Abstract
Background and Aims
The Fibrosis-4 (FIB-4) index has been used to predict liver fibrosis in various liver diseases, including nonalcoholic fatty liver disease (NAFLD). Because the FIB-4 formula uses age, different cutoff values may be required for different age groups, making the interpretation difficult. To avoid the influence of age, we attempted to create a new score, the Fibrosis-3 (FIB-3) index.
Methods
The FIB-3 index was created using a training cohort of 735 NAFLD cases using aspartate aminotransferase, alanine amino transferase, and platelet for predicting fibrosis. The abilities of the FIB-3 and FIB-4 indices were compared among different age groups in the training cohort and validation cohort with 324 patients. The FIB-3 index was also compared with other liver fibrosis indices.
Results
The area under the receiver operating characteristic curve (AUROC) values of the FIB-3 and FIB-4 indices for predicting F3–F4 fibrosis were 0.764 and 0.762, respectively, in the training cohort. No difference in the AUROC values was observed between the 2 indices in the validation cohort. The differences in the accuracies of FIB-3 between elderly and nonelderly patients were 0.140 and 0.178, respectively, in each cohort and were smaller than those of FIB-4 index (0.199 and 0.336, respectively). Analysis using a joined cohort revealed that the AUROC of FIB-3 for predicting F3–F4 fibrosis (0.774) was the highest among the 5 fibrosis scores examined and was comparable to that of FIB-4.
Conclusion
The FIB-3 index is an improved version of the FIB-4 index and can effectively predict liver fibrosis in patients with NAFLD.
Keywords: FIB-3 Index, FIB-4 Index, Liver Fibrosis, Nonalcoholic Fatty Liver Disease, Age
Introduction
The Fibrosis-4 (FIB-4) index was created as an indicator of liver fibrosis in patients with human immunodeficiency virus/hepatitis C virus coinfection, and its usefulness is well known in clinical practice.1 The FIB-4 index can be used to predict liver fibrosis in patients with nonalcoholic fatty liver disease (NAFLD).2, 3, 4, 5, 6 Additionally, we reported that the FIB-4-T grade, which is calculated using the FIB-4 index value and tumor factors, can be used for predicting the prognosis of patients with hepatocellular carcinoma (HCC).7 However, because the FIB-4 index includes age in the formula, there have been discussions that the optimum cutoff values may differ between young and elderly patients.8
Several attempts have been made to establish a more universal score that does not include age as a factor. We have reported that the AP20 (AST-3PLT+20) score, which uses aspartate aminotransferase (AST) and platelet (PLT) levels and is calculated as AST (IU/L) − 3 × PLT (×104/μL) + 20, is useful for predicting the development of non-B/non-C HCC in patients with diabetes mellitus9 and for predicting liver fibrosis up to F3. However, because AST is typically low in patients with advanced F4 fibrosis, the AP20 score could not predict F4 fibrosis and was found to be inferior to the FIB-4 index for this prediction. Furthermore, the FIB-4 index is considered superior or equal to the other indices, albumin platelet product (APP)10 and AST to Platelet Ratio Index (APRI)11 in terms of its ability to predict fibrosis above F3.
In this study, we developed a novel sensitive fibrosis prediction score in NAFLD that does not use age as a factor.
Materials and Methods
Patients
Among 777 patients with NAFLD with fibrosis data confirmed by liver biopsy (from September 1999 to June 2019) at Kawasaki Medical School, 42 patients with high levels of AST or alanine aminotransferase (ALT) (>200 IU/L) or PLT of more than 40 × 104/μL were excluded, and 735 patients were enrolled as a training cohort (Table 1). Among 366 patients with NAFLD (RELPEC Study Group) with a confirmed diagnosis of fibrosis by liver biopsy, 42 patients with high levels of AST or ALT (>200 IU/L) or PLT of more than 40 × 104/μL were excluded, and 324 patients were used to test the predictive ability as a validation cohort. Liver biopsies were performed after receiving informed consent from the patients. The patients were informed regarding the purpose of the biopsies, biopsy methods, and probabilities of complications. Liver biopsy was performed percutaneously under ultrasound guidance using a 17G needle. Pathological diagnosis was obtained by a pathologist and a hepatologist. When the opinions differed, they were discussed, and final diagnosis was made.
Table. 1.
Patients’ Characteristics
| Factor | Group | Training cohort (N = 735) | Validation cohort (N = 324) | Joined cohort (N = 1059) |
|---|---|---|---|---|
| Age | 54 [14, 82] | 57 [15, 87] | 55 [14, 87] | |
| Gender (%) | M | 387 (52.6) | 165 (50.9) | 552 (52.1) |
| F (%) | 0 | 82 (11.2) | 111 (34.3) | 193 (18.2) |
| 1 | 252 (34.3) | 105 (32.4) | 357 (33.7) | |
| 2 | 150 (20.4) | 61 (18.8) | 211 (19.9) | |
| 3 | 204 (27.8) | 40 (12.3) | 244 (23.0) | |
| 4 | 47 (6.4) | 7 (2.2) | 54 (5.1) | |
| A (%) | 0 | 63 (8.6) | 96 (29.6) | 159 (15.0) |
| 1 | 347 (47.2) | 128 (39.5) | 475 (44.9) | |
| 2 | 257 (35.0) | 89 (27.5) | 346 (32.7) | |
| 3 | 68 (9.3) | 11 (3.4) | 79 (7.5) | |
| ALB (g/dL) | 4.4 [2.5, 5.4] | 4.5 [1.7, 5.1] | 4.4 [1.7, 5.4] | |
| PLT (104/μL) | 20.4 [3.7, 39.9] | 23.4 [4.5, 38.5] | 21.2 [3.7, 39.9] | |
| AST (IU/L) | 37 [12, 198] | 44 [13, 177] | 40 [12, 198] | |
| ALT (IU/L) | 58 [10, 199] | 65 [9, 198] | 60 [9, 199] |
Data are expressed as median [range] or number.
A, the degree of inflammation’s activity of the hepatitis; ALB, albumin; F, the degree of fibrosis.
The mean age ± standard deviation of the entire cohort was 53.0 ± 14.9 years.
The study protocol complied with the ethical guidelines of the World Medical Association and the Declaration of Helsinki and was approved by the institutional review boards of the institutions involved (authorization number #3-202 and #2022-0086).
Construction of a New Score
To create a new prediction formula, we used AST, ALT, and PLT as explanatory variables, which are the constituent factors of the FIB-4 index, and set “liver fibrosis F3 or more” as an objective variable in a logistic regression analysis using the training set. AST and ALT were log-transformed to make them closer to a normal distribution. We created a new score using the obtained regression coefficient and called it the “original FIB-3 index.” Additionally, to make the index more useful in clinical practice, we adjusted the coefficients and intercepts so that the calculated score directly indicates the fibrosis stage and created a new liver fibrosis prediction formula, the FIB-3 index.
Fibrosis Prediction Ability of the FIB-3 Index
We verified the fibrosis prediction ability of the FIB-3 index by comparing it with that of the FIB-4 index using the receiver operating characteristic (ROC) curve analysis. The analysis was performed to predict F3–F4 fibrosis.
To determine the effects of age on fibrosis prediction, the accuracy of the FIB-3 and FIB-4 indices was compared among patients aged 60 years or older and younger patients.
The prediction ability of the FIB-3 index was compared with that of other fibrosis scores, including AP20,9 APP,10 APRI,11 and FIB-4 index, to investigate the usefulness of the FIB-3 index using a joined cohort.
Statistics
Continuous variables are presented as medians and interquartile ranges. The Mann-Whitney U-test was used to compare the median values. Categorical variables were analyzed using Fisher’s exact test. Cutoff values in ROC analysis were determined using Youden’s index. Differences in P values of less than .05 were considered statistically significant. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan)12 and a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).13
Results
Construction of the FIB-3 Index
We performed a logistic regression analysis using ln AST, ln ALT, and PLT as the descriptive factors and “fibrosis level F3 or higher” as the objective variable. The odds ratios of ln AST, ln ALT, and PLT were 3.82, 1.30, and 0.896, respectively; their 95% confidence intervals were 2.72–5.36, 1.03–1.64, and 0.87–0.924, respectively; and their regression coefficients were 0.47, −0.19, and −0.017, respectively. Based on these data, we developed a new score for predicting “fibrosis level F3 or higher” and called this score the “original FIB-3 index.” The formula is as follows:
To make the original FIB-3 more accessible, the formula was modified as follows so that the fibrosis stage directory coincided with the new score, FIB-3 index.
We subtracted 0.18 from the original FIB-3 index formula, multiplied by 10.8, and then each coefficient was rounded.
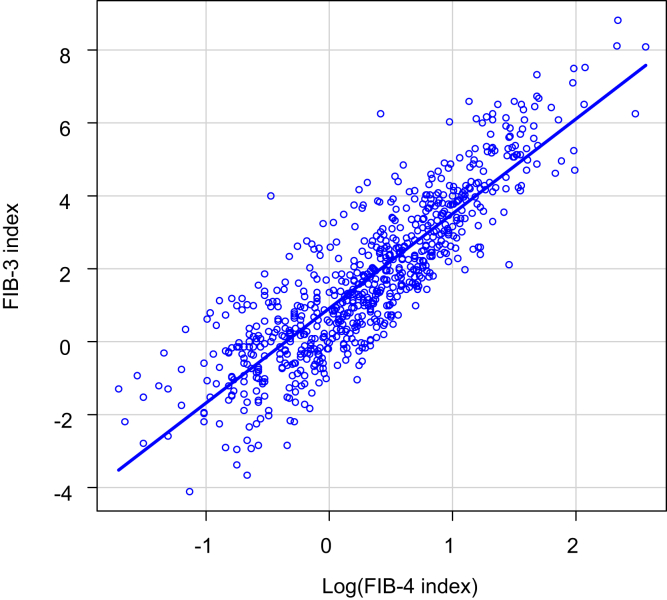
Correlation Between the FIB-3 and FIB-4 Indices
The correlation between the FIB-3 and FIB-4 indices in the training cohort is shown in Figure 1. ln (FIB-4 index), The logarithm of the FIB-4 index, was used because the FIB-3 index was normally distributed, and the FIB-4 index was distributed close to the chi-square distribution. The correlation coefficient between the FIB-3 index and ln (FIB-4 index) was 0.876 (95% confidence interval: 0.861–0.889; P < .0001). From this correlation, the following regression equation was derived between the FIB-3 and FIB-4 indices:
Figure 1.
Correlation between the FIB-3 and FIB-4 indices. The correlation coefficient between the FIB-3 index and ln (FIB-4 index) was 0.867 (95% confidence interval: 0.848–0.884) (P < .0001). The regression equation between the FIB-3 and FIB-4 indices was calculated: FIB-3 index = 2.69 log (FIB-4 index) + 0.86. The commonly used cutoff values of the FIB-4 index for mild, moderate, and severe fibrosis are 1.3, 2.67, and 3.25, respectively, which correspond to those of the FIB-3 index (1.57, 3.50, and 4.03, respectively). The logarithm of the FIB-4 index was used because the FIB-3 index was normally distributed and the FIB-4 index was distributed close to the chi-square distribution.
The cutoff values of the FIB-3 index were set based on the commonly used cutoff values of the FIB-4 index.14,15 The cutoff values of the FIB-4 index for mild, moderate, and severe fibrosis are 1.3, 2.67, and 3.25, respectively, which correspond to 1.57, 3.50, and 4.03, respectively, of the FIB-3 index.
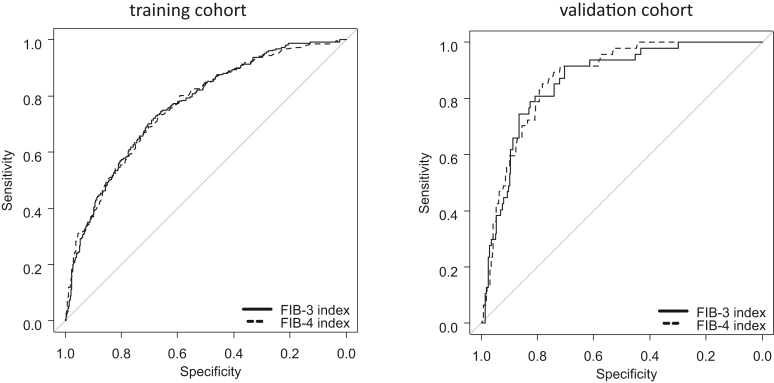
The area under the ROC curve (AUROC) comparison of the ability to predict fibrosis above F3 between the FIB-3 and FIB-4 indices (training and validation cohorts) is shown in Figure 2. In the comparison of the ability to predict fibrosis above F3 between the FIB-3 and FIB-4 indices using ROC analysis, the AUROC values/sensitivities/specificities/cutoff values were 0.764/0.701/0.707/1.892 and 0.762/0.685/0.711/1.580, respectively, in the training cohort and 0.863/0.915/0.704/2.119 and 0.872/0.851/0.783/1.970, respectively, in the validation cohort. The predictive ability of fibrosis in ROC analysis between the FIB-3 index and FIB-4 index was not significant in either the training cohort (P = .832) or the vascularization cohort (P = .361). In both cohorts, the FIB-3 index was as predictive as the FIB-4 index.
Figure 2.
Predictive ability of the FIB-3 and FIB-4 indices for F3–F4 fibrosis. In both cohorts, no difference in the ability to diagnose fibrosis was observed between the FIB-3 and FIB-4 indices. The area under the receiver operating characteristic curve values of the FIB-3 and FIB-4 indices in the training cohort were 0.764 and 0.762, respectively (P = .832), and those in the validation cohort were 0.863 and 0.872, respectively (P = .361).
The Ability to Predict Fibrosis of the FIB-3 and FIB-4 Indices in Elderly and Nonelderly Patients
To examine the effect of age, the patients were divided into 2 groups—those older than 60 years and those younger than 60 years—and the ability to predict fibrosis of the FIB-3 and FIB-4 indices was examined. To analyze the effect of age, 55 years of age (median age in our cohort) was the ideal cutoff. However, a high false-positive rate of the FIB-4 index was reported in elderly patients older than 65 years, so we set the cutoff age at 60 years in the present study.
In this age-specific study, the FIB-3 index showed a smaller difference in accuracy between ages than the FIB-4 index in both cohorts. The differences between the FIB-3 and FIB-4 indices in the training cohort were 0.140 and 0.199, respectively, and those in the validation cohort were 0.178 and 0.336, respectively (Table 2).
Table. 2.
Comparison of the Difference of Accuracy by Age Group in Fibrosis Prediction: F3 or More
| Score | Age | FIB-4 index Cutoff: 1.63 |
FIB-3 index Cutoff: 1.89 |
||
|---|---|---|---|---|---|
| Accuracy: estimate value (95% CI) | Difference | Accuracy: estimate value (95% CI) | Difference | ||
| Training cohort | All | 0.706 (0.672–0.739) | 0.702 (0.668–0.735) | ||
| ≥60 | 0.579 (0.517–0.639) | 0.199 | 0.613 (0.551–0.672) | 0.140 | |
| <60 | 0.778 (0.738–0.815) | 0.753 (0.711–0.791) | |||
| Validation cohort | All | 0.722 (0.670–0.770) | 0.704 (0.651–0.753) | ||
| ≥60 | 0.526 (0.438–0.612) | 0.336 | 0.600 (0.512–0.683) | 0.178 | |
| <60 | 0.862 (0.805–0.908) | 0.778 (0.712–0.835) | |||
The difference in the FIB-3 index is shown in bold to indicate that the difference in ACCURACY between those aged 60 and over and those aged under 60 is smaller for the FIB-3 index than for the FIB-4 index.
CI, confidential interval.
Details of the statistics for all cohorts are presented in Table A1. As shown in the table, the FIB-3 index showed less difference in sensitivity, specificity, and accuracy among the age groups than the FIB-4 index.
Comparison With Other Fibrosis Scores
Using a joined cohort (N = 1059), we compared the predictive ability of the FIB-3 index with that of various fibrosis scores, namely, AP20, APP, APRI, and FIB-4 index (Table 3). The FIB-3 index showed the best AUROC (0.774) among all indices for predicting F3 fibrosis or higher (Table 3). The FIB-3 index showed significantly better AUROC than AP20 (P < .0001), APP (P = .0022), and APRI (P = .0008); however, no significant difference was observed between the FIB-3 and FIB-4 indices (P = .883).
Table. 3.
League Table Showing Comparisons Among Each Fibrosis Score in all Cases (N=1059)
| Fibrosis score | Cutoff value (sensitivity/specificity [%]) | AUROC | 95% CI | AP20 | APP | APRI | FIB-4 index | FIB-3 index |
|---|---|---|---|---|---|---|---|---|
| AP20 (AST-3PLT+20) | 2.2 (71.5/66.6) | 0.745 | 0.713–0.777 | |||||
| APP (albumin platelet product) | 8.732 (68.1/67.3) | 0.730 | 0.696–0.764 | P = .352 | ||||
| APRI (AST platelet ratio index) | 2.184 (70.1/69.0) | 0.749 | 0.718–0.781 | P = .238 | P = .254 | |||
| FIB-4 index | 1.630 (70.1/71.5) | 0.773 | 0.742–0.804 | P = .024 | P = .0018 | P = .082 | ||
| FIB-3 index | 1.892 (73.5/69.0) | 0.774 | 0.744-0.805 | P <.0001 | P = .0022 | P = .0008 | P = .883 |
The FIB-3 index showed the best AUROC (0.774) among all indices for predicting F3 fibrosis or higher. The FIB-3 index showed significantly better AUROC than AP20 (p < 0.0001), APP (p = 0.0022), and APRI (p = 0.0008); however, no significant difference was observed between the FIB-3 and FIB-4 indices (p = 0.883).
CI, confidential interval.
FIB-3 and FIB-4 Indices in Different Fibrosis Stages
The FIB-3 and FIB-4 index values in the joined cohort for each fibrosis stage are shown in Figure 3. The FIB-3 index increased linearly as fibrosis progressed, and the mean values for each fibrosis stage were 0.18, 1.06, 1.97, 2.96, and 4.34 for F0, F1, F2, F3, and F4, respectively (Figure 3). The FIB-3 index was almost consistent with the number of each stage. The accuracy of the FIB-3 index for predicting fibrosis above F3 in the joined cohort was 0.703 with a sensitivity of 0.735 and specificity of 0.690 at a cutoff value of 1.892.
Figure 3.
FIB-3 and FIB-4 indices at each fibrosis stage. The FIB-3 index increased linearly as fibrosis progressed, and the mean values ± standard deviations for each fibrosis stage were 0.18 ± 1.67, 1.06 ± 1.99, 1.96 ± 2.11, 2.96 ± 2.00, and 4.34 ± 1.53 for F0, F1, F2, F3, and F4, respectively. In contrast, the FIB-4 index increased exponentially as the fibrosis stage progressed, and the mean values ± standard deviations for each fibrosis stage were 1.12 ± 0.76, 1.34 ± 1.32, 1.85 ± 1.50, 2.38 ± 1.52, and 4.08 ± 2.20 for F0, F1, F2, F3, and F4, respectively.
In contrast, the FIB-4 index increased exponentially as the fibrosis stage progressed, and the mean values for each fibrosis stage were 1.12, 1.34, 1.85, 2.38, and 4.08 for F0, F1, F2, F3, and F4, respectively.
Discussion
In this study, we developed a new liver fibrosis prediction score, the FIB-3 index, which showed a good ability to predict fibrosis comparable to that of the FIB-4 index. Note that the effect of age on the prediction ability of the FIB-3 index was less than that on the prediction ability of the FIB-4 index. The difference in the accuracy in predicting fibrosis in patients over 60 years of age and in those younger than 60 years was smaller in the FIB-3 index than that in the FIB-4 index. It indicates that changing the cutoff value of the FIB-3 index according to the age of the patients is unnecessary, as is the case with the FIB-4 index. Additionally, the FIB-3 index showed significantly better predictive ability than AP20, APP, and APRI. Therefore, the FIB-3 index could be used as a representative index to predict liver fibrosis in clinical practice.
In this study, the FIB-3 index, which does not use age as a factor, did not differ from the FIB-4 index in predicting fibrosis when we examined an entire cohort. Age is included in the FIB-4 index because it improves the prediction ability; however, it seems to have too much effect on predicting fibrosis than expected. One possible reason is that age might not be linearly correlated with the extent of liver fibrosis, and the FIB-4 index could not fit to patients with NAFLD in various age groups. Actually, the FIB-4 index was originally constructed for human immunodeficiency virus/hepatitis C virus-coinfected patients of relatively young age (mean age ± standard deviation, 40 ± 7 years).1 In contrast, the mean age of patients with NAFLD in this study was 53.0 years, and the standard deviation was much larger (14.9 years). To avoid this unwanted effect of age, we included only PLT, AST, and ALT in the FIB-3 index. Although the FIB-3 index does not include age in the formula, PLT and ALT tended to decrease with age, and they seem to mildly complement the effect of age on liver fibrosis (Figure A1A, B and C).
The FIB-3 index showed the highest AUROC value among the existing well-known fibrosis prediction scores and was not influenced by patient age. Additionally, the variables used in the index are PLT, AST, and ALT, which are measured routinely in clinical practice, meaning that the FIB-3 index could be widely used by physicians, including nonhepatologists. This index is essential in terms of enclosing high-risk patients for non-B/non-C HCC because liver fibrosis is a risk factor for HCC and the number of patients with NAFLD who might have liver fibrosis is too large to be screened by hepatologists alone.
The FIB-3 index has another advantage in clinical practice. Unlike the FIB-4 index, which increases exponentially as the fibrosis stage progresses, the FIB-3 index score increases linearly and indicates the approximate fibrosis stage.
Although the ability of the FIB-3 index to predict fibrosis is high, this index should be used with caution in elderly patients. As we observed for the FIB-4 index, the accuracy of the FIB-3 index for predicting fibrosis in patients aged over 60 years was lower than that in patients aged under 60 years. Therefore, these indices should be used as a reference, and imaging examination, such as abdominal ultrasonography, is better to be introduced more frequently in elderly patients. In contrast, the FIB-3 index is an exceptional predictor of hepatic fibrosis in young patients. Recently, the number of NAFLD cases among young individuals has been increasing; therefore, the FIB-3 index must be useful for screening liver fibrosis and will have a significant impact on the field.
This study has several limitations. First, this was a retrospective study. Second, the extent of liver fibrosis was diagnosed using needle biopsy, and there must have been a sampling bias. Third, the study was limited to Japanese patients. Additionally, this study was conducted only for NAFLD. Examining whether this index can be applied to viral liver diseases is necessary as a next step.
Nevertheless, the FIB-3 index, a new prediction score for liver fibrosis that does not include age as a factor, can diagnose liver fibrosis effectively and could be widely used in future clinical practice. Further validation studies should be conducted in the future.
Acknowledgments:
The authors gratefully acknowledge all doctors who collaborated on this project by collecting data on the patients registered in their respective hospitals.
Footnotes
Authors' Contributions: Conceptualization: Atsushi Hiraoka and Takashi Kumada; data curation: Miwa Kawanaka, Hidenori Toyoda, Toshifumi Tada, Atsushi Hiraoka, Toru Ishikawa, Akiko Wakuta, Nozomi Miyake, Shiho Murakami, Shohei Shiota, and Takashi Kumada; methodology: Takashi Kumada; writing (original draft): Kazuya Kariyama; writing (review and editing): Kazuhiro Nouso.
Conflicts of Interest: The authors disclose no conflicts.
Funding: The authors report no funding.
Ethical Statement: The corresponding author, on behalf of all authors, jointly and severally, certifies that their institution has approved the protocol for any investigation involving humans or animals and that all experimentation was conducted in conformity with ethical and humane principles of research.
Date Transparency Statement: The data, analytical methods, and research materials used in this study are not available to other researchers.
Material associated with this article can be found in the online version at https://doi.org/10.1016/j.gastha.2022.07.012.
Supplementary Materials
Correlation between age and the factors that construct the FIB-3 index. (a) Correlation between age and platelet (correlation coefficient = −0.369; 95% CI = −0.43 to −0.304; P < .0001). (b) Correlation between age and aspartate transaminase (AST) (correlation coefficient = 0.0791; 95% CI = 0.00679–0.151; P =.0321). (c) Correlation between age and alanine transaminase (ALT) (correlation coefficient = −0.339; 95% CI = −0.401 to −0.273; P = 3.39e-21). The training cohort was used for the analyses. The lines in the figures indicate the regression lines. Note that platelet and ALT decline with increasing age.
References
- 1.Sterling R.K., Lissen E., Clumeck N., et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 2.Shah A.G., Lydecker A., Murray K., et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104–1112. doi: 10.1016/j.cgh.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sumida Y., Yoneda M., Hyogo H., et al. Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol. 2012;12:2. doi: 10.1186/1471-230X-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nones R.B., Ivantes C.P., Pedroso M.L.A. Can FIB4 and NAFLD fibrosis scores help endocrinologists refer patients with non-alcoholic fat liver disease to a hepatologist? Arch Endocrinol Metab. 2017;61:276–281. doi: 10.1590/2359-3997000000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takeuchi H., Sugimoto K., Oshiro H., et al. Liver fibrosis: noninvasive assessment using supersonic shear imaging and FIB4 index in patients with non-alcoholic fatty liver disease. J Med Ultrason (2001) 2018;45:243–249. doi: 10.1007/s10396-017-0840-3. [DOI] [PubMed] [Google Scholar]
- 6.Poynard T., Munteanu M., Charlotte F., et al. Diagnostic performance of a new noninvasive test for nonalcoholic steatohepatitis using a simplified histological reference. Eur J Gastroenterol Hepatol. 2018;30:569–577. doi: 10.1097/MEG.0000000000001064. [DOI] [PubMed] [Google Scholar]
- 7.Kariyama K., Nouso K., Toyoda H., et al. Utility of FIB4-T as a prognostic factor for hepatocellular carcinoma. Cancers (Basel) 2019;11:203. doi: 10.3390/cancers11020203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishiba H., Sumida Y., Tanaka S., et al. The novel cutoff points for the FIB4 index categorized by age increase the diagnostic accuracy in NAFLD: a multi-center study. J Gastroenterol. 2018;53:1216–1224. doi: 10.1007/s00535-018-1474-y. [DOI] [PubMed] [Google Scholar]
- 9.Kariyama K., Nouso K., Wakuta A., et al. [Simple scoring index for early detection of non-B, non-C hepatocellular carcinoma in patients with diabetes mellitus] Kanzo. 2019;60:156–158. [Google Scholar]
- 10.Fujita K., Yamasaki K., Morishita A., et al. Albumin platelet product as a novel score for liver fibrosis stage and prognosis. Sci Rep. 2021;11(1):5345. doi: 10.1038/s41598-021-84719-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wai C.T., Greenson J.K., Fontana R.M., et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 12.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.R development core team RFfSC. R: a language and environment for statistical computing. The R Foundation for Statistical Computing; Vienna, Austria: 2005. [Google Scholar]
- 14.Sun W., Cui H., Li N., et al. Comparison of FIB-4 index, NAFLD fibrosis score and BARD score for prediction of advanced fibrosis in adult patients with non-alcoholic fatty liver disease: a meta-analysis study. Hepatol Res. 2016;46(9):862–870. doi: 10.1111/hepr.12647. [DOI] [PubMed] [Google Scholar]
- 15.Kawata N., Takahashi H., Iwane S., et al. FIB-4 index-based surveillance for advanced liver fibrosis in diabetes patients. Diabetol Int. 2020;12(1):118–125. doi: 10.1007/s13340-020-00453-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correlation between age and the factors that construct the FIB-3 index. (a) Correlation between age and platelet (correlation coefficient = −0.369; 95% CI = −0.43 to −0.304; P < .0001). (b) Correlation between age and aspartate transaminase (AST) (correlation coefficient = 0.0791; 95% CI = 0.00679–0.151; P =.0321). (c) Correlation between age and alanine transaminase (ALT) (correlation coefficient = −0.339; 95% CI = −0.401 to −0.273; P = 3.39e-21). The training cohort was used for the analyses. The lines in the figures indicate the regression lines. Note that platelet and ALT decline with increasing age.