnetic iron oxide nanoparticle (IONP) synthesis to applications: present and future. Materials 2020, 13 (20), 4644. 10.3390/ma13204644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J.; Dutta T.; Kim K. H.; Rawat M.; Samddar P.; Kumar P. 'Green’ synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J. Nanobiotechnology 2018, 16 (1), 84. 10.1186/s12951-018-0408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda A.; Akpobolokemi T.; Chung E.; Ren G.; Raimi-Abraham B. T. pH Alteration in Plant-Mediated Green Synthesis and Its Resultant Impact on Antimicrobial Properties of Silver Nanoparticles (AgNPs). Antibiotics (Basel) 2022, 11 (11), 1592. 10.3390/antibiotics11111592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharaf M.; Sewid A. H.; Hamouda H. I.; Elharrif M. G.; El-Demerdash A. S.; Alharthi A.; Hashim N.; Hamad A. A.; Selim S.; Alkhalifah D. H. M.; Hozzein W. N.; Abdalla M.; Saber T. Rhamnolipid-Coated Iron Oxide Nanoparticles as a Novel Multitarget Candidate against Major Foodborne E. coli Serotypes and Methicillin-Resistant S. aureus. Microbiology Spectrum 2022, 10 (4), e00250-22 10.1128/spectrum.00250-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail A. S.; Mady A. H.; Tawfik S. M. Synthesis, Characterization and Biological Activity of Iron (III) Oxide and Titanium (IV) Oxide Nanoparticle Dispersed Polyester Resin Nanocomposites. Arabian Journal for Science and Engineering 2020, 45 (1), 197–203. 10.1007/s13369-019-04209-7. [DOI] [Google Scholar]
- Khalid H. F.; Tehseen B.; Sarwar Y.; Hussain S. Z.; Khan W. S.; Raza Z. A.; Bajwa S. Z.; Kanaras A. G.; Hussain I.; Rehman A. Biosurfactant coated silver and iron oxide nanoparticles with enhanced anti-biofilm and anti-adhesive properties. Journal of Hazardous Materials 2019, 364, 441–448. 10.1016/j.jhazmat.2018.10.049. [DOI] [PubMed] [Google Scholar]
- Moshafi M. H.; Ranjbar M.; Ilbeigi G. Biotemplate of albumen for synthesized iron oxide quantum dots nanoparticles (QDNPs) and investigation of antibacterial effect against pathogenic microbial strains. Int. J. Nanomed. 2019, 14, 3273–3282. 10.2147/IJN.S202462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulhady Y. A. M.; El-Shazly M. M.; El-Kased R. F. Evaluation of antibacterial activity and toxic metal removal of chemically synthesized magnetic iron oxide titanium coated nanoparticles and application in bacterial treatment. Journal of Environmental Science and Health - Part A Toxic/Hazardous Substances and Environmental Engineering 2018, 53 (3), 205–212. 10.1080/10934529.2017.1387012. [DOI] [PubMed] [Google Scholar]
- Taylor E. N.; Kummer K. M.; Durmus N. G.; Leuba K.; Tarquinio K. M.; Webster T. J. Superparamagnetic iron oxide nanoparticles (SPION) for the treatment of antibiotic-resistant biofilms. Small 2012, 8 (19), 3016–3027. 10.1002/smll.201200575. [DOI] [PubMed] [Google Scholar]
- Abdulsada F. M.; Hussein N. N.; Sulaiman G. M.; Al Ali A.; Alhujaily M. Evaluation of the Antibacterial Properties of Iron Oxide, Polyethylene Glycol, and Gentamicin Conjugated Nanoparticles against Some Multidrug-Resistant Bacteria. Journal of Functional Biomaterials 2022, 13 (3), 138. 10.3390/jfb13030138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armenia I.; Marcone G. L.; Berini F.; Orlandi V. T.; Pirrone C.; Martegani E.; Gornati R.; Bernardini G.; Marinelli F., Magnetic nanoconjugated teicoplanin: A novel tool for bacterial infection site targeting. Frontiers in Microbiology 2018, 9 ( (OCT), ). 10.3389/fmicb.2018.02270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielyan L.; Hovhannisyan A.; Gevorgyan V.; Ananyan M.; Trchounian A. Antibacterial effects of iron oxide (Fe 3 O 4) nanoparticles: distinguishing concentration-dependent effects with different bacterial cells growth and membrane-associated mechanisms. Appl. Microbiol. Biotechnol. 2019, 103 (6), 2773–2782. 10.1007/s00253-019-09653-x. [DOI] [PubMed] [Google Scholar]
- Arokiyaraj S.; Saravanan M.; Udaya Prakash N. K.; Valan Arasu M.; Vijayakumar B.; Vincent S. Enhanced antibacterial activity of iron oxide magnetic nanoparticles treated with Argemone mexicana L. leaf extract: An in vitro study. Mater. Res. Bull. 2013, 48 (9), 3323–3327. 10.1016/j.materresbull.2013.05.059. [DOI] [Google Scholar]
- Arshad M.; Alyousef A.; Ahmad I.; Khan J. M.; Shahzad S. A., Low temperature synthesis of superparamagnetic iron oxide (Fe3O4) nanoparticles and their ROS mediated inhibition of biofilm formed by food-associated bacteria. Frontiers in Microbiology 2018, 9 (). 10.3389/fmicb.2018.02567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya P.; Neogi S. Gentamicin coated iron oxide nanoparticles as novel antibacterial agents. Materials Research Express 2017, 4 (9), 095005 10.1088/2053-1591/aa8652. [DOI] [Google Scholar]
- Prabhu Y. T.; Rao K. V.; Kumari B. S.; Kumar V. S. S.; Pavani T. Synthesis of Fe3O4 nanoparticles and its antibacterial application. International Nano Letters 2015, 5 (2), 85–92. 10.1007/s40089-015-0141-z. [DOI] [Google Scholar]
- Philip S.; Kuriakose S. Studies on the antibacterial activity of water-soluble iron oxide nanoparticle- β-cyclodextrin aggregates against selected human pathogenic bacteria. Nano-Structures and Nano-Objects 2018, 16, 347–353. 10.1016/j.nanoso.2018.09.008. [DOI] [Google Scholar]
- Khan S.; Shah Z. H.; Riaz S.; Ahmad N.; Islam S.; Raza M. A.; Naseem S. Antimicrobial activity of citric acid functionalized iron oxide nanoparticles – Superparamagnetic effect. Ceram. Int. 2020, 46 (8), 10942–10951. 10.1016/j.ceramint.2020.01.109. [DOI] [Google Scholar]
- Brahmbhatt P. N.; Bharucha S. R.; Bhatt A.; Dave M. S. The Synthesis, Characterization, and Antimicrobial Activity of Magnetite (Fe3O4) Nanoparticles by the Sol–Gel Method. Eng. Proc. 2023, 56 (1), 293. 10.3390/ASEC2023-15950. [DOI] [Google Scholar]
- Ismail R. A.; Sulaiman G. M.; Abdulrahman S. A.; Marzoog T. R. Antibacterial activity of magnetic iron oxide nanoparticles synthesized by laser ablation in liquid. Materials Science and Engineering: C 2015, 53, 286–297. 10.1016/j.msec.2015.04.047. [DOI] [PubMed] [Google Scholar]
- Arias L. S.; Pessan J. P.; Vieira A. P. M.; Lima T. M. T. d.; Delbem A. C. B.; Monteiro D. R. Iron oxide nanoparticles for biomedical applications: a perspective on synthesis, drugs, antimicrobial activity, and toxicity. Antibiotics 2018, 7 (2), 46. 10.3390/antibiotics7020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakha M.; Pal S.; Samantarrai D.; Panigrahi T. K.; Mallick B. C.; Pramanik K.; Mallick B.; Jha S. Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle-bacteria interface. Sci. Rep. 2015, 5 (1), 14813 10.1038/srep14813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W.; Wang M.; Xiong C.; Xie D.; Chen Q.; Chu X.; Qiu X.; Li Y.; Xiao X. Synthesis, surface modification, and applications of magnetic iron oxide nanoparticles. J. Mater. Res. 2019, 34 (11), 1828–1844. 10.1557/jmr.2019.129. [DOI] [Google Scholar]
- Swoboda J. G.; Campbell J.; Meredith T. C.; Walker S. Wall Teichoic Acid Function, Biosynthesis, and Inhibition. Chembiochem: a European journal of chemical biology 2010, 11 (1), 35–35. 10.1002/cbic.200900557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lacerda Coriolano D.; de Souza J. B.; Bueno E. V.; Medeiros S. M. d. F. R. d. S.; Cavalcanti I. D. L.; Cavalcanti I. M. F. Antibacterial and antibiofilm potential of silver nanoparticles against antibiotic-sensitive and multidrug-resistant Pseudomonas aeruginosa strains. Brazilian Journal of Microbiology 2021, 52, 267–278. 10.1007/s42770-020-00406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam A.; Ahmed A. S.; Oves M.; Khan M. S.; Habib S. S.; Memic A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: a comparative study. International journal of nanomedicine 2012, 6003–6009. 10.2147/IJN.S35347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai K.; Tsuruta D. What are reactive oxygen species, free radicals, and oxidative stress in skin diseases?. International journal of molecular sciences 2021, 22 (19), 10799. 10.3390/ijms221910799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. D.; Huang B.-W.; Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cellular signalling 2012, 24 (5), 981–990. 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wydra R. J.; Oliver C. E.; Anderson K. W.; Dziubla T. D.; Hilt J. Z. Accelerated generation of free radicals by iron oxide nanoparticles in the presence of an alternating magnetic field. RSC Adv. 2015, 5 (24), 18888–18893. 10.1039/C4RA13564D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiggundu R.; Lusaya E.; Seni J.; Waswa J. P.; Kakooza F.; Tjipura D.; Kikule K.; Muiva C.; Joshi M. P.; Stergachis A.; Kitutu F. E.; Konduri N. Identifying and addressing challenges to antimicrobial use surveillance in the human health sector in low- and middle-income countries: experiences and lessons learned from Tanzania and Uganda. Antimicrobial Resistance and Infection Control 2023, 12 (1), 1–8. 10.1186/s13756-023-01213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdal Dayem A.; Hossain M.; Lee S.; Kim K.; Saha S.; Yang G.-M.; Choi H.; Cho S.-G. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. International Journal of Molecular Sciences 2017, 18 (1), 120. 10.3390/ijms18010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik A.; Khan R.; Solanki P. R.; Pandey P.; Alam J.; Ahmad S.; Malhotra B. D. Iron oxide nanoparticles–chitosan composite based glucose biosensor. Biosens. Bioelectron. 2008, 24 (4), 676–683. 10.1016/j.bios.2008.06.032. [DOI] [PubMed] [Google Scholar]
- Chatterjee S.; Bandyopadhyay A.; Sarkar K. Effect of iron oxide and gold nanoparticles on bacterial growth leading towards biological application. J. Nanobiotechnol. 2011, 9 (1), 1–7. 10.1186/1477-3155-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Current K. M.; Dissanayake N. M.; Obare S. O. Effect of Iron Oxide Nanoparticles and Amoxicillin on Bacterial Growth in the Presence of Dissolved Organic Carbon. Biomedicines 2017, Vol. 5, Page 55 2017, 5 (3), 55–55. 10.3390/biomedicines5030055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Ma Z.; Xing J.; Liu H. Preparation and characterization of amino–silane modified superparamagnetic silica nanospheres. J. Magn. Magn. Mater. 2004, 270 (1–2), 1–6. 10.1016/j.jmmm.2003.07.006. [DOI] [Google Scholar]
- Yilmaz E.; Soylak M., Functionalized nanomaterials for sample preparation methods. In Handbook of Nanomaterials in analytical chemistry; Elsevier: 2020; pp 375–413. [Google Scholar]
- Riaz S.; Bashir M.; Naseem S. Iron Oxide Nanoparticles Prepared by Modified Co-Precipitation Method. IEEE Trans. Magn. 2014, 50 (1), 1–4. 10.1109/TMAG.2013.2277614. [DOI] [Google Scholar]
- Pallela P. N. V. K.; Ummey S.; Ruddaraju L. K.; Gadi S.; Cherukuri C. S. L.; Barla S.; Pammi S. V. N. Antibacterial efficacy of green synthesized α-Fe2O3 nanoparticles using Sida cordifolia plant extract. Heliyon 2019, 5 (11), e02765 10.1016/j.heliyon.2019.e02765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufus A.; Sreeju N.; Philip D. Synthesis of biogenic hematite (α-Fe2O3) nanoparticles for antibacterial and nanofluid applications. RSC Adv. 2016, 6 (96), 94206–94217. 10.1039/C6RA20240C. [DOI] [Google Scholar]
- Patra J. K.; Baek K. H. Green biosynthesis of magnetic iron oxide (Fe3O4) nanoparticles using the aqueous extracts of food processing wastes under photo-catalyzed condition and investigation of their antimicrobial and antioxidant activity. Journal of Photochemistry and Photobiology B: Biology 2017, 173, 291–300. 10.1016/j.jphotobiol.2017.05.045. [DOI] [PubMed] [Google Scholar]
- Toropova Y. G.; Zelinskaya I. A.; Gorshkova M. N.; Motorina D. S.; Korolev D. V.; Velikonivtsev F. S.; Gareev K. G. Albumin covering maintains endothelial function upon magnetic iron oxide nanoparticles intravenous injection in rats. J. Biomed. Mater. Res., Part A 2021, 109 (10), 2017–2026. 10.1002/jbm.a.37193. [DOI] [PubMed] [Google Scholar]
- Sathishkumar G.; Logeshwaran V.; Sarathbabu S.; Jha P. K.; Jeyaraj M.; Rajkuberan C.; Senthilkumar N.; Sivaramakrishnan S. Green synthesis of magnetic Fe3O4 nanoparticles using Couroupita guianensis Aubl. fruit extract for their antibacterial and cytotoxicity activities. Taylor & Francis 2018, 46 (3), 589–598. 10.1080/21691401.2017.1332635. [DOI] [PubMed] [Google Scholar]
- Huq M. A.; Ashrafudoulla M.; Rahman M. M.; Balusamy S. R.; Akter S. Green synthesis and potential antibacterial applications of bioactive silver nanoparticles: A review. Polymers 2022, 14 (4), 742. 10.3390/polym14040742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A.; Das B. K.; Roy A.; Mandal B.; Chandra G. Antibacterial activity of some medicinal plant extracts. Journal of natural medicines 2008, 62, 259–262. 10.1007/s11418-007-0216-x. [DOI] [PubMed] [Google Scholar]
- Alaiya M. A.; Odeniyi M. A. Utilisation of Mangifera indica plant extracts and parts in antimicrobial formulations and as a pharmaceutical excipient: a review. Future Journal of Pharmaceutical Sciences 2023, 9 (1), 29. 10.1186/s43094-023-00479-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A.; Vishvakarma R.; Sharma P.; Sharma S.; Vimal A., Green Synthesis of Metal-Oxide Nanoparticles from Fruits and Their Waste Materials for Diverse Applications. In Nanomaterials from Agricultural and Horticultural Products; Springer: 2023; pp 81–119. [Google Scholar]
- Longmire M.; Choyke P. L.; Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine 2008, 3, 703. 10.2217/17435889.3.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad E. N.; Salem S. S.; Mohamed A. A.; El-Dougdoug W. Environmental Impacts of Ecofriendly Iron Oxide Nanoparticles on Dyes Removal and Antibacterial Activity. Appl. Biochem. Biotechnol. 2022, 194 (12), 6053–6067. 10.1007/s12010-022-04105-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G.; Liu Z.; Lin R.; Li E.; Mao Z.; Ling J.; Yang Y.; Yin W.-B.; Xie B. Biosynthesis of Antibiotic Leucinostatins in Bio-control Fungus Purpureocillium lilacinum and Their Inhibition on Phytophthora Revealed by Genome Mining. PLOS Pathogens 2016, 12 (7), e1005685 10.1371/journal.ppat.1005685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montemartini Corte A.; Liotta M.; Venturi C. B.; Calegari L. Antibacterial activity of Penicillium spp. strains isolated in extreme environments. Polar Biology 2000, 23 (4), 294–297. 10.1007/s003000050447. [DOI] [Google Scholar]
- Al-Aamri M. S.; Al-Abousi N. M.; Al-Jabri S. S.; Alam T.; Khan S. A. Chemical composition and in-vitro antioxidant and antimicrobial activity of the essential oil of Citrus aurantifolia L. leaves grown in Eastern Oman. J. Taibah Univ Med. Sci. 2018, 13 (2), 108–112. 10.1016/j.jtumed.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Otibi F.; Moria G. A.; Alharbi R. I.; Yassin M. T.; Al-Askar A. A. The Antifungal Properties of Tamarix aphylla Extract against Some Plant Pathogenic Fungi. Microorganisms 2023, 11 (1), 127. 10.3390/microorganisms11010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroun M. F.; Al-Kayali R. S. Synergistic effect of Thymbra spicata L. extracts with antibiotics against multidrug- resistant Staphylococcus aureus and Klebsiella pneumoniae strains. Iranian Journal of Basic Medical Sciences 2016, 19 (11), 1193–1200. [PMC free article] [PubMed] [Google Scholar]
- Antimicrobial activity of Lawsonia inermis leaf extracts against some human pathogens. International Journal of Current Microbiology and Applied Sciences 2013, 2 (5), 342–349. [Google Scholar]
- Naseer S.; Hussain S.; Naeem N.; Pervaiz M.; Rahman M., The phytochemistry and medicinal value of Psidium guajava (guava). Clinical Phytoscience 2018, 4 ( (1), ). 10.1186/s40816-018-0093-8 [DOI] [Google Scholar]
- Zakariya N. A.; Majeed S.; Jusof W. H. W. Investigation of antioxidant and antibacterial activity of iron oxide nanoparticles (IONPS) synthesized from the aqueous extract of Penicillium spp. Sensors International 2022, 3, 100164. 10.1016/j.sintl.2022.100164. [DOI] [Google Scholar]
- Mohandoss N.; Renganathan S.; Subramaniyan V.; Nagarajan P.; Elavarasan V.; Subramaniyan P.; Vijayakumar S. Investigation of Biofabricated Iron Oxide Nanoparticles for Antimicrobial and Anticancer Efficiencies. Applied Sciences (Switzerland) 2022, 12 (24), 12986. 10.3390/app122412986. [DOI] [Google Scholar]
- Ahmad W.; Khan A. U.; Shams S.; Qin L.; Yuan Q.; Ahmad A.; Wei Y.; Khan Z. U. H.; Ullah S.; Rahman A. U. Eco-benign approach to synthesize spherical iron oxide nanoparticles: A new insight in photocatalytic and biomedical applications. Journal of Photochemistry and Photobiology B: Biology 2020, 205, 111821. 10.1016/j.jphotobiol.2020.111821. [DOI] [PubMed] [Google Scholar]
- Erci F.; Cakir-Koc R. Rapid green synthesis of noncytotoxic iron oxide nanoparticles using aqueous leaf extract of Thymbra spicata and evaluation of their antibacterial, antibiofilm, and antioxidant activity. Inorganic and Nano-Metal Chemistry 2021, 51 (5), 683–692. 10.1080/24701556.2020.1802754. [DOI] [Google Scholar]
- Chauhan S.; Upadhyay L. S. B. Biosynthesis of iron oxide nanoparticles using plant derivatives of Lawsonia inermis (Henna) and its surface modification for biomedical application. Nanotechnology for Environmental Engineering 2019, 4 (1), 8 10.1007/s41204-019-0055-5. [DOI] [Google Scholar]
- Madubuonu N.; Aisida S. O.; Ali A.; Ahmad I.; Zhao T. k.; Botha S.; Maaza M.; Ezema F. I. Biosynthesis of iron oxide nanoparticles via a composite of Psidium guavaja-Moringa oleifera and their antibacterial and photocatalytic study. Journal of Photochemistry and Photobiology B: Biology 2019, 199, 111601. 10.1016/j.jphotobiol.2019.111601. [DOI] [PubMed] [Google Scholar]
- Ramalingam V.; Dhinesh P.; Sundaramahalingam S.; Rajaram R. Green fabrication of iron oxide nanoparticles using grey mangrove Avicennia marina for antibiofilm activity and in vitro toxicity. Surfaces and Interfaces 2019, 15, 70–77. 10.1016/j.surfin.2019.01.008. [DOI] [Google Scholar]
- Patra J. K.; Ali M. S.; Oh I. G.; Baek K. H. Proteasome inhibitory, antioxidant, and synergistic antibacterial and anticandidal activity of green biosynthesized magnetic Fe3O4 nanoparticles using the aqueous extract of corn (Zea mays L.) ear leaves. Artificial Cells, Nanomedicine and Biotechnology 2017, 45 (2), 349–356. 10.3109/21691401.2016.1153484. [DOI] [PubMed] [Google Scholar]
- Bao N.; Gupta A. Self-assembly of superparamagnetic nanoparticles. J. Mater. Res. 2011, 26 (2), 111–121. 10.1557/jmr.2010.25. [DOI] [Google Scholar]
- Bustamante-Torres M.; Romero-Fierro D.; Estrella-Nuñez J.; Arcentales-Vera B.; Chichande-Proaño E.; Bucio E. Polymeric composite of magnetite iron oxide nanoparticles and their application in biomedicine: a review. Polymers 2022, 14 (4), 752. 10.3390/polym14040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zúñiga-Miranda J.; Guerra J.; Mueller A.; Mayorga-Ramos A.; Carrera-Pacheco S. E.; Barba-Ostria C.; Heredia-Moya J.; Guamán L. P. Iron oxide nanoparticles: green synthesis and their antimicrobial activity. Nanomaterials 2023, 13 (22), 2919. 10.3390/nano13222919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W.; Guo Z.; Gao F.; Gao Q.; Wang D.; Liaw B. S.; Cai Q.; Sun X.; Wang X.; Zhao L. Shape-, size- and structure-controlled synthesis and biocompatibility of iron oxide nanoparticles for magnetic theranostics. Theranostics 2018, 8 (12), 3284–3284. 10.7150/thno.25220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharani K.; Jegatha Christy A.; Sagadevan S.; Nehru L. C. Photocatalytic and antibacterial performance of iron oxide nanoparticles formed by the combustion method. Chem. Phys. Lett. 2021, 771, 138524–138524. 10.1016/j.cplett.2021.138524. [DOI] [Google Scholar]
- De Jong W. H.; Borm P. J. A. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3 (2), 133–133. 10.2147/IJN.S596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi S. A. A.; Saleh A. M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharmaceutical Journal: SPJ. 2018, 26 (1), 64–64. 10.1016/j.jsps.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen Inbaraj B.; Tsai T. Y.; Chen B. H. Synthesis, characterization and antibacterial activity of superparamagnetic nanoparticles modified with glycol chitosan. Science and Technology of Advanced Materials 2012, 13 (1), 015002. 10.1088/1468-6996/13/1/015002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.; Kumon R. E.; Cui J.; Deng C. X. The Size of Sonoporation Pores on the Cell Membrane. Ultrasound in Medicine & Biology 2009, 35 (10), 1756–1760. 10.1016/j.ultrasmedbio.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow M.; Taylor A.; Fuentes-Caparrós A. M.; Sharkey J.; Daniels L. M.; Mandal P.; Park B. K.; Murray P.; Rosseinsky M. J.; Adams D. J. SPIONs for cell labelling and tracking using MRI: magnetite or maghemite?. Biomaterials science 2018, 6 (1), 101–106. 10.1039/C7BM00515F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi M.; Sahraian M. A.; Shokrgozar M. A.; Laurent S. Superparamagnetic iron oxide nanoparticles: promises for diagnosis and treatment of multiple sclerosis. ACS chemical neuroscience 2011, 2 (3), 118–140. 10.1021/cn100100e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Q.; Liu Y.; Huang J.; Chen K.; Huang J.; Xiao K. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci. Rep. 2018, 8 (1), 1–13. 10.1038/s41598-018-19628-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong S.; Quinto C. A.; Zhang L.; Mohindra P.; Bao G. Size-dependent heating of magnetic iron oxide nanoparticles. ACS Nano 2017, 11 (7), 6808–6816. 10.1021/acsnano.7b01762. [DOI] [PubMed] [Google Scholar]
- Freis B.; Ramirez M. D. L. A.; Kiefer C.; Harlepp S.; Iacovita C.; Henoumont C.; Affolter-Zbaraszczuk C.; Meyer F.; Mertz D.; Boos A.; et al. Effect of the Size and Shape of Dendronized Iron Oxide Nanoparticles Bearing a Targeting Ligand on MRI, Magnetic Hyperthermia, and Photothermia Properties—From Suspension to In Vitro Studies. Pharmaceutics 2023, 15 (4), 1104. 10.3390/pharmaceutics15041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shineh G.; Mobaraki M.; Afzali E.; Alakija F.; Velisdeh Z. J.; Mills D. K. Antimicrobial Metal and Metal Oxide Nanoparticles in Bone Tissue Repair. Biomedical Materials & Devices 2024, 2, 918. 10.1007/s44174-024-00159-3. [DOI] [Google Scholar]
- Aadinath W.; Muthuvijayan V. Antibacterial and angiogenic potential of iron oxide nanoparticles-stabilized acrylate-based scaffolds for bone tissue engineering applications. Colloids Surf., B 2023, 231, 113572 10.1016/j.colsurfb.2023.113572. [DOI] [PubMed] [Google Scholar]
- Vahini M.; Rakesh S. S.; Subashini R.; Loganathan S.; Prakash D. G. In vitro biological assessment of green synthesized iron oxide nanoparticles using Anastatica hierochuntica (Rose of Jericho). Biomass Conversion and Biorefinery 2023, 1–11. 10.1007/s13399-023-04018-x. [DOI] [Google Scholar]
- Uniyal S.; Choudhary K.; Sachdev S.; Kumar S. Nano-bio fusion: Advancing biomedical applications and biosensing with functional nanomaterials. Optics & Laser Technology 2024, 168, 109938 10.1016/j.optlastec.2023.109938. [DOI] [Google Scholar]
- Ioncica M.-C.; Bandyopadhyay S.; Bali N.; Socoliuc V.; Bernad S. I. Investigation of Cubic and Spherical IONPs’ Rheological Characteristics and Aggregation Patterns from the Perspective of Magnetic Targeting. Magnetochemistry 2023, 9 (4), 99. 10.3390/magnetochemistry9040099. [DOI] [Google Scholar]
- Shahbazi F.; Ahmadi R.; Noghani M.; Karimi G. Antibacterial activity of the Iron-Zinc Oxide nanoparticles synthesized via electric discharge method. International Journal of Nano Dimension 2023, 14 (1), 60–72. 10.22034/IJND.2022.1962974.2158. [DOI] [Google Scholar]
- Sayed F. A.; Eissa N. G.; Shen Y.; Hunstad D. A.; Wooley K. L.; Elsabahy M. Morphologic design of nanostructures for enhanced antimicrobial activity. J. Nanobiotechnology 2022, 20 (1), 536. 10.1186/s12951-022-01733-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine R.; Hasan A.; Primavera R.; Wilson R. J.; Thakor A. S.; Kevadiya B. D. Cellular uptake and retention of nanoparticles: Insights on particle properties and interaction with cellular components. Materials Today Communications 2020, 25, 101692 10.1016/j.mtcomm.2020.101692. [DOI] [Google Scholar]
- Song Y.; Elsabahy M.; Collins C. A.; Khan S.; Li R.; Hreha T. N.; Shen Y.; Lin Y.-N.; Letteri R. A.; Su L.; et al. Morphologic design of silver-bearing sugar-based polymer nanoparticles for uroepithelial cell binding and antimicrobial delivery. Nano Lett. 2021, 21 (12), 4990–4998. 10.1021/acs.nanolett.1c00776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabahy M.; Song Y.; Eissa N. G.; Khan S.; Hamad M. A.; Wooley K. L. Morphologic design of sugar-based polymer nanoparticles for delivery of antidiabetic peptides. J. Controlled Release 2021, 334, 1–10. 10.1016/j.jconrel.2021.04.006. [DOI] [PubMed] [Google Scholar]
- Cheon J. Y.; Kim S. J.; Rhee Y. H.; Kwon O. H.; Park W. H. Shape-dependent antimicrobial activities of silver nanoparticles. International journal of nanomedicine 2019, 14, 2773–2780. 10.2147/IJN.S196472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S.; Diyali S.; Vinothini G.; Perumalsamy B.; Balakrishnan G.; Ramasamy T.; Dharumadurai D.; Biswas B. Synthesis, morphological analysis, antibacterial activity of iron oxide nanoparticles and the cytotoxic effect on lung cancer cell line. Heliyon 2020, 6 (9), e04953 10.1016/j.heliyon.2020.e04953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo P. H. F. D.; Garcia M. T.; Figueiredo-Godoi L. M. A.; Lage A. C. P.; Silva N. S. D.; Junqueira J. C. Metal Nanoparticles to Combat Candida albicans Infections: An Update. Microorganisms 2023, 11 (1), 138. 10.3390/microorganisms11010138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B.; Wang Z.; Almutairi L.; Huang S.; Kim M.-H. Harnessing iron-oxide nanoparticles towards the improved bactericidal activity of macrophage against Staphylococcus aureus. Nanomedicine: Nanotechnology, Biology and Medicine 2020, 24, 102158 10.1016/j.nano.2020.102158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal N.; Seifan M.; Ebrahiminezhad A.; Berenjian A. The Effect of Iron Oxide Nanoparticles on the Menaquinone-7 Isomer Composition and Synthesis of the Biologically Significant All-Trans Isomer. Nanomaterials 2023, 13 (12), 1825. 10.3390/nano13121825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W.; Guo Z.; Gao F.; Gao Q.; Wang D.; Liaw B.-S.; Cai Q.; Sun X.; Wang X.; Zhao L. Shape-, size- and structure-controlled synthesis and biocompatibility of iron oxide nanoparticles for magnetic theranostics. Theranostics 2018, 8 (12), 3284–3307. 10.7150/thno.25220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Ouay B.; Stellacci F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano today 2015, 10 (3), 339–354. 10.1016/j.nantod.2015.04.002. [DOI] [Google Scholar]
- Agarwal H.; Nakara A.; Shanmugam V. K. Anti-inflammatory mechanism of various metal and metal oxide nanoparticles synthesized using plant extracts: A review. Biomedicine & Pharmacotherapy 2019, 109, 2561–2572. 10.1016/j.biopha.2018.11.116. [DOI] [PubMed] [Google Scholar]
- Waghchaure R. H.; Adole V. A. Biosynthesis of metal and metal oxide nanoparticles using various parts of plants for antibacterial, antifungal and anticancer activity: A review. Journal of the Indian Chemical Society 2023, 100, 100987 10.1016/j.jics.2023.100987. [DOI] [Google Scholar]
- Gautam S.; Bansal D.; Bhatnagar D.; Sharma C.; Goyal N., Synthesis of iron-based nanoparticles by chemical methods and their biomedical applications. In Oxides for Medical Applications; Elsevier, 2023; pp 167–195. [Google Scholar]
- Chang H.; Kim B. H.; Lim S. G.; Baek H.; Park J.; Hyeon T. Role of the precursor composition in the synthesis of metal ferrite nanoparticles. Inorg. Chem. 2021, 60 (7), 4261–4268. 10.1021/acs.inorgchem.0c03567. [DOI] [PubMed] [Google Scholar]
- Sharifi Dehsari H.; Halda Ribeiro A.; Ersoz B.; Tremel W.; Jakob G.; Asadi K. Effect of precursor concentration on size evolution of iron oxide nanoparticles. CrystEngComm 2017, 19 (44), 6694–6702. 10.1039/C7CE01406F. [DOI] [Google Scholar]
- Wang G.; Cui M.; Qiu Y.; Miao Y.; Ma H.; Zhang H.; Zhang Y.; Liu X.; Yi J.; Peng M.; et al. FeCO3 as a novel precursor for controllable synthesis of monodisperse iron oxide nanoparticles via solution thermal decomposition. Micro & Nano Letters 2021, 16 (11), 552–557. 10.1049/mna2.12085. [DOI] [Google Scholar]
- Thakur A.; Kumar A.; Kaya S.; Marzouki R.; Zhang F.; Guo L. Recent Advancements in Surface Modification, Characterization and Functionalization for Enhancing the Biocompatibility and Corrosion Resistance of Biomedical Implants. Coatings 2022, 12 (10), 1459. 10.3390/coatings12101459. [DOI] [Google Scholar]
- Zhu N.; Ji H.; Yu P.; Niu J.; Farooq M.; Akram M. W.; Udego I.; Li H.; Niu X. Surface modification of magnetic iron oxide nanoparticles. Nanomaterials 2018, 8 (10), 810. 10.3390/nano8100810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd Elrahman A. A.; Mansour F. R. Targeted magnetic iron oxide nanoparticles: Preparation, functionalization and biomedical application. Journal of Drug Delivery Science and Technology 2019, 52, 702–712. 10.1016/j.jddst.2019.05.030. [DOI] [Google Scholar]
- Abakumov M. A.; Semkina A. S.; Skorikov A. S.; Vishnevskiy D. A.; Ivanova A. V.; Mironova E.; Davydova G. A.; Majouga A. G.; Chekhonin V. P. Toxicity of iron oxide nanoparticles: Size and coating effects. Journal of Biochemical and Molecular Toxicology 2018, 32 (12), e22225–e22225. 10.1002/jbt.22225. [DOI] [PubMed] [Google Scholar]
- Anush K.; Shushanik K.; Susanna T.; Ashkhen H. Antibacterial effect of silver and iron oxide nanoparticles in combination with antibiotics on E. coli K12. BioNanoScience 2019, 9, 587–596. 10.1007/s12668-019-00640-0. [DOI] [Google Scholar]
- Khashan K. S.; Sulaiman G. M.; Mahdi R. Preparation of iron oxide nanoparticles-decorated carbon nanotube using laser ablation in liquid and their antimicrobial activity. Artificial cells, nanomedicine, and biotechnology, Taylor & Francis 2017, 45 (8), 1699–1709. 10.1080/21691401.2017.1282498. [DOI] [PubMed] [Google Scholar]
- Gabrielyan L.; Badalyan H.; Gevorgyan V.; Trchounian A. Comparable antibacterial effects and action mechanisms of silver and iron oxide nanoparticles on Escherichia coli and Salmonella typhimurium. Scientific Reports 2020, 10 (1), 1–12. 10.1038/s41598-020-70211-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. M.; Jeong H. J.; Kim S. L.; Kim E. M.; Kim D. W.; Lim S. T.; Jang K. Y.; Jeong Y. Y.; Nah J. W.; Sohn M. H. SPION-loaded chitosan–linoleic acid nanoparticles to target hepatocytes. Int. J. Pharm. 2009, 371 (1–2), 163–169. 10.1016/j.ijpharm.2008.12.021. [DOI] [PubMed] [Google Scholar]
- Velusamy P.; Chia-Hung S.; Shritama A.; Kumar G. V.; Jeyanthi V.; Pandian K. Synthesis of oleic acid coated iron oxide nanoparticles and its role in anti-biofilm activity against clinical isolates of bacterial pathogens. Journal of the Taiwan Institute of Chemical Engineers 2016, 59, 450–456. 10.1016/j.jtice.2015.07.018. [DOI] [Google Scholar]
- Xiao L.; Huang H.; Fan S.; Zheng B.; Wu J.; Zhang J.; Pi J.; Xu J. F. Ferroptosis: A mixed blessing for infectious diseases. Front Pharmacol 2022, 13, 992734 10.3389/fphar.2022.992734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradkar P. N.; De Domenico I.; Durchfort N.; Zohn I.; Kaplan J.; Ward D. M. Iron depletion limits intracellular bacterial growth in macrophages. Blood, The Journal of the American Society of Hematology 2008, 112 (3), 866–874. 10.1182/blood-2007-12-126854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.-M.; Rock C. O. Membrane lipid homeostasis in bacteria. Nature Reviews Microbiology 2008, 6 (3), 222–233. 10.1038/nrmicro1839. [DOI] [PubMed] [Google Scholar]
- Ozdal M.; Gurkok S. Recent advances in nanoparticles as antibacterial agent. ADMET and DMPK 2018, 10 (2), 115–129. 10.5599/admet.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linklater D. P.; Baulin V. A.; Le Guével X.; Fleury J.-B.; Hanssen E.; Nguyen H. P.; Juodkazis S.; Bryant G.; Crawford R.; Stoodley P. Antibacterial Action of Nanoparticles by Lethal Stretching of Bacterial Cell Membranes. Advanced Materials 2020, 32 (52), 2005679 10.1002/adma.202005679. [DOI] [PubMed] [Google Scholar]
- Nosaka Y.; Nosaka A. Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 2017, 117 (17), 11302–11336. 10.1021/acs.chemrev.7b00161. [DOI] [PubMed] [Google Scholar]
- Vaishampayan A.; Grohmann E. Antimicrobials functioning through ros-mediated mechanisms: Current insights. Microorganisms 2022, 10 (1), 61. 10.3390/microorganisms10010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.; Wang C.; Wang N.; Li J.; Zhu Y.; Zai J.; Fu J.; Hao Y. Photogenerated reactive oxygen species and hyperthermia by Cu3SnS4 nanoflakes for advanced photocatalytic and photothermal antibacterial therapy. J. Nanobiotechnol. 2022, 20 (1), 195. 10.1186/s12951-022-01403-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touati D. Iron and oxidative stress in bacteria. Archives of biochemistry and biophysics 2000, 373 (1), 1–6. 10.1006/abbi.1999.1518. [DOI] [PubMed] [Google Scholar]
- Seixas A. F.; Quendera A. P.; Sousa J. P.; Silva A. F.; Arraiano C. M.; Andrade J. M. Bacterial response to oxidative stress and RNA oxidation. Frontiers in Genetics 2022, 12, 821535 10.3389/fgene.2021.821535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedernikova I. Magnetic nanoparticles: Advantages of using, methods for preparation, characterization, application in pharmacy. Review Journal of Chemistry 2015, 5 (3), 256–280. 10.1134/S2079978015030036. [DOI] [Google Scholar]
- Anik M. I.; Mahmud N.; Masud A. A.; Khan M. I.; Islam M. N.; Uddin S.; Hossain M. K. Role of reactive oxygen species in aging and age-related diseases: a review. ACS Applied Bio Materials 2022, 5 (9), 4028–4054. 10.1021/acsabm.2c00411. [DOI] [PubMed] [Google Scholar]
- Canaparo R.; Foglietta F.; Limongi T.; Serpe L. Biomedical Applications of Reactive Oxygen Species Generation by Metal Nanoparticles. Materials 2021, 14 (1), 1–14. 10.3390/ma14010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande O. A.; Mohiuddin S. S.. Biochemistry, Oxidative Phosphorylation; StatPearls, 2022. [PubMed] [Google Scholar]
- Dunn J.; Grider M. H.. Physiology, Adenosine Triphosphate; StatPearls, 2023. [PubMed] [Google Scholar]
- Tirichen H.; Yaigoub H.; Xu W.; Wu C.; Li R.; Li Y. Mitochondrial Reactive Oxygen Species and Their Contribution in Chronic Kidney Disease Progression Through Oxidative Stress. Frontiers in Physiology 2021, 12, 398–398. 10.3389/fphys.2021.627837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R. Z.; Jiang S.; Zhang L.; Yu Z. B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44 (1), 3–15. 10.3892/ijmm.2019.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorov D. B.; Juhaszova M.; Sollott S. J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94 (3), 909–909. 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Reyes I.; Cuezva J. M. The H+-ATP synthase: A gate to ROS-mediated cell death or cell survival. Biochimica et Biophysica Acta (BBA) - Bioenergetics 2014, 1837 (7), 1099–1112. 10.1016/j.bbabio.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Dissanayake N. M.; Current K. M.; Obare S. O. Mutagenic Effects of Iron Oxide Nanoparticles on Biological Cells. International Journal of Molecular Sciences 2015, 16 (10), 23482–23482. 10.3390/ijms161023482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari M. O.; Parveen N.; Ahmad M. F.; Wani A. L.; Afrin S.; Rahman Y.; Jameel S.; Khan Y. A.; Siddique H. R.; Tabish M.; Shadab G. G. H. A. Evaluation of DNA interaction, genotoxicity and oxidative stress induced by iron oxide nanoparticles both in vitro and in vivo: attenuation by thymoquinone. Scientific Reports 2019, 9 (1), 1–14. 10.1038/s41598-019-43188-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radeloff K.; Ramos Tirado M.; Haddad D.; Breuer K.; Muller J.; Hochmuth S.; Hackenberg S.; Scherzad A.; Kleinsasser N.; Radeloff A. Superparamagnetic Iron Oxide Particles (VSOPs) Show Genotoxic Effects but No Functional Impact on Human Adipose Tissue-Derived Stromal Cells (ASCs). Materials 2021, 14 (2), 263. 10.3390/ma14020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren E.; Zhang C.; Li D.; Pang X.; Liu G., Leveraging metal oxide nanoparticles for bacteria tracing and eradicating. In VIEW; John Wiley and Sons Inc, 2020; Vol. 1. [Google Scholar]
- Huang Y.; Jiang J.; Wang Y.; Chen J.; Xi J. Nanozymes as Enzyme Inhibitors. Int. J. Nanomed. 2021, 16, 1143–1155. 10.2147/IJN.S294871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.; Hu C.; Shao L. The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. 10.2147/IJN.S121956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha S.-H.; Hong J.; McGuffie M.; Yeom B.; Vanepps J. S.; Kotov N. A. Shape-Dependent Biomimetic Inhibition of Enzyme by Nanoparticles and Their Antibacterial Activity. ACS Nano 2015, 9 (9), 9097–9105. 10.1021/acsnano.5b03247. [DOI] [PubMed] [Google Scholar]
- Gupta D.; Singh A.; Khan A. U. Nanoparticles as Efflux Pump and Biofilm Inhibitor to Rejuvenate Bactericidal Effect of Conventional Antibiotics. Nanoscale Res. Lett. 2017, 12 (1), 454 10.1186/s11671-017-2222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasani A.; Madhi M.; Gholizadeh P.; Shahbazi Mojarrad J.; Ahangarzadeh Rezaee M.; Zarrini G.; Samadi Kafil H., Metal nanoparticles and consequences on multi-drug resistant bacteria: reviving their role. SN Applied Sciences 2019, 1 ( (4), ). 10.1007/s42452-019-0344-4 [DOI] [Google Scholar]
- Molina-Hernandez J. B.; Aceto A.; Bucciarelli T.; Paludi D.; Valbonetti L.; Zilli K.; Scotti L.; Chaves-López C. The membrane depolarization and increase intracellular calcium level produced by silver nanoclusters are responsible for bacterial death. Sci. Rep. 2021, 11 (1), 21557 10.1038/s41598-021-00545-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy H. M.; Shekewy S.; Elhusseiny F. A.; Elmekawy A. Enhanced Biocompatibility by Evaluating the Cytotoxic and Genotoxic Effects of Magnetic Iron Oxide Nanoparticles and Chitosan on Hepatocellular Carcinoma Cells (HCC). Cell Biochem. Biophys. 2024, 1–16. 10.1007/s12013-024-01256-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L.; Lopez K.; Fritz S.; Easterling C. P.; Krawchuck J. A.; Poerwoprajitno A. R.; Xu W. Determination of the optical interference of iron oxide nanoparticles in fluorometric cytotoxicity assays. Heliyon 2024, 10 (3), e25378 10.1016/j.heliyon.2024.e25378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodan-Bărbulescu C.; Watz C.-G.; Moacă E.-A.; Faur A.-C.; Dehelean C.-A.; Faur F. I.; Grigoriţă L. O.; Maghiari A. L.; Tuţac P.; Duţă C.; et al. A Preliminary Report Regarding the Morphological Changes of Nano-Enabled Pharmaceutical Formulation on Human Lung Carcinoma Monolayer and 3D Bronchial Microtissue. Medicina 2024, 60 (2), 208. 10.3390/medicina60020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou M.; Melagraki G.; Lynch I.; Afantitis A. In Vitro Toxicological Insights from the Biomedical Applications of Iron Carbide Nanoparticles in Tumor Theranostics: A Systematic Review and Meta-Analysis. Nanomaterials 2024, 14 (9), 734. 10.3390/nano14090734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakil M. S.; Hasan M. A.; Sarker S. R. Iron Oxide Nanoparticles for Breast Cancer Theranostics. Curr. Drug Metab 2019, 20 (6), 446–456. 10.2174/1389200220666181122105043. [DOI] [PubMed] [Google Scholar]
- Daviu N.; Portilla Y.; Gomez de Cedron M.; Ramirez de Molina A.; Barber D. F. DMSA-coated IONPs trigger oxidative stress, mitochondrial metabolic reprograming and changes in mitochondrial disposition, hindering cell cycle progression of cancer cells. Biomaterials 2024, 304, 122409 10.1016/j.biomaterials.2023.122409. [DOI] [PubMed] [Google Scholar]
- Majeed S.; Mohd Rozi N. A. B.; Danish M.; Mohamad Ibrahim M. N.; Joel E. L. In vitro apoptosis and molecular response of engineered green iron oxide nanoparticles with l-arginine in MDA-MB-231 breast cancer cells. Journal of Drug Delivery Science and Technology 2023, 80, 104185 10.1016/j.jddst.2023.104185. [DOI] [Google Scholar]
- Rahman M. Magnetic resonance imaging and iron-oxide nanoparticles in the era of personalized medicine. Nanotheranostics 2023, 7 (4), 424. 10.7150/ntno.86467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setia A.; Mehata A. K.; Vikas; Malik A. K.; Viswanadh M. K.; Muthu M. S. Theranostic magnetic nanoparticles: Synthesis, properties, toxicity, and emerging trends for biomedical applications. Journal of Drug Delivery Science and Technology 2023, 81, 104295 10.1016/j.jddst.2023.104295. [DOI] [Google Scholar]
- Wibowo N. A.; Kurniawan C.; Kusumahastuti D. K.; Setiawan A.; Suharyadi E. Potential of Tunneling Magnetoresistance Coupled to Iron Oxide Nanoparticles as a Novel Transducer for Biosensors-on-Chip. J. Electrochem. Soc. 2024, 171 (1), 017512 10.1149/1945-7111/ad1f35. [DOI] [Google Scholar]
- Falsini S.; Colzi I.; Dainelli M.; Parigi E.; Salvatici M. C.; Papini A.; Talbot D.; Abou-Hassan A.; Gonnelli C.; Ristori S. Impact of airborne iron oxide nanoparticles on Tillandsia usneoides as a model plant to assess pollution in heavy traffic areas. Chemosphere 2024, 355, 141765 10.1016/j.chemosphere.2024.141765. [DOI] [PubMed] [Google Scholar]
- Arami H.; Khandhar A.; Liggitt D.; Krishnan K. M. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 2015, 44 (23), 8576–8607. 10.1039/C5CS00541H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X.; Wang S.; Ma S.; Han K.; Li X. Quantitative prediction of flow dynamics and mechanical retention of surface-altered red blood cells through a splenic slit. Phys. Fluids 2021, 33 (5), 051902 10.1063/5.0050747. [DOI] [Google Scholar]
- Ilium L.; Davis S.; Wilson C.; Thomas N.; Frier M.; Hardy J. Blood clearance and organ deposition of intravenously administered colloidal particles. The effects of particle size, nature and shape. International journal of pharmaceutics 1982, 12 (2–3), 135–146. 10.1016/0378-5173(82)90113-2. [DOI] [Google Scholar]
- Patil U. S.; Adireddy S.; Jaiswal A.; Mandava S.; Lee B. R.; Chrisey D. B. In vitro/in vivo toxicity evaluation and quantification of iron oxide nanoparticles. International journal of molecular sciences 2015, 16 (10), 24417–24450. 10.3390/ijms161024417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Huwaij R.; Al-Assaf S. F.; Mousli F.; Kutkut M. S.; Al-Bashtawi A. Perceptive review on properties of iron oxide nanoparticles and their antimicrobial and anticancer activity. Sys. Rev. Pharm. 2020, 11 (8), 418–431. [Google Scholar]
- Dandan Doganci M.; Doganci E.; Balci H.; Cetin M. Antibacterial and cytotoxic performance of methenamine-based poly (lactic acid)/poly (ethylene glycol)(PLA/PEG) composite films. J. Appl. Polym. Sci. 2024, 141 (21), e55412 10.1002/app.55412. [DOI] [Google Scholar]
- Nakipoglu M.; Tezcaner A.; Contag C. H.; Annabi N.; Ashammakhi N. Bioadhesives with antimicrobial properties. Adv. Mater. 2023, 35 (49), 2300840 10.1002/adma.202300840. [DOI] [PubMed] [Google Scholar]
- Rehman A.; Khan S.; Sun F.; Peng Z.; Feng K.; Wang N.; Jia Y.; Pan Z.; He S.; Wang L.; et al. Exploring the nano-wonders: unveiling the role of Nanoparticles in enhancing salinity and drought tolerance in plants. Frontiers in Plant Science 2024, 14, 1324176 10.3389/fpls.2023.1324176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdy N. M.; Boseila A. A.; Ramadan A.; Basalious E. B. Iron oxide nanoparticles-plant insignia synthesis with favorable biomedical activities and less toxicity, in the “era of the-green”: a systematic review. Pharmaceutics 2022, 14 (4), 844. 10.3390/pharmaceutics14040844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raheem M. A.; Rahim M. A.; Gul I.; Zhong X.; Xiao C.; Zhang H.; Wei J.; He Q.; Hassan M.; Zhang C. Y.; et al. Advances in nanoparticles-based approaches in cancer theranostics. OpenNano 2023, 12, 100152 10.1016/j.onano.2023.100152. [DOI] [Google Scholar]
- Hancharova M.; Halicka-Stępień K.; Dupla A.; Lesiak A.; Sołoducho J.; Cabaj J. Antimicrobial activity of metal-based nanoparticles: a mini-review. BioMetals 2024, 37, 773. 10.1007/s10534-023-00573-y. [DOI] [PubMed] [Google Scholar]
- Aliya S.; Rethinasabapathy M.; Yoo J.; Kim E.; Chung J.-Y.; Cha J.-H.; Huh Y. S. Phytogenic fabrication of iron oxide nanoparticles and evaluation of their in vitro antibacterial and cytotoxic activity. Arabian Journal of Chemistry 2023, 16 (6), 104703 10.1016/j.arabjc.2023.104703. [DOI] [Google Scholar]
- Tao Z.; Zhou Q.; Zheng T.; Mo F.; Ouyang S. Iron oxide nanoparticles in the soil environment: Adsorption, transformation, and environmental risk. Journal of Hazardous Materials 2023, 459, 132107 10.1016/j.jhazmat.2023.132107. [DOI] [PubMed] [Google Scholar]
- Kamble A. Iron Oxide Nanoparticles: An Alternative against Drug-Resistant Uropathogens. World J. Pharm. Res. 2022, 11 (9), 451–476. 10.20959/wjpr20229-24659. [DOI] [Google Scholar]
- Rashki S.; Asgarpour K.; Tarrahimofrad H.; Hashemipour M.; Ebrahimi M. S.; Fathizadeh H.; Khorshidi A.; Khan H.; Marzhoseyni Z.; Salavati-Niasari M.; et al. Chitosan-based nanoparticles against bacterial infections. Carbohydr. Polym. 2021, 251, 117108 10.1016/j.carbpol.2020.117108. [DOI] [PubMed] [Google Scholar]
- Fu P. P.; Xia Q.; Hwang H.-M.; Ray P. C.; Yu H. Mechanisms of nanotoxicity: generation of reactive oxygen species. Journal of food and drug analysis 2014, 22 (1), 64–75. 10.1016/j.jfda.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min Y.; Suminda G. G. D.; Heo Y.; Kim M.; Ghosh M.; Son Y.-O. Metal-based nanoparticles and their relevant consequences on cytotoxicity cascade and induced oxidative stress. Antioxidants 2023, 12 (3), 703. 10.3390/antiox12030703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.-L.; Fan Y.-G.; Zhao L.-X.; Zhang Q.; Wang Z.-Y. The metal ion hypothesis of Alzheimer’s disease and the anti-neuroinflammatory effect of metal chelators. Bioorganic Chemistry 2023, 131, 106301 10.1016/j.bioorg.2022.106301. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Hou S. Recent progress in the effect of magnetic iron oxide nanoparticles on cells and extracellular vesicles. Cell Death Discovery 2023, 9 (1), 195. 10.1038/s41420-023-01490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supardianningsih S.; Susiani S.; Nugraha M. Green Synthesis of ZnO Nanoparticles Using Potato Extracts (Solanum tuberosum) As an Antibacterial Agent in the Active Packaging For Food Products. KnE Engineering 2024, 6 (1), 420–428. 10.18502/keg.v6i1.15410. [DOI] [Google Scholar]
- Güell F.; Galdámez-Martínez A.; Martínez-Alanis P. R.; Catto A. C.; da Silva L. F.; Mastelaro V. R.; Santana G.; Dutt A. ZnO-based nanomaterials approach for photocatalytic and sensing applications: recent progress and trends. Materials Advances 2023, 4, 3685. 10.1039/D3MA00227F. [DOI] [Google Scholar]
- Zhan Y.; Hu H.; Yu Y.; Chen C.; Zhang J.; Jarnda K. V.; Ding P. Therapeutic strategies for drug-resistant Pseudomonas aeruginosa: Metal and metal oxide nanoparticles. J. Biomed. Mater. Res., Part A 2024, 112, 1343. 10.1002/jbm.a.37677. [DOI] [PubMed] [Google Scholar]
- Kapoor D. U.; Patel R. J.; Gaur M.; Parikh S.; Prajapati B. G. Metallic and metal oxide nanoparticles in treating Pseudomonas aeruginosa infections. Journal of Drug Delivery Science and Technology 2024, 91, 105290 10.1016/j.jddst.2023.105290. [DOI] [Google Scholar]
- Ali A.; Bairagi S.; Ganie S. A.; Ahmed S. Polysaccharides and proteins based bionanocomposites as smart packaging materials: From fabrication to food packaging applications a review. Int. J. Biol. Macromol. 2023, 252, 126534 10.1016/j.ijbiomac.2023.126534. [DOI] [PubMed] [Google Scholar]
- Tornero-Gutiérrez F.; Ortiz-Ramírez J. A.; López-Romero E.; Cuéllar-Cruz M. Materials used to prevent adhesion, growth, and biofilm formation of Candida species. Medical Mycology 2023, 61 (7), myad065 10.1093/mmy/myad065. [DOI] [PubMed] [Google Scholar]
- Hashim A. F.; Alghuthaymi M. A.; Vasil’kov A. Y.; Abd-Elsalam K. A. Polymer inorganic nanocomposites: A sustainable antimicrobial agents. Advances and Applications Through Fungal Nanobiotechnology 2016, 265–289. 10.1007/978-3-319-42990-8_13. [DOI] [Google Scholar]
- Al-Hakkani M. F.; Gouda G. A.; Hassan S. H.; Nagiub A. M. Echinacea purpurea mediated hematite nanoparticles (α-HNPs) biofabrication, characterization, physicochemical properties, and its in-vitro biocompatibility evaluation. Surfaces and Interfaces 2021, 24, 101113 10.1016/j.surfin.2021.101113. [DOI] [Google Scholar]
- Ghosh S.; Singh B. P.; Webster T. J., Nanoparticle-impregnated biopolymers as novel antimicrobial nanofilms. In Biopolymer-based nano films; Elsevier, 2021; pp 269–309. [Google Scholar]
- Jafarizadeh-Malmiri H.; Sayyar Z.; Anarjan N.; Berenjian A.. Nanobiotechnology in food: Concepts, applications and perspectives; Springer, 2019. [Google Scholar]
- Pucci C.; Degl'Innocenti A.; Belenli Gumus M.; Ciofani G. Superparamagnetic iron oxide nanoparticles for magnetic hyperthermia: recent advancements, molecular effects, and future directions in the omics era. Biomaterials Science 2022, 10 (9), 2103–2121. 10.1039/D1BM01963E. [DOI] [PubMed] [Google Scholar]
- Nabil G.; Bhise K.; Sau S.; Atef M.; El-Banna H. A.; Iyer A. K. Nano-engineered delivery systems for cancer imaging and therapy: Recent advances, future direction and patent evaluation. Drug Discovery Today 2019, 24 (2), 462–491. 10.1016/j.drudis.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]




