Abstract
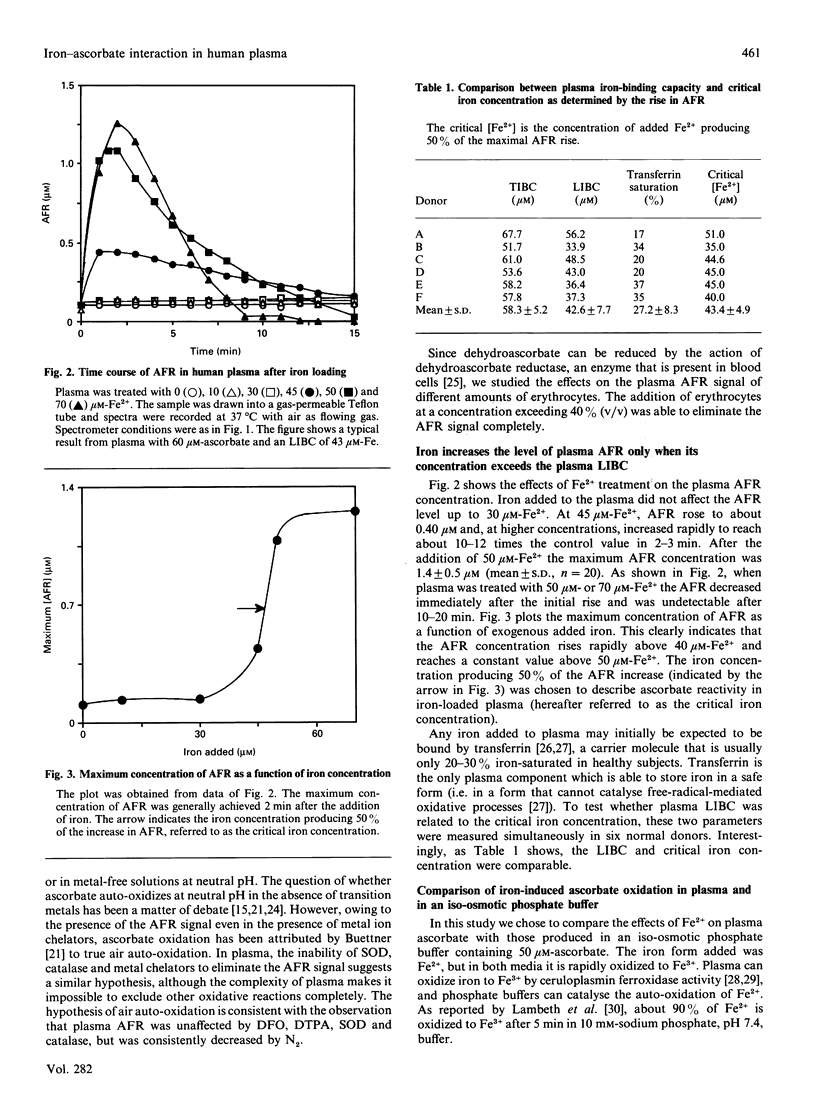
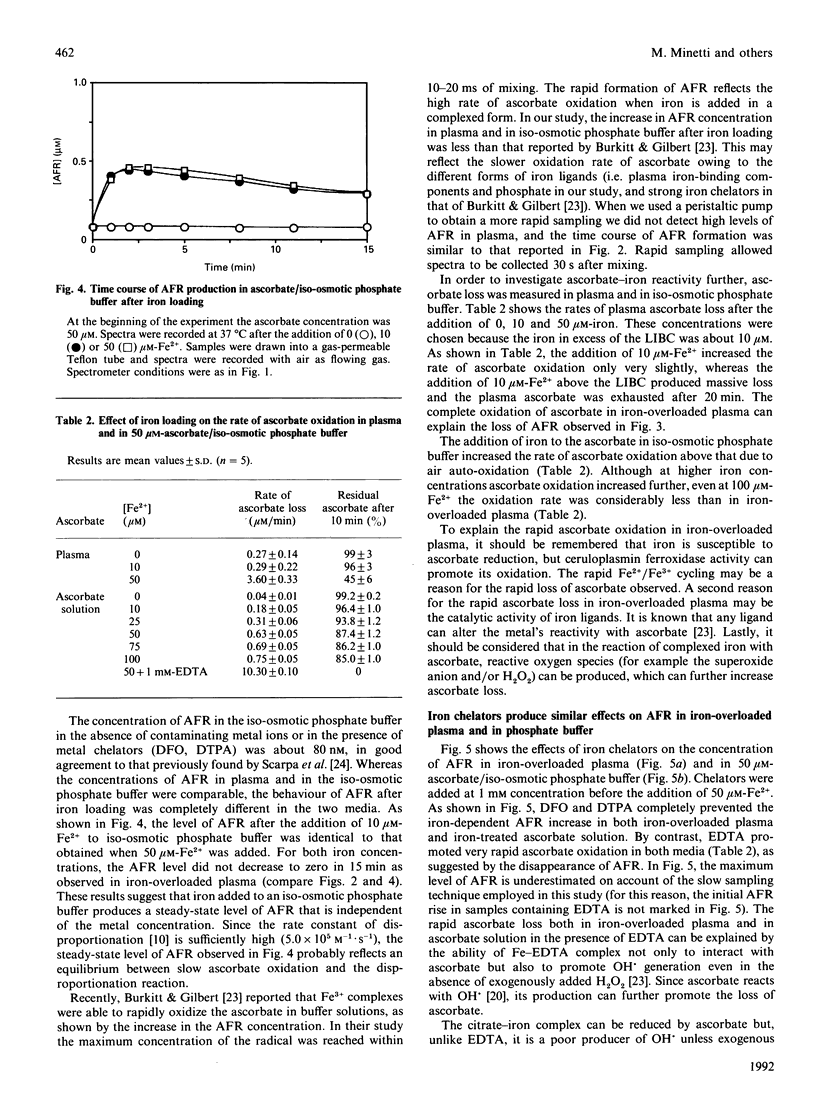
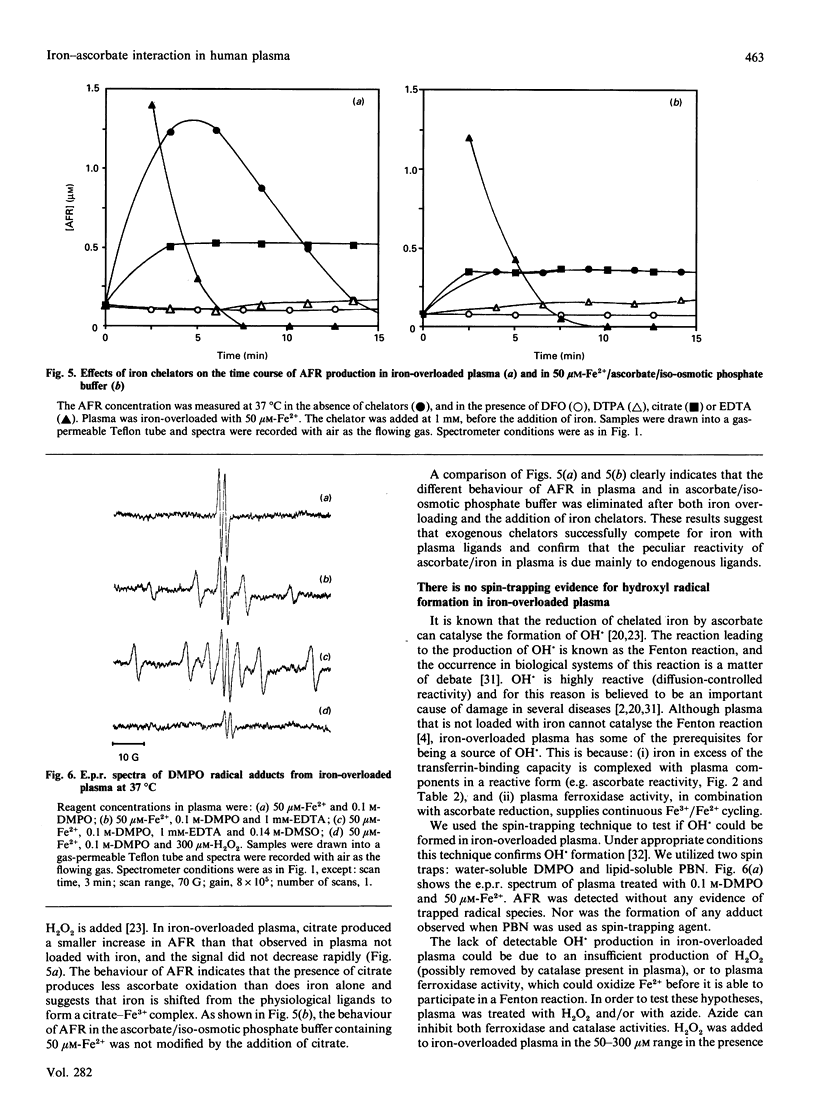
A study was made of the interaction of plasma ascorbate and ascorbate free radical (AFR) with exogenously added iron. The quantitative determination of AFR has the advantage that transient increases in ascorbate oxidation can be directly monitored by e.p.r. spectroscopy. An AFR signal was found in the plasma of all donors and was unaffected by superoxide dismutase, catalase and the strong iron chelator deferoxamine. These findings and the rapid decrease in AFR under a nitrogen atmosphere suggest that plasma AFR is probably a result of air auto-oxidation. Iron loading of plasma did not affect the intensity of the AFR signal until the iron concentration approached or exceeded the plasma latent iron-binding capacity. In iron-overloaded plasma, the intensity of the AFR signal increased to about 10 times the normal level before decreasing rapidly to undetectable levels after 15-20 min. Determination of plasma ascorbate showed that the disappearance of AFR was due to a complete loss of the vitamin. When 50 microM-ascorbate was loaded with iron in iso-osmotic phosphate buffer there was an increase in the AFR signal, independent of the iron concentration, which was stable at least for 15 min. Thus the rate of ascorbate loss in the iso-osmotic phosphate buffer was considerably lower than in iron-overloaded plasma. The addition of different iron chelators produced comparable effects on the intensity of the AFR signal in both iron-overloaded plasma and ascorbate solution. These results suggest that the characteristic behaviour of plasma AFR after iron loading is due to its specific iron-binding capacity and to plasma ferroxidase activity. The ferroxidase activity of plasma is important to promote the transfer of Fe2+ into transferrin without a transient ascorbate oxidation. Spin-trapping studies with 5,5-dimethyl-1-pyrroline N-oxide and N-t-butyl-alpha-phenylnitrone revealed that iron-overloaded plasma was unable to produce spin-trap adducts even in the presence of 50-300 microM-hydrogen peroxide or 100 microM-azide. Evidence of OH. radical formation was obtained only after the addition of EDTA. Therefore, iron-overloaded plasma itself does not produce a Fenton reaction and, if ascorbate does indeed have a free-radical-mediated pro-oxidant role, it is not detectable in plasma by spin-trapping experiments.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P., Brown E. B. Structure and function of transferrin. Prog Hematol. 1975;9:25–56. [PubMed] [Google Scholar]
- Ames B. N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983 Sep 23;221(4617):1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- Aruoma O. I., Halliwell B. Superoxide-dependent and ascorbate-dependent formation of hydroxyl radicals from hydrogen peroxide in the presence of iron. Are lactoferrin and transferrin promoters of hydroxyl-radical generation? Biochem J. 1987 Jan 1;241(1):273–278. doi: 10.1042/bj2410273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolann B. J., Ulvik R. J. On the limited ability of superoxide to release iron from ferritin. Eur J Biochem. 1990 Nov 13;193(3):899–904. doi: 10.1111/j.1432-1033.1990.tb19415.x. [DOI] [PubMed] [Google Scholar]
- Boyer R. F., McCleary C. J. Superoxide ion as a primary reductant in ascorbate-mediated ferritin iron release. Free Radic Biol Med. 1987;3(6):389–395. doi: 10.1016/0891-5849(87)90017-7. [DOI] [PubMed] [Google Scholar]
- Britigan B. E., Hassett D. J., Rosen G. M., Hamill D. R., Cohen M. S. Neutrophil degranulation inhibits potential hydroxyl-radical formation. Relative impact of myeloperoxidase and lactoferrin release on hydroxyl-radical production by iron-supplemented neutrophils assessed by spin-trapping techniques. Biochem J. 1989 Dec 1;264(2):447–455. doi: 10.1042/bj2640447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner G. R. Ascorbate autoxidation in the presence of iron and copper chelates. Free Radic Res Commun. 1986;1(6):349–353. doi: 10.3109/10715768609051638. [DOI] [PubMed] [Google Scholar]
- Buettner G. R. Ascorbate oxidation: UV absorbance of ascorbate and ESR spectroscopy of the ascorbyl radical as assays for iron. Free Radic Res Commun. 1990;10(1-2):5–9. doi: 10.3109/10715769009145927. [DOI] [PubMed] [Google Scholar]
- Buettner G. R. In the absence of catalytic metals ascorbate does not autoxidize at pH 7: ascorbate as a test for catalytic metals. J Biochem Biophys Methods. 1988 May;16(1):27–40. doi: 10.1016/0165-022x(88)90100-5. [DOI] [PubMed] [Google Scholar]
- Buettner G. R., Oberley L. W. Considerations in the spin trapping of superoxide and hydroxyl radical in aqueous systems using 5,5-dimethyl-1-pyrroline-1-oxide. Biochem Biophys Res Commun. 1978 Jul 14;83(1):69–74. doi: 10.1016/0006-291x(78)90398-4. [DOI] [PubMed] [Google Scholar]
- Burkitt M. J., Gilbert B. C. Model studies of the iron-catalysed Haber-Weiss cycle and the ascorbate-driven Fenton reaction. Free Radic Res Commun. 1990;10(4-5):265–280. doi: 10.3109/10715769009149895. [DOI] [PubMed] [Google Scholar]
- Chidambaram M. V., Barnes G., Frieden E. Ceruloplasmin and the reactions forming diferric transferrin. FEBS Lett. 1983 Aug 8;159(1-2):137–140. doi: 10.1016/0014-5793(83)80432-3. [DOI] [PubMed] [Google Scholar]
- Cohen A., Cohen I. J., Schwartz E. Scurvy and altered iron stores in thalassemia major. N Engl J Med. 1981 Jan 15;304(3):158–160. doi: 10.1056/NEJM198101153040307. [DOI] [PubMed] [Google Scholar]
- Cross C. E., Halliwell B., Borish E. T., Pryor W. A., Ames B. N., Saul R. L., McCord J. M., Harman D. Oxygen radicals and human disease. Ann Intern Med. 1987 Oct;107(4):526–545. doi: 10.7326/0003-4819-107-4-526. [DOI] [PubMed] [Google Scholar]
- Frei B., England L., Ames B. N. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6377–6381. doi: 10.1073/pnas.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg H., Grebing C., Löw H. NADH-monodehydroascorbate reductase in human erythrocyte membranes. Biochem Int. 1983 Jan;6(1):1–9. [PubMed] [Google Scholar]
- Grootveld M., Bell J. D., Halliwell B., Aruoma O. I., Bomford A., Sadler P. J. Non-transferrin-bound iron in plasma or serum from patients with idiopathic hemochromatosis. Characterization by high performance liquid chromatography and nuclear magnetic resonance spectroscopy. J Biol Chem. 1989 Mar 15;264(8):4417–4422. [PubMed] [Google Scholar]
- Gutteridge J. M. Fate of oxygen free radicals in extracellular fluids. Biochem Soc Trans. 1982 Apr;10(2):72–73. doi: 10.1042/bst0100072. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Iron and oxygen: a biologically damaging mixture. Acta Paediatr Scand Suppl. 1989;361:78–85. doi: 10.1111/apa.1989.78.s361.78. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Iron promoters of the Fenton reaction and lipid peroxidation can be released from haemoglobin by peroxides. FEBS Lett. 1986 Jun 9;201(2):291–295. doi: 10.1016/0014-5793(86)80626-3. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Rowley D. A., Griffiths E., Halliwell B. Low-molecular-weight iron complexes and oxygen radical reactions in idiopathic haemochromatosis. Clin Sci (Lond) 1985 Apr;68(4):463–467. doi: 10.1042/cs0680463. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys. 1986 May 1;246(2):501–514. doi: 10.1016/0003-9861(86)90305-x. [DOI] [PubMed] [Google Scholar]
- Halliwell B. How to characterize a biological antioxidant. Free Radic Res Commun. 1990;9(1):1–32. doi: 10.3109/10715769009148569. [DOI] [PubMed] [Google Scholar]
- Hershko C., Weatherall D. J. Iron-chelating therapy. Crit Rev Clin Lab Sci. 1988;26(4):303–345. doi: 10.3109/10408368809105894. [DOI] [PubMed] [Google Scholar]
- Jain S. K., Williams D. M. Reduced levels of plasma ascorbic acid (vitamin C) in sickle cell disease patients: its possible role in the oxidant damage to sickle cells in vivo. Clin Chim Acta. 1985 Jul 15;149(2-3):257–261. doi: 10.1016/0009-8981(85)90339-0. [DOI] [PubMed] [Google Scholar]
- Lambeth D. O., Ericson G. R., Yorek M. A., Ray P. D. Implications for in vitro studies of the autoxidation of ferrous ion and the iron-catalyzed autoxidation of dithiothreitol. Biochim Biophys Acta. 1982 Dec 17;719(3):501–508. doi: 10.1016/0304-4165(82)90239-2. [DOI] [PubMed] [Google Scholar]
- Osaki S., Johnson D. A., Frieden E. The possible significance of the ferrous oxidase activity of ceruloplasmin in normal human serum. J Biol Chem. 1966 Jun 25;241(12):2746–2751. [PubMed] [Google Scholar]
- Rosen G. M., Halpern H. J. Spin trapping biologically generated free radicals: correlating formation with cellular injury. Methods Enzymol. 1990;186:611–621. doi: 10.1016/0076-6879(90)86156-p. [DOI] [PubMed] [Google Scholar]
- Rowley D. A., Halliwell B. Formation of hydroxyl radicals from hydrogen peroxide and iron salts by superoxide- and ascorbate-dependent mechanisms: relevance to the pathology of rheumatoid disease. Clin Sci (Lond) 1983 Jun;64(6):649–653. doi: 10.1042/cs0640649. [DOI] [PubMed] [Google Scholar]
- Scarpa M., Stevanato R., Viglino P., Rigo A. Superoxide ion as active intermediate in the autoxidation of ascorbate by molecular oxygen. Effect of superoxide dismutase. J Biol Chem. 1983 Jun 10;258(11):6695–6697. [PubMed] [Google Scholar]
- Silcock J. M., Dodd N. J. Electron spin resonance study of changes during development of solid Yoshida tumour. I: Ascorbyl radical. Br J Cancer. 1976 Nov;34(5):550–555. doi: 10.1038/bjc.1976.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Hider R. C., Porter J. B. A direct method for quantification of non-transferrin-bound iron. Anal Biochem. 1990 May 1;186(2):320–323. doi: 10.1016/0003-2697(90)90088-q. [DOI] [PubMed] [Google Scholar]
- Stocks J., Gutteridge J. M., Sharp R. J., Dormandy T. L. The inhibition of lipid autoxidation by human serum and its relation to serum proteins and alpha-tocopherol. Clin Sci Mol Med. 1974 Sep;47(3):223–233. doi: 10.1042/cs0470223. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C. Comparison of superoxide with other reducing agents in the biological production of hydroxyl radicals. Biochem J. 1979 Aug 15;182(2):625–628. doi: 10.1042/bj1820625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C. Hydroxyl radical production in body fluids. Roles of metal ions, ascorbate and superoxide. Biochem J. 1981 Jul 15;198(1):125–131. doi: 10.1042/bj1980125. [DOI] [PMC free article] [PubMed] [Google Scholar]


