Abstract
Background and Aims
Immune checkpoint inhibitor therapy causes numerous immune-related adverse events, including autoimmune pancreatic injury (AIPI), which results in rapid organ atrophy. We profiled the clinico-radiological features, short-term natural history, and response to steroids of AIPI.
Methods
We retrospectively reviewed medical records of 229/11,165 (2.1%) adult patients with AIPI. One hundred and ten out of 229 (48%) had abdominal computerized tomography (CT) scan at lipase elevation; data of 110 without pancreatic metastases were analyzed. We analyzed serial CT-based pancreas volumetry data in 48 patients with AIPI (32 with normal CT and 16 with pancreatitis on CT at lipase elevation). We examined impact of steroids on pain and disease course.
Results
In AIPI (n = 229), median lipase elevation was 4x upper limit of normal (range: 3–40x). The injury was more often asymptomatic than painful (143/229 (62%) vs 86/229 (38%), P < .000). Majority (83/110 (75%) had normal CT, often in painless vs painful disease: 51/57 (90%) vs 32/53 (60%), P < .001) 25% had interstitial pancreatitis. On serial pancreas volumetry, marked volume (cc) loss occurred 1 year after vs 3 months before lipase elevation in both normal CT (median 81.6 vs 61.3, P = .00) and pancreatitis on CT groups (91.8 vs 60.5, P = .00), ≥20% volume loss occurred in 47% vs 73%, respectively (P = .08). Steroids, when used did not mitigate pain, biochemical relapse, pancreas volume loss or 1-year diabetes incidence (7.2%).
Conclusion
Autoimmune pancreatic injury (AIPI) is uniquely characterized by painless lipase elevation, normal pancreas on CT and rapid pancreatic volume loss on follow-up. Steroids do not appear to have a role in management.
Keywords: CTLA-4, PD-L1, Immune-Related Adverse Events, Type 3 AIP, Steroids, Pancreatic Atrophy, Chronic Pancreatitis
See editorial on page 440.
Introduction
Immune checkpoints inhibitors target regulators of the immune system, namely cytotoxic T lymphocyte-associated protein 4, programmed cell death protein 1 (PD-1), and programmed cell death ligand-1 and facilitate an unmitigated T cell mediated immune response by stimulating T cell activation and proliferation. This stirring up of a highly efficacious antitumor activity predisposes to inflammatory toxicities referred to as immune-related adverse events (irAEs) that affect any organ system, the precise mechanism of which are yet to be fully understood.1,2 Pancreatic injury is one such IrAE of immune checkpoint inhibitor therapy (ICI-℞) characterized by inflammation of the organ due to a nonspecific inflammatory T cell–mediated immune response consequent to checkpoint blockade.3
Many forms of autoimmune injury to the pancreas are known. Those targeting islet cells cause various forms of type 1 diabetes. Autoimmune pancreatitis (AIP) is a generic term for exocrine pancreatic injury secondary to presumed autoimmunity. At the 2010 Honolulu Consensus Conference, the terminology of type 1 and type 2 AIP was adopted for the then known 2 pancreatitis that were deemed to be autoimmune in nature.4 While type 1 AIP is a pancreatic manifestation of immunoglobulin G4-related disease (IgG4-RD), type 2 AIP is a pancreas specific and pancreatic duct-centric disease sometimes associated with inflammatory bowel disease. Both these types of AIP are exquisitely steroid responsive. Following prior convention, autoimmune pancreatic injury (AIPI) induced by ICI-℞, which is distinct from type 1 and type 2 AIP, is referred to as type 3 AIP.3 It has an estimated incidence of 0.6%–4% among those on ICI-℞ and almost always occurs in association with other irAEs.3,5 While we previously demonstrated that this form of injury leads to significant rapid organ atrophy,6 herein, we delineate the clinical profile, response to steroids and short-term outcomes of subjects with type 3 AIP which clearly sets it apart from other forms of pancreatitis.
Study Design and Methods
The study was approved by the University of Texas MD Anderson Cancer Center Institutional Review Board.
Inclusion Criteria
Our sample comprised all adult patients that received ICI-℞ in our study period (January 2010–2020) with a serum lipase elevation ≥3 × upper limit of normal (ULN) with or without pain, that occurred during or up 2 years after exposure to the first dose of ICI-℞ with no other identifiable cause for pancreatitis. Patients with primary pancreatic cancer or pancreatic metastases from another primary were excluded. Seventy seven were included in a prior publication.7 Based on presentation, this group was subdivided into 2 groups viz. symptomatic (abdominal pain, nausea, vomiting) and asymptomatic type 3 AIP. In patients with computerized tomography (CT) imaging additional subgroups were identified (see section below on imaging and organ volumetry)
Data Abstraction
A detailed review of the electronic medical records was conducted.
Clinical presentation, demographic variables, and oncologic characteristics namely, cancer type, ICI type, duration and number of doses were collected. Lipase values at presentation were noted. Since the ULN for lipase varied over time, data are presented as fold increase over ULN. Additionally, data pertaining to risk factors for pancreatitis, presence of diabetes at diagnosis and follow-up were abstracted.
Data on management in this cohort were collected and categorized as follows: 1. Active management: A) withholding ICI, B) administration of steroids, C) both A and B. 2. No management.
Biochemical Outcomes
Since majority of cases were asymptomatic at presentation and follow-up, subsequent elevated lipase values were taken as evidence of disease relapse. These data were classified as follows: a) Persistent remission: persistently normal lipase ie, biochemical resolution up to 2 years after initial pancreatitis. b) Early relapse: lipase ≥ 3x ULN ≤ 90 days of initial pancreatitis. c). Late relapse: lipase ≥ 3x ULN > 90 days up to 2 years after initial pancreatitis, and d). Smoldering pancreatitis: persistent low grade (between > 1x and < 3x ULN) lipase abnormalities.
Functional Gland Failure at Follow-Up
Electronic medical records were reviewed thoroughly to obtain diagnosis codes to determine endocrine and exocrine insufficiency at last follow-up after type 3 AIP.
Subgroup Analysis of Cohort With Painful Type 3 AIP
We retrospectively evaluated patients with symptomatic type 3 AIP and the role of steroids in management of this cohort. Based on the visual analog scale (VAS 1–10) scores, the pain was retrospectively categorized as mild (score 0–3), moderate (score 4–6), and severe (score ≥ 7). Information on the use of narcotics for acute pain was obtained from reviewing the medications administered during the hospitalization encounter. Pre-existing narcotic use for cancer pain was noted.
Imaging Evaluation
We recorded and reviewed the pancreatic findings in patients that had an abdominal CT scan at the first elevation of lipase ≥ 3x ULN (n = 110/229). We compared the cohorts with CT at lipase elevation to those without CT and found them to be comparable (data not shown).
Serial Pancreas Volumetry
Of the 110 patients that had CT scan at the time of first elevation of lipase ≥ 3x ULN, we previously reported serial pancreas volumetry before, during and 1 year after lipase elevation in a subset of 48 subjects6 where we have shown that type 3 AIP is frequently associated with pancreatic volume loss. Methodology for pancreas volumetry as detailed in our prior publication was re-employed.6
It however, remains unclear if subjects with normal CT at presentation would experience volume loss. In other words, does painless lipase elevation with normal CT indicate an acute pancreatic injury? To answer this question and delineate the clinical profile of this AIPI, we compared volume loss based on clinical presentation and CT findings in pancreatitis subgroups, ie in those with normal CT at lipase elevation (n = 32) vs evidence of pancreatitis at lipase elevation (n = 16). Our study also accounted for 22 control subjects who received ICI-℞ but had normal lipase values to see if volume loss only followed lipase elevation and wasn’t a nonspecific drug effect. Lastly, we also compared organ volume loss in those with and without pain as well as those with and without exposure to steroids.
Statistical Analyses
All the results are expressed as mean (standard deviation) or median (interquartile range [IQR]) as appropriate. The Pearson’s χ2 test was used to compare categorical variables. The 2-tailed t-test was used to compare continuous variables. Pancreatic volumes were compared across various time points using a paired t-test or if the data were skewed, a Wilcoxon Rank Sum test was used instead. The ratio of pancreas to splenic attenuation was analyzed similarly. A P value of ≤ .05 indicated statistical significance.
Results
Type 3 AIP
The majority of our sample consisted of Caucasian females (129/229, 56%) in their seventh decade of life (Table 1). Genitourinary cancer was the most frequently observed cancer type. Approximately 62% of this sample received PD1 blockade therapy. The median number of doses and days from immune checkpoint inhibitor exposure to elevated lipase was 3 and 169 days, respectively.
Table 1.
Clinical Profile of all Type 3 AIP Patients and Subjects in Serial Pancreas Volumetry Cohort
| Characteristics | Type 3 AIP (n = 229) |
Serial pancreas volumetry (n = 48) | |
|---|---|---|---|
| Symptomatica (n = 86) | Asymptomatica (n = 143) | ||
| Median age in years (IQR) | 61 (49–69) | 61 (54–68) | 55 (47–67) |
| Male N (%) | 34 (39.5%) | 66 (46%) | 17 (35%) |
| Cancer type (%) | |||
| Genitourinary | 24 (28%) | 56 (39%) | 19 (40%) |
| Melanoma | 23 (27%) | 28 (20%) | 16 (33%) |
| Other | 39 (45%) | 59 (41%) | 12 (27%) |
| ICI Agent (%) | |||
| CTLA-4 | 8 (9%) | 9 (6%) | 5 (11%) |
| PD-1 | 55 (64%) | 89 (62%) | 28 (58%) |
| Combined | 23 (27%) | 45 (32%) | 15 (31%) |
| ICI doses before elevated lipase (IQR) | 4 (2–6) | 3 (2%–8%) | 5 (2–10) |
| Median days from ICI to type3 AIP (IQR) | 133 (49–329) | 189 (58–366) | 245 (84–435) |
| ICI discontinued (%) | 61 (71%) | 69 (48%) | 27 (56%) |
| Alcohol consumption | 36 (42%) | 66 (46%) | 22 (46%) |
| Smoking history | 48 (56%) | 69 (48%) | 25 (52%) |
| Diabetes before pancreatitis | 14 (16%) | 42 (29%) | 15 (31%) |
| Drug allergy | 53 (62%) | 75 (52%) | 22 (46%) |
| Prior history of pancreatitis | 3 (3.5%) | 3 (2%) | 2 (4%) |
| Abnormal CT on presentation (%) | 15 (17%) | 12 (8%) | 8 (17%) |
AIP, Autoimmune pancreatitis; CTLA-4, cytotoxic T lymphocyte-associated protein 4; IQR, inter quartile range; PD-1, programmed cell death receptor-1; PD-L1, programmed cell death ligand-1.
We note no significant differences between symptomatic and asymptomatic group of patients with type 3 AIP.
Features of Acute Pancreatic Injury
Median lipase elevation at presentation was 4x ULN (range 3x–40x). Only almost a quarter of this cohort had epigastric pain that radiated to the back on presentation (Table 2). Pain varied in severity from mild to severe on the visual analog scale and almost 50% of patients required narcotics for management. Approximately half of this group had accompanying symptoms of nausea and bilious emesis. The median duration of pain was 5 days (IQR 2,12). At lipase elevation, 75% had normal CT (findings are discussed in detail later).
Table 2.
Clinical Presentation of Patients With Symptomatic Type 3 AIP
| Characteristics | Population (n = 86) |
|---|---|
| Pain location | |
| Generalized | 39 (45%) |
| Epigastric | 20 (23%) |
| other | 27 (31%) |
| Pain severity | |
| 0–3 | 22 (26%) |
| 4–6 | 9 (11%) |
| 7–10 | 23 (27%) |
| Unknown | 32 (37%) |
| Median (d) pain duration | 5 (2–12) |
| Pain radiating into back | 18 (21%) |
| Nausea and/or emesis | 47 (55%) |
| Narcotics for relief | 43 (50%) |
| Intravenous fluids | 53 (62%) |
Management of Type 3 AIP
ICI-℞ was held in 139/229(61%) (Table 3). Steroid therapy was administered in 74/229 (32%). Thirty out of 229(13%) of this group was administered steroids for other irAEs. Sixty out of 229 (26%) had no intervention. Forty four out of 229(19%) had ICI held and were given steroids. Those with no intervention were more likely to be asymptomatic compared to those with active intervention (82% vs 52%, P < .0001).
Table 3.
Interventions at Diagnosis in Type 3 AIP and Biochemical Outcomes
| Lipase on follow-up N (% of row total) |
Persistently normal lipasea N (%) |
Early (< 90 d) lipase elevation N (%) |
Late (> 90 d) lipase elevation N (%) |
Persistent lipase elevation N (%) |
|---|---|---|---|---|
| Intervention (n) (% of all patients) | ||||
| ICI-℞ held + No steroids (n = 95, 42%) | 58 (41%) | 8 (33%) | 4 (18%) | 25 (60%) |
| ICI-℞ held + steroids given (n = 44, 19%) | 25 (18%) | 6 (25%) | 4 (18%) | 9 (21%) |
| ICI not held + No steroid (n = 60, 26%) | 36 (26%) | 9 (38%) | 12 (55%) | 3 (7%) |
| Steroid given for another irAEs (n = 30, 13%) | 22 (15%) | 1 (4%) | 2 (9%) | 5 (12%) |
| Total (n = 229, 100%) | 141 (62%) | 24 (10%) | 22 (10%) | 42 (18%) |
Persistent biochemical resolution occurred more often when ICI-℞ was held (58/95) vs no intervention (36/60, P = .89) or with steroid and held ICI-℞ (25/44, P = .64) steroid treatment for another irAEs (22/30, P = .22) and steroid treatment whatever the cause (47/74, P = .99).
Follow-up Biochemical Outcomes (Based on Lipase Levels)
141/229 (62%) had complete and persistent remission (normal lipase), 24/229 (10%) developed early relapse, 22/229 (10%) developed late relapse, and 42/229 (18%) had smoldering, persistent, asymptomatic, low-grade lipase abnormalities (Table 3). we found no statistically significant difference between those in who ICI-℞ was administered, leading to complete and persistent remission, and those without any intervention (P = .89) or steroid and held ICI-℞ (P = .64), and steroid group (P = .99). Early or late relapses and low-grade elevations occurred equally frequently regardless of intervention.
Functional Gland Failure on Follow-Up
Seventeen out of 229 (7.4%) were noted to have diabetes within 2 years after pancreatitis. This was determined using diagnosis codes and electronic medical records. There were insufficient data (fecal elastase levels, pancreatic enzyme use, or symptoms) to systematically assess proportion of patients with exocrine failure.
Painful Type 3 AIP
We performed a subgroup analysis on the symptomatic group (n = 86) to assess the role of steroids in the management of pain secondary to type 3 AIP (Tables 2 and 4). A majority in this group were Caucasian (79%) males (39.5%) with a history of smoking (56%) and with stage IV tumors (77%). The most common cancer types were melanoma and genitourinary cancer. Majority of this cohort received PD1 blockade monotherapy (64%). In terms of pain, almost half the patients reported a generalized abdominal pain with accompanying nausea and/emesis in 55%. Only 18 patients reported radiation of pain into the back. The median duration of pain in days was 5 (IQR 2–12). Almost half the patients received narcotics for management and reported relief with the same. Forty-six point five percent and 63% received steroids and intravenous fluids, respectively.
Table 4.
Role of Steroids in Management of Pain in Type 3 AIP
| Characteristics | All patients with abdominal pain (n = 86) | Patients that received steroids (n = 43) | Patients who did not receive steroids (n = 43) | P Value |
|---|---|---|---|---|
| Duration of abdominal pain (d) | .50 | |||
| 0–14 | 67 (78%) | 33 (76.7%) | 34 (79%) | |
| 15–30 | 13 (15%) | 8 (18.6%) | 5 (12%) | |
| > 30 | 6 (7%) | 2 (4.6%) | 4 (9%) | |
| Abdominal pain severity | .19 | |||
| 0–3 | 54 (63%) | 31 (72%) | 23 (53%) | |
| 4–6 | 9 (10%) | 3 (7%) | 6 (14%) | |
| 7–10 | 23 (27%) | 9 (21%) | 14 (33%) | |
| Time (days) to resume ICI | .62 | |||
| < 30 d | 5 (6%) | 3 (7%) | 2 (4.6%) | |
| ≥ 30 d | 13 (15%) | 5 (12%) | 8 (18.6%) | |
| Did not resume ICI | 68 (79%) | 35 (81%) | 33 (76.7%) | |
| Recurrence of type 3 AIP | .56 | |||
| Yes | 3 (3.5%) | 2 (5%) | 1 (2%) | |
| No | 83 (96.5%) | 41 (95%) | 42 (98%) |
Compared to those who did not receive steroids among patients with symptomatic type 3 AIP, the administration of steroids did not mitigate pain severity (P = .19) or duration (P = .39). Steroid use neither significantly expedited resumption of ICI (P = .62), nor prevented recurrence of type 3 AIP (P = .56) in our cohort (Table 3).
Imaging Findings
One hundred and ten patients had a CT at the time of type 3 AIP (after exclusion of those that had pancreatic metastasis). Of these, ∼ 75% (n = 83) had a normal appearing pancreas. Among patients in our imaging cohort of type 3 AIP, diffuse peripancreatic stranding was the most common abnormal CT finding at the time of enzyme elevation followed by diffuse pancreatic enlargement. Feathery appearance of the pancreas was lost in 21% of patients. An enhancing peripancreatic rim, seen in other forms of AIP, was not seen in type 3 AIP.
Serial Volumetry
Pancreatitis subgroups
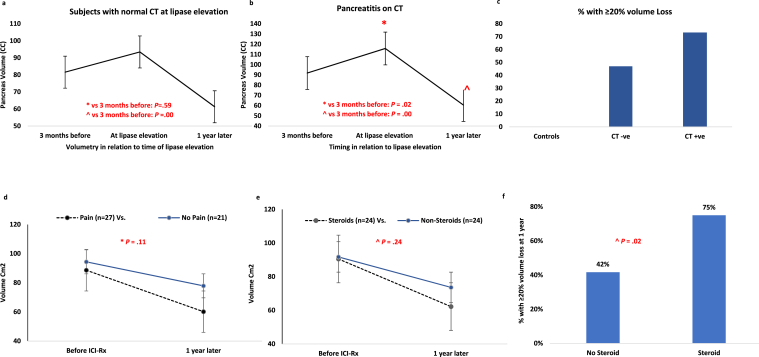
We have previously shown that type 3 AIP patients suffer pancreas volume loss.6 Overall, 54 % of those with type 3 AIP demonstrated ≥ 20% volume loss at 1 year. For this study, we reanalyzed the data by CT findings to understand the significance of imaging abnormality at the time of injury. On serial pancreas volumetry, marked pancreatic volume (cc) loss occurred 1 year after vs 3 months before lipase elevation in both normal CT (median 81.6 vs 61.3, P = .00) and pancreatitis on CT groups (91.8 vs 60.5, P = .00). Greater than 20% volume loss occurring in 47% vs 75%, respectively (P = .08) (Figure 1A–C). Looked at another way, in subjects with lipase elevation and normal CT, > 20% volume loss at 1 year was seen in 47% vs 73% of those with CT changes of pancreatitis (P = .08). In comparison, the 22 patients with ICI exposure without pancreatitis (controls) did not develop volume loss (Figure 1C) and none of them developed ≥ 20% pancreatic volume loss (P = .00 vs CT subgroups in type 3 AIP).
Figure 1.
(A) Serial pancreatic volume (median, cm3) in relation to time of lipase elevation among patients with normal CT at presentation of type 3 AIP. (B) Serial pancreatic volume (median, cm3) in relation to time of lipase elevation among patients with pancreatitis on CT at presentation of type 3 AIP. (C) Percentage of patients with ≥ 20% pancreatic volume loss at 1 year with normal CT and pancreatitis on CT at presentation of type 3 AIP in comparison to controls. (D) Serial pancreatic volume (median, cm3) in relation to before and 1 year after ICI exposure among patients with and without pain at presentation of type 3 AIP. (E) Serial pancreatic volume (median, cm3) in relation to before and 1 year after ICI exposure among patients with and without steroid exposure for type 3 AIP. (F) Percentage of patients with ≥ 20% pancreatic volume loss at 1 year with or without steroid exposure at presentation of type 3 AIP.
Subgroups based on clinical presentation and management
While the subgroups of patients with and without pain and steroid exposure were small, we note an overall decrease in organ volume regardless of clinical presentation or management (Figure 1D–E). We also found a significantly greater volume loss in those with steroid exposure compared to those without. (P = .02) (Figure 1F).
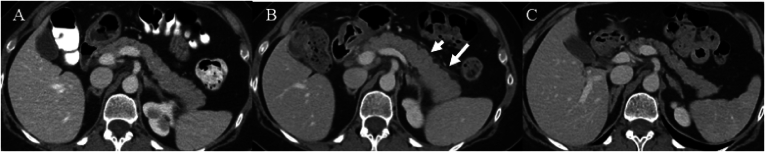
Our cohort showed no other imaging findings of chronic pancreatic damage such as fatty replacement, calcification, or ductal dilatation (Figure 2).
Figure 2.
Imaging changes over time in type 3 AIP. Serial contrast-enhanced CTs of the abdomen and 1 representative patient before the start of immunotherapy in 4 months before the diagnosis of pancreatitis (A) at the time of diagnosis of immune mediated pancreatitis (B) and 1 year after the diagnosis of pancreatitis (C). (B) shows diffuse enlargement of the entire pancreas associated with peripancreatic stranding (arrows). (C) shows pancreatic volume loss without ductal dilatation or calcification or fatty replacement.
Discussion
In this study, we provide a comprehensive profile of clinical presentation, radiologic characteristics, and short-term natural history of a large cohort of patients with type 3 AIP. We demonstrate that ICI-℞–induced AIPI (type 3 AIP) is uniquely characterized by painless lipase elevation, normal pancreas on CT, and rapid pancreatic atrophy on follow-up. While, by definition, it is a result of excessive autoimmunity, it is distinct from both classic chronic pancreatitis and the 2 known types of AIP.8 We identified substantial novel observations pertaining to definition of pancreatitis vs injury and steroid responsiveness that have implications for management of type 3 AIP, considering presumed utility of systemic immunosuppression with steroids in irAEs due to ICI-℞.
In keeping with prior reports9 we confirm incidence of type 3 AIP in ICI-exposed subjects (∼2.1%) and demographics (slight female predominance, 50% Caucasian, in the sixth to seventh decade of life). As expected, patients have advanced malignancies, genitourinary cancer being the most frequent. We also confirm previous observations that the vast majority (∼ 60%) of patients are asymptomatic and that pancreatic volume loss occurs frequently in this form of pancreatic injury.6
The important novel findings of this study are the infrequency of CT abnormalities at onset, the short-lived course of pain and the inability of steroids to impact the short-term course of type 3 AIP. The clinical profile also points to a novel mechanism of pancreatic injury.
While it has been previously described that type 3 AIP is predominantly asymptomatic, what is striking in our study is that CT abnormalities at presentation occur in a small minority (25%), especially if asymptomatic (10%). When abnormal, it shows mild pancreatitis. Therefore, a CT scan may have a limited role in management of type 3 AIP. It is not indicated in asymptomatic lipase elevation. In symptomatic patients it may be used to identify other causes of pain especially due to other irAEs, but it is not helpful to manage pancreatitis.
Extrapolating from the above noted numbers, over 50% of type 3 AIP have only an elevation of lipase without pain or CT abnormalities and yet suffer volume loss on follow-up without calcification or duct dilatation. Thus, majority of patients with type 3 AIP do not meet traditional criteria for “acute” pancreatitis at onset and on follow-up do not meet radiological criteria for “chronic pancreatitis”.10,11
We recognize acute pancreatic injury by lipase elevation > 3x ULN, severe upper abdominal pain, and inflammatory changes in the pancreas on CT. Majority of patients with acute pancreatic injury have at least 2, if not all 3 features. Permanent (chronic) pancreatic damage is inferred when we see one or more of pancreatic calcification, segmental ductal dilatation, or pancreatic volume loss (atrophy). Apart from being a sequela of acute pancreatic injury, pancreatic volume loss can also be due to other causes, including older age, ductal obstruction, and sudden loss of insulin (type 1 diabetes mellitus). We have previously shown that type 3 AIP is frequently associated with pancreatic volume loss. But it is unclear if subjects with normal CT at presentation would experience volume loss. In other words, does painless lipase elevation with normal CT indicate acute pancreatic injury?
In a previous study we showed that ICI-exposed subjects with no enzyme elevation and < 3x enzyme elevation experience no or far less volume loss (indicating significant pancreatic injury), suggesting that volume loss is a postinflammatory pancreatic injury consequent of ICI. Volume loss occurring in subjects with ≥ 3x enzyme elevation, but no symptoms and normal CT suggests an alternate mechanism of chronic inflammatory pancreatic injury in type 3 AIP.
While the etiopathogenesis of this chronic disease process remains unknown, rare irAEs have previously been reported to occur up to 2 years after the first infusion, suggesting persistence of the biological impact of the drug long after its clearance.12,13 Gland atrophy occurs at least as early as 1 year after onset, which is when we studied it. Could type 3 AIP be predominantly due to periacinar stromal injury with lipase elevations a result of minimal collateral acinar injury? In patients with more pronounced acinar damage, does a more typical picture of pancreatitis occur? This may be supported by the fact that CT findings of pancreatitis are 3 times more common in those with pain than asymptomatic subjects (Figure 2). Furthermore, recent anecdotal reports demonstrate preserved normal lobular pancreatic architecture with inflammatory cell infiltration of the stroma and focal acinar atrophy associated with a predominantly cluster of differentiation 8+ T lymphocyte-predominant infiltrate on histopathology.14,15
Pancreatitis is typically understood as a painful disease. The VAS is routinely used to assess pain and is performed and documented by nursing staff. Despite the retrospective nature of our study, we were able to assess the severity and course of pain in type 3 AIP by tracking the VAS scores at initial presentation and subsequent visits. Our study of the subgroup who presented with painful type 3 AIP suggests that the pain is often short lived and mild in severity. As other irAEs of ICIs respond to steroids, they are often used to treat pain of ICI pancreatitis. In our sample, steroids did not prove to be helpful to mitigate acute pain or prevent future volume loss.
Recurrence of enzyme elevation occurred in the majority regardless of type of intervention at pancreatitis. Some patients developed pancreatitis while on steroids for other reasons, an unusual occurrence in classic AIP. When steroid therapy was the sole intervention, it hardly induced or maintained remission. The most effective intervention for inducing biochemical remission was withholding ICI-℞ and the addition of steroids did not prove to be any more beneficial.
The major limitation of our study is its retrospective nature with attendant lack of details in some aspects, especially development of diabetes and steatorrhea. Nevertheless, the medical record documentation was sufficient to provide a clear profile of the earlier stages of the disease. We plan to prospectively study the development of exocrine and endocrine dysfunction in patients with type 3 AIP. Despite our efforts to obtain autopsy related pancreas data, the lack of histopathological information remains a barrier.
Conclusion
In summary, type 3 AIP is a chronic drug-induced immune mediated inflammatory injury to the pancreas which is mild in severity (as traditionally defined) yet leads to a rapid organ atrophy. This course is not mitigated by use of steroids. Its recognition should be of interest to the study of the more traditional forms of chronic pancreatic injury.
Acknowledgments:
Dr Kristy Broch from Imaging Physics generously accommodated our research and provided access to RayStation database and AI software to perform accurate pancreas volumetry.
Authors' Contributions:
Anusha Shirwaikar Thomas: data analysis and interpretation, drafting of the manuscript, critical revision of the manuscript for intellectual content. Michael Abreo: acquisition of data; data analysis; drafting of the manuscript. Sayed Ahmed Sayed: data analysis and interpretation. Sai Prasada Rao Manikonda, Mostafa Eyada, Aaron Issac, Fiyinfoluwa Abraham, Jake Sheraj Jacob: acquisition of data. Sireesha Yedururi: data analysis and interpretation, critical revision of the manuscript for intellectual content. Yinghong Wang: critical revision of the manuscript for intellectual content. Suresh Chari: study concept and design; analysis and interpretation of data; critical revision of the manuscript for intellectual content.
Footnotes
Conflicts of Interest: The authors disclose no conflicts.
Funding: The authors report no funding.
Ethical Statement: This study was approved by the University of Texas MD Anderson Cancer Center Institutional Review Board. The article guarantor is Suresh T. Chari, MD.
Data Transparency Statement: Data, analytic methods, and study materials will not be made available to other researchers.
Reporting Guidelines: Not applicable.
Material associated with this article can be found in the online version at https://doi.org/10.1016/j.gastha.2023.11.020.
Supplementary Materials
Figure A1.
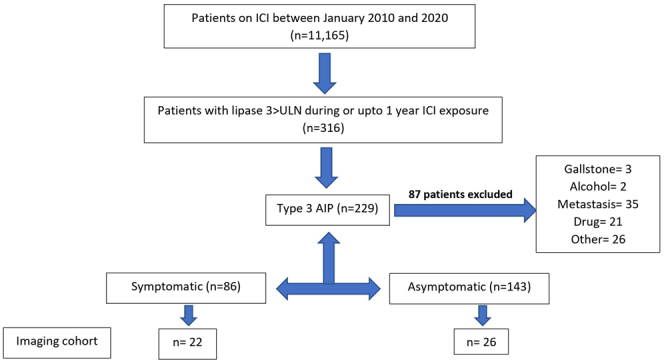
Patient selection diagram. ∗Three hundred sixteen patients were found to have > 3 ULN (upper limit of normal) lipase with/without pain. Eighty seven patients were excluded due to other etiology for pancreatitis (gallstone-3, alcohol-2, metastasis-35, drug-21, other-26).
References
- 1.Sury K., Perazella M.A., Shirali A.C. Cardiorenal complications of immune checkpoint inhibitors. Nat Rev Nephrol. 2018;14:571–588. doi: 10.1038/s41581-018-0035-1. [DOI] [PubMed] [Google Scholar]
- 2.Ramos-Casals M., Brahmer J.R., Callahan M.K., et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6:38. doi: 10.1038/s41572-020-0160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sayed Ahmed A., Abreo M., Thomas A., et al. Type 3 autoimmune pancreatitis (immune checkpoint inhibitor-induced pancreatitis) Curr Opin Gastroenterol. 2022;38:516–520. doi: 10.1097/MOG.0000000000000873. [DOI] [PubMed] [Google Scholar]
- 4.Chari S.T., Kloeppel G., Zhang L., et al. Histopathologic and clinical subtypes of autoimmune pancreatitis: the honolulu consensus document. Pancreatology. 2010;10(6):664–672. doi: 10.1159/000318809. [DOI] [PubMed] [Google Scholar]
- 5.Shirwaikar Thomas A., Wang Y. In: Managing immunotherapy related organ toxicities: a practical guide. Wang Y., editor. Springer International Publishing; Cham: 2022. Gastroenterology (GI) pp. 81–96. [Google Scholar]
- 6.Thomas A.S., Abreo M., Sayed S.A., et al. Autoimmune pancreatitis secondary to immune checkpoint inhibitor therapy (type 3 AIP): insights into a new disease from serial pancreatic imaging. Gastroenterology. 2023;164:154–155. doi: 10.1053/j.gastro.2022.09.042. [DOI] [PubMed] [Google Scholar]
- 7.Abu-Sbeih H., Tang T., Lu Y., et al. Clinical characteristics and outcomes of immune checkpoint inhibitor-induced pancreatic injury. J Immunother Cancer. 2019;7:31. doi: 10.1186/s40425-019-0502-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shirwaikar Thomas A., Takahashi N., Levy M.J., et al. Picking a zebra among horses: more difficult than you think! Gastroenterology. 2023;164:34–41.e1. doi: 10.1053/j.gastro.2022.10.027. [DOI] [PubMed] [Google Scholar]
- 9.Michot J.M., Bigenwald C., Champiat S., et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–148. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Banks P.A., Bollen T.L., Dervenis C., et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 11.Vege S.S., Chari S.T. Chronic pancreatitis. N Engl J Med. 2022;386:869–878. doi: 10.1056/NEJMcp1809396. [DOI] [PubMed] [Google Scholar]
- 12.Robert C., Schachter J., Long G.V., et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 13.Weber J.S., Kähler K.C., Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–2697. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 14.Shi W., Tan B., Li Y., et al. The diagnosis of immune-related pancreatitis disguised as multifocal lesions on MRI by endoscopic ultrasound-guided fine-needle biopsy: a case report. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.933595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikemoto J., Ishii Y., Serikawa M., et al. Pembrolizumab-induced focal pancreatitis diagnosed by endoscopic ultrasound-guided fine-needle aspiration. Intern Med. 2022;61(16):2463–2469. doi: 10.2169/internalmedicine.8507-21. [DOI] [PMC free article] [PubMed] [Google Scholar]