Abstract
Background
Little is known about the bleeding risk associated with cangrelor use in patients with myocardial infarction (MI) who are exposed to an oral P2Y12 inhibitor before coronary angiography.
Methods
Cangrelor in Acute MI: Effectiveness and Outcomes (CAMEO) is an observational registry studying platelet inhibition for patients with MI. Upstream oral P2Y12 inhibition was defined as receipt of an oral P2Y12 inhibitor within 24 hours before hospitalization or in-hospital before angiography. Among cangrelor-treated patients, we compared bleeding after cangrelor use through 7 days postdischarge between patients with and without upstream oral P2Y12 inhibitor exposure.
Results
Among 1802 cangrelor-treated patients with MI, 385 (21.4%) received upstream oral P2Y12 inhibitor treatment. Of these, 101 patients (33.8%) started cangrelor within 1 hour, 103 (34.4%) between 1 and 3 hours, and 95 (31.8%), >3 hours after in-hospital oral P2Y12 inhibitor administration; the remaining received an oral P2Y12 inhibitor before hospitalization. There was no statistically significant difference in rates of bleeding among cangrelor-treated patients with and without upstream oral P2Y12 inhibitor exposure (6.5% vs 8.8%; adjusted odds ratio [OR], 0.62; 95% CI, 0.38-1.01). Bleeding was observed in 5.0%, 10.7%, and 3.2% of patients treated with cangrelor <1, 1 to 3, and >3 hours after the last oral PY12 inhibitor dose, respectively; bleeding rates were not statistically different between groups (1-3 hours vs <1 hour: adjusted OR, 2.70; 95% CI, 0.87-8.32; >3 hours vs <1 hour: adjusted OR, 0.65; 95% CI, 0.15-2.85).
Conclusions
Bleeding risk was not observed to be significantly higher after cangrelor treatment in patients with and without upstream oral P2Y12 inhibitor exposure.
Keywords: bleeding, cangrelor, myocardial infarction, P2Y12 inhibitor
Central Illustration
Highlights
-
•
Our study represents a real-world Switching Antiplatelet-5 study.
-
•
Cangrelor administration after a P2Y12 inhibitor is not associated with increased bleeding.
-
•
Timing of the upstream P2Y12 inhibitor is not associated with an increased bleeding risk.
Introduction
Cangrelor is an intravenous platelet P2Y12 antagonist characterized by a rapid onset of action with potent P2Y12 inhibitory effects and is approved for use in patients who have not been pretreated with an oral P2Y12 inhibitor.1, 2, 3 In patients with acute myocardial infarction (MI), it is often used when the patient has inadequate time for onset of effect of an oral P2Y12 inhibitor or when there is concern for inadequate absorption of an oral P2Y12 inhibitor, such as in patients presenting with cardiac arrest or cardiogenic shock.4 However, upstream use of an oral P2Y12 inhibitor commonly occurs in clinical practice, and little is known about the bleeding risk associated with cangrelor treatment in patients who have received an upstream oral P2Y12 inhibitor.5
The timing of cangrelor administration relative to the last upstream oral P2Y12 inhibitor dose may influence bleeding risk. Although drug-drug interactions did not occur when ticagrelor was given during cangrelor infusion,6, 7, 8 administering cangrelor to patients pretreated with ticagrelor 1 hour in advance of the infusion had an additive platelet inhibitory effect.9 After discontinuation of cangrelor, platelet inhibition remained suppressed by ticagrelor at the same level achieved with the added use of cangrelor. There are few data regarding the safety of pretreatment (or upstream treatment) with clopidogrel or prasugrel before cangrelor. Indeed, data from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR) demonstrated that <1% of cangrelor-treated patients had received either of these agents (33.4% received ticagrelor upstream) before percutaneous coronary intervention (PCI).10 Hence, there are little real-world data for the use of these agents upstream of cangrelor administration.
Cangrelor in Acute Myocardial Infarction: Effectiveness and Outcomes (CAMEO) is an ongoing registry designed to examine antiplatelet selection strategies and cangrelor use patterns among patients with acute myocardial infarction with (ST segment–elevated myocardial infarction [STEMI]) or without (non–ST segment–elevated myocardial infarction [NSTEMI]) ST segment elevation who undergo coronary angiography in real-world practice.11 Using data from the CAMEO registry, we sought to examine the real-world utilization of upstream oral P2Y12 inhibitors (either as home medications or given in-hospital) with cangrelor. In this analysis, we compared the patient and procedural characteristics of cangrelor-treated patients who received an upstream oral P2Y12 inhibitor with those of who did not; examined the association between upstream oral P2Y12 inhibitor use and bleeding risk among cangrelor-treated patients; evaluated whether bleeding risk differed based on the timing of cangrelor initiation relative to the last upstream oral P2Y12 inhibitor dose or on the basis of MI type (STEMI or NSTEMI); and assessed whether bleeding rates observed with use of the higher-potency P2Y12 agents ticagrelor and prasugrel differed from those observed with clopidogrel in this clinical setting.
Methods
The CAMEO registry began enrolling patients in October 2019 and is an ongoing study at 12 U.S. centers that meet the following criteria: (1) capability to perform PCI and coronary artery bypass grafting; (2) minimum of 10 MI patients treated monthly; and (3) minimum use of cangrelor in at least 2 patients with MI monthly. Each hospital obtained approval from their local institutional review board before enrolling patients.
Study design and population
Site selection and study design has been previously described.11 In brief, each hospital began participation in phase 1 of the registry by retrospectively collecting data on ∼50 consecutive patients within the 4 months before site activation. These patients met the following criteria: (1) aged 18 years or older; (2) underwent coronary angiography for STEMI or NSTEMI; and (3) received any P2Y12 inhibitor (cangrelor or oral) during the first 48 hours after hospitalization for MI. After completion of phase 1, each hospital proceeded to phase 2, in which data were collected in a 2:1 ratio for patients with MI treated with cangrelor and those not treated with cangrelor. Phase 2 was designed to focus on the evaluation of patients treated with cangrelor while compiling a contemporary control cohort.
This analysis focused on patients who received a cangrelor infusion started in the catheterization laboratory, stratified by upstream P2Y12 inhibitor use (Central Illustration). Upstream P2Y12 inhibitor use was defined as treatment with a P2Y12 inhibitor at home with the last dose taken within 24 hours of hospital admission or in-hospital administration of a P2Y12 inhibitor before coronary angiography. In addition, coronary angiography had to be started within 24 hours of admission (with home use of a P2Y12 inhibitor) or within 24 hours after in-hospital administration of an oral P2Y12 inhibitor. All qualified patients from phases 1 and 2 were included in this analysis. We excluded patients who were missing date and time for coronary angiography (n = 27).
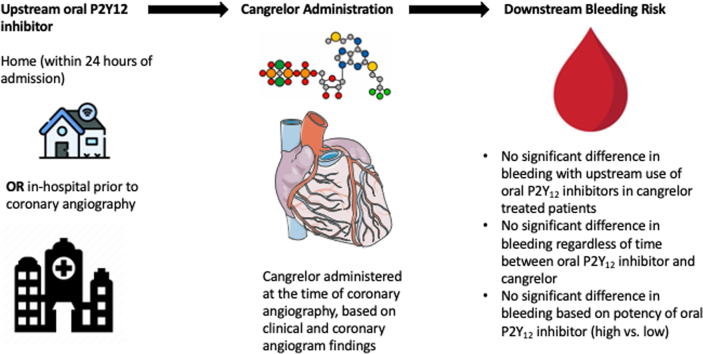
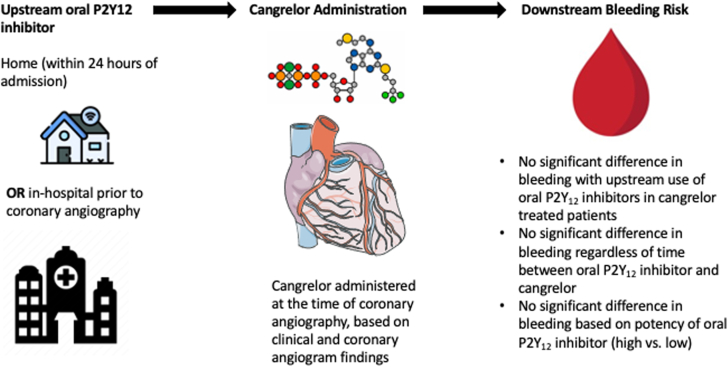
Central Illustration.
Schema of study design of real-world use of upstream oral P2Y12 inhibitors before cangrelor administration at the time of coronary angiography. To treat patients with upstream oral P2Y12 inhibitors, they can be administered within 24 hours of hospital admission or in-hospital administration of a P2Y12 inhibitor before coronary angiography. In addition, coronary angiography had to be started within 24 hours of admission (with home use of a P2Y12 inhibitor) or within 24 hours after in-hospital administration of an oral P2Y12 inhibitor. The illustration describes the bleeding outcomes.
Data collection
Trained personnel at each hospital abstracted patient-level data into a web-based electronic data collection tool. Patient demographic characteristics, medical history, MI admission features, medications taken within 24 hours before hospital arrival, in-hospital medications, predefined in-hospital laboratory values and imaging data, and coronary angiography and PCI data were collected. Adverse clinical events, including bleeding events, were collected during hospitalization and for up to 7 days after discharge. Bleeding events were defined as any event associated with a hemoglobin drop ≥ 3 gm/dL; any event requiring blood transfusion (platelet or red blood cell); or any bleeding event that required an intervention or surgery to stop bleeding, such as surgical closures, exploration of the arteriotomy site, balloon angioplasty to seal an arterial tear, or endoscopy with cautery of a gastrointestinal bleed.12 Bleeding was defined as major if the hemoglobin drop was ≥3 gm/dL, if a surgical intervention was required, an intravenous vasoactive agent was required, or if the patient required transfusion.
Statistical analysis
We compared baseline patient characteristics, home medications, clinical presentations, and procedural characteristics among cangrelor-treated patients who received upstream P2Y12 inhibitor therapy with those of who did not. Categorical variables were reported as counts and frequencies and continuous variables as medians (IQR). The χ2 tests were performed to examine for statistically significant differences between frequencies in the 2 cohorts. Wilcoxon rank sum tests were used to compare continuous variables across the 2 cohorts. Similarly, we compared the baseline characteristics of patients receiving upstream oral P2Y12 inhibitor administration <1, 1 to 3, and >3 hours before cangrelor administration. Kruskal-Wallis tests were used for continuous variables in this analysis.
Then, we examined the association between upstream oral P2Y12 inhibitor use and clinical bleeding using logistic regression. We compared cangrelor-treated patients who received upstream oral P2Y12 inhibitor therapy with those who did not and reported unadjusted and adjusted odds ratios (OR) and 95% CI. Furthermore, we adjusted for modified Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA guidelines (CRUSADE) score, race, age, and arterial access site and assessed bleeding risk stratified by MI type (NSTEMI vs STEMI).
We also used logistic regression to assess the association between bleeding events and the amount of time elapsed between in-hospital P2Y12 inhibitor administration and the beginning of the first cangrelor infusion, stratified into 3 groups: <1 hour (reference), 1 to 3 hours, and >3 hours. We calculated both unadjusted and adjusted OR and 95% CI and adjusted for the modified CRUSADE score, race, and age.
Finally, we assessed factors associated with upstream use of an oral P2Y12 inhibitor among cangrelor-treated patients. We used a stepwise regression with entry criteria of α = 0.20 and exit criteria of α = 0.05. For each characteristic, the OR and 95% CI were calculated.
Two-tailed P values were used and P values of <.05 were considered significant. Analyses were performed using SAS version 9.4 (SAS Institute) and R version 4.1.1.13
Results
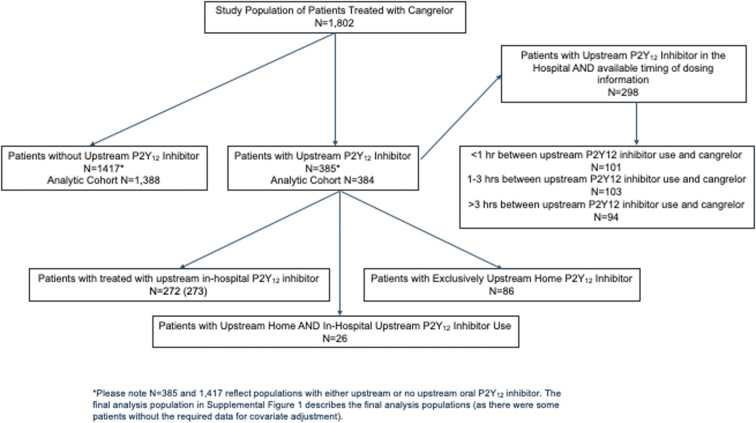
Among the 1802 patients with cangrelor initiated during coronary angiography, 384 (21.3 %) patients received an upstream oral P2Y12 inhibitor (Supplemental Figure S1). Among these, 32.7% of patients were taking an oral P2Y12 inhibitor before hospital admission. The most frequent upstream oral P2Y12 inhibitor used was ticagrelor (92.2%), followed by clopidogrel (6.8%) and prasugrel (1.0%). For the 86 patients who were only treated with a P2Y12 inhibitor at home, 46 (53.5%) used clopidogrel, 3 (3.5%) were administered prasugrel, and 37 (43.0%) took ticagrelor.
Table 1 compares the differences in patient and procedural characteristics between patients who received an upstream oral P2Y12 inhibitor before a cangrelor infusion and those patients who did not receive upstream therapy. Cangrelor-treated patients who received upstream treatment were more likely to be of Black race or Hispanic ethnicity and to present with STEMI or a history of PCI, MI, peripheral artery disease (PAD), diabetes, or ejection fraction of <40% than those without upstream treatment (all P < .05). Cangrelor-treated patients who received upstream treatment were also significantly more likely to be transferred from another hospital and to experience active chest discomfort. There were no observed differences in parenteral antithrombotic medication use, but upstream P2Y12-treated patients were more likely to undergo placement of a mechanical circulatory support device (Table 1).
Table 1.
Baseline patient and procedural characteristics between patients who received an upstream P2Y12 inhibitor vs those without upstream P2Y12 inhibitor therapy.
| Upstream P2Y12 inhibitor use (n = 385a) | No upstream P2Y12 inhibitor use (n = 1417a) | P | |
|---|---|---|---|
| Demographic characteristics | |||
| Age, y | 62.0 (56.0-71.0) | 64.0 (55.0-73.0) | .431 |
| Female | 103 (26.8) | 431 (30.4) | .163 |
| Black | 125 (32.6) | 299 (21.4) | <.001 |
| Hispanic | 71 (18.5) | 173 (12.5) | .003 |
| Clinical history | |||
| STEMI | 240 (62.3) | 796 (56.2) | .030 |
| Previous MI | 107 (27.8) | 220 (15.7) | <.001 |
| Previous PCI | 122 (31.7) | 253 (18.1) | <.001 |
| Previous CABG | 27 (7.0) | 69 (4.9) | .108 |
| Stroke or TIA | 29 (7.6) | 111 (7.9) | .808 |
| PAD | 36 (9.4) | 66 (4.7) | <.001 |
| Previous HF | 45 (11.7) | 150 (10.7) | .587 |
| Atrial fibrillation/flutter | 29 (7.5) | 106 (7.6) | .980 |
| Diabetes | 154 (40.0) | 470 (33.6) | .019 |
| Dialysis | 13 (3.4) | 30 (2.1) | .162 |
| Current/recent smoker | 122 (31.7) | 407 (29.1) | .327 |
| Hospitalized or transfused for bleeding in past year | 2 (0.5) | 18 (1.3) | .279 |
| Home oral anticoagulant use | 34 (8.8) | 116 (8.3) | .738 |
| In-hospital features | |||
| Transfer in from another hospital | 175 (45.5) | 470 (33.6) | <.001 |
| Admission hemoglobin, g/dL | 14.0 (12.6-15.2) | 14.0 (12.4-15.1) | .547 |
| Admission platelets, 109/L | 237.5 (201.5-288.5) | 242.0 (201.0-290.0) | .483 |
| Radial artery access | 180 (46.8) | 891 (64.0) | <.001 |
| Femoral artery access | 201 (52.2) | 490 (35.2) | <.001 |
| LVEFa <40% | 126 (33.3) | 370 (26.9) | .013 |
| PCI performed | 379 (98.4) | 1347 (96.4) | .039 |
| Signs/symptoms present at the time of PCI | |||
| Active chest discomfort | 78 (20.6) | 208 (15.4) | .017 |
| Sustained VT/VF | 14 (3.7) | 37 (2.7) | .336 |
| Cardiogenic shock | 41 (10.8) | 110 (8.2) | .107 |
| Cardiac arrest | 10 (2.6) | 45 (3.3) | .492 |
| Thrombus visualized | 193 (50.9) | 610 (45.3) | .052 |
| Bypass graft treated | 10 (2.6) | 32 (2.4) | .769 |
| Multivessel PCI performed | 60 (15.8) | 217 (16.1) | .896 |
| Thrombectomy | 64 (16.9) | 186 (13.8) | .133 |
| Mechanical circulatory support | 60 (15.8) | 161 (12.0) | .046 |
| Concomitant antithrombotic medication use | |||
| Parenteral anticoagulant | 337 (87.5) | 1229 (87.8) | .867 |
| Glycoprotein IIb/IIIa inhibitor | 1 (2.9) | 45 (3.2) | .720 |
| Thrombolytics | 8 (2.1) | 16 (1.1) | .159 |
| Oral anticoagulants | 46 (11.9) | 141 (10.1) | .289 |
Values are median (IQR) or n (%).
CABG, coronary artery bypass grafting; HF, heart failure; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; STEMI, ST segment–elevated myocardial infarction; TIA, transient ischemic attack; VF, ventricular fibrillation; VT, ventricular tachycardia.
Please note that n = 385 and 1417 reflect populations with either upstream or no upstream oral P2Y12 inhibitor. The final analysis population in Supplemental Figure S1 describes the final analysis populations (because there were some patients without the required data for covariate adjustment).
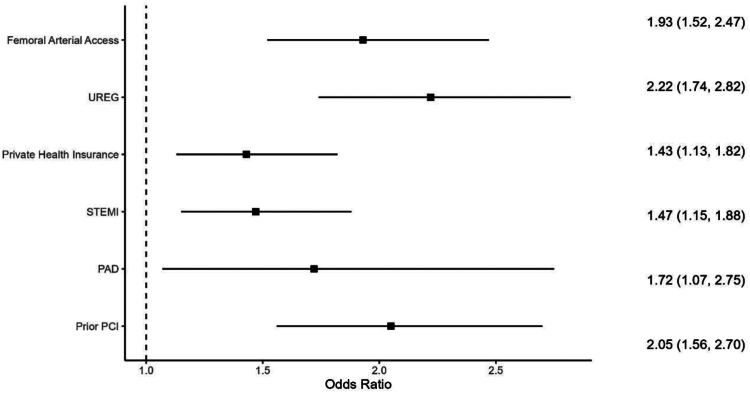
Figure 1 describes the factors associated with upstream use of an oral P2Y12 inhibitor among cangrelor-treated patients. Patients treated with cangrelor and an upstream oral P2Y12 inhibitor treatment were more likely to be from an underrepresented racial or ethnic group or to have private health insurance. In addition, patients treated with cangrelor and an upstream oral P2Y12 inhibitor treatment were more likely to present with STEMI, undergo femoral artery access, have had a previous PCI, or have PAD compared with cangrelor-treated patients without upstream pretreatment.
Figure 1.
Factors associated with upstream use of an oral P2Y12inhibitor among cangrelor-treated patients. Patients treated with cangrelor and an upstream oral P2Y12 inhibitor treatment were more likely to be from an underrepresented racial or ethnic group (UREG) or to have private health insurance. In addition, patients treated with cangrelor and an upstream oral P2Y12 inhibitor treatment were more likely to present with ST segment–elevated myocardial infarction (STEMI), undergo femoral artery access, have had a previous percutaneous coronary intervention (PCI), or have experienced peripheral artery disease (PAD) compared with cangrelor-treated patients without upstream pretreatment. An odds ratio (OR) of >1 is likely to be associated with pretreatment and an OR of <1 is likely not be associated with pretreatment.
Table 2 demonstrates the association between upstream P2Y12 inhibitor therapy and bleeding events among cangrelor-treated patients. There were no statistically significant differences in observed or adjusted bleeding rates associated with cangrelor use between patients with and without upstream P2Y12 inhibitor exposure (6.5% vs 8.8%; adjusted OR, 0.62; 95% CI, 0.38-1.01). The upstream treatment group consisted of 384 patients. Among the 86 patients who were treated with a P2Y12 inhibitor exclusively at home, 6 patients (7.0%) experienced a bleeding event. There were also 272 patients exclusively treated only with an in-hospital P2Y12 inhibitor, 18 of whom (6.6%) experienced a bleeding event. Finally, 1 bleeding event was observed among 26 patients (3.85%) who were administered P2Y12 therapy both at home and in the hospital.
Table 2.
The association between upstream oral P2Y12 inhibitor therapy and bleeding
| Outcome | n/N (%) | Unadjusted odds ratio (95% CI) | P value | Adjusted odds ratio (95% CI) | P value |
|---|---|---|---|---|---|
| Bleed event - pretreatment | 25/384 (6.5%) | 0.72 (0.46-1.13) | .154 | 0.62 (0.38-1.01) | .053 |
| Bleed event - no pretreatment | 122/1388 (8.8%) | ||||
| Bleed event - high potency | 19/277 (6.86%) | 1.24 (0.48-3.19) | .656 | 2.00 (0.69-5.81) | .205 |
| Bleed event - low potency | 6/107 (5.61%) |
Among 298 cangrelor-treated patients who received a P2Y12 inhibitor upstream in-hospital (Supplemental Figure S1), 101 patients (33.8%) started cangrelor within 1 hour, 103 (34.4%) between 1 and 3 hours, and 94 (31.5%), >3 hours after the in-hospital oral P2Y12 inhibitor dose. Patients presenting with STEMI, Black patients, and smokers were more likely to comprise those with shorter durations between last upstream oral P2Y12 inhibitor dose and cangrelor initiation (Supplemental Table S1). Bleeding event rates were 5.0%, 10.7%, and 3.2% among patients with 0- to 1-hour, 1- to 3-hours, and >3-hour gaps between the last upstream P2Y12 inhibitor use and cangrelor initiation, respectively; these differences were not statistically significant with or without multivariable adjustment (Table 3). Table 4 summarizes that the relationship between upstream oral P2Y12 inhibitor exposure and bleeding events was not statistically different between patients with STEMI and those with NSTEMI treated with cangrelor. Table 2 describes the association between the use of upstream high potency (ticagrelor or prasugrel) and low potency (clopidogrel) P2Y12 inhibitors and bleeding risk among cangrelor-treated patients with MI. There was no statistically significant difference after adjustment in the risk of bleeding among cangrelor-treated patients who received an upstream high potency versus low potency oral P2Y12 inhibitor (adjusted OR, 2.00; 95% CI, 0.69-5.81).
Table 3.
The association between timing of upstream oral P2Y12 inhibitor therapy and cangrelor initiation and risk of bleeding.
| Time group, h | Bleed eventsa, n/N (%) | Unadjusted odds ratio (95% CI) | P | Adjusted odds ratio (95% CI) | P |
|---|---|---|---|---|---|
| 0-1 | 5/101 (5.0) | Ref. | Ref. | ||
| 1-3 | 11/103 (10.7) | 2.30 (0.77-6.86) | .137 | 2.70 (0.87-8.32) | .084 |
| >3 | 3/94 (3.2) | 0.63 (0.15-2.73) | .539 | 0.65 (0.15-2.85) | .566 |
Number of bleeding events/number of patients who received an upstream oral P2Y12 inhibitor in the particular time group.
Table 4.
The association between upstream oral P2Y12 inhibitor therapy and bleeding, stratified by MI type.
| Outcome | MI type | Pretreatment, n/N (%) | No pretreatment, n/N (%) | Unadjusted odds ratio (95% CI) | P | Adjusted odds ratio (95% CI) | P |
|---|---|---|---|---|---|---|---|
| Bleed event | STEMI | 26/240 (8.3) | 82/785 (10.5) | 0.78 (0.47-1.30) | .340 | 0.80 (0.46-1.40) | .439 |
| NSTEMI | 5/144 (3.5) | 40/603 (6.6) | 0.51 (0.20-1.31) | .159 | 0.46 (0.17-1.24) | .112 |
MI, myocardial infarction; NSTEMI, non–ST segment–elevated myocardial infarction; STEMI, ST segment–elevated myocardial infarction.
Discussion
In this analysis of the CAMEO Registry, we examined the use of cangrelor among patients with MI who had received oral P2Y12 inhibitor drugs before cardiac catheterization and sought to understand the association of upstream P2Y12 inhibitor drug use with bleeding risk. Cangrelor-treated patients who received an upstream oral P2Y12 inhibitor were more likely to present with a significant cardiovascular history, including a history of PCIs, MIs, and diagnoses of PAD and diabetes when compared with patients who received cangrelor without upstream oral P2Y12 inhibitors. Ticagrelor was the most frequently used P2Y12 drug in this registry, representing >90% of those receiving both a P2Y12 drug and cangrelor. Nearly 34% of cangrelor-treated patients with in-hospital upstream treatment had received an oral P2Y12 inhibitor within an hour of initiating cangrelor therapy. No statistically significant association between bleeding and upstream oral P2Y12 inhibitor use among cangrelor-treated patients was observed, regardless of MI type, P2Y12 inhibitor potency, or timing of cangrelor administration relative to oral P2Y12 use.
Given the urgency in timing of invasive management for MI, particularly in patients presenting with STEMI, interventional operators may not know at the onset of catheterization the following: (1) whether a patient has been taking an oral P2Y12 inhibitor chronically or has been recently loaded with one (particularly in the case of interhospital transfers); (2) whether a patient has adequately absorbed an oral P2Y12 inhibitor (in the case of cardiogenic shock or cardiac arrest); or (3) whether an oral P2Y12 inhibitor was given early enough for therapeutically adequate platelet inhibition to have occurred. In these cases, the use of cangrelor in addition to an oral P2Y12 inhibitor may be beneficial to ensure adequate platelet inhibition at the time of catheterization. However, use of cangrelor in combination with an upstream oral P2Y12 inhibitor raises concerns of increased bleeding risk; current Food and Drug Administration-approved product instructions stipulate that cangrelor is intended for use in patients who have not been treated with an oral P2Y12 inhibitor.14 In this regard, our observations are reassuring in having found no significant increased bleeding risk associated with cangrelor therapy in patients who received an upstream oral P2Y12 inhibitor. We did note that there were higher rates of bleeding in patients who received an oral P2Y12 inhibitor 1 to 3 hours before cangrelor treatment compared with patients who received upstream oral P2Y12 inhibitors either 0 to 1 hours or >3 hours before cangrelor therapy. Although these rates did not statistically differ, these relationships should continue to be explored.
The recently published Switching Antiplatelet-5 trial by Franchi et al9 randomized participants to receive a ticagrelor loading dose followed after 1 hour by cangrelor bolus and infusion or a ticagrelor loading dose followed after 1 hour by placebo bolus and infusion. The study demonstrated that there was no significant difference in platelet reaction units 2 hours after infusion discontinuation for cangrelor versus placebo despite significant reductions in platelet reaction units with the addition of cangrelor versus placebo at 30 minutes (P = .001) and 1 hour (P = .005) after the cangrelor bolus. This study demonstrated that among patients who receive a 180-mg oral loading dose of ticagrelor 1 hour earlier, cangrelor further enhances P2Y12 inhibitory effects up to 1 hour after initiation of therapy compared with placebo. It is important to note that after discontinuation of cangrelor, platelet reactivity remain markedly suppressed by ticagrelor at the same level achieved with the added use of cangrelor. Our study complements Switching Antiplatelet-5 demonstration of an additive platelet inhibitory effect when administering cangrelor after ticagrelor by showing no additional risk of bleeding with this therapeutic combination.
Although several studies have examined the use of clopidogrel during cangrelor infusion, few data exist about upstream treatment with thienopyridine drugs; this is related to the fact that thienopyridine drugs compete for the same ADP-binding site, and because binding of clopidogrel and prasugrel are irreversible, concomitant use of cangrelor with either of these agents may be problematic. Schneider et al15 demonstrated that clopidogrel administration either 0.5 or 1 hour before discontinuation of cangrelor did not prevent recovery of platelet reactivity as effectively as clopidogrel administration at the time of infusion discontinuation. Similarly, Steinhubl et al16 found a 600-mg load of clopidogrel given concomitantly with cangrelor was associated with a weaker antiplatelet effect than the same clopidogrel load given just after cangrelor infusion discontinuation. These observations have likely increased use of ticagrelor among cangrelor-treated patients because ticagrelor does not compete with cangrelor for the same allosteric binding site as clopidogrel and prasugrel.17 In an analysis from SCAAR,9 nearly a third of cangrelor-treated patients received ticagrelor upstream of cangrelor (before PCI), whereas very few cangrelor-treated patients received clopidogrel (1%) or prasugrel (0%) upstream. This study did not analyze the bleeding outcomes of cangrelor-treated patients treated with upstream P2Y12 inhibitors.
We also examined whether certain clinical factors were associated with cangrelor administration in patients who had received an upstream oral P2Y12 inhibitor. Patients treated with cangrelor and an upstream oral P2Y12 inhibitor treatment were more likely to have underwent a previous PCI or a diagnosis of PAD compared with cangrelor-treated patients without an upstream oral P2Y12 inhibitor. These findings are not surprising because patients with a significant history of PCIs or with PAD are more likely to be prescribed chronic antiplatelet therapy. In addition, we observed that a STEMI presentation was associated with upstream administration of P2Y12 inhibitor therapy, consistent with guidelines recommending higher potency dual antiplatelet therapy for patients with acute STEMI. Finally, we found that underrepresented racial or ethnic groups were more likely to receive upstream oral P2Y12 inhibitors in addition to cangrelor therapy. Further work will be needed to understand the relationship among race, ethnicity, and cangrelor therapy.
There are several important limitations to this study. As a registry report, our findings provide exploratory observations describing current patterns of clinical care and identifying opportunities for future study in a sample of patients with MI treated at hospitals of varying size and capabilities. The observed bleeding events, the main outcome of interest in this analysis, occurred with relatively low frequency; thus, a larger study would be helpful to narrow confidence interval ranges and confirm our findings. In addition, the overall sample size is smaller than that of large nationwide registries and is limited to use from centers with cangrelor on formulary. There are centers around the United States that do not have cangrelor on formulary, so these results would not be generalizable to those centers. We included only patients who received an upstream oral P2Y12 inhibitor either at home within 24 hours of hospital admission or an in-hospital P2Y12 inhibitor before cardiac catheterization. There were patients who may have received an oral P2Y12 inhibitor just prior to 24 hours before admission who were excluded but may have received cangrelor within a window of time that could have created overlapping antiplatelet effects. In addition, we do not know from the registry the rationale for use of cangrelor. Moreover, we could not determine which bleeding events were directly related to cangrelor use, but could state only whether they occurred during or after infusion. However, there are instances when bleeding event may have started during cangrelor infusion but were not clinically detected until after the infusion was discontinued. Thus, we were not able to describe why some operators chose to use cangrelor in patients who had upstream P2Y12 loading, contrary to current recommendations for cangrelor use. Finally, because this was an observational study, there may be residual confounding.
Conclusions
Bleeding risk was not observed to be significantly higher in cangrelor-treated patients who received upstream oral P2Y12 inhibitor medication compared with similar patients who did not receive upstream oral P2Y12 inhibitor therapy.
Acknowledgments
Declaration of competing interest
Jennifer Rymer discloses research funding from Chiesi, Idorsia, Pfizer, and Abiomed. Deepak Bhatt discloses the following relationships—advisory board: Angiowave, Bayer, Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, High Enroll, Janssen, Level Ex, McKinsey, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, and Stasys; board of directors: Angiowave (stock options), Boston VA Research Institute, Bristol Myers Squibb (stock), DRS.LINQ (stock options), High Enroll (stock), Society of Cardiovascular Patient Care, and TobeSoft; chair: Inaugural Chair, American Heart Association Quality Oversight Committee; consultant: Broadview Ventures and Hims; data monitoring committees: Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi-Sankyo; for the ABILITY-DM trial, funded by Concept Medical), Novartis, Population Health Research Institute; and Rutgers University (for the NIH-funded MINT Trial); honoraria: American College of Cardiology; Chair, ACC Accreditation Oversight Committee, Arnold and Porter law firm (work related to Sanofi/Bristol Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor-in-Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor-in-Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Oakstone CME (Course Director, Comprehensive Review of Interventional Cardiology), Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees), Wiley (steering committee); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Patent: Sotagliflozin (named on a patent for sotagliflozin assigned to Brigham and Women's Hospital who assigned to Lexicon; neither I nor Brigham and Women’s Hospital receive any income from this patent); research funding: Abbott, Acesion Pharma, Afimmune, Aker Biomarine, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CinCor, Cleerly, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, Youngene, and 89Bio; royalties: Elsevier (Editor, Braunwald’s Heart Disease); site co-investigator: Abbott, Biotronik, Boston Scientific, CSI, Endotronix, St. Jude Medical (now Abbott), Philips, SpectraWAVE, Svelte, and Vascular Solutions; trustee: American College of Cardiology; unfunded research: FlowCo, Takeda. Dominick Angiolillo declares that he has received consulting fees or honoraria from Abbott, Amgen, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol Myers Squibb, Chiesi, Daiichi-Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, Novartis, PhaseBio, PLx Pharma, Pfizer, Sanofi, and Ventura, outside the present work; has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi-Sankyo, Eisai, Eli Lilly, Gilead, Janssen, Matsutani Chemical Industry, Merck, Novartis, Osprey Medical, Renal Guard Solutions, and Scott R. MacKenzie Foundation.Kirk Garratt discloses the following relationships—advisory board: Abbott Vascular, SHL Telemedicine, and LifeCuff; equity: LifeCuff (significant). Ron Waksman reports serving on the advisory boards of Abbott Vascular, Boston Scientific, Medtronic, Philips IGT, and Pi-Cardia; being a consultant for Abbott Vascular, Biotronik, Boston Scientific, Cordis, Medtronic, Philips IGT, Pi-Cardia Ltd, Swiss Interventional Systems/SIS Medical AG, Transmural Systems, and Venus MedTech; receiving institutional grant support from Amgen, Biotronik, Boston Scientific, Chiesi, Medtronic, and Philips IGT; and being an investor in MedAlliance and Transmural Systems. Ajay Kirtane reported institutional funding given to Columbia University and/or the Cardiovascular Research Foundation from Medtronic, Boston Scientific, Abbott Vascular, Amgen, Abiomed, Cardiovascular Systems Inc, CathWorks, Siemens Healthineers, Philips, ReCor Medical, Neurotronics, Biotronik, Chiesi Farmaceutici, Bolt Medical, Magenta Medical, Canon Medical Systems, SoniVie, Shockwave Medical, and Merck and receiving personal fees from Neurotronics, Medtronic, Boston Scientific, Abbott Vascular, Abiomed, Cardiovascular Systems Inc, Interventional Medical Device Systems, CathWorks, Siemens Healthineers, Philips, ReCor Medical, Chiesi Farmaceutici, OpSens Medical, ZOLL Medical, and Regeneron Pharmaceuticals. Richard Bach reports institutional funding for clinical trials from Cytokinetics, BMS, and CSL Behring; consulting fees from NGM Biopharmaceuticals and Novo Nordisk. Magnus Ohman has received research funding from Abiomed and Chiesi; and has received consulting honoraria from Cara Therapeutics, Cytokinetics, Imbria Pharmaceuticals, Otsuka Pharmaceutical, Milestone Pharmaceuticals, Pfizer, and XyloCor Therapeutics. Schuyler Jones reports research grants from Bayer, Boehringer Ingelheim, Janssen, Merck, Novartis, National Institute on Aging, Patient-Centered Outcomes Research Institute. Tracy Wang reports research grants to the Duke Clinical Research Institute from Abbott, AstraZeneca, Bristol Myers Squibb, Boston Scientific, Artivion (formerly Cryolife), Chiesi, Merck, Portola, and Regeneron and consulting honoraria from AstraZeneca, Bristol Myers Squibb, Artivion (formerly Cryolife), CSL Behring, and Novartis. No other disclosures were reported.
Funding sources
This work was funded by a research grant to the Duke Clinical Research Institute, supported by Chiesi.
Ethics statement and patient consent
Each hospital obtained approval from their local institutional review board before enrolling patients.
Footnotes
To access the supplementary material accompanying this article, visit the online version of the Journal of the Society for Cardiovascular Angiography & Interventions at 10.1016/j.jscai.2023.101202.
Supplementary material
Supplemental Figure.
References
- 1.Bhatt D.L., Stone G.W., Mahaffey K.W., et al. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med. 2013;368(14):1303–1313. doi: 10.1056/NEJMoa1300815. [DOI] [PubMed] [Google Scholar]
- 2.Angiolillo D.J., Galli M., Collet J.P., Kastrati A., O’Donoghue M.L. Antiplatelet therapy after percutaneous coronary intervention. EuroIntervention. 2022;17(17):e1371–e1396. doi: 10.4244/EIJ-D-21-00904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawton J.S., Tamis-Holland J.E., Bangalore S., et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(3):e18–e114. doi: 10.1161/CIR.0000000000001038. [DOI] [PubMed] [Google Scholar]
- 4.Franchi F., Rollini F., Angiolillo D.J. Antithrombotic therapy for patients with STEMI undergoing primary PCI. Nat Rev Cardiol. 2017;14(6):361–379. doi: 10.1038/nrcardio.2017.18. [DOI] [PubMed] [Google Scholar]
- 5.Capodanno D., Angiolillo D.J. Timing, selection, modulation, and duration of P2Y12 inhibitors for patients with acute coronary syndromes undergoing PCI. J Am Coll Cardiol Interv. 2023;16(1):1–18. doi: 10.1016/j.jcin.2022.10.023. [DOI] [PubMed] [Google Scholar]
- 6.Schneider D.J., Agarwal Z., Seecheran N., Keating F.K., Gogo P. Pharmacodynamic effects during the transition between cangrelor and ticagrelor. J Am Coll Cardiol Interv. 2014;7(4):435–442. doi: 10.1016/j.jcin.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 7.Franchi F., Rollini F., Rivas A., et al. Platelet inhibition with cangrelor and crushed ticagrelor in patients with ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Circulation. 2019;139(14):1661–1670. doi: 10.1161/CIRCULATIONAHA.118.038317. [DOI] [PubMed] [Google Scholar]
- 8.Mohammad M.A., Andell P., Koul S., et al. Cangrelor in combination with ticagrelor provides consistent and potent P2Y12-inhibition during and after primary percutaneous coronary intervention in real-world patients with ST-segment-elevation myocardial infarction. Platelets. 2017;28(4):414–416. doi: 10.1080/09537104.2016.1246714. [DOI] [PubMed] [Google Scholar]
- 9.Franchi F., Ortega-Paz L., Rollini F., et al. Cangrelor in patients with coronary artery disease pre-treated with ticagrelor: the Switching Antiplatelet (SWAP)-5 study. J Am Coll Cardiol Interv. 2023;16(1):36–46. doi: 10.1016/j.jcin.2022.10.034. [DOI] [PubMed] [Google Scholar]
- 10.Grimfjärd Per, Lagerqvist Bo, Erlinge David, et al. Clinical use of cangrelor: nationwide experience from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR) Eur Heart J Cardiovasc Pharmacother. 2019;5(3):151–157. doi: 10.1093/ehjcvp/pvz002. [DOI] [PubMed] [Google Scholar]
- 11.Rymer J.A., Bhatt D.L., Angiolillo D.J., et al. Cangrelor use patterns and transition to oral P2Y12 inhibitors among patients with myocardial infarction: initial results from the CAMEO Registry. J Am Heart Assoc. 2022;11(11) doi: 10.1161/JAHA.121.024513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desai N.R., Kennedy K.F., Cohen D.J., et al. Contemporary risk model for inhospital major bleeding for patients with acute myocardial infarction: the acute coronary treatment and intervention outcomes network (ACTION) registry®-Get With The Guidelines (GWTG)®. Am Heart J. 2017;194:16–24. doi: 10.1016/j.ahj.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 13.R Core Team . R Foundation for Statistical Computing; 2021. R: A Language and Environment for Statistical Computing.https://www.R-project.org/ [Google Scholar]
- 14.Center for Drug Evaluation and Research https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/204958Orig1s000OtherR.pdf
- 15.Schneider D.J., Agarwal Z., Seecheran N., Gogo P. Pharmacodynamic effects when clopidogrel is given before cangrelor discontinuation. J Interv Cardiol. 2015;28(5):415–419. doi: 10.1111/joic.12229. [DOI] [PubMed] [Google Scholar]
- 16.Steinhubl S.R., Oh J.J., Oestreich J.H., Ferraris S., Charnigo R., Akers W.S. Transitioning patients from cangrelor to clopidogrel: pharmacodynamic evidence of a competitive effect. Thromb Res. 2008;121(4):527–534. doi: 10.1016/j.thromres.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 17.Husted S., van Giezen J.J. Ticagrelor: the first reversibly binding oral P2Y12 receptor antagonist. Cardiovasc Ther. 2009;27(4):259–274. doi: 10.1111/j.1755-5922.2009.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.