The complication risk of pericardiocentesis is relatively low (<2%).1 Performing a pericardiocentesis, even in the setting of tamponade, in patients with pulmonary hypertension remains controversial because of concern for hemodynamic collapse and elevated mortality in some studies.2 Despite the risks in this context, coronary perforation during pericardiocentesis is reported to be rare (<1%).3 However, this specific problem represents one of the most serious and immediate mechanical complications requiring rapid recognition.
Case Presentation
We present the case of a 53-year-old woman with a past medical history of type 2 diabetes, hypothyroidism, asthma, endometrial cancer (status post total abdominal hysterectomy with bilateral salpingo-oophorectomy), and chronic myeloid leukemia who was started on dasatinib recently. The patient had a recent diagnosis of pulmonary hypertension and presented with increasing shortness of breath for the past 2 months. A physical examination revealed severe respiratory distress, distant heart sounds, bilateral wet crackles, and +2 pitting edema. She required bilevel inspiratory positive airway pressure support to maintain her oxygen saturation above 88% upon admission.
The differential diagnosis included new onset heart failure, pulmonary embolism, pneumonia, and adverse effects of dasatinib, including pleural effusion and newly diagnosed pulmonary hypertension.
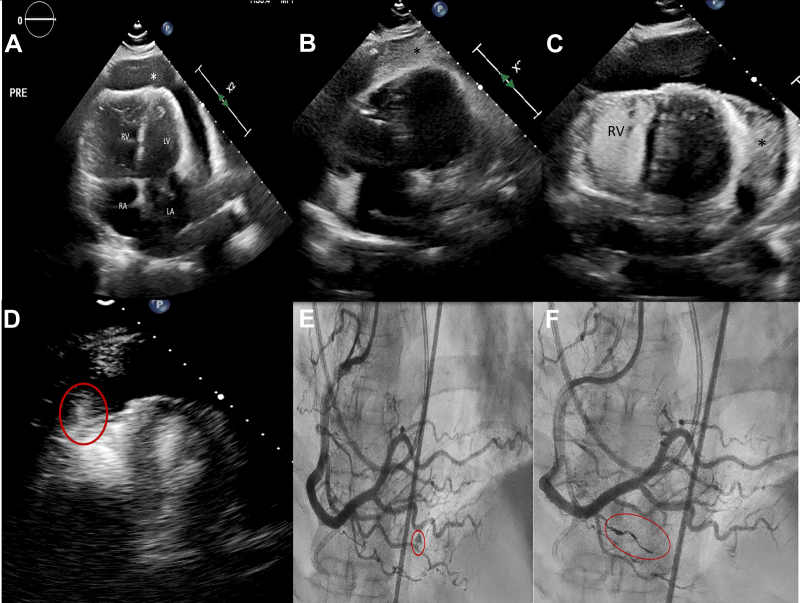
The work-up comprised negative procalcitonin and SARS-CoV2 screening. Chest x-rays showed bilateral pleural effusions. A chest computed tomography scan was negative for pulmonary embolism; however, it demonstrated a large pericardial effusion. Echocardiography confirmed a large circumferential pericardial effusion (Figure 1A), with signs of impending tamponade, and severely elevated pulmonary artery systolic pressure (80 mm Hg) with a central venous pressure of 15 mm Hg.
Figure 1.
Echocardiogram images and coronary angiogram images showing the progress of the case. (A) Four-chamber echocardiogram view showing enlarged RA, enlarged RV, LA, LV, and the pericardial effusion (∗). (B) Agitated saline (∗) in 4-chamber echocardiogram view, confirming the placement of the drain in the pericardium. (C) Agitated saline injected in the RV with no crossing to the pericardium (∗). Thrombosed pericardial drain at the LV anterolateral wall. (D) Perflutren lipid microspheres entering into the pericardium from the apical segment of the RV (red circle). (E) RCA angiogram demonstrating perforation of a branch of the acute marginal artery (red circle). (F) RCA angiogram after coiling demonstrating successful embolization of the perforated branch. LA, left atrium; LV, left ventricle; RA, right atrium; RCA, right coronary artery; RV, right ventricle.
Approach
The patient was taken to the catheterization laboratory for pulmonary artery catheter and subxiphoid pericardial drain placement. The goal was to slowly drain the pericardial fluids under close hemodynamic monitoring. The right heart pressures were as follows: right atrium, 15 mm Hg; right ventricle (RV), 107/10 mm Hg; pulmonary artery (PA), 107/38 mm Hg; mean PA, 61 mm Hg; and wedge pressure, 15 mm Hg. PA oxygen saturation was 62%. Echocardiography and fluoroscopic-guided long microneedle puncture were used to perform the pericardiocentesis through the subxyphoid approach. The initial fluid aspirated was clear yellow and became bloody very quickly. The positions of the needle tip and later the 6-F pigtail drain catheter in the pericardial space were confirmed by echocardiography using agitated saline injection (Figure 1B). The opening pressure in the pericardium was 18 mm Hg.
A total of 50 mL of sanguineous fluid was drained, followed by a rapid drop in blood pressure with worsening respiratory distress and increase in the size of the pericardial effusion. The patient had a cardiac arrest with pulseless electrical activity that required chest compressions for approximately 30 seconds and pressor support for resuscitation. There was return of spontaneous circulation with a return of consciousness.
At this point, cardiac perforation was a leading concern. Cardiothoracic surgery was notified for possible need of emergency surgery. Agitated saline injection in the RV did not demonstrate any bubbles crossing to the pericardium (Figure 1C). Echocardiography with perflutren lipid microspheres (Definity, Lantheus) showed echo-contrast crossing from what appeared to be the distal RV into the pericardial space (Figure 1D). However, the blood drained from the pericardium appeared “bright red” and had 90% oxygen saturation.
Of note, the aspiration of bloody pericardial fluid was continued with return to the patient of the fluid using a femoral venous sheath. Unfortunately, the subxiphoid pericardial drain appeared to become thrombosed and could not drain any more fluid despite large size effusion on echocardiography. An apical pericardial drain was then placed and pericardial drainage with autotransfusion was continued, which resulted in significant improvement of the patient’s hemodynamics. After 5 to 10 minutes of monitoring in the catheterization laboratory, the pericardial effusion began accumulating again.
Given the high oxygen saturation of the effusion, a coronary angiogram was performed, which showed a perforation of the distal acute marginal branch of the right coronary artery (Figure 1E). A small dose of systemic heparin (3000 units) was administered. Using a 6-F AL 0.75 guiding catheter for support, the branch was wired with a workhorse wire, and an Asahi Corsair ProXS catheter was advanced just proximal to the perforation site. A Penumbra Ruby coronary coil (2.0 mm × 10 cm) was advanced to the perforation site for embolization through the microcatheter (Figure 1F). Systemic protamine was administered. The embolization was successful, and there was no recurrence of the pericardial effusion. The drain was removed after 48 hours, and the patient was discharged within a week from admission.
Discussion
Coronary artery perforation is a known rare complication of pericardiocentesis. Kanda et al4 published a similar case that was managed by coronary coiling; however, in their case, the pericardiocentesis was performed by an emergency department physician emergently during field resuscitation using point-of-care ultrasound guidance. In our case, we could not determine exactly the main reason that led to this rare complication despite using echocardiogram-guided needle access followed by soft tip wire and pigtail catheter placement. A possible explanation is the hyperdynamic motion of the RV in the setting of severe pulmonary hypertension. The agitated saline injection in the RV was helpful to rule out rupture of the free wall. The use of perflutren lipid microspheres was helpful to identify the location of the bleeding over the RV apex. Unfortunately, this injection does not differentiate the source of the bleeding because the microspheres are distributed in the left ventricle, RV, and coronary circulation. An elevated oxygen saturation of the pericardial blood was a key indicator to determine the source of the bleeding from the coronary circulation, which was confirmed by coronary angiogram and managed emergently by focal coil embolization. In this case as well, the amount of drained blood from the pericardium was significantly large (1.5-2 L), which was managed with autotransfusion.
Unfortunately, the preventive measures to avoid such a serious complication as the use of echocardiogram during pericardiocentesis and soft tip wire were not adequate to preclude a coronary perforation in our case. The elected management of the coronary perforation was embolization by coiling because of the small size and distal location of the perforated vessel. The other option of prolonged cycles of occluding balloon inflation could have been attempted but given the distal location was not tried.
Conclusion
Despite its rare occurrence, coronary perforation during pericardiocentesis should be recognized urgently because of the possible fatal consequences. The use of oxygen saturation and echocardiography are key modalities to define the etiology of an enlarging effusion despite drain placement. Familiarity with coronary coils is valuable in this context.
Acknowledgments
Declaration of competing interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics statement
The author(s) have adhered to the relevant ethical guidelines.
Footnotes
To access the supplementary material accompanying this article, visit the online version of the Journal of the Society for Cardiovascular Angiography & Interventions at 10.1016/j.jscai.2022.100409.
Supplementary material
References
- 1.Tsang T.S.M., Enriquez-Sarano M., Freeman W.K., et al. Consecutive 1127 therapeutic echocardiographically guided pericardiocenteses: clinical profile, practice patterns, and outcomes spanning 21 years. Mayo Clin Proc. 2002;77(5):429–436. doi: 10.4065/77.5.429. [DOI] [PubMed] [Google Scholar]
- 2.Case B.C., Yang M., Kagan C.M., et al. Safety and feasibility of performing pericardiocentesis on patients with significant pulmonary hypertension. Cardiovasc Revasc Med. 2019;20(12):1090–1095. doi: 10.1016/j.carrev.2019.09.022. [DOI] [PubMed] [Google Scholar]
- 3.Kumar R., Sinha A., Lin M., et al. Complications of pericardiocentesis: a clinical synopsis. Int J Crit Illn Inj Sci. 2015;5(3):206–212. doi: 10.4103/2229-5151.165007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanda D., Takumi T., Sonoda T., et al. Coronary artery perforation secondary to lifesaving pericardiocentesis for cardiac tamponade: a case report. BMC Cardiovasc Disord. 2021;21(1):55. doi: 10.1186/s12872-021-01875-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.