Abstract
Background
Permanent pacemaker implantation (PPI) after transcatheter aortic valve replacement (TAVR) is relatively frequent, and its impact on outcomes during follow-up remains a matter of discussion. Previous meta-analyses have yielded conflicting results.
Methods
To compare late outcomes in patients after TAVR with and without PPI, PubMed/MEDLINE, Embase, and Google Scholar were searched for studies that reported rates of mortality/survival, rehospitalization for heart failure (HF), stroke, and/or endocarditis accompanied by at least 1 Kaplan-Meier curve for any of these outcomes. We adopted a 2-stage approach to reconstruct individual patient data on the basis of the published Kaplan-Meier graphs.
Results
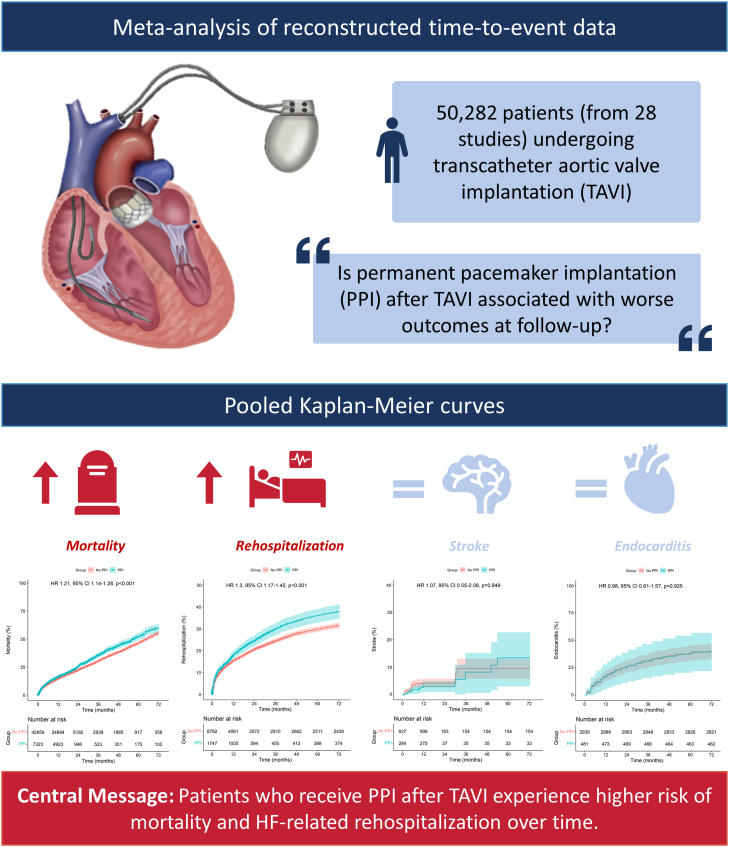
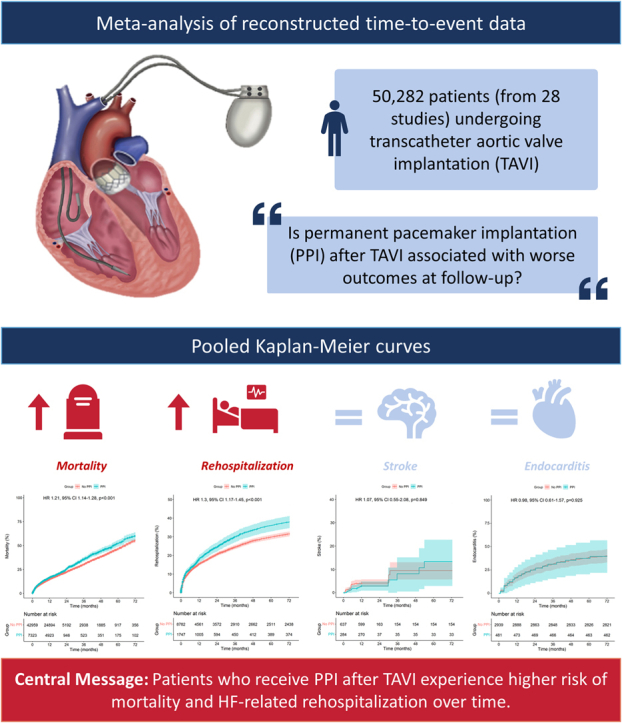
Twenty-eight studies with Kaplan-Meier curves met our eligibility criteria and included a total of 50,282 patients (7232 who underwent PPI and 42,959 who did not undergo PPI). Patients who underwent PPI after TAVR had a significantly higher risk of mortality (hazard ratio [HR], 1.21; 95% CI, 1.14-1.28; P < .001) and HF-related rehospitalization (HR, 1.30; 95% CI, 1.17-1.45; P < .001) over time. We did not observe statistically significant differences in the incidence of stroke (HR, 1.07; 95% CI, 0.55-2.08; P = .849) and endocarditis (HR, 0.98; 95% CI, 0.61-1.57; P = .925) during follow-up.
Conclusions
Patients who undergo PPI after TAVR experience higher risk of mortality and HF-related rehospitalization over time. These findings provide support for the implementation of procedural strategies to prevent heart conduction disorder and, thus, avoid PPI at the time of TAVR.
Keywords: aortic valve, cardiac surgical procedures, cardiovascular surgical procedures, heart valve prosthesis implantation, meta-analysis, transcatheter aortic valve replacement
Central Illustration
Highlights
-
•
Pacemaker implantation after transcatheter aortic valve replacement is associated with higher risk of all-cause mortality
-
•
Pacemaker implantation after transcatheter aortic valve replacement carries a higher risk of rehospitalization for heart failure
-
•
There was no higher risk of stroke or endocarditis associated with permanent pacemaker implantation after transcatheter aortic valve replacement
-
•
Efforts to prevent conduction abnormalities requiring permanent pacemaker implantation after transcatheter aortic valve replacement should be made
Introduction
Over the last 20 years, transcatheter aortic valve replacement (TAVR) has become the treatment of choice for patients at prohibitive/high or intermediate surgical risk,1 and, more recently, new clinical trials have demonstrated its benefit (or noninferiority) in patients at low surgical risk.2,3 Among several post-TAVR complications, conduction abnormalities requiring permanent pacemaker implantation (PPI) are considered relatively common and are associated with clinical and technical factors.4
When compared with surgical aortic valve replacement, the occurrence of post-TAVR PPI has been reported to be higher.5 However, the impact of PPI after TAVR during follow-up remains a matter of discussion. Previous meta-analyses have yielded conflicting results.6, 7, 8, 9
Despite the good quality of these meta-analyses, most authors pool their data using mostly random-effects models to produce incidence rate ratios, odds ratios, or risk ratios as summary measures. Time-to-event outcomes are not easily incorporated into traditional meta-analyses. Researchers have resorted to pooling median survival times, incidence rate ratios, or event rates estimated from survival estimates at given time points or made direct estimates of hazard ratios (HRs) across the studies. All these approaches have been shown to be limiting and unsatisfactory because they do not allow the production of pooled Kaplan-Meier curves and fail to recognize some of the central tenets of survival analysis, such as censoring and the proportional hazards assumption.10 In response to the inconsistent reporting that resulted from these diverging approaches, the “curve approach” has emerged as the current standard for meta-analysis of aggregated time-to-event data.11 This approach reconstructs individual patient data (IPD) on the basis of published Kaplan-Meier graphs from the included studies.
The objective of this study was to evaluate the association between PPI after TAVR and the risk of all-cause mortality, rehospitalization for heart failure (HF), stroke, and endocarditis during follow-up. To address this objective, we performed a pooled analysis of Kaplan-Meier–estimated IPD from studies comparing the outcomes of patients who underwent PPI after TAVR with those of patients who did not undergo PPI after TAVR.
Materials and methods
Eligibility criteria, databases, and search strategy
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting statement.12 Using the Population, Interventions, Comparison, Outcome, and Study design strategy, studies were included if the following criteria were fulfilled:
-
1.
The population comprised adults with aortic valve disease, requiring TAVR.
-
2.
There was a group of patients who underwent PPI after TAVR.
-
3.
There was a group of patients who did not undergo PPI after TAVR.
-
4.
The outcomes studied included survival/mortality, HF-related rehospitalization, stroke, and/or endocarditis, with at least 1 of these outcomes with Kaplan-Meier curves.
-
5.
The study design was retrospective/prospective, randomized/nonrandomized, and mono/multicentric, with matched/unmatched populations.
The following sources were searched for articles meeting our inclusion criteria and published on or before December 31, 2021: PubMed/MEDLINE, Embase, Google Scholar, and the reference lists of relevant articles. We searched for the following terms: (“TAVI” OR “transcatheter aortic valve implantation” OR “transcatheter aortic valve replacement” OR “TAVR”) AND (“PPI” OR “permanent pacemaker implantation” OR “artificial pacemakers” OR “artificial cardiac pacemaker”). Studies were selected by 2 independent reviewers (T.S. and J.V.E.). When there was disagreement, a third reviewer (M.P.S.) made the decision to include or exclude the study.
Assessment of risk of bias
The Risk of Bias in Non-Randomized Studies of Interventions tool was systematically used to assess the included studies for risk of bias.13 Two independent reviewers (P.T. and O.E.) assessed the risk for bias. When there was a disagreement, a third reviewer (M.P.S.) checked the data and made the final decision.
Statistical analysis
We used the 2-stage approach as described by Liu et al14 based on the R package “IPDfromKM” (version 0.1.10). In the first stage, raw data coordinates (time and survival probability) were extracted from each treatment arm in each of the Kaplan-Meier curves. In the second stage, the data coordinates were processed on the basis of the raw data coordinates from the first stage in conjunction with the numbers at risk at given time points, and IPD were reconstructed. Finally, the reconstructed IPD from all the studies were merged to create the study data set. The cumulative incidence of each outcome at follow-up in both arms (with and without PPI after TAVR) was visually assessed using Kaplan-Meier estimates with the R packages “survival” (version 3.2-13) and “survminer” (version 0.4.9). HRs with 95% confidence intervals (CIs) for the difference between both the treatment arms were calculated using a Cox regression model with the R package “coxphw” (version 4.0.2). In this study, an HR of >1 (with a P value of <.05) indicated a higher risk for a certain outcome after TAVR. We accounted for statistical heterogeneity among the included studies with a random intercept parameter. The proportionality of the hazards of each Cox model was checked with the Grambsch-Therneau test and diagnostic plots based on Schoenfeld residuals. One-study-removed sensitivity analyses were planned to avoid excessive impact of disproportionally larger samples on pooled results. As an extra step, when statistically significant differences for any outcomes were found, HRs and 95% CI values were derived from each Kaplan-Meier curve and pooled using a random-effects model. All analyses were completed with R Statistical Software version 4.1.1 (Foundation for Statistical Computing).
Results
Study selection and characteristics
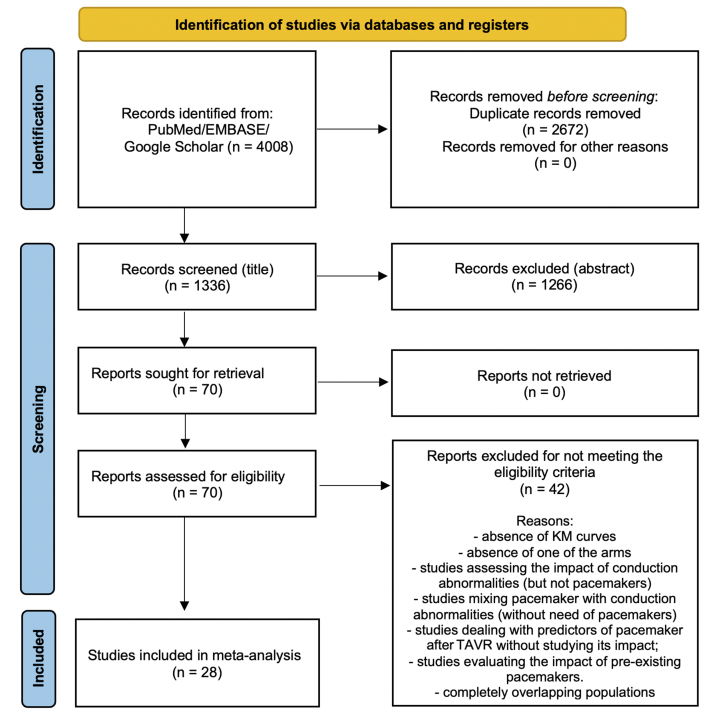
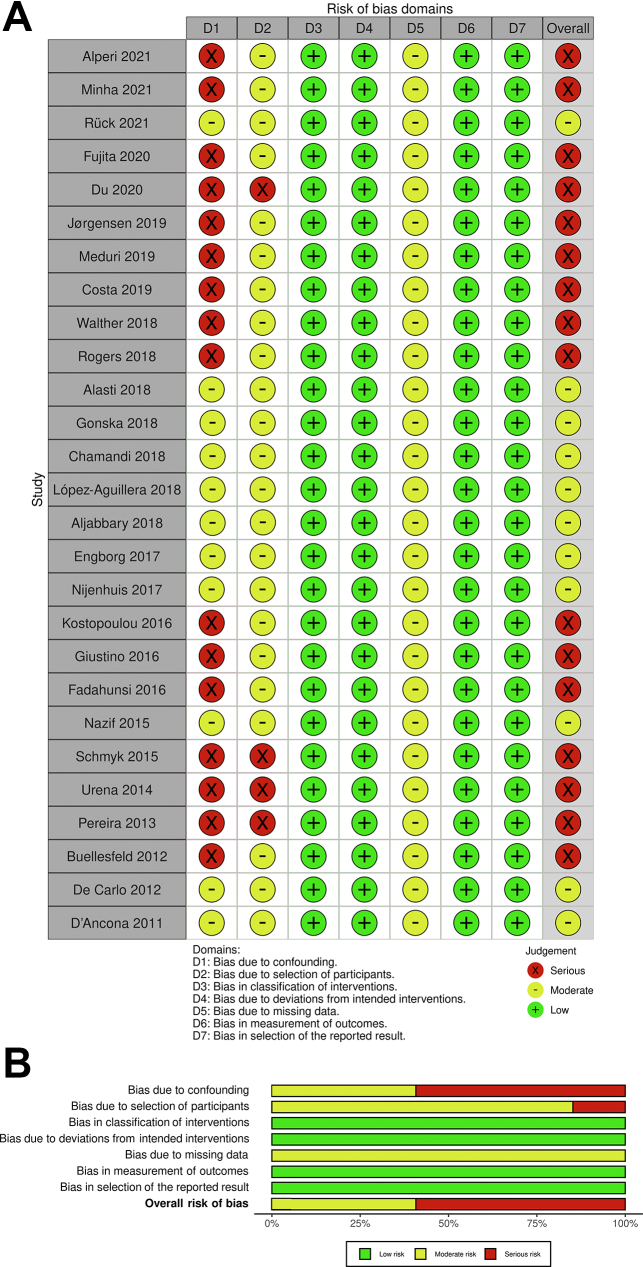
After excluding duplicates and noneligible studies, 28 studies15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42 met our eligibility criteria (Figure 1). Almost all the studies were nonrandomized and observational, whereas 14 studies were multicentric and 17 studies included prospective populations (Table 1). A total of 50,282 patients were included in the original studies (7232 patients who underwent PPI after TAVR and 42,959 patients who did not undergo PPI after TAVR). The overall incidence of PPI after TAVR was 14.4% and ranged from 6.4% to 32.8%. All the studies included in our meta-analysis excluded patients with preexisting pacemakers. Table 2 shows the characteristics of the patients included in the studies. Figure 2 shows qualitative assessment of the studies with the Risk of Bias in Non-Randomized Studies of Interventions tool. We have some concerns related to missing data and confounding factors; for example, very few studies reported the type of pacemaker implanted and its pacing mode (Supplemental Table S1), presence of left ventricle outflow tract calcification, prosthesis-patient mismatch, or paravalvular leakage after TAVR (all factors which may affect the outcomes).
Figure 1.
Flow diagram of studies included in data search. A total of 4008 records were identified, 70 full-text articles were assessed for eligibility, and 28 articles were included in the study. KM, Kaplan-Meier; TAVR, transcatheter aortic valve replacement.
Table 1.
Studies included.
| Reference, year | Design | Total patients (N) | PPI n/N (%) | BEV (%) | SEV (%) | MEV (%) |
|---|---|---|---|---|---|---|
| Alperi et al,15 2021 | O, P, M | 1987 | 128/1987 (6.4) | 40.5 | 59.5 | 0 |
| Minha et al,16 2021 | O, R, M | 1377 | 276/1377 (20.0) | 36.2 | 63.8 | 0 |
| Rück et al,17 2021 | O, R, M | 3420 | 481/3420 (14.1) | 38.4 | NR | NR |
| Fujita et al,18 2020 | O, P, M | 20,872 | 3459/20,872 (16.6) | 53.7 | 37.5 | NR |
| Du et al,19 2020 | O, R, S | 256 | 38/256 (14.8) | 0 | 100 | 0 |
| Jørgensen et al,20 2019 | O, P, S | 816 | 132/816 (16.2) | 9.4 | 82.6 | 8 |
| Meduri et al,21 2019 | P, M | 864 | 245/864 (28.4) | NR | NR | NR |
| Costa et al,22 2019 | O, P, S | 1116 | 145/1116 (13) | 27.2 | 72.5 | NR |
| Walther et al,23 2018 | O, P, M | 196 | 33/196 (16.7) | 0 | 100 | 0 |
| Rogers et al,24 2018 | O, R, S | 614 | 145/614 (23.6) | 77.8 | 22.1 | 0 |
| Alasti et al,25 2018 | O, P, S | 152 | 38/152 (25.0) | 0 | 0 | 100 |
| Gonska et al,26 2018 | O, R, S | 612 | 168/612 (27.5) | 58.8 | 4.4 | 36.8 |
| Chamandi et al,27 2018 | O, P, M | 1629 | 322/1629 (19.8) | 43.8 | 53.9 | NR |
| López-Aguillera et al,28 2018 | O, P, S | 217 | 39/217 (17.9) | NR | NR | NR |
| Aljabbary et al,29 2018 | O, R, M | 1263 | 186/1263 (14.7) | NR | NR | NR |
| Engborg et al,30 2017 | O, P, S | 128 | 41/128 (32) | 21.9 | 78.1 | 0 |
| Nijenhuis et al,31 2017 | O, R, S | 155 | 37/155 (24) | NR | NR | NR |
| Kostopoulou et al,32 2016 | O, P, S | 30 | 8/30 (26.7) | NR | NR | NR |
| Giustino et al,33 2016 | O, R, M | 947 | 145/947 (13.2) | 47.9 | 52.1 | 0 |
| Fadahunsi et al,34 2016 | O, R, M | 9785 | 651/9785 (6.7) | 88.8 | 11.2 | 0 |
| Nazif et al,35 2015 | RCT, M | 1973 | 173/1973 (8.8) | NR | NR | NR |
| Mouillet et al,36 2015 | O, P, M | 833 | 252/833 (30.3) | 0 | 100 | 0 |
| Schymik et al,37 2015 | O, P, S | 634 | 69/634 (10.8) | 80.8 | 19.2 | 0 |
| Urena et al,38 2014 | O, P, M | 1556 | 239/1556 (15.4) | 55 | 45 | 0 |
| Pereira et al,39 2013 | O, R, S | 58 | 19/58 (32.8) | NR | NR | NR |
| Buellesfeld et al,40 2012 | O, P, M | 305 | 98/305 (32.1) | 10.5 | 89.5 | 0 |
| De Carlo et al,41 2012 | O, P, M | 275 | 66/275 (24) | 0 | 100 | 0 |
| D’Ancona et al,42 2011 | O, P, S | 322 | 20/322 (6.2) | 100 | 0 | 0 |
BEV, balloon-expandable valve; M, multicentric; MEV, mechanically expandable valve; NR, nonreported; O, observational; P, prospective; PPI, permanent pacemaker implantation; R, retrospective; RCT, randomized controlled trial; S, single-center study; SEV, self-expandable valve.
Table 2.
Characteristics of the patients included in the studies.
| Reference, year | Age (y), mean PPI/no PPI | Female sex (%) PPI/no PPI | Diabetes mellitus (%) PPI/no PPI | CAD (%) PPI/no PPI | AF (%) PPI/no PPI | STS score (mean/median) PPI/no PPI | LVEF (%) (mean) PPI/no PPI |
|---|---|---|---|---|---|---|---|
| Alperi et al,15 2021 | 80.2/77.4a | 46.5/43.6 | 26.4/25.4 | NR | 25.4/20.5 | 6.0/6.1 | 54.2/53.0 |
| Minha et al,16 2021 | NR | NR | NR | NR | NR | NR | NR |
| Rück et al,17 2021 | 81.7/81.2 | 43.9/51.4 | 32.8/28.2 | 30.4/29.8 | 43.7/39.6 | NR | NR |
| Fujita et al,18 2020 | NR | NR | NR | NR | NR | NR | NR |
| Du et al,19 2020 | 76.6/75.5 | 39.5/42.7 | 21.5/21.6 | 21.1/8.7 | 18.4/16.1 | 6.5/7.2 | 55.6/52.6 |
| Jørgensen et al,20 2019 | 80/81 | 54.5/48.7 | 21.2/20.2 | 39.4/51.0 | 39.4/32.5 | 3.4/3.3 | 60/55 |
| Meduri et al,21 2019 | 83/82 | 50/51 | 34/30 | 72/70 | 4.6/3.1 | 6.8/6.5 | NR |
| Costa et al,22 2019 | 82/82 | 55.9/58.6 | 23.4/20.1 | 23.4/20.1 | 17.2/16.2 | 4.4/4.4 | 54.3/53.2 |
| Walther et al,23 2018 | 82.1/83.3 | 69.7/78.8 | 42.4/26.7 | 66.7/58.2 | 30.3/20.6 | 6.1/5.7 | 54.9/60.7a |
| Rogers et al,24 2018 | 82.3/82.7 | 53.2/53.9 | 35.8/31.8 | 68.4/72.5 | 47.8/33.4a | 8.0/8.8a | 55.1/53.8 |
| Alasti et al,25 2018 | 84.6/83.4 | 41.0/58.0 | 8.0/22.0 | 21.0/12.0 | 18.0/30.0 | NR | 61.1/58.8 |
| Gonska et al,26 2018 | 81.1/80.1 | 47.6/55.0 | 25.6/31.5 | 63.5/60.8 | 39.3/34.7 | 6.7/6.6 | 58.0/57.1 |
| Chamandi et al,27 2018 | 82/81 | 43.8/41.2 | 32.3/32.5 | 44.1/42.0 | 21.2/21.4 | 7.4/6.9 | 57/56 |
| López-Aguillera et al,28 2018 | 78/78 | 41/55 | 33.3/28.6 | 25.6/34.2 | 23.0/25.3 | 10.7/11.6 | 62/58 |
| Aljabbary et al,29 2018 | 82.4/82.4 | 46.7/47.1 | 45.4/47.4 | 74.5/72.0 | 24.3/26.2 | 8.9/8.4 | NR |
| Engborg et al,30 2017 | 82.1/79.9 | 42/61a | 17/18 | 22/15 | 32/22 | 15.5/17.9b | 53.4/48.9 |
| Nijenhuis et al,31 2017 | 81/80 | 41/53 | 32/22 | 68/54 | 57/34a | 7/5 | 59/59 |
| Kostopoulou et al,32 2016 | 78/82 | 60/34 | NR | NR | 10/17 | NR | 51/49 |
| Giustino et al,33 2016 | 82.2/80.8 | 40.2/50.6 | 28.8/28.6 | 24.4/30.5 | 24.7/22.1 | 8.1/8.7 | 52.3/52.2 |
| Fadahunsi et al,34 2016 | 84/84 | 47.8/53.1a | 36.4/34.7 | 38.4/34.8 | 39.6/36.9 | 7.3/6.7a | 57/58 |
| Nazif et al,35 2015 | 84.8/84.2 | 53.8/51.4 | 35.8/36.3 | 80.9/75.8 | 22.8/23.6 | 11.5/11.3 | 53.5/53.9 |
| Mouillet et al,36 2015 | 81.6/82.4 | 36.1/43.5a | 25.5/24.8 | 48.2/45.8 | 30.9/26.9 | 12.2/14.9a | 53.8/52.7 |
| Schymik et al,37 2015 | NR | NR | NR | NR | NR | NR | NR |
| Urena et al,38 2014 | 81/80 | 53.6/52.2 | 28.0/31.7 | 46.9/58.1a | 25.9/28.2 | 7.2/7.7 | 56/55 |
| Pereira et al,39 2013 | NR | NR | NR | NR | NR | NR | NR |
| Buellesfeld et al,40 2012 | 82.5/82.6 | 53.1/61.4 | 28.6/23.7 | 58.1/52.7 | 20.4/22/7 | 27.7/22.7a,b | 49.2/52.2 |
| De Carlo et al,41 2012 | 82.3/81.9 | 43.9/56.4 | NR | NR | NR | 23.1/22.1b | 52.1/51.7 |
| D’Ancona et al,42 2011 | 82.4/79.1 | 60/67 | 20/25 | 30/62a | 25/29 | 13.4/18.8a | 50.5/50.5 |
AF, atrial fibrillation; CAD, coronary artery disease; LVEF, left ventricular ejection fraction; NR, nonreported; PPI, permanent pacemaker implantation; STS, Society of Thoracic Surgeons.
P < .05.
EuroSCORE.
Figure 2.
Risk of bias summary. The Risk of Bias in Non-Randomized Studies of Interventions tool with (A) traffic lights and (B) summary plots.
Analysis of adverse events
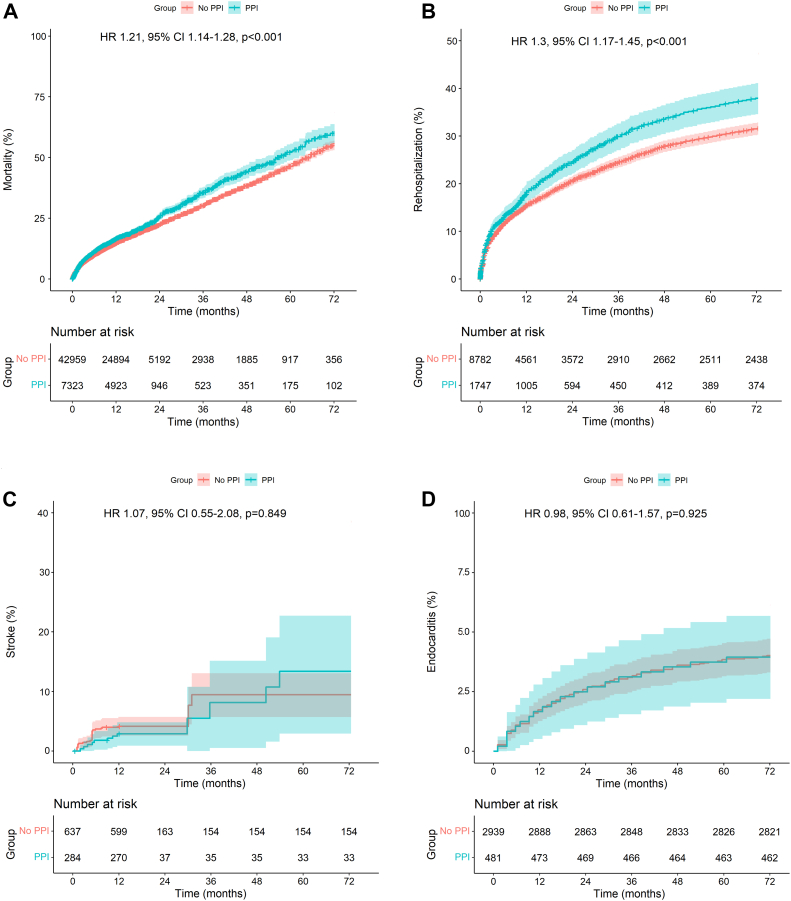
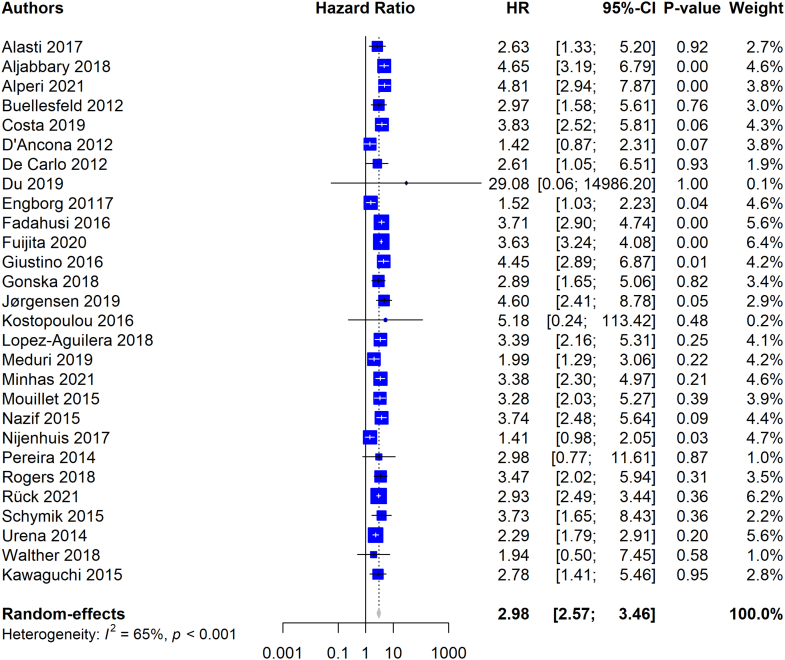
Figure 3A depicts the pooled Kaplan-Meier curve for the cumulative risk of all-cause mortality in all the included studies reporting PPI. The data of 50,282 patients (PPI, 7323 patients; no PPI, 42,959 patients) from 28 studies were pooled. PPI after TAVR was associated with a statistically significantly higher risk of mortality during follow-up (HR, 1.30; 95% CI, 1.17-1.45; P < .001). Pooling the data using a random-effects model also revealed higher risk of all-cause death in the group with PPI (Supplemental Figure S1).
Figure 3.
Pooled Kaplan-Meier curves showing the cumulative risk of all-cause mortality, rehospitalization, stroke, and endocarditis after transcatheter aortic valve replacement with and without PPI. (A and B) Shows the impact of PPI on mortality and rehospitalization, whereas (C and D) does not show any statistically significant differences. HR, hazard ratio; PPI, permanent pacemaker implantation.
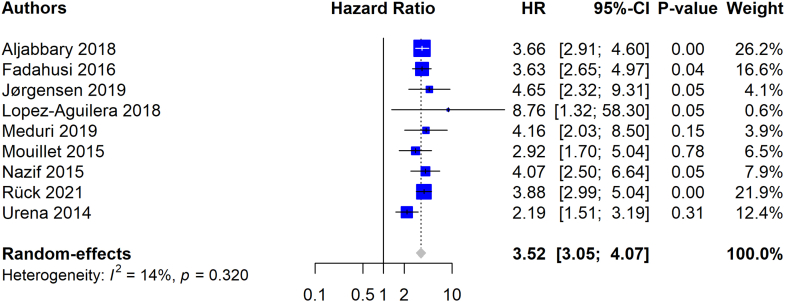
Figure 3B depicts the pooled Kaplan-Meier curve for the cumulative risk of HF-related rehospitalization. The data of 20,314 patients (PPI, 2398 patients; no PPI, 17,916 patients) from 9 studies were pooled. PPI after TAVR was associated with a statistically significantly higher risk of HF-related rehospitalization during follow-up (HR, 1.30; 95% CI, 1.17-1.45; P < .001). Pooling the data using a random-effects model also revealed higher risk of HF-related hospitalization in the group with PPI (Supplemental Figure S2).
Figure 3C depicts the pooled Kaplan-Meier curve for the cumulative risk of stroke. The data of 921 patients (PPI, 284 patients; no PPI, 637 patients) from 2 studies were pooled. Patients who underwent PPI had a risk of stroke comparable with that in patients who did not undergo PPI (HR, 1.07; 95% CI, 0.55-2.08; P = .849).
Figure 3D depicts the Kaplan-Meier curve for the cumulative risk of endocarditis. The data of 3420 patients (PPI, 481 patients; no PPI, 2939 patients) were reconstructed. Patients who underwent PPI had a risk of endocarditis comparable with that in patients who did not undergo PPI (HR, 0.98; 95% CI, 0.61-1.57; P = .925).
Sensitivity analysis
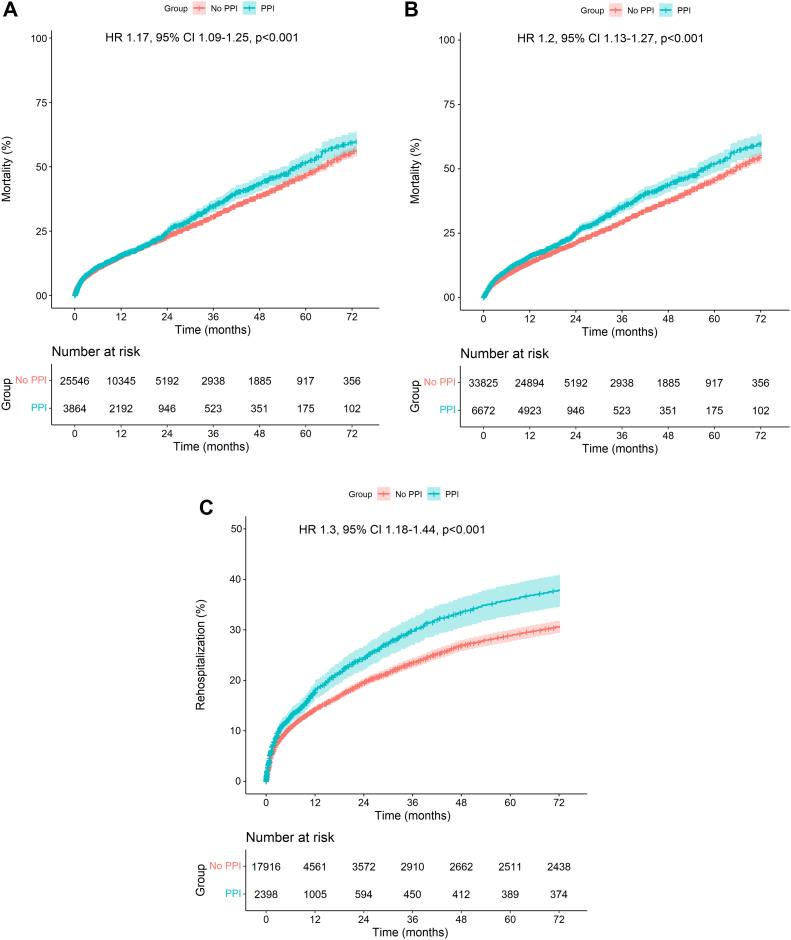
Because Fujita et al18 and Fadahunsi et al.34 contributed a disproportionately large number of patients to the mortality analyses (n = 20,872 [41.5%] and n = 9785 [19.5%], respectively), a sensitivity analysis was conducted, excluding these studies subsequently. Exclusion of the former left the data of 29,410 patients (no PPI, 25,546 patients; PPI, 3864 patients) from the remaining studies available for analysis of mortality. Exclusion of the latter left the data of 40,497 patients (no PPI, 33,825 patients; PPI, 6672 patients) from the remaining studies available for analysis of mortality. As depicted in Figure 4A and B, the sensitivity analyses confirmed the association of PPI with higher risk of mortality (HR, 1.17; 95% CI, 1.09-1.25; P < .001 and HR, 1.20; 95% CI, 1.13-1.27; P < .001).
Figure 4.
Sensitivity analysis (1-study-removed analysis). (A-C) All Kaplan-Meier curves shows the consistent impact of PPI on mortality and rehospitalization despite the removal of some studies from the analyses. HR, hazard ratio; PPI, permanent pacemaker implantation.
In the analysis of HF-related rehospitalization, Fadahunsi et al34 also contributed a disproportionately large number of patients (n = 9785, 48.2%). Thus, a sensitivity analysis was conducted. Exclusion of this study left the data of 10,529 patients (no PPI, 8782 patients; PPI, 1747 patients) from the remaining studies available for analysis of rehospitalization. As depicted in Figure 4C, the sensitivity analysis confirmed the association of PPI with higher risk of rehospitalization (HR, 1.30; 95% CI, 1.18-1.44; P < .001).
Discussion
To our knowledge, this is the first meta-analysis of reconstructed time-to-event data comparing late outcomes of TAVR with and without PPI. Our study complements and expands on the most recent meta-analysis published by Zito et al6 by doing the following:
-
1.
Adding new recently published studies.
-
2.
Adding pooled Kaplan-Meier curves to be visualized with HRs (instead of risk ratios) with longer follow-up for all-cause mortality and HF-related rehospitalization, respecting the principles of survival analysis, such as censoring and the proportional hazards assumption.
-
3.
Confirming the higher risk of all-cause mortality and HF-related rehospitalization in those who underwent PPI after TAVR.
-
4.
Adding endocarditis as an outcome of interest and showing the absence of any additional risk regarding this outcome.
The main finding of this study is that PPI after TAVR is associated with increased risk of all-cause death and HF-related hospitalization (Central Illustration). Ventricular dyssynchrony associated with right ventricular (RV) pacing43 may contribute to the higher risk of all-cause death and HF-related rehospitalization among patients who underwent PPI after TAVR. In this population, absence of mechanical synchrony after afterload relief (induced by preprocedural left ventricle hypertrophy) combined with ventricular dyssychrony (electrically induced by RV pacing) may not only hamper the postprocedural normalization of cardiac function but also induce further decline in function, which may account for the adverse clinical outcomes associated with chronic RV pacing after TAVR.43 Further studies are needed to determine whether single-chamber/dual-chamber pacemakers and pacing mode may yield different clinical outcomes during follow-up.
Central Illustration.
Impact of permanent pacemaker implantation after transcatheter aortic valve implantation. HF, heart failure; HR, hazard ratio; PPI, permanent pacemaker implantation; TAVR, transcatheter aortic valve replacement.
One of the limitations of our study is the lack of reporting of types of pacemaker and pacing mode in the original studies, which precluded us from analyzing these aspects as modulating factors of the pooled results by means of sensitivity analyses. Furthermore, the lack of data regarding pacing rates and dependency precluded us from evaluating the impact of these variables on outcomes. Additionally, the lack of real IPD (ours is Kaplan-Meier–derived IPD) regarding dependent and independent variables prevented us from establishing whether PPI after TAVR is an independent predictor of worse outcomes.
Conclusions
Patients who undergo PPI after TAVR experience higher risk of mortality and HF-related rehospitalization over time. These findings provide support for the implementation of procedural strategies to prevent heart conduction disorder and, thus, avoid PPI at the time of TAVR.
Acknowledgments
Dr Sá receives support from The Thoracic Surgery Foundation (charitable arm of The Society of Thoracic Surgeons) through the Thoracic Surgery Foundation Every Heartbeat Matters Global Structural Heart Fellowship Award for the project “Structural Heart/Minimally Invasive Cardiac Surgery.”
Declaration of competing interest
Dr Clavel has a computed tomography core laboratory contract with Edwards Lifesciences, for which she receives no direct compensation, and has received a research grant from Medtronic. Dr Pibarot has echocardiography core laboratory contracts with Edwards Lifesciences, for which he receives no direct compensation. Dr Ramlawi has received financial support from Medtronic, Corcym, and AtriCure. Authors Pompeu Sá, Jacquemyn, Sun, Van den Eynde, Tasoudis, Erten, Sicouri, and Torregrossa reported no financial interests.
Funding sources
This work was supported by the Sharpe-Strumia Research Foundation (Bryn Mawr Hospital).
Ethics statement
The research reported has adhered to the relevant ethical guidelines.
Footnotes
To access the supplementary material accompanying this article, visit the online version of the Journal of the Society for Cardiovascular Angiography & Interventions at 10.1016/j.jscai.2022.100434.
Supplementary material
Supplementary Figure 1.
Supplementary Figure 2.
References
- 1.Vahanian A., Beyersdorf F., Praz F., et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2022;43(7):561–632. doi: 10.1093/eurheartj/ehab395. [DOI] [PubMed] [Google Scholar]
- 2.Mack M.J., Leon M.B., Thourani V.H., et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380(18):1695–1705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 3.Popma J.J., Deeb G.M., Yakubov S.J., et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380(18):1706–1715. doi: 10.1056/NEJMoa1816885. [DOI] [PubMed] [Google Scholar]
- 4.Khatri P.J., Webb J.G., Rodés-Cabau J., et al. Adverse effects associated with transcatheter aortic valve implantation: a meta-analysis of contemporary studies. Ann Intern Med. 2013;158(1):35–46. doi: 10.7326/0003-4819-158-1-201301010-00007. [DOI] [PubMed] [Google Scholar]
- 5.Gleason T.G., Reardon M.J., Popma J.J., et al. 5-year outcomes of self-expanding transcatheter versus surgical aortic valve replacement in high-risk patients. J Am Coll Cardiol. 2018;72(22):2687–2696. doi: 10.1016/j.jacc.2018.08.2146. [DOI] [PubMed] [Google Scholar]
- 6.Zito A., Princi G., Lombardi M., et al. Long-term clinical impact of permanent pacemaker implantation in patients undergoing transcatheter aortic valve implantation: a systematic review and meta-analysis. Europace. 2022;24(7):1127–1136. doi: 10.1093/europace/euac008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faroux L., Chen S., Muntané-Carol G., et al. Clinical impact of conduction disturbances in transcatheter aortic valve replacement recipients: a systematic review and meta-analysis. Eur Heart J. 2020;41(29):2771–2781. doi: 10.1093/eurheartj/ehz924. [DOI] [PubMed] [Google Scholar]
- 8.Mohananey D., Jobanputra Y., Kumar A., et al. Clinical and echocardiographic outcomes following permanent pacemaker implantation after transcatheter aortic valve replacement: meta-analysis and meta-regression. Circ Cardiovasc Interv. 2017;10(7) doi: 10.1161/CIRCINTERVENTIONS.117.005046. [DOI] [PubMed] [Google Scholar]
- 9.Regueiro A., Abdul-Jawad Altisent O., Del Trigo M., et al. Impact of new-onset left bundle branch block and periprocedural permanent pacemaker implantation on clinical outcomes in patients undergoing transcatheter aortic valve replacement: a systematic review and meta-analysis. Circ Cardiovasc Interv. 2016;9(5) doi: 10.1161/CIRCINTERVENTIONS.115.003635. [DOI] [PubMed] [Google Scholar]
- 10.Guyot P., Ades A.E., Ouwens M.J., Welton N.J. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. doi: 10.1186/1471-2288-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei Y., Royston P. Reconstructing time-to-event data from published Kaplan-Meier curves. Stata J. 2017;17(4):786–802. [PMC free article] [PubMed] [Google Scholar]
- 12.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sterne J.A.C., Hernán M.A., Reeves B.C., et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu N., Zhou Y., Lee J.J. IPDfromKM: reconstruct individual patient data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2021;21(1):111. doi: 10.1186/s12874-021-01308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alperi A., Rodés-Cabau J., Simonato M., et al. Permanent pacemaker implantation following valve-in-valve transcatheter aortic valve replacement: VIVID registry. J Am Coll Cardiol. 2021;77(18):2263–2273. doi: 10.1016/j.jacc.2021.03.228. [DOI] [PubMed] [Google Scholar]
- 16.Minha S., Yarkoni Y., Segev A., et al. Comparison of permanent pacemaker implantation rate after first and second generation of transcatheter aortic valve implantation-a retrospective cohort study. Catheter Cardiovasc Interv. 2021;98(7):E990–E999. doi: 10.1002/ccd.29891. [DOI] [PubMed] [Google Scholar]
- 17.Rück A., Saleh N., Glaser N. Outcomes following permanent pacemaker implantation after transcatheter aortic valve replacement: SWEDEHEART observational study. JACC Cardiovasc Interv. 2021;14(19):2173–2181. doi: 10.1016/j.jcin.2021.07.043. [DOI] [PubMed] [Google Scholar]
- 18.Fujita B., Schmidt T., Bleiziffer S., et al. Impact of new pacemaker implantation following surgical and transcatheter aortic valve replacement on 1-year outcome. Eur J Cardiothorac Surg. 2020;57(1):151–159. doi: 10.1093/ejcts/ezz168. [DOI] [PubMed] [Google Scholar]
- 19.Du F., Zhu Q., Jiang J., Chen H., Liu X., Wang J. Incidence and predictors of permanent pacemaker implantation in patients who underwent transcatheter aortic valve replacement: observation of a Chinese population. Cardiology. 2020;145(1):27–34. doi: 10.1159/000502792. [DOI] [PubMed] [Google Scholar]
- 20.Jørgensen T.H., De Backer O., Gerds T.A., Bieliauskas G., Svendsen J.H., Søndergaard L. Mortality and heart failure hospitalization in patients with conduction abnormalities after transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2019;12(1):52–61. doi: 10.1016/j.jcin.2018.10.053. [DOI] [PubMed] [Google Scholar]
- 21.Meduri C.U., Kereiakes D.J., Rajagopal V., et al. Pacemaker implantation and dependency after transcatheter aortic valve replacement in the REPRISE III Trial. J Am Heart Assoc. 2019;8(21) doi: 10.1161/JAHA.119.012594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costa G., Zappulla P., Barbanti M., et al. Pacemaker dependency after transcatheter aortic valve implantation: incidence, predictors and long-term outcomes. EuroIntervention. 2019;15(10):875–883. doi: 10.4244/EIJ-D-18-01060. [DOI] [PubMed] [Google Scholar]
- 23.Walther T., Manoharan G., Linke A., et al. Incidence of new-onset left bundle branch block and predictors of new permanent pacemaker following transcatheter aortic valve replacement with the Portico™ valve. Eur J Cardiothorac Surg. 2018;54(3):467–474. doi: 10.1093/ejcts/ezy078. [DOI] [PubMed] [Google Scholar]
- 24.Rogers T., Devraj M., Thomaides A., et al. Utility of invasive electrophysiology studies in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation. Am J Cardiol. 2018;121(11):1351–1357. doi: 10.1016/j.amjcard.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 25.Alasti M., Rashid H., Rangasamy K., et al. Long-term pacemaker dependency and impact of pacing on mortality following transcatheter aortic valve replacement with the LOTUS valve. Catheter Cardiovasc Interv. 2018;92(4):777–782. doi: 10.1002/ccd.27463. [DOI] [PubMed] [Google Scholar]
- 26.Gonska B., Keßler M., Wöhrle J., Rottbauer W., Seeger J. Influence of permanent pacemaker implantation after transcatheter aortic valve implantation with new-generation devices. Neth Heart J. 2018;26(12):620–627. doi: 10.1007/s12471-018-1194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chamandi C., Barbanti M., Munoz-Garcia A., et al. Long-term outcomes in patients with new permanent pacemaker implantation following transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2018;11(3):301–310. doi: 10.1016/j.jcin.2017.10.032. [DOI] [PubMed] [Google Scholar]
- 28.López-Aguilera J., Segura Saint-Gerons J.M., Sánchez Fernández J., et al. Long-term clinical impact of permanent cardiac pacing after transcatheter aortic valve implantation with the CoreValve prosthesis: a single center experience. Europace. 2018;20(6):993–1000. doi: 10.1093/europace/eux046. [DOI] [PubMed] [Google Scholar]
- 29.Aljabbary T., Qiu F., Masih S., et al. Association of clinical and economic outcomes with permanent pacemaker implantation after transcatheter aortic valve replacement. JAMA Netw Open. 2018;1(1) doi: 10.1001/jamanetworkopen.2018.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engborg J., Riechel-Sarup C., Gerke O., et al. Effect of permanent pacemaker on mortality after transcatheter aortic valve replacement. Scand Cardiovasc J. 2017;51(1):40–46. doi: 10.1080/14017431.2016.1236982. [DOI] [PubMed] [Google Scholar]
- 31.Nijenhuis V.J., Van Dijk V.F., Chaldoupi S.M., Balt J.C., Ten Berg J.M. Severe conduction defects requiring permanent pacemaker implantation in patients with a new-onset left bundle branch block after transcatheter aortic valve implantation. Europace. 2017;19(6):1015–1021. doi: 10.1093/europace/euw174. [DOI] [PubMed] [Google Scholar]
- 32.Kostopoulou A., Karyofillis P., Livanis E., et al. Permanent pacing after transcatheter aortic valve implantation of a CoreValve prosthesis as determined by electrocardiographic and electrophysiological predictors: a single-centre experience. Europace. 2016;18(1):131–137. doi: 10.1093/europace/euv137. [DOI] [PubMed] [Google Scholar]
- 33.Giustino G., Van der Boon R.M., Molina-Martin de Nicolas J., et al. Impact of permanent pacemaker on mortality after transcatheter aortic valve implantation: the PRAGMATIC (Pooled Rotterdam-Milan-Toulouse in Collaboration) Pacemaker substudy. EuroIntervention. 2016;12(9):1185–1193. doi: 10.4244/EIJV12I9A192. [DOI] [PubMed] [Google Scholar]
- 34.Fadahunsi O.O., Olowoyeye A., Ukaigwe A., et al. Incidence, predictors, and outcomes of permanent pacemaker implantation following transcatheter aortic valve replacement: analysis from the U.S. Society of Thoracic Surgeons/American College of Cardiology TVT Registry. JACC Cardiovasc Interv. 2016;9(21):2189–2199. doi: 10.1016/j.jcin.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 35.Nazif T.M., Dizon J.M., Hahn R.T., et al. Predictors and clinical outcomes of permanent pacemaker implantation after transcatheter aortic valve replacement: the PARTNER (placement of aortic transcatheter valves) trial and registry. JACC Cardiovasc Interv. 2015;8(1 Pt A):60–69. doi: 10.1016/j.jcin.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 36.Mouillet G., Lellouche N., Yamamoto M., et al. Outcomes following pacemaker implantation after transcatheter aortic valve implantation with CoreValve® devices: results from the France 2 Registry. Catheter Cardiovasc Interv. 2015;86(3):E158–E166. doi: 10.1002/ccd.25818. [DOI] [PubMed] [Google Scholar]
- 37.Schymik G., Tzamalis P., Bramlage P., et al. Clinical impact of a new left bundle branch block following TAVI implantation: 1-year results of the TAVIK cohort. Clin Res Cardiol. 2015;104(4):351–362. doi: 10.1007/s00392-014-0791-2. [DOI] [PubMed] [Google Scholar]
- 38.Urena M., Webb J.G., Tamburino C., et al. Permanent pacemaker implantation after transcatheter aortic valve implantation: impact on late clinical outcomes and left ventricular function. Circulation. 2014;129(11):1233–1243. doi: 10.1161/CIRCULATIONAHA.113.005479. [DOI] [PubMed] [Google Scholar]
- 39.Pereira E., Ferreira N., Caeiro D., et al. Transcatheter aortic valve implantation and requirements of pacing over time. Pacing Clin Electrophysiol. 2013;36(5):559–569. doi: 10.1111/pace.12104. [DOI] [PubMed] [Google Scholar]
- 40.Buellesfeld L., Stortecky S., Heg D., et al. Impact of permanent pacemaker implantation on clinical outcome among patients undergoing transcatheter aortic valve implantation. J Am Coll Cardiol. 2012;60(6):493–501. doi: 10.1016/j.jacc.2012.03.054. [DOI] [PubMed] [Google Scholar]
- 41.De Carlo M., Giannini C., Bedogni F., et al. Safety of a conservative strategy of permanent pacemaker implantation after transcatheter aortic CoreValve implantation. Am Heart J. 2012;163(3):492–499. doi: 10.1016/j.ahj.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 42.D’Ancona G., Pasic M., Unbehaun A., Hetzer R. Permanent pacemaker implantation after transapical transcatheter aortic valve implantation. Interact Cardiovasc Thorac Surg. 2011;13:373–376. doi: 10.1510/icvts.2011.274456. [DOI] [PubMed] [Google Scholar]
- 43.Nadeem F., Tsushima T., Ladas T.P., et al. Impact of right ventricular pacing in patients who underwent implantation of permanent pacemaker after transcatheter aortic valve implantation. Am J Cardiol. 2018;122(10):1712–1717. doi: 10.1016/j.amjcard.2018.07.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.