Abstract
Pinus halepensis Mill. is a Mediterranean aromatic plant largely used, in addition to its nutritional value, in traditional medicine as antiseptic, antifungal, antituberculotic, and antirheumatic. Thus, the objective of this work was to appraise the antioxidant and cytotoxic activity of the essential oil (EO) of P. halepensis from Tunisia on cancer cell cultures, along with chemical composition evaluation by GC–MS. To attain the best yield and also highest quality in extraction of the EOs, conventional hydrodistillation (HD) and novel microwave-assisted extraction (MAE) methods have been performed and compared. The antioxidant activity was evaluated through the inhibition of 2,2-diphenyl-1-picrylhydrazyl (DPPH)• radicals. The cytotoxic activity in prostate cancer cells (LNCaP and PC3) and cervical cancer cells (HeLa) of EO was evaluated by the MTT assay and effect on the cell cycle by flow cytometry analysis. A total of 38 and 37 components were identified from HD (HD-EO)- and MAE (MAE-EO)-extracted EOs, respectively, which were dominated by hydrocarbon compounds (HD-EO = 86.65%; MAE-EO = 77.36%), especially monoterpenes (HD-EO = 32.11%; MAE-EO = 21.55%) and sesquiterpenes (HD-EO = 44.29%; MAE-EO = 61.32%). Both extracted EOs showed significant antioxidant activity, as shown by the inhibition of DPPH• radicals [IC50 (HD-EO) = 4102.30 ± 159.73 μg mL–1 and IC50 (MAE-EO) = 3430.13 ± 78.46 μg mL–1]. Also, the EOs exhibited substantial (p < 0.001) antiproliferative activities with G0-G1 arrest on PC3, LNCaP, and HeLa cells by yielding very low IC50 values more conspicuous in MAE-EO with respective IC50 values of 25.70 ± 6.58, 14.97 ± 3.21, and 14.55 ± 2.30 μg mL–1. This finding points out for the first time that the EO of P. halepensis Mill. from Tunisia can be an effective natural antitumor agent with more pronounced activity when extracted with the MAE method that, after further in vivo studies, can be harnessed as a putative phytopharmaceutical for prostate and cervical cancer treatment.
Introduction
With 19.3 million new cases and about 10 million cancer deaths predicted for 2020, cancer is one of the leading causes of death worldwide. By 2040, there would be 28.4 million new cases, up 47% from 2020, according to predictions.1 Cervical cancer ranks fourth among tumors in women’s cancer incidence,2 accounting for over 342.0 deaths in women in 2020.1 Prostate cancer is the second most frequent type of cancer in men, accounting for over 375.0 deaths worldwide.1
Several in vivo and in vitro studies have pointed out putative anticancer activities for numerous essential oils (EOs) against many tumor cell lines. Hence, the prostate cancer PC3 cells are sensitive to EOs from Pinus wallichiana,3Hypericum hircinum L. subsp. majus,4 and Solanum erianthum.5 Likewise, the EO of Machilus mushaensis shows anticancer activity in cell culture, as cytotoxic activity was observed on oral, colon, liver, melanoma, leukemic, and lung cancer cells.6 Other researchers have described cytotoxic activities of EO from Plectranthus stems on lung metastasis of melanoma tumors .7 According to Bayala et al., the SF-767 cells, derived from a resistant glioblastoma, have their survival significantly decreased when exposed to Lippia multiflora or Ocimum basilicum.8 These data indicate that EOs could be a significant source of new molecules. Also, isolation of these EOs and the characterization of their biological effects are necessary steps to develop new strategies to fight cancer.
Besides, recent advances in in silico techniques have significantly contributed not only to enhance the understanding of the mechanisms of action of EOs but also to aid in the design and development of more effective natural products for therapeutic use. Computational methods such as molecular docking, quantitative structure–activity relationship (QSAR) modeling, and molecular dynamics simulations are commonly used to predict the biological activity of EO components and to understand their interactions with biological targets. Aouf et al.9 applied in silico methods to investigate the antimicrobial activity of EO and their components.
For example, molecular docking studies have been employed to predict the binding affinity of EO constituents for various enzymes and receptors involved in cancer, inflammation, and microbial infections. QSAR modeling helps in understanding the relationship between the chemical structures of EO components and their observed biological activities. Molecular dynamics simulations provide insights into the stability and behavior of these interactions at the molecular level. Jena Sudipta et al.10 have used molecular docking and QSAR studies to explore the anticancer potential of EO compounds from various plants.
Aleppo pine, or Pinus halepensis Mill., is native to the Mediterranean region. It dominates forest forms in semiarid and arid regions, encompassing around 3.5 million hectares.11
Historically, this plant has been widely used in traditional medicine across various cultures. In Arab countries, the seeds of P. halepensis Mill. are traditionally used to prepare sweet pudding. Furthermore, its EO has been employed for its antiseptic, antituberculotic, and antirheumatic properties. The resin and extracts of P. halepensis Mill. have been used to treat wounds, respiratory ailments, and skin infections.12−14 Moreover, various therapeutic properties have been identified for EO from P. halepensis, such as anticoagulant,15 anti-inflammatory,15−17 antimicrobial,18,19 antibacterial,14,20,21 antifungal,22−25 larvicidal,26 antioxidant,15,20,27,28 and anticancer.11,29
The relevance of studying the EO of P. halepensis Mill. lies in its rich chemical composition, which includes a variety of bioactive compounds such as monoterpenes and sesquiterpenes. These compounds are known for their antioxidant, anti-inflammatory, and anticancer activities. Investigating the EO’s biological activities can lead to the discovery of new natural compounds with potential therapeutic applications, particularly in the treatment of cancer and other serious diseases. Understanding the mechanisms by which these bioactive compounds exert their effects can contribute to the development of novel phytopharmaceuticals.30
Since EOs are concentrated hydrophobic solutions containing volatile chemical compounds from plants, the solvent used for the extraction and the chemical methods could modify the composition of the EOs. Currently, a variety of techniques, including solvent extraction, supercritical fluid extraction, and conventional hydrodistillation (HD), are used to extract EOs.31 A number of drawbacks accompany these techniques, including intense heat, the need for comparatively large amounts of hazardous and polluting solvents, and issues with costly electrical equipment.32
On the other hand, novel microwave-assisted extraction (MAE) has been recognized as an important alternative in separation and extraction processes with several advantages compared to other extraction methods, mainly including reduction of extraction time, improvement of extract quality, support for low operating costs and energy consumption, and low CO2 emissions.33−36
The goal of this study is to characterize and analyze the physical and chemical properties of the EOs of the P. halepensis Mill. needles obtained by the novel MAE method and to compare their yield and quality with those obtained by conventional HD. Furthermore, antioxidant and cytotoxic activities of Pinus EOs obtained from both HD and MAE methods have been enhanced and correlated to the quality of both obtained EOs. To our knowledge, no work has been performed for the anticarcinogenic activity of EO from P. halepensis Mill. needles growing in northern Tunisia, against prostate and cervical cancer cells. The present study serves as the first-hand evidence on the fact that this aromatic plant potentially serves as an effective alternative for prostate and cervical cancer future natural treatment.
Results and Discussion
Physical Properties and Chemical Composition of P. halepensis Mill. EO
Table 1 shows, for each of the two extraction methods employed, the extraction yield as well as the physical characteristics (appearance and refractive index of the P. halepensis Mill. EO).37
Table 1. Yields and Physical Properties of EOs of P. halepensis Mill. Extracted by HD and MAE.
| HDa | MAEb | |
|---|---|---|
| material weight (g) | 150 | 150 |
| time of boiling (min) | 30 | 5 |
| time for extraction (min) | 180 | 20 |
| solvent or evaporation | water | water |
| volume of water (mL) | 600 | 150 |
| yield (%) | 0.91 | 0.32 |
| appearance | yellow | pale yellow |
| refractive index 20 °C | 1.4923 | 1.4998 |
HD, hydrodistillation extraction.
MAE, microwave-assisted extraction.
Extraction with MAE began much earlier when compared to HD, with 5 min versus 30 min, respectively. This is because microwaves require more efficient heat flow.38,39 Furthermore, the extraction time is significantly decreased when MAE is used.40
The EO yields obtained from P. halepensis Mill. according to the various isolation techniques are 0.32% for MAE and 0.91% for HD, respectively. This yield was higher than the ones that Hmamouchi et al.19 and Dob et al.41 recorded (0.44 and 0.52%, respectively).
The yield variability from needles, stems, and cones was determined by Amri et al.22 to be 0.85, 0.60, and 0.60%, respectively. According to additional research, the production of EOs from fresh aerial parts of the P. halepensis Mill. ranged from 0.13 to 0.63% at ten locations in West and Northern Algeria.14 Numerous other factors, such as the extraction method, time, temperature, type of solvent, and raw material treatment procedures (powder, drying, etc.), can also affect the yield of the P. halepensis Mill. oil extraction.42
P. halepensis Mill. EOs have a refractive index value of 1.3–1.7 at 20 °C, according to the European Pharmacopoeia [Ph. EUR., 2012]. The refractive index determines the oil quality on a global scale. Higher refractive index EOs are known to be of greater quality than lower refractive index EOs.43 The refractive index and the quality of the extracted EOs are unaffected by the ensemble of extraction techniques.
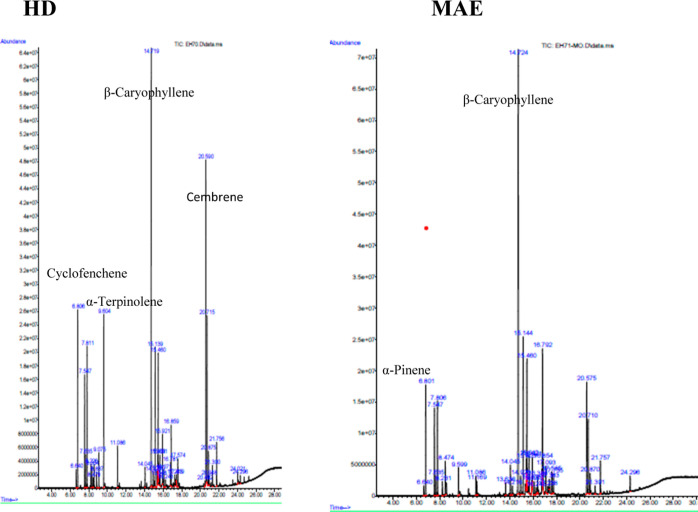
Based on their qualitative chemical structure, a comparative analysis of the two P. halepensis Mill. EOs isolated from the needles was also carried out. Figure 1 displays the chromatograms, and Table 2 lists the chemical compositions.
Figure 1.
GC–MS chromatograms of the P. halepensis Mill. EOs extracted by HD and MAE.
Table 2. Chemical Composition of P. halepensis Mill. Needle EOs Obtained by HD and MAE Processesa.
| compounds | formula | RT | RI | relative
area (%) |
|
|---|---|---|---|---|---|
| HD | MAE | ||||
| β-thujene | C10H16 | 6.640 | 930 | 0.73 | 0.48 |
| α-pinene | C10H16 | 6.801 | 937 | 6.01 | |
| camphene | C10H16 | 6.806 | 952 | 8.05 | |
| sabinene | C10H16 | 7.547 | 975 | 4.60 | 4.47 |
| β-pinene | C10H16 | 7.635 | 979 | 1.17 | 0.92 |
| β-myrcene | C10H16 | 7.806 | 991 | 5.70 | 4.62 |
| α-phellandrene | C10H16 | 8.226 | 1004 | 0.83 | 0.64 |
| 4-carene | C10H16 | 8.334 | 1011 | 0.75 | |
| p-cymene | C10H14 | 8.479 | 1025 | 0.33 | 1.50 |
| β-ocimene | C10H16 | 8.837 | 1038 | 0.55 | |
| γ-terpinene | C10H16 | 9.075 | 1060 | 1.22 | |
| α-terpinolene | C10H16 | 9.604 | 1078 | 6.78 | 1.42 |
| terpinen-4-ol | C10H18O | 11.086 | 1176 | 1.40 | 0.85 |
| p-cymenol | C10H14O | 11.169 | 1183 | 0.64 | |
| α-cubebene | C15H24 | 13.636 | 1351 | 0.53 | |
| α-copaene | C15H24 | 14.045 | 1376 | 0.70 | 1.29 |
| β-cubebene | C15H24 | 14.216 | 1389 | 0.44 | |
| β-caryophyllene | C15H24 | 14.719 | 1405 | 22.20 | 31.50 |
| β-sesquiphellandrene | C15H24 | 14.926 | 0.52 | 0.76 | |
| α-caryophyllene | C15H24 | 15.139 | 1454 | 5.28 | 7.80 |
| γ-muurolene | C15H24 | 15.330 | 1464 | 0.70 | |
| phenylethyl pivalate | C13H18O2 | 15.403 | 0.59 | 0.82 | |
| phenethyl oxalate | 15.460 | 4.84 | 6.29 | ||
| β-cadinene | C15H24 | 15.548 | 0.28 | ||
| α-muurolene | C15H24 | 15.641 | 1499 | 1.20 | 2.08 |
| cubebol | C15H26O | 15.875 | 1515 | 0.43 | |
| δ-cadinene | C15H24 | 15.921 | 1511 | 1.60 | 1.04 |
| α-cedrene | C15H24 | 16.071 | 1410 | 0.57 | 0.53 |
| elemol | C15H26O | 16.243 | 1548 | 0.25 | 0.37 |
| compounds | formula | RT | RI | relative
area (%) |
|
|---|---|---|---|---|---|
| HD | MAE | ||||
| geranylgeraniol | C20H34O | 16.388 | 0.68 | ||
| caryophyllene oxide | C15H24O | 16.781 | 1581 | 0.74 | 5.77 |
| guaiol | C15H26O | 16.859 | 1597 | 2.07 | 1.46 |
| α-humulene epoxide II | C15H24O | 17.093 | 1602 | 1.11 | |
| cadine-1,4-diene | C15H24 | 17.243 | 0.36 | 0.35 | |
| caryophyllenol II | C15H24O | 17.383 | 1655 | 0.39 | |
| cis-muurola-3,5-diene | C15H24 | 17.419 | 0.24 | ||
| α-gurjunene | C15H24 | 17.574 | 1409 | 1.50 | |
| eremophilene | C15H24 | 17.585 | 1.50 | ||
| isoshyobunone | C15H24O | 17.735 | 0.89 | ||
| andien-beta | C19H28O | 20.492 | 0.24 | ||
| cembrene | C20H32 | 20.590 | 1921 | 13.24 | 5.23 |
| β-gurjenene | C15H24 | 20.715 | 1430 | 6.08 | 3.43 |
| 3E-cembrene A | C20H32 | 20.870 | 1954 | 1.17 | 0.91 |
| cembrene A | C20H32 | 20.948 | 1962 | 0.30 | |
| trace | 21.300 | 0.89 | 0.43 | ||
| isocembrol | C20H34O | 21.756 | 1928 | 1.65 | 1.72 |
| methyl 8,15-pimaradien-18-oate | C21H34O2 | 24.021 | 0.40 | ||
| methyl dehydroabietate | C21H30O2 | 24.296 | 0.28 | 0.69 | |
| total | 100 | 99.99 | |||
| hydrocarbon compounds | 86.65 | 77.36 | |||
| oxygenated compounds | 12.46 | 22.11 | |||
| monoterpene hydrocarbons | 30.71 | 20.06 | |||
| oxygenated monoterpenes | 1.40 | 1.49 | |||
| sesquiterpene hydrocarbons | 41.23 | 50.9 | |||
| oxygenated sesquiterpenes | 3.06 | 10.42 | |||
| diterpene hydrocarbons | 14.71 | 6.40 | |||
| oxygenated diterpenes | 1.89 | 2.40 | |||
| esters | 6.11 | 7.80 | |||
RT, retention time; RI, retention index; HD, hydrodistillation extraction; and MAE, microwave-assisted extraction,
With 100 and 99.99% of the total EOs, P. halepensis Mill. EOs extracted by HD and MAE revealed 38 and 37 volatile chemicals, respectively. The main components of the EO recovered using the HD technique were β-caryophyllene (22.20%), cembrene (13.24%), and cyclofenchene (8.05%). Nevertheless, β-caryophyllene (31.50%), α-caryophyllene (7.80%), and phenethyl isovalerate (6.29%) were the dominant constituents of the obtained EO using the MAE method.
The primary volatile component in both EOs which was from P. halepensis Mill. grown in Tunisia is β-caryophyllene, with a greater concentration seen in the MAE method. Samples taken from Tunisia,44,45 Algeria,14,46 Marocco,20,47 France,48 and Greece26 have all shown similar results. Furthermore, α-terpinolene (6.78%) is present in EO extracted by HD; this quantity is reduced with MAE (1.42%). Conversely, HD-EO has a lower content of caryophyllene oxide (0.74%) compared with MAE (5.77%).
Eleven compounds, including camphene (8.05%), 4-carene (0.75%), β-ocimene (0.55%), γ-terpinene (1.22%), γ-muurolene (0.70%), β-cadinene (0.28%), β-bisabolene (0.24%), α-gurjunene (1.50%), andien-beta (0.24%), cembrene A (0.30%), and methyl 8,15-pimaradien-18-oate (0.40%) are the only compounds found in the EO isolated by HD. Conversely, only ten constituents are specifically present in EO isolated by MAE such as α-pinene (6.01%), p-cymenol (0.64%), α-cubebene (0.53%), β-cubebene (0.44%), cubebol (0.43%), elemol (0.68%), α-humulene epoxide II (1.11%), caryophyllenol II (0.39%), cis-α-bisabolene (1.50%), and α-cadinol (0.89%). Consequently, a change of the extraction method may cause certain compounds to disappear or modify the abundances of some compounds.49
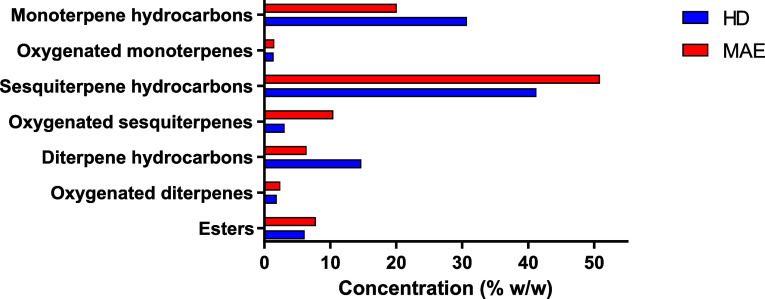
A comparison of the compound chemical classes obtained in both EOs is shown in Figure 2. Significant differences can be observed. MAE gave an EO more concentrated in oxygenated compounds such as terpinen-4-ol, phenethyl isovalerate, caryophyllene oxide, and guaiol (22.11%) compared to the HD method (12.46%).
Figure 2.
Comparison the chemical classes of compounds in EO of P. halepensis Mill. leaves obtained by HD and MAE extraction methods.
Besides, MAE induced the highest percentage of sesquiterpenes (61.32%) compared to HD (44.29%). However, EO extracted by the HD method was found to be richer in monoterpene (33%) than MAE-extracted EO (21.98%).
Previous studies showed that the concentration of oxygenated compounds and sesquiterpenes increased with the MAE technique. According to the current results, there was also a decrease in monoterpene content as compared to the HD approach.50−52 Contrarily, Wang et al.53 found that the oxygenated compounds of the EO obtained by HD were higher than that MAE method.
When comparing the EO extracted by MAE to that extracted by the HD method, the largest concentration of oxygenated molecules is found in the former. This is most likely because MAE includes potential cost savings and efficiency improvements for industrial economic perspectives as the MAE process requires less water than HD in order to decrease degradation of the volatile potent compounds due to oxidation or hydrolysis, which are considered undesirable chemical reactions.36 Additionally, Filly et al.54 described that oxygenated compounds have more dipole moments compared to monoterpene hydrocarbons. This causes them to interact more strongly with microwaves and thus they are easier to extract than monoterpene hydrocarbons.
As shown in previous works,55 the EO’s chemical composition varies and is dependent on various factors, including plant part, maturity, geographic location, climatic conditions, harvesting period, and especially extraction process methods.
Antioxidant Potential of P. halepensis Mill. EO
The antioxidant capacities of P. halepensis Mill. EOs extracted by MAE or HD were measured using 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity assays. The percentage of inhibition as the EO concentration increases is displayed in Figure 3.
Figure 3.
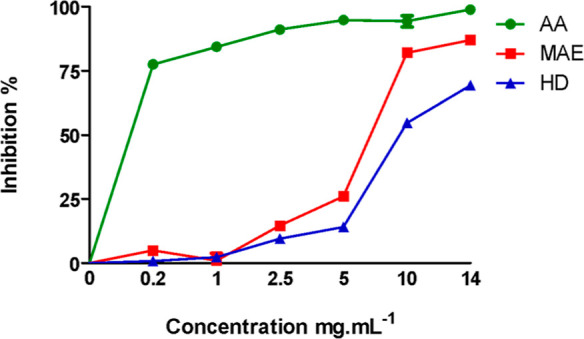
Radical scavenging ability of P. halepensis Mill. EOs was assessed by HD and MAE using different concentrations and ascorbic acid (AA) as control. MAE, microwave extraction; HD, hydrodistillation; AA, ascorbic acid.
Both EOs were able to reduce DPPH• in a concentration-dependent manner. The results obtained were compared to those of standard ascorbic acid (Table 3).
Table 3. Antioxidant Activity Values of EOs Obtained by MAE and HD and Ascorbic Acida.
| DPPH radical scavenging assay activity, IC50 (μg mL–1) | |
|---|---|
| MAE | 3430.132 ± 78.464 |
| HD | 4102.301 ± 159.730 |
| ascorbic acid | 1.501 ± 1.698 |
DPPH: 2,2-diphenyl-1-picrylhydrazyl. IC50 μg mL–1. Data is shown as the mean ± standard deviation (SD) and N = three independent experiments.
The results given in Table 3 showed that the EO obtained by MAE presented a higher antiradical effect as calculated by IC50 = 3430.13 ± 78.46 μg mL–1 compared to that obtained by the HD method IC50 = 4102.30 ± 159.73 μg mL–1. Similar results regarding the EO’s antioxidant activity were also reported by Guo et al.56 and Araujo et al.,57 which reported that the DPPH• free radical scavenging capacity of the EO obtained using microwave extraction was higher than that of the conventional technique.
Similar findings were noticed by Chouchan for the extraction of Mentha spicata, who demonstrates that the EO using SFMAE has better biological activity in terms of antioxidant potential when compared to the EO extracted by the HD method. Consequently, microwave extraction preserves the biological integrity of the product, alleviating concerns that electromagnetic waves could compromise its medicinal value.58 The antioxidant activity not only could be attributed to the presence of the oxygenated compounds which are directly proportional to the antioxidant capacity of the EO59 but also could be related to the amount of sesquiterpene β-caryophyllene as earlier described.60 Indeed, previous studied showed that the antioxidant activity of β-caryophyllene in two test methods was 1.25 μM for DPPH and 3.23 μM for FRAP.61
Cytotoxic Activity of P. halepensis Mill. EO
Several studies have investigated the chemical composition and biological activities of EOs extracted from P. halepensis Mill. and related species. For instance, Bouzenna et al.44 found that the EO of P. halepensis Mill. from Tunisia contained significant amounts of β-caryophyllene and α-pinene which exhibited strong antioxidant and anti-inflammatory activities. Similarly, research by Djerrad et al.46 highlighted the presence of monoterpenes and sesquiterpenes in the EO, which demonstrated notable antimicrobial and cytotoxic effects against various cancer cell lines.
Currently, there has never been any research done on the cytotoxic activity of EO from P. halepensis Mill. needles against the human cancer cells PC3 (prostate), LNCaP (prostate), and HeLa (cervical). To assess the antiproliferative effects of the EOs obtained by MAE and HD, the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction test was performed on these cell lines (Figure 4). Cell viability experiments pointed out that both EOs are potent cytotoxic agents inducing dose-dependent cell cytotoxicity in three tumor cell lines. The median inhibitory concentration IC50 values for each cancer cell line are displayed in Table 4.
Figure 4.
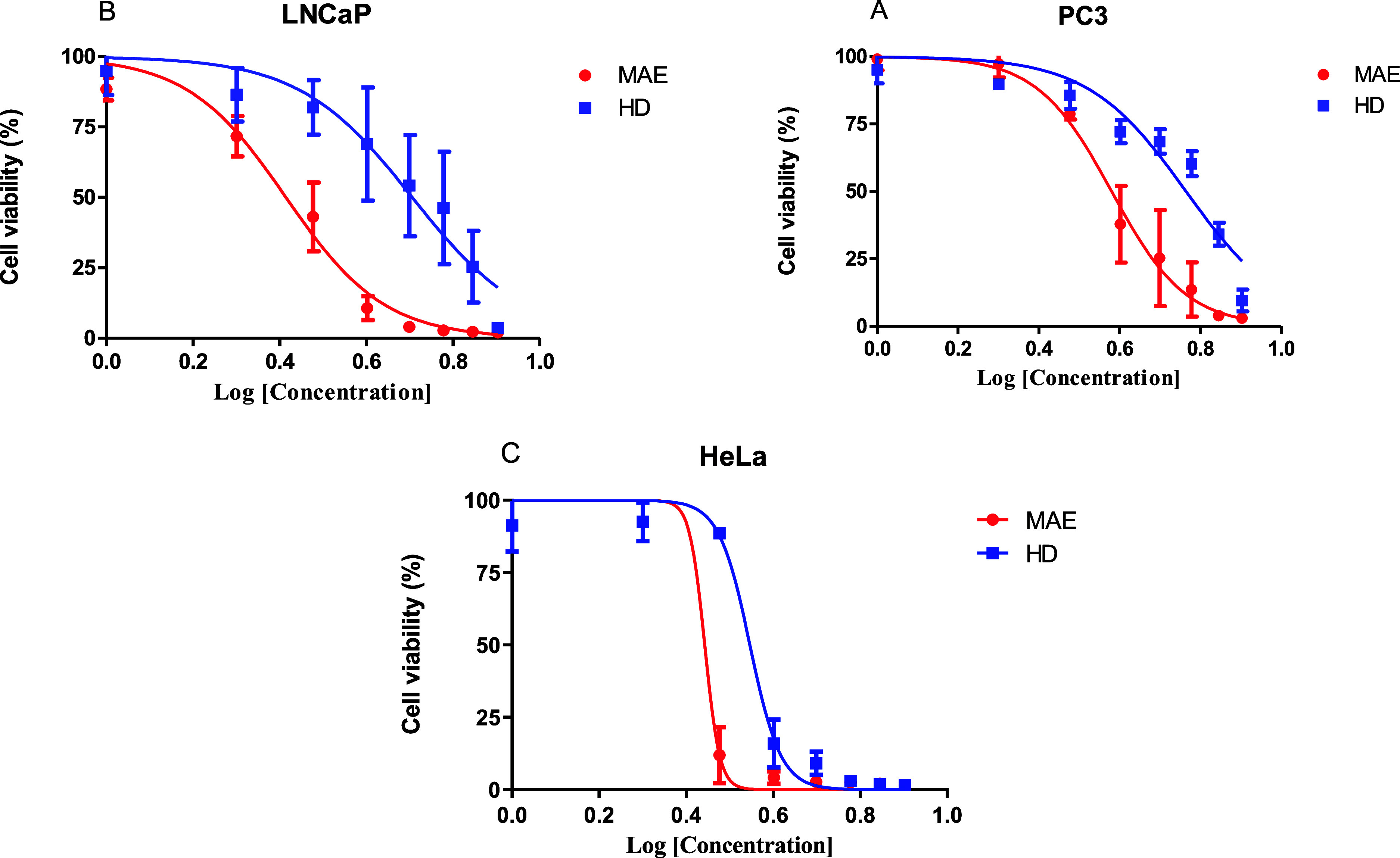
Dose-dependent cytotoxic activity of P. halepensis Mill. EO was assessed by HD and MAE on various cell lines. (A) PC3, (B) LNCaP, and (C) HeLa. The cell lines were treated for 48 h at indicated concentrations expressed in log (μg mL–1). Values are expressed as mean ± SD and N = three independent experiments and assays are carried out in octuplicate.
Table 4. IC50 (Mean ± SD, n = 3) Values of P. halepensis Mill EO on Three Tumor Cell Linesa.
| PC3 | LNCaP | HeLa | |
|---|---|---|---|
| HD | 65.19 ± 2.61 | 49.34 ± 19.49 | 23.05 ± 2.68 |
| MAE | 25.70 ± 6.58 | 14.97 ± 3.21 | 14.55 ± 2.30 |
IC50 (μg mL–1): half maximal inhibitory concentration.
Based on the three cancer cell lines tested, the data show that the MAE-EO was more potent than the HD one, with IC50 values ranging from 14.55 ± 2.30 to 25.70 ± 6.58 μg mL–1 versus 23.05 ± 2.68 to 65.19 ± 2.61 μg mL–1, respectively (Table 4). According to the National Central Institute of the United States, plant extracts with a cytotoxicity showing an IC50 < 30 μg mL–1 could be considered as potential active ingredients for the development of anticancer drugs.62 Therefore, EO obtained from P. halepensis Mill. obtained by MAE with lower IC50 can be considered for further analyses for identifying promising agents for anticancer therapy. The cytotoxicity of this EO can be attributed to the main compound β-caryophyllene, which exhibited cytotoxic activity against PC3, HCT 116, HT-29, ME-180, K562, and PANC-1 cancer cell lines in a previous study.61
Three lipid fractions obtained from P. halepensis Mill. seeds (neutral lipids, glycolipids, and phospholipids) were studied against human cancer cells A549 (lung), HCT 15 (umbilical), and HL60 (myeloma) by the MTT assay at three concentrations (1, 10, and 100 μg mL–1). An absence of inhibition was noted in the three cancer cells. A concentration of 100 μg mL–1 and a low inhibition effect of 10 to 15% cell proliferation were observed in A549 cells.11 These results are in agreement with a previous study, effect of seeds oil of P. halepensis Mill. against U78 cells.29
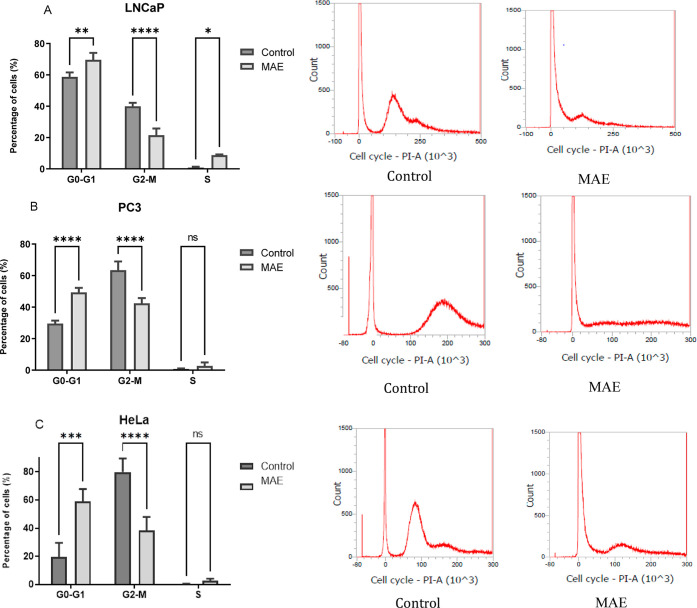
To decipher how EOs obtained by MAE could decrease the cell viability, flow cytometry analysis was performed on the 3 cell lines derived from human tumors. The results are shown in Figure 4 and Table S1.
In the 3 cell lines, P. halepensis Mill. EO increases the proportion of cells in the G0-G1 phase when comparing treated versus untreated cells (70 versus 59% for LNCaP cells, p < 0.01; 50 versus 30% for PC3 cells, p < 0.0001; and 59 versus 20%, p < 0.001 for HeLa cells). Conversely, EO decreases the percentage of cells in the G2-M phase when comparing treated versus untreated cells (21 versus 39% for LNCaP cells, p < 0.0001; 42 versus 64% for PC3 cells, p < 0.0001; and 38 versus 80%, p < 0.0001 for HeLa cells) (Figure 5). Our results indicated that the EO of P halepensis Mill. induces the cytotoxic effect by stopping the cell cycle in the G0-G1 phase in human prostate and cervical cancer cell lines. β-Caryophyllene isolated from Chrysanthemum boreale (Korea) was characterized with anticancer properties which induce the cytotoxic effect in human lung cancer cell lines by stopping the cell cycle in the G1 phase by downregulating cyclin D1, cyclin E, cyclin-dependent protein kinase (CDK)-2, -4, and -6, and RB phosphorylation and by upregulating p21CIP1/WAF1 and p27KIP1.63
Figure 5.
Effect of the P. halepensis Mill. EO extracted by MAE treatment on the cell cycle in cancer for 48 h. (A) LNCaP, (B) PC3, and (C) HeLa. All data are statistically significant (mean ± SD, N = three independent experiments, *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 versus the control group).
EOs of P. halepensis Mill. are mainly enriched in β-caryophyllene. Evidence points out the cytotoxic potential of β-caryophyllene to several cancer cells; this dose-dependent effect ranges from antiproliferative to lethal cytotoxicity. β-Caryophyllene is a “dietary cannabinoid” by the interaction with cannabinoid receptor type 2 (CB2).64 Indeed, the modulation of CB2 has been associated with the cytotoxic potential of BCP (Anticancer Potential of Cannabinoids, Terpenes, and Flavonoids Present in Cannabis). Other mechanisms described include the regulation of different pathways as ROS generation and MAPK activation via JAK1/STAT3, NF-kB, and PI3K/AKT/mTOR/S6K1 and the regulation of apoptotic signaling.65 In addition, the mechanisms of β-caryophyllene cytotoxicity on cancer cells include membrane swelling and remodeling by upregulation of cholesterol and lipid biosynthesis pathway genes (β-caryophyllene enhances the transcriptional upregulation of cholesterol biosynthesis in breast cancer cells).
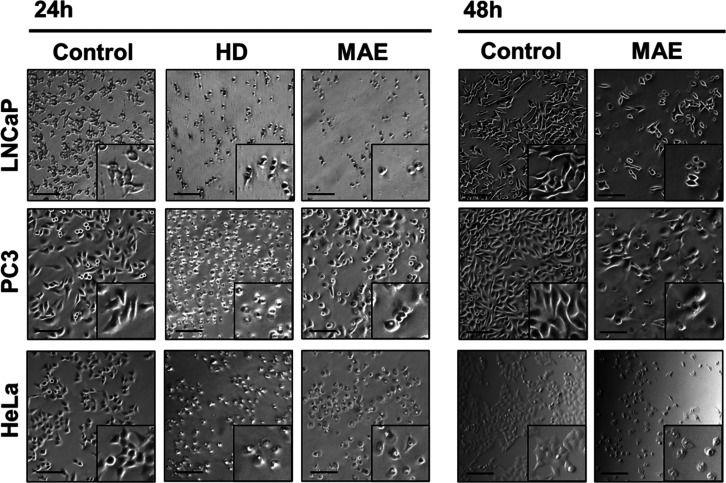
Figure 6 shows representative photos depicting the effect of MAE- and HD-extracted P. halepensis Mill. EOs on PCa cell lines.
Figure 6.
Representative images of PCa cell lines treated with ethanol (control) and EOs of P. halepensis Mill. extracted by HD and MAE at IC50 value and incubated for 24 or 48 h (scale bars, 50 μm).
The EO of P. halepensis Mill. has been extensively studied for its chemical composition and biological activities, with various studies highlighting its rich content of monoterpenes and sesquiterpenes, such as β-caryophyllene and α-pinene, which correlated with its biological activities. Likewise, the obtained finding was in accordance with the recently published research indicating that the EO of Tunisian P. halepensis Mill. needles is rich in β-caryophyllene (41.28%) and α-pinene (14.52%) and possesses a significant antiproliferative effect against human breast cancer (MCF-7) and human colon adenocarcinoma (HT-29) cell lines with IC50 values of 30 ± 2.26 and 26.67 ± 1.31 μg mL–1, respectively.30
In a similar manner, a study by Fekih et al.14 on the EO of P. halepensis Mill. from Algeria reported a similar composition to that found in Tunisian samples, with significant amounts of β-caryophyllene and α-pinene, which exhibited strong antimicrobial and antioxidant activities.
Comparing these findings with other aromatic plants, the EOs of P. wallichiana and Pinus sylvestris have also shown substantial bioactivity. P. wallichiana EO, rich in α-pinene and β-pinene, exhibited strong antiproliferative effects on human prostate cancer cells.66 Moreover, the EO of P. sylvestris, containing compounds like limonene and β-caryophyllene, demonstrated significant antioxidant and anti-inflammatory properties.67
The consistency in the presence of certain bioactive compounds such as β-caryophyllene and α-pinene across different species of Pinus highlights the potential of these oils as sources of natural therapeutic agents. However, the specific composition and concentration of these compounds can vary significantly depending on the geographic location, environmental factors, extraction method, and part of the plant used. These factors underscore the importance of comprehensive studies.
In conclusion, our contribution gives for the first time a comprehensive evaluation of the chemical composition and antioxidant and cytotoxic activities of Pinus EOs extracted with conventional HD and novel MAE methods. The chemical composition points out the richness of both EO extracts in piperitone and terpenes. MAE-EO exhibited a significant antiproliferative effect in human cancer cell lines (LNCaP, PC3, and HeLa), more pronounced than that of HD-EO. The antioxidant capacity of both EOs showed a significant inhibition of DPPH• radicals and the cytotoxic activities on prostate (LNCaP and PC3) and cervical (HeLa) cancer cells are significant. Besides, EOs also prevent the migration of grown cancer cells and lead to the arrest of their cell cycle in the G0-G1phase. Overall, the in vitro screening of antioxidant and cytotoxic activities of EOs of P. halepensis Mill. from Tunisia constitutes a scientific basis to illuminate the mechanisms of action of phytochemicals on human cancer cells in vivo for future application of EOs of P. halepensis Mill. from Tunisia in tumor treatment.
Experimental Section
Plant Material and EO Extraction
Plant Material
In January 2018, fresh P. halepensis Mill. needles, which are not endangered or protected, were collected from Rimel forest in Bizerte located in northern Tunisia (latitude 37° 17′ 48″ N; longitude 10° 0′ 2″ E). The taxonomical identification was confirmed by Pr BOUGHANMI Naziha of the Faculty of Sciences of Bizerte, Tunisia, at a regional INRGREF (National Research Institute of Rural Engineering, Water, and Forests) station.
HD Extraction
European Pharmacopeia states that a 1000 mL round-bottom flask of a traditional Clevenger-type apparatus was filled with 600 mL of distilled water and 150 g of fresh needles. After that, HD was done for 3 h or until no more EO could be reused. The extraction temperature (100 °C) is the same as the boiling point of water. The obtained EO was conserved at 4 °C and protected from light until analysis.
Microwave-Assisted Extraction
Microwave extraction was carried out in a Milestone microwave laboratory oven (NEOS, Milestone, Italy). It is a multimode 2.45 GHz reactor that can output power in increments of 10 up to a maximum of 900 W. The software controlled the power, temperature, time, and stirring rate during the experiment. 150 mL of distilled water and 150 g of fresh needles were combined in a 2 L Pyrex glass cylinder. The MAE process was split into two stages: the sample was heated in the first step to almost the boiling point (100 °C), and the oil was then distilled in the second step. A 500 W microwave power and a 250 rpm stirring rate were maintained during the 20 min microwave extraction. At the end of the experiment, EO was gathered and kept in a brown glass vial at 4 °C for further chemical and biological analysis.
Yield Extraction
Using both methods, the extraction yield of EOs from P. halepensis Mill. was determined to be as follows (eq 1)
| 1 |
Refractive Index
The AOAC methods68 were used to determine the EO’s refractive index obtained by the two methods from the needles of P. halepensis Mill., and the temperature was 20 °C.
Chemical Composition
Gas Chromatography–Mass Spectrometry Analysis
Mass spectrometry analyses of EO were performed on an Agilent model 6890N gas chromatograph, coupled with an Agilent MSD 5973N model. This setup was equipped with an Agilent J&W Scientific capillary HP-5MS column (30 × 0.25 mm, a film thickness of 0.25 μm). The temperatures of the injector and detector were maintained at 250 and 300 °C, respectively. The volume of 1 μL of the EO solution, diluted in n-hexane at a ratio of 1/10 (v/v), was injected in split mode with a split ratio of 1/10. Helium (He) was carried as the carrier gas at a flow rate of 1 mL min–1. Temperature programming of the oven was initiated with a 3 min hold at 60 °C followed by a gradual increase to 300 °C and held at 300 °C for 37 min. The temperatures of source and quadrupole remained fixed at 200 and 150 °C, respectively. Mass scanning was conducted from 50 to 500 m/z with an ionizing voltage of 70 eV.
Identification of Components
The main compounds in the EO of P. halepensis Mill. were performed on the basis of retention indices determined by coinjection with reference to a homologous series of n-alkanes, under identical experimental conditions. The identification was performed by comparing of their mass spectra with those from commercial libraries NIST17 and with data from mass spectrum literature; the oil constituents were determined.69
Antioxidant Activity
Based on the radical scavenging effect of the stable DPPH (Sigma-Aldrich, USA) free radical activity, as reported by Brand-Williams et al.,70 the total antioxidant property of the EOs of P. halepensis Mill. was evaluated. The samples were first diluted in methanol (1 mg mL–1). After the DPPH solution was added to the samples and properly mixed, the reaction was allowed to occur for 30 min. The absorbance at 517 nm was measured after 30 min incubation time at room temperature.
Additionally, the DPPH solution in methanol was also tested in the absence of any antioxidant (control). The formula below was used to determine the radical scavenging activity (eq 2)
| 2 |
with Acontrol as the absorbance of the DPPH solution in methanol and Asample as the absorbance of the test sample.
By measuring the effective concentration at which free radicals are scavenged by 50%, or the IC50 value, the scavenging activity was expressed. Nonlinear regression was used to calculate the various IC50 values from graph drawing. The positive control in this experiment was ascorbic acid.
Cell Culture
We have selected three human cancer cell lines that represent common models for prostate and cervical cancers, allowing for the evaluation of the EO efficacy across different cancer types. The human cancer cell lines used were PC3 (derived from bone metastasis of prostate cancer, androgen-insensitive),71 LNCaP (derived from lymph node metastasis of prostate, androgen-responsive),72 and HeLa (derived from a cervical tumor).73 The culture medium used for LNCaP and PC3 cells was RPMI 1640 (Invitrogen, Carlsbad, CA). DMEM was used as a culture medium for HeLa cells (Invitrogen, Carlsbad, CA). The Genetics, Reproduction, and Development Institute possessed all of these cell lines. The cells were cultured as monolayer adherent cultures in 75 cm2 tissue culture flasks at 37 °C in a humidified atmosphere with 5% CO2. The medium was supplemented with 10% fetal calf serum (FCS, Biowest, Nuaillé, France), 1% streptomycin (Invitrogen, Oslo, Norway), and 1% penicillin.
Cytotoxicity Assay
The cytotoxicity and IC50 values of the EOs were ascertained by the MTT assays (Sigma-Aldrich, USA).74 In 96-well plates, cancer cell lines were plated at a density of 2 × 104 cells per well. The cell lines were treated with the EOs at varying concentrations for 48 h after the first 24 h, with ethanol (max 1/1000) serving as the solvent.75 Following treatments, cancer cells were incubated for 4 h at 37 °C with 5 mg mL–1 of MTT solution. Utilizing a Multiskan GO (Thermo Scientific, USA) microplate reader spectrophotometer, the absorbance at 570 nm was measured to investigate the viability of the cells. The proportion of cell viability relative to the 100% control group was used to express it.
The MTT assay was performed in triplicate for each concentration of EO, and each experiment was repeated three times independently for each cell line to ensure the reliability and reproducibility of the results.
Cell Cycle Analysis
Cancer cells were initially placed into 6-well plates, incubated at a concentration of 35 × 104 for a duration of 24 h at 37 °C. Prior to the addition of the test compound, EO was processed and applied at a concentration corresponding to the IC50 value. The treated cells were then allowed to incubate for 48 h. After the treatment period, the cell lines were trypsinized, centrifuged, fixed in 4% (v/v) formaldehyde solution in PBS for 15 min at room temperature, and subsequently washed with PBS to prepared them in suspension as described by Bayala et al.76 This step allows for the centrifugation of cancer cells and the removal of supernatant. After that, 0.2 mL of the FxCycle PI/RNase staining solution (Invitrogen, Carlsbad, CA) was added to each tube and thoroughly mixed. The samples were then incubated for 30 min at room temperature and protected from light. Cancer cells were then subjected to flow cytometry for cell cycle analysis using FACS with excitation at 488 nm, and the emissions were collected through a 585/42 bandpass filter. Flow cytometry analysis was performed at GReD.
Statistical Analysis
GraphPad Prism was used for all statistical analyses, with each experiment’s mean ± SD calculated from a minimum of three separate experiments. Two-way RM ANOVA was used to analyze the differences between the control and treated groups and they were considered significant, and the differences were p < 0.05.
Acknowledgments
All authors acknowledge the financial support through Carthage University, Ministry of Higher Education and Scientific Research, Tunisia.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.4c05123.
Table of the effect of MAE-extracted P. halepensis Mill EO on the cancerous cell cycle (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Sung H.; Ferlay J.; Siegel R. L.; Laversanne M.; Soerjomataram I.; Jemal A.; Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-Cancer J. Clin. 2021, 71 (3), 209–249. 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Bray F.; Ferlay J.; Soerjomataram I.; Siegel R. L.; Torre L. A.; Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-Cancer J. Clin. 2018, 68 (6), 394–424. 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Yousuf Dar M.; Shah W. A.; Mubashir S.; Rather M. A. Chromatographic analysis, anti-proliferative and radical scavenging activity of Pinus wallichina essential oil growing in high altitude areas of Kashmir, India. Phytomedicine 2012, 19 (13), 1228–1233. 10.1016/j.phymed.2012.07.015. [DOI] [PubMed] [Google Scholar]
- Quassinti L.; Lupidi G.; Maggi F.; Sagratini G.; Papa F.; Vittori S.; Bianco A.; Bramucci M. Antioxidant and antiproliferative activity of Hypericum hircinum L. subsp. majus (Aiton) N. Robson essential oil. Nat. Prod. Res. 2013, 27 (10), 862–868. 10.1080/14786419.2012.677044. [DOI] [PubMed] [Google Scholar]
- Essien E.; Ogunwande I.; Setzer W.; Ekundayo O. Chemical composition, antimicrobial, and cytotoxicity studies on S. erianthum and S. macranthum essential oils. Pharm. Biol. 2012, 50 (4), 474–480. 10.3109/13880209.2011.614623. [DOI] [PubMed] [Google Scholar]
- Su Y.-C.; Ho C.-L. Composition, in-vitro anticancer, and antimicrobial activities of the leaf essential oil of Machilus mushaensis from Taiwan. Nat. Prod. Commun. 2013, 8 (2), 273–275. 10.1177/1934578x1300800236. [DOI] [PubMed] [Google Scholar]
- Manjamalai A.; Grace V. M. B. The chemotherapeutic effect of essential oil of Plectranthus amboinicus (Lour) on lung metastasis developed by B16F-10 cell line in C57BL/6 mice. Cancer Invest. 2013, 31 (1), 74–82. 10.3109/07357907.2012.749268. [DOI] [PubMed] [Google Scholar]
- Bayala B.; Bassole I. H. N.; Gnoula C.; Nebie R.; Yonli A.; Morel L.; Figueredo G.; Nikiema J.-B.; Lobaccaro J.-M. A.; Simpore J. Chemical composition, antioxidant, anti-inflammatory and anti-proliferative activities of essential oils of plants from Burkina Faso. PLoS One 2014, 9 (3), e92122 10.1371/journal.pone.0092122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aouf A.; Bouaouina S.; Abdelgawad M. A.; Abourehab M. A.; Farouk A. In silico study for Algerian essential oils as antimicrobial agents against multidrug-resistant bacteria isolated from pus samples. Antibiotics 2022, 11 (10), 1317. 10.3390/antibiotics11101317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda P. C.; Jena S.; Ray A.; Sahoo A.; Das P. K.; Kamila P. K.; Kar S. K.; Nayak S. Anti-proliferative activity of Piper trioicum leaf essential oil based on phytoconstituent analysis, molecular docking and in silico ADMET approaches. Comb. Chem. High Throughput Screening 2023, 26 (1), 183–190. 10.2174/1386207325666211222113239. [DOI] [PubMed] [Google Scholar]
- Kadri N.; Khettal B.; Adjebli A.; Cresteil T.; Yahiaoui-Zaidi R.; Barragan-Montero V.; Montero J.-L. Antiangiogenic activity of neutral lipids, glycolipids, and phospholipids fractions of Pinus halepensis Mill. seeds. Ind. Crops Prod. 2014, 54, 6–12. 10.1016/j.indcrop.2013.12.051. [DOI] [Google Scholar]
- Macchioni F.; Cioni P.; Flamini G.; Morelli I.; Maccioni S.; Ansaldi M. Chemical composition of essential oils from needles, branches and cones of Pinus pinea, P. halepensis, P. pinaster and P. nigra from central Italy. Flavour Fragrance J. 2003, 18 (2), 139–143. 10.1002/ffj.1178. [DOI] [Google Scholar]
- Cheikh-Rouhou S.; Hentati B.; Besbes S.; Blecker C.; Deroanne C.; Attia H. Chemical composition and lipid fraction characteristics of Aleppo pine (Pinus halepensis Mill.) seeds cultivated in Tunisia. Food Sci. Technol. Int. 2006, 12 (5), 407–415. 10.1177/1082013206069910. [DOI] [Google Scholar]
- Fekih N.; Allali H.; Merghache S.; Chaïb F.; Merghache D.; El Amine M.; Djabou N.; Muselli A.; Tabti B.; Costa J. Chemical composition and antibacterial activity of Pinus halepensis Miller growing in West Northern of Algeria. Asian Pac. J. Trop. Dis. 2014, 4 (2), 97–103. 10.1016/S2222-1808(14)60323-6. [DOI] [Google Scholar]
- Abbou A.; Kadri N.; Debbache N.; Dairi S.; Remini H.; Dahmoune F.; Berkani F.; Adel K.; Belbahi A.; Madani K. Effect of precipitation solvent on some biological activities of polysaccharides from Pinus halepensis Mill. seeds. Int. J. Biol. Macromol. 2019, 141, 663–670. 10.1016/j.ijbiomac.2019.08.266. [DOI] [PubMed] [Google Scholar]
- Basholli-Salihu M.; Schuster R.; Hajdari A.; Mulla D.; Viernstein H.; Mustafa B.; Mueller M. Phytochemical composition, anti-inflammatory activity and cytotoxic effects of essential oils from three Pinus spp. Pharm. Biol. 2017, 55 (1), 1553–1560. 10.1080/13880209.2017.1309555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotroni E.; Simirioti E.; Kikionis S.; Sfiniadakis I.; Siamidi A.; Karalis V.; Vitsos A.; Vlachou M.; Ioannou E.; Roussis V.; et al. In Vivo evaluation of the anti-inflammatory activity of electrospun micro/nanofibrous patches loaded with pinus halepensis bark extract on hairless mice skin. Materials 2019, 12 (16), 2596. 10.3390/ma12162596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy M. E.; Kherallah I. E.; Badawy M. E.; Salem M. Z.; Yousef H. A. Chemical composition and activity of bark and leaf extracts of Pinus halepensis and Olea europaea grown in AL-Jabel AL-Akhdar region, Libya against some plant phytopathogens. J. Appl. Biotechnol. Bioeng. 2017, 3, 331–342. 10.15406/jabb.2017.03.00067. [DOI] [Google Scholar]
- Hmamouchi M.Chemical and antimicrobial properties of essential oils of five Moroccan Pinaceae. In The Antimicrobial/Biological Activity of Essential Oils; Allured Publishing Corporation, 2005. [Google Scholar]
- Bouyahya A.; Belmehdi O.; Abrini J.; Dakka N.; Bakri Y. Chemical composition of Mentha suaveolens and Pinus halepensis essential oils and their antibacterial and antioxidant activities. Asian Pac. J. Trop. Med. 2019, 12 (3), 117. 10.4103/1995-7645.254937. [DOI] [Google Scholar]
- Raho G. B. Antibacterial potential of essential oils of the needles of Pinus halepensis against Staphylococcus aureus and Escherichia coli. J. Coastal Life Med. 2014, 2 (8), 651–655. 10.12980/JCLM.2.2014APJTD-2014-0041. [DOI] [Google Scholar]
- Amri I.; Hamrouni L.; Hanana M.; Gargouri S.; Fezzani T.; Jamoussi B. Chemical composition, physico-chemical properties, antifungal and herbicidal activities of Pinus halepensis Miller essential oils. Biol. Agric. Hortic. 2013, 29 (2), 91–106. 10.1080/01448765.2013.764486. [DOI] [Google Scholar]
- Hamrouni L.; Hanana M.; Amri I.; Romane A. E.; Gargouri S.; Jamoussi B. Allelopathic effects of essential oils of Pinus halepensis Miller: chemical composition and study of their antifungal and herbicidal activities. Arch. Phytopathol. Plant Prot. 2015, 48 (2), 145–158. 10.1080/03235408.2014.884667. [DOI] [Google Scholar]
- Amri I.; Lamia H.; Gargouri S.; Hanana M.; Mahfoudhi M.; Fezzani T.; Ezzeddine F.; Jamoussi B. Chemical composition and biological activities of essential oils of Pinus patula. Nat. Prod. Commun. 2011, 6 (10), 1531–1536. 10.1177/1934578x1100601031. [DOI] [PubMed] [Google Scholar]
- Mohamed A. A.; Behiry S. I.; Ali H. M.; EL-Hefny M.; Salem M. Z.; Ashmawy N. A. Phytochemical compounds of branches from P. halepensis oily liquid extract and S. terebinthifolius essential oil and their potential antifungal activity. Processes 2020, 8 (3), 330. 10.3390/pr8030330. [DOI] [Google Scholar]
- Mitić Z. S.; Jovanović B.; JovanovićStojanović-RadićMihajilov-KrstevJovanovićNikolić S. Č.Z. Z. T. N. M. B. M.; Marin P. D.; Zlatković B. K.; Stojanović G. S.; Nikolić B. M.; Marin P. D.; Zlatković B. K.; Stojanović G. S. Essential oils of Pinus halepensis and P. heldreichii: Chemical composition, antimicrobial and insect larvicidal activity. Ind. Crops Prod. 2019, 140, 111702. 10.1016/j.indcrop.2019.111702. [DOI] [Google Scholar]
- Salim H.; Rimawi W. H.; Shaheen S.; Mjahed A. Phytochemical analysis and antibacterial activity of extracts from Palestinian Aleppo pine seeds, bark and cones. Asian J. Chem. 2019, 31 (1), 143–147. 10.14233/ajchem.2019.21633. [DOI] [Google Scholar]
- Meziti H.; Bouriche H.; Kada S.; Demirtas I.; Kizil M.; Senator A.; Garrido G. Phytochemical analysis, and antioxidant, anti-hemolytic and genoprotective effects of Quercus ilex L. and Pinus halepensis Mill. methanolic extracts. J. Pharm. Pharmacogn. Res. 2019, 7, 260–272. 10.56499/jppres19.569_7.4.260. [DOI] [Google Scholar]
- Rigane G.; Jebali J.; Ghazghazi H.; Riguene H.; Laarbi Khouja M.; Ben Salem R. Chemical composition and biological activities of pinus halepensis Mill. oil. Rev. Roum. Chim. 2019, 64 (11), 999–1006. 10.33224/rrch/2019.64.11.09. [DOI] [Google Scholar]
- Dakhlaoui S.; Bourgou S.; Bachkouel S.; Ben Mansour R.; Ben Jemaa M.; Jallouli S.; Megdiche-Ksouri W.; Hessini K.; Msaada K. Essential oil composition and biological activities of Aleppo pine (Pinus halepensis Miller) needles collected from different Tunisian regions. Int. J. Environ. Health Res. 2023, 33 (1), 83–97. 10.1080/09603123.2021.2005001. [DOI] [PubMed] [Google Scholar]
- Riela S.; Bruno M.; Formisano C.; Rigano D.; Rosselli S.; Saladino M. L.; Senatore F. Effects of solvent-free microwave extraction on the chemical composition of essential oil of Calamintha nepeta (L.) Savi compared with the conventional production method. J. Sep. Sci. 2008, 31 (6–7), 1110–1117. 10.1002/jssc.200700425. [DOI] [PubMed] [Google Scholar]
- Vinatoru M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001, 8 (3), 303–313. 10.1016/S1350-4177(01)00071-2. [DOI] [PubMed] [Google Scholar]
- Kaufmann B.; Christen P. Recent extraction techniques for natural products: microwave-assisted extraction and pressurised solvent extraction. Phytochem. Anal. 2002, 13 (2), 105–113. 10.1002/pca.631. [DOI] [PubMed] [Google Scholar]
- Wang L.; Weller C. L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17 (6), 300–312. 10.1016/j.tifs.2005.12.004. [DOI] [Google Scholar]
- Ferhat M. A.; Meklati B. Y.; Smadja J.; Chemat F. An improved microwave Clevenger apparatus for distillation of essential oils from orange peel. J. Chromatogr. A 2006, 1112 (1–2), 121–126. 10.1016/j.chroma.2005.12.030. [DOI] [PubMed] [Google Scholar]
- Lucchesi M. E.; Chemat F.; Smadja J. Solvent-free microwave extraction of essential oil from aromatic herbs: comparison with conventional hydro-distillation. J. Chromatogr. A 2004, 1043 (2), 323–327. 10.1016/j.chroma.2004.05.083. [DOI] [PubMed] [Google Scholar]
- de l’Europe C.Pharmacopée Européenne; Conseil de l’Europe, 1996. [Google Scholar]
- Golmakani M.-T.; Rezaei K. Comparison of microwave-assisted hydrodistillation withthe traditional hydrodistillation method in the extraction of essential oils from Thymus vulgaris L. Food Chem. 2008, 109 (4), 925–930. 10.1016/j.foodchem.2007.12.084. [DOI] [PubMed] [Google Scholar]
- Moradi S.; Fazlali A.; Hamedi H. Microwave-assisted hydro-distillation of essential oil from rosemary: Comparison with traditional distillation. Avicenna J. Med. Biotechnol. 2018, 10 (1), 22. [PMC free article] [PubMed] [Google Scholar]
- Bustamante J.; van Stempvoort S.; García-Gallarreta M.; Houghton J. A.; Briers H. K.; Budarin V. L.; Matharu A. S.; Clark J. H. Microwave assisted hydro-distillation of essential oils from wet citrus peel waste. J. Cleaner Prod. 2016, 137, 598–605. 10.1016/j.jclepro.2016.07.108. [DOI] [Google Scholar]
- Dob T.; Berramdane T.; Chelghoum C. Essential oil composition of Pinus halepensis Mill. from three different regions of Algeria. J. Essent. Oil Res. 2007, 19 (1), 40–43. 10.1080/10412905.2007.9699226. [DOI] [Google Scholar]
- Argo B.; Hermanto M.; Andriani D.; Rosadhani J.. The effect of ginger oil extraction using Microwave Assisted Hydro-distillation (MAHD) method on zingiberene content. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: 2020; p 012002.
- Wibowo S.; Komarayati S. Sifat Fisiko Kimia Minyak Cupresus (Cupressus Benthamii) Asal Aek Nauli, Parapat Sumatera Utara. Jurnal Penelitian Hasil Hutan 2015, 33 (2), 93–103. 10.20886/jphh.2015.33.2.93-103. [DOI] [Google Scholar]
- Bouzenna H.; Hfaiedh N.; Bouaziz M.; Giroux-Metges M.-A.; Elfeki A.; Talarmin H. Cytoprotective effects of essential oil of Pinus halepensis L. against aspirin-induced toxicity in IEC-6 cells. Arch. Physiol. Biochem. 2017, 123 (5), 364–370. 10.1080/13813455.2017.1347689. [DOI] [PubMed] [Google Scholar]
- Ameur E.; Sarra M. E.; Takoua k.; Mariem K.; Nabil A.; Lynen F.; Larbi K. M. Chemical composition of five Tunisian Pinus Species’ essential oils and effect of their blends on Otitis infection. Ind. Crops Prod. 2022, 180, 114688. 10.1016/j.indcrop.2022.114688. [DOI] [Google Scholar]
- Djerrad Z.; Djouahri A.; Kadik L. Variability of Pinus halepensis Mill. Essential oils and their antioxidant activities depending on the stage of growth during vegetative cycle. Chem. Biodiversity 2017, 14 (4), e1600340 10.1002/cbdv.201600340. [DOI] [PubMed] [Google Scholar]
- Postu P. A.; Sadiki F. Z.; El Idrissi M.; Cioanca O.; Trifan A.; Hancianu M.; Hritcu L. Pinus halepensis essential oil attenuates the toxic Alzheimer’s amyloid beta (1–42)-induced memory impairment and oxidative stress in the rat hippocampus. Biomed. Pharmacother. 2019, 112, 108673. 10.1016/j.biopha.2019.108673. [DOI] [PubMed] [Google Scholar]
- Santonja M.; Bousquet-Mélou A.; Greff S.; Ormeño E.; Fernandez C. Allelopathic effects of volatile organic compounds released from Pinus halepensis needles and roots. Ecol. Evol. 2019, 9 (14), 8201–8213. 10.1002/ece3.5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso-Ugarte G. A.; Juárez-Becerra G. P.; SosaMorales M. E.; López-Malo A. Microwave-assisted extraction of essential oils from herbs. J. Microw. Power Electromagn. Energy. 2013, 47 (1), 63–72. 10.1080/08327823.2013.11689846. [DOI] [PubMed] [Google Scholar]
- Ferhat M. A.; Meklati B. Y.; Chemat F. Comparison of different isolation methods of essential oil from Citrus fruits: cold pressing, hydrodistillation and microwave ‘dry’distillation. Flavour Fragrance J. 2007, 22 (6), 494–504. 10.1002/ffj.1829. [DOI] [Google Scholar]
- Lucchesi M. E.; Smadja J.; Bradshaw S.; Louw W.; Chemat F. Solvent free microwave extraction of Elletaria cardamomum L.: A multivariate study of a new technique for the extraction of essential oil. J. Food Eng. 2007, 79 (3), 1079–1086. 10.1016/j.jfoodeng.2006.03.029. [DOI] [Google Scholar]
- Bendahou M.; Muselli A.; Grignon-Dubois M.; Benyoucef M.; Desjobert J.-M.; Bernardini A.-F.; Costa J. Antimicrobial activity and chemical composition of Origanum glandulosum Desf. essential oil and extract obtained by microwave extraction: Comparison with hydrodistillation. Food Chem. 2008, 106 (1), 132–139. 10.1016/j.foodchem.2007.05.050. [DOI] [Google Scholar]
- Wang Z.; Ding L.; Li T.; Zhou X.; Wang L.; Zhang H.; Liu L.; Li Y.; Liu Z.; Wang H.; et al. Improved solvent-free microwave extraction of essential oil from dried Cuminum cyminum L. and Zanthoxylum bungeanum Maxim. J. Chromatogr. A 2006, 1102 (1–2), 11–17. 10.1016/j.chroma.2005.10.032. [DOI] [PubMed] [Google Scholar]
- Filly A.; Fernandez X.; Minuti M.; Visinoni F.; Cravotto G.; Chemat F. Solvent-free microwave extraction of essential oil from aromatic herbs: from laboratory to pilot and industrial scale. Food Chem. 2014, 150, 193–198. 10.1016/j.foodchem.2013.10.139. [DOI] [PubMed] [Google Scholar]
- Farhat A.; Ginies C.; Romdhane M.; Chemat F. Eco-friendly and cleaner process for isolation of essential oil using microwave energy: experimental and theoretical study. J. Chromatogr. A 2009, 1216 (26), 5077–5085. 10.1016/j.chroma.2009.04.084. [DOI] [PubMed] [Google Scholar]
- Guo Y.; Li Y.; Li Z.; Jiang L.; Cao X.; Gao W.; Wang J.; Luo D.; Chen F. Deep eutectic solvent-homogenate based microwave-assisted hydrodistillation of essential oil from Litsea cubeba (Lour.) Pers. fruits and its chemical composition and biological activity. J. Chromatogr. A 2021, 1646, 462089. 10.1016/j.chroma.2021.462089. [DOI] [PubMed] [Google Scholar]
- Araujo A. R.; Périno S.; Fernandez X.; Cunha C.; Rodrigues M.; Ribeiro M. P.; Jordao L.; Silva L. A.; Rodilla J.; Coutinho P.; et al. Solvent-free microwave extraction of Thymus Mastichina essential oil: Influence on their chemical composition and on the antioxidant and antimicrobial activities. Pharmaceuticals 2021, 14 (8), 709. 10.3390/ph14080709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh Chouhan K. B.; Tandey R.; Sen K. K.; Mehta R.; Mandal V. A unique model of gravity assisted solvent free microwave based extraction of essential oil from mentha leaves ensuring biorefinery of leftover waste biomass for extraction of nutraceuticals: Towards cleaner and greener technology. J. Cleaner Prod. 2019, 225, 587–598. 10.1016/j.jclepro.2019.03.325. [DOI] [Google Scholar]
- Meullemiestre A.; Petitcolas E.; Maache-Rezzoug Z.; Ginies C.; Chemat F.; Rezzoug S.-A. Isolation of volatils from maritime pine sawdust waste by different processes: Ultrasound, microwave, turbo hydrodistillation, and hydrodistillation. Wood Mater. Sci. Eng. 2014, 9 (2), 76–83. 10.1080/17480272.2014.881915. [DOI] [Google Scholar]
- Dar M. Y.; Shah W. A.; Rather M. A.; Qurishi Y.; Hamid A.; Qurishi M. Chemical composition, in vitro cytotoxic and antioxidant activities of the essential oil and major constituents of Cymbopogon jawarancusa (Kashmir). Food Chem. 2011, 129 (4), 1606–1611. 10.1016/j.foodchem.2011.06.016. [DOI] [Google Scholar]
- Dahham S. S.; Tabana Y. M.; Iqbal M. A.; Ahamed M. B.; Ezzat M. O.; Majid A. S.; Majid A. M. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20 (7), 11808–11829. 10.3390/molecules200711808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suffness M.Assays related to cancer drug discovery. In Methods in Plant Biochemistry: Assays for Bioactivity; Academic Press, 1990; Vol. 6, pp 71–133. [Google Scholar]
- Chung K.-S.; Hong J. Y.; Lee J.-H.; Lee H.-J.; Park J. Y.; Choi J.-H.; Park H.-J.; Hong J.; Lee K.-T. β-Caryophyllene in the Essential Oil from Chrysanthemum Boreale Induces G1 Phase Cell Cycle Arrest in Human Lung Cancer Cells. Molecules 2019, 24 (20), 3754. 10.3390/molecules24203754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertsch J.; Leonti M.; Raduner S.; Racz I.; Chen J.-Z.; Xie X.-Q.; Altmann K.-H.; Karsak M.; Zimmer A. Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. U.S.A. 2008, 105 (26), 9099–9104. 10.1073/pnas.0803601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidyt K.; Fiedorowicz A.; Strządała L.; Szumny A. β-caryophyllene and β-caryophyllene oxide—natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5 (10), 3007–3017. 10.1002/cam4.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayala B.; Bassole I. H.; Scifo R.; Gnoula C.; Morel L.; Lobaccaro J.-M. A.; Simpore J. Anticancer activity of essential oils and their chemical components-a review. Am. J. Cancer Res. 2014, 4 (6), 591. [PMC free article] [PubMed] [Google Scholar]
- Mitić Z. S.; Jovanović B.; JovanovićMihajilov-KrstevStojanović-RadićCvetkovićMitrović S. Č.T. Z. Z. V. J. T. L.; Marin P. D.; Zlatković B. K.; Stojanović G. S.; Mitrović T. L.; Marin P. D.; Zlatković B. K.; Stojanović G. S. Comparative study of the essential oils of four Pinus species: Chemical composition, antimicrobial and insect larvicidal activity. Ind. Crops Prod. 2018, 111, 55–62. 10.1016/j.indcrop.2017.10.004. [DOI] [Google Scholar]
- Bates J.AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Virginia, USA, 1994. [Google Scholar]
- Adams R. P.Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation Carol Stream: Illinois, 2007; Vol. 456. [Google Scholar]
- Brand-Williams W.; Cuvelier M.; Berset C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. 10.1016/s0023-6438(95)80008-5. [DOI] [Google Scholar]
- Kaighn M.; Narayan K. S.; Ohnuki Y.; Lechner J. F.; Jones L. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Invest. Urol. 1979, 17 (1), 16–23. [PubMed] [Google Scholar]
- Horoszewicz J. S.; Leong S. S.; Chu T. M.; Wajsman Z. L.; Friedman M.; Papsidero L.; Kim U.; Chai L. S.; Kakati S.; Arya S. K.; Sandberg A. A. The LNCaP cell line-a new model for studies on human prostatic carcinoma. Prog. Clin. Biol. Res. 1980, 37, 115–132. [PubMed] [Google Scholar]
- Culliton B. J. HeLa (for Henrietta lacks). Science 1974, 184 (4143), 1268. 10.1126/science.184.4143.1268. [DOI] [PubMed] [Google Scholar]
- Sylvester P. W.Optimization of the tetrazolium dye (MTT) colorimetric assay for cellular growth and viability. In Drug Design and Discovery; Springer, 2011; pp 157–168. [DOI] [PubMed] [Google Scholar]
- Legault J.; Pichette A. Potentiating effect of β-caryophyllene on anticancer activity of α-humulene, isocaryophyllene and paclitaxel. J. Pharm. Pharmacol. 2010, 59 (12), 1643–1647. 10.1211/jpp.59.12.0005. [DOI] [PubMed] [Google Scholar]
- Bayala B.; Nadembega C.; Guenn&eacu S.; Buñ J.; Mahouk&egr T.; Wendkuuni F.; Yonli A.; Baron S.; Figueredo G.; A Lobacca J. M.; et al. Chemical Composition, Antioxidant and Cytotoxic Activities of Hyptis suaveolens (L.) Poit. Essential Oil on Prostate and Cervical Cancers Cells. Pak. J. Biol. Sci. 2020, 23 (9), 1184–1192. 10.3923/pjbs.2020.1184.1192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.