Abstract
Background
Coronary calcification impairs stent delivery and optimal expansion, a significant predictor of subsequent stent thrombosis and restenosis. Current calcium ablative technologies may be limited by guidewire bias and periprocedural complications. Intravascular lithotripsy (IVL) delivers acoustic pressure waves to modify calcium, enhance vessel compliance, and optimize stent deployment. The Disrupt CAD III study demonstrated high (92.4%) procedural success and low (7.8%) 30-day major adverse cardiac event (MACE) rates following IVL, but longer term follow-up is required to determine the durability of clinical benefit and the late impact of optimized stent implantation associated with IVL. This analysis evaluates 1-year outcomes from the Disrupt CAD III study.
Methods
Disrupt CAD III (NCT03595176) was a prospective, single-arm approval study designed to assess the safety and effectiveness of IVL as an adjunct to coronary stenting in de novo, severely calcified coronary lesions (n = 384). MACE was defined as the composite of cardiac death, myocardial infarction (MI), or ischemia-driven target vessel revascularization; target lesion failure was defined as cardiac death, MI, or ischemia-driven target lesion revascularization (ID-TLR).
Results
At 1 year, MACE occurred in 13.8% of patients (cardiac death: 1.1%, MI: 10.5%, ischemia-driven target vessel revascularization: 6.0%) and target lesion failure occurred in 11.9% (ID-TLR: 4.3%), both driven by non-Q-wave MI (9.2%). Stent thrombosis (definite or probable) occurred in 1.1% of patients (including 1 event [0.3%] beyond 30 days).
Conclusions
Disrupt CAD III represents the largest long-term (1-year) analysis of coronary IVL to date. IVL treatment prior to coronary stent implantation in severely calcified lesions was associated with low 1-year rates of MACE, ID-TLR, and stent thrombosis.
Keywords: Coronary artery disease, Calcification, Percutaneous coronary intervention
Central Illustration
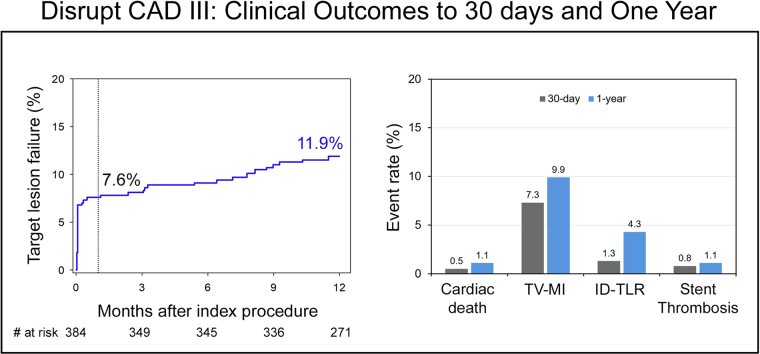
Target lesion failure (TLF), defined as the composite of cardiac death, target vessel myocardial infarction (TV-MI), or ischemia-driven target lesion revascularization (ID-TLR), was 7.6% at 30 days and 11.9% at 1 year. The 1-year ID-TLR was 4.3%, and stent thrombosis (definite or probable) occurred in 1.1% of patients with only one event beyond 30 days.
Highlights
-
•
Disrupt CAD III is the largest long-term (1-year) coronary intravascular lithotripsy study
-
•
Intravascular lithotripsy benefit on calcium modification and subsequent stent expansion is sustained to 1 year
-
•
Major adverse cardiac events and target vessel revascularization rates were consistent across multiple subgroups
Introduction
Percutaneous coronary intervention (PCI) with drug-eluting stent (DES) implantation is the most frequent mode of coronary artery revascularization. Advanced age and an increasing frequency of diabetes mellitus, hypertension, and renal insufficiency contribute to an increasing prevalence and severity of coronary artery calcification,1, 2, 3 the presence of which is associated with worse outcomes.3, 4, 5, 6, 7 Coronary artery calcification may limit stent delivery and prevent optimal stent expansion,8 the absence of which is a powerful predictor of subsequent stent thrombosis (ST) and restenosis.7,9, 10, 11, 12, 13
The Disrupt CAD III study reported favorable early safety and effectiveness results following intravascular lithotripsy (IVL) pretreatment of severely calcified coronary stenoses prior to DES implantation. High rates of procedural success with low rates of angiographic and clinical procedural complications were observed. In the optical coherence tomography (OCT) substudy of Disrupt CAD III, the large minimal stent area (MSA) and degree (%) of stent expansion achieved despite the severity of target lesion calcification were associated with low rates of major adverse cardiovascular events (MACE) at 30 days.14 However, longer term follow-up is required to determine the durability of clinical benefit and the late impact of optimized stent implantation associated with IVL. In this context, we report the 1-year outcomes from the Disrupt CAD III study.
Methods
Study design and oversight
The present report represents the prespecified 1-year analysis from the Disrupt CAD III study. The Disrupt CAD III (NCT03595176) study design, major inclusion and exclusion criteria, endpoints, definitions, and 30-day results have been previously described in detail.14,15 In summary, Disrupt CAD III was a prospective, single-arm, multicenter, clinical study designed to evaluate the safety and effectiveness of coronary IVL as an adjunct to stent deployment in severely calcified de novo coronary lesions. Disrupt CAD III was designed to support US regulatory approval for the Shockwave IVL system and was carried out under an investigational device exemption from the US Food and Drug Administration. The study protocol was approved by the institutional review board at each participating center, and all patients signed written, informed consent. The sponsor (Shockwave Medical Inc) funded the study and participated in site selection and management as well as in data collection and analysis. The principal investigators and study chair had unrestricted access to the data, prepared the manuscript, controlled the decision to publish, and vouch for the accuracy and completeness of the reported data.
Study patients
Disrupt CAD III enrolled 431 patients at 47 sites in 4 countries (United States, United Kingdom, France, and Germany) between January 2019 and March 2020. Among those were 47 roll-in patients, leaving 384 patients in the intention-to-treat dataset for analysis.14 Patients presenting with stable or unstable angina or silent ischemia and severely calcified de novo coronary artery lesions undergoing PCI were eligible for enrollment. Target lesions not previously treated with any interventional procedure were by visual assessment required to be less than 40 mm in length with a reference vessel diameter of 2.5 to 4.0 mm and with diameter stenosis of ≥70% and <100% or ≥50% and <70% with evidence of ischemia by noninvasive stress testing or by functional testing at the time of coronary angiography (instantaneous wave free ratio <0.90 or fractional flow reserve ≤0.80).
Study device and procedure
The IVL system and coronary IVL catheter and their technique for use have been described.14, 15, 16, 17 The coronary IVL system includes 2 lithotripsy emitters incorporated into the shaft of a 12-mm-long rapid exchange balloon catheter available in 2.5-, 3.0-, 3.5-, and 4.0-mm diameters. Each catheter can provide up to 80 total IVL pulses, and the balloon position can be adjusted with overlap to provide complete coverage of longer lesions. Following IVL, noncompliant (NC) balloon dilatation was performed in lesions with residual stenosis ≥50%, after which DES were implanted. High-pressure (>16 atm) postdilatation with an NC balloon was then routinely performed. Dual antiplatelet therapy was prescribed per current guidelines for a minimum of 6 months unless chronic oral anticoagulation was also administered in which case aspirin could be discontinued within 30 days after PCI.18 Follow-up was performed by clinic or telephone visit at 30 days and at 6, 12, and 24 months and is presently complete for all patients through 12 months.
Data management
Independent angiographic tomography and OCT core laboratories (Cardiovascular Research Foundation) performed quantitative and qualitative analysis of all images. Severe calcification was defined angiographically as radiopaque densities noted either without cardiac motion involving both sides of the arterial wall extending 15 mm or more or by intravascular imaging (intravascular ultrasound or OCT) as a calcium angle of 270° or more. Calcium length (calcified segment length) was defined as the total length of visible continuous fluoroscopic calcium within the lesion and reference vessel segments. All MACE, target lesion failure (TLF), and ST events were adjudicated by an independent clinical events committee (CEC, Cardiovascular Research Foundation). An independent data safety monitoring board (Cardiovascular Research Foundation) reviewed data related to safety, data integrity, and overall conduct of the study on a periodic basis, and each time recommended the study continue without modification.
Study endpoints
The study endpoints have been previously described.14,15 The primary effectiveness endpoint was procedural success. The primary study safety endpoint was freedom from MACE at 30 days. The primary safety and effectiveness endpoints as well as in-hospital and 30-day outcomes have been previously reported and are presented here for context.14 All 1-year outcome measures were secondary endpoints. MACE was defined as the CEC-adjudicated composite of cardiac death, any myocardial infarction (MI), or ischemia-driven target vessel revascularization (ID-TVR). TLF was defined as cardiac death, target vessel MI (TV-MI), or ischemia-driven target lesion revascularization (ID-TLR). Periprocedural MI was defined as peak post-PCI CK-MB level >3× the upper limit of normal (ULN), identical to the MI definition from the predicate ORBIT II study that was used for US regulatory approval of orbital atherectomy.19 Nonprocedural MI was defined by the Fourth Universal Definition of MI (UDMI).14,15 Sensitivity analyses included MACE at 1 year using alternative contemporary periprocedural MI definitions, specifically the Fourth UDMI Type 4a (cardiac troponin [cTn] level >5× the 99th percentile of the upper reference limit with either new ischemic ECG changes, new pathological Q waves, evidence of new loss of viable myocardium or angiographic findings consistent with a procedural flow-limiting complication),20 and the Society for Cardiovascular Angiography and Interventions definition (CK-MB level ≥10× ULN or cTn ≥70× ULN).21
Statistical analysis
All principal analyses were performed in the intent-to-treat population. In-hospital and 30-day results are presented as binomial proportions with the number of events previously reported.14 Continuous data are presented as mean ± standard deviation, and categorical variables are presented as percentages and frequencies. Kaplan-Meier estimates were used to construct survival curves for time-to-event variables at 1 year, with no prespecified formal hypothesis testing for outcomes at 1 year. Level of statistical significance was defined as P < .05 without adjustment for multiplicity. The following subgroups were evaluated for consistency of MACE and TVR at 1 year: age, sex, diabetes mellitus, renal insufficiency, prior coronary artery bypass graft, reference vessel diameter, lesion length, and bifurcation lesions. The independent predictors of MACE and ID-TVR at 1 year were determined by multivariable logistic regression using stepwise selection with a P < .1 threshold for entry into the model and a P < .05 level of significance to stay in the final model. Covariates were selected a priori from historical relatedness to adverse events after calcified lesion PCI. Covariates entered into each model appear in the footnote of the corresponding results table. All statistical analyses were carried out using SAS software, version 9.4 (SAS Institute).
Results
Patients and procedures
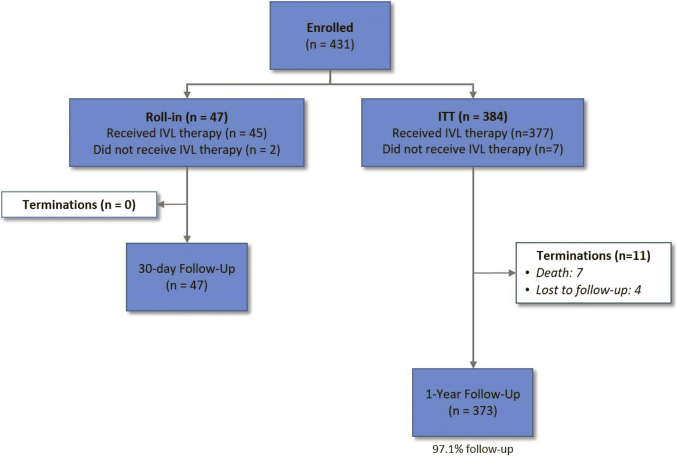
From January 2019 to March 2020, 431 patients were enrolled at 47 sites in 4 countries. Among these were 47 roll-in patients, leaving 384 patients in the intention-to-treat dataset for primary and secondary endpoint analyses. Baseline and procedural characteristics have been previously reported14; mean age was 71.2 ± 8.6 years, 76.6% were male, diabetes mellitus was present in 40.1%, and renal insufficiency (estimated glomerular filtration rate <60 mL/min/1.73 m2) was present in 26.4% of patients (Supplemental Table S1 and Table 2). By quantitative coronary angiography, the average target lesion length was 26.0 ± 11.7 mm, the calcified arterial segment length was 47.9 ± 18.8 mm, and 100% of lesions were classified as severely calcified by the angiographic core laboratory (Supplemental Table S1). Procedural details have been previously reported (15) and are shown in Supplemental Table S2. Follow-up through 1 year was complete in 97.1% of patients (Fig. 1).
Table 2.
Sub-group analyses for MACE and TVR at 1 year.
| Variable | N | MACE |
TVR |
||
|---|---|---|---|---|---|
| N events (KM estimate, %) | Log-rank P value | N events (KM estimate, %) | Log-rank P value | ||
| Age (median) | |||||
| ≤71 years | 199 | 27 (13.8) | .99 | 12 (6.2) | .82 |
| >71 years | 185 | 25 (13.8) | 10 (5.7) | ||
| Sex | |||||
| Male | 294 | 40 (13.8) | .97 | 17 (6.0) | .94 |
| Female | 90 | 12 (13.8) | 5 (6.1) | ||
| Diabetes | |||||
| Yes | 136 | 23 (17.2) | .17 | 11 (8.3) | .15 |
| No | 247 | 29 (11.9) | 11 (4.7) | ||
| Renal insufficiency | |||||
| eGFR <60 mL/min/1.73 m2 | 101 | 15 (14.9) | .55 | 7 (7.1) | .56 |
| eGFR ≥60 mL/min/1.73 m2 | 282 | 36 (13.1) | 15 (5.6) | ||
| Prior CABG | |||||
| Yes | 36 | 5 (14.6) | .99 | 4 (11.8) | .15 |
| No | 348 | 47 (13.7) | 18 (5.3) | ||
| RVD | |||||
| ≤3.0 mm | 196 | 26 (13.4) | .91 | 13 (6.8) | .44 |
| >3.0 mm | 185 | 24 (13.3) | 9 (5.2) | ||
| Lesion length | |||||
| <25 mm | 190 | 17 (9.0) | .02 | 7 (3.7) | .08 |
| ≥25 mm | 191 | 33 (17.6) | 15 (8.3) | ||
| Bifurcation lesion | |||||
| Yes | 116 | 21 (18.2) | .08 | 8 (7.1) | .49 |
| No | 268 | 31 (11.8) | 14 (5.5) | ||
CABG, coronary artery bypass graft; eGFR, estimated glomerular filtration rate using the MDRD formula, Modification of Diet in Renal Disease formula; KM, Kaplan-Meier; MACE, major adverse cardiovascular events; RVD, reference vessel diameter; TVR, target vessel revascularization.
Fig. 1.
Patient flow through 1 year. The first subject enrolled at each site was considered a roll-in patient and was not included in the ITT analysis cohort. ITT, intention-to-treat; IVL, intravascular lithotripsy.
One-year outcomes
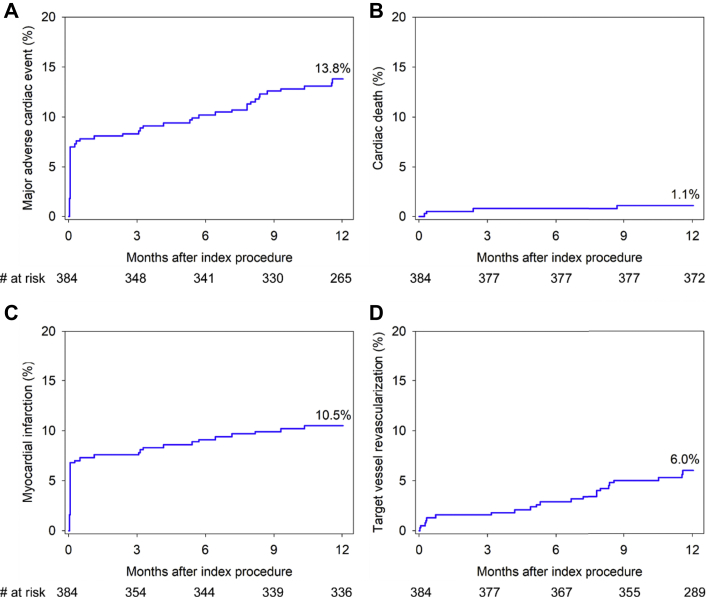
The composite endpoint of MACE occurred in 13.8% of patients (including cardiac death in 1.1%, MI in 10.5%, and ID-TVR in 6.0%) (Fig. 2, Table 1). All-cause mortality within 1 year occurred in 7 patients (1.8%), one of whom did not receive IVL therapy. Sensitivity analyses using alternative periprocedural MI definitions resulted in a similar 1-year MACE rate using the Fourth UDMI20 (14.3%) and a lower rate using the Society for Cardiovascular Angiography and Interventions definition for clinically relevant MI21 (8.5%). The 1-year ID-TLR rate was 4.3%, and ST (definite or probable) occurred in 1.1% of patients, with only one event beyond 30 days (late ST rate of 0.3%).
Central Illustration.
Target lesion failure (TLF), defined as the composite of cardiac death, target vessel myocardial infarction (TV-MI), or ischemia-driven target lesion revascularization (ID-TLR), was 7.6% at 30 days and 11.9% at 1 year. The 1-year ID-TLR was 4.3%, and stent thrombosis (definite or probable) occurred in 1.1% of patients with only one event beyond 30 days.
Fig. 2.
Clinical outcomes through 1 year. Cumulative event rates through 1 year in patients enrolled in the Disrupt CAD III study. (A) MACE, (B) cardiac death, (C) myocardial infarction, and (D) ischemia-driven target vessel revascularization. MACE, major adverse cardiac event.
Table 1.
Clinical outcomes through 1-year follow-up.
| Outcome | In-hospital | 30-day | 1-year |
|---|---|---|---|
| MACE | 27 (7.0) | 30 (7.8) | 52 (13.8) |
| Cardiac death | 1 (0.3) | 2 (0.5) | 4 (1.1) |
| All myocardial infarction | 26 (6.8) | 28 (7.3) | 40 (10.5) |
| Non-Q-wave myocardial infarction | 22 (5.7) | 23 (6.0) | 35 (9.2) |
| Q-wave myocardial infarction | 4 (1.0) | 6 (1.6) | 6 (1.6) |
| Target vessel revascularization | 2 (0.5) | 6 (1.6) | 22 (6.0) |
| All-cause death | 1 (0.3) | 2 (0.5) | 7 (1.8) |
| Cardiac | 1 (0.3) | 2 (0.5) | 4 (1.1) |
| Noncardiac | 0 (0.0) | 0 (0.0) | 2 (0.5) |
| Vascular | 0 (0.0) | 0 (0.0) | 1 (0.3) |
| Target lesion failure | 26 (6.8) | 29 (7.6) | 45 (11.9) |
| Cardiac death | 1 (0.3) | 2 (0.5) | 4 (1.1) |
| TV-MI | 26 (6.8) | 28 (7.3) | 38 (9.9) |
| ID-TLR | 1 (0.3) | 5 (1.3) | 16 (4.3) |
| All revascularization | 2 (0.5) | 10 (2.6) | 45 (12.3) |
| Target vessel | 2 (0.5) | 6 (1.6) | 22 (6.0) |
| ID-TVR | 2 (0.5) | 6 (1.6) | 22 (6.0) |
| ID-TLR | 1 (0.3) | 5 (1.3) | 16 (4.3) |
| Non-ID-TVR | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Non-ID-TLR | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Nontarget vessel | 0 (0.0) | 6 (1.6) | 29 (8.0) |
| Stent thrombosis (definite or probable) | 0 (0.0) | 3 (0.8) | 4 (1.1) |
| Definite | 0 (0.0) | 3 (0.8) | 4 (1.1) |
| Probable | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Values are n (%). In-hospital (n = 384) and 30-day (n = 383) rates are based on binomial proportions. One-year rates are Kaplan-Meier estimates.
ID-TLR, ischemia-driven target lesion revascularization; ID-TVR, ischemia-driven target vessel revascularization; TV-MI, target vessel myocardial infarction.
Subgroup analyses demonstrated significantly greater MACE at 1 year in lesions ≥25 mm versus <25 mm in length (17.6% vs 9.0%, P = .02). The increased MACE rate in the long-lesion cohort was driven primarily by non–Q-wave MI (NQWMI), which accounted for 75.8% of all MACE in this cohort. MACE and ID-TVR rates at 1 year were similar in all other clinical and angiographic subgroups (Table 2). Predictors of MACE and ID-TVR at 1 year are shown in Table 3. Univariate analysis results for MACE and ID-TVR at 1 year are shown in Supplemental Tables S3 and S4. By multivariable logistic regression, bifurcation lesion, prior MI, and current/former smoker were the only independent predictors of MACE at 1 year, whereas prior MI was the only predictor of ID-TVR at 1 year.
Table 3.
Independent predictors of 1-year adverse events.
| Clinical event | OR (95% CI) | P value |
|---|---|---|
| MACE | ||
| Bifurcation (yes vs no) | 2.69 (1.32-5.47) | .006 |
| Prior MI (yes vs no) | 2.22 (1.01-4.87) | .048 |
| Current or former smoker (yes vs no) | 2.21 (1.01-4.78) | .045 |
| TVR | ||
| Prior MI (yes vs no) | 4.07 (1.20-13.77) | .024 |
The independent predictors of MACE, and TVR, at 1-year were determined by multivariable logistic regression using stepwise selection with a P < .1 univariate threshold for entry and a P < .05 level of significance to stay in the final model. The following variables were entered into the models: age (71 years), sex, prior MI, lesion length per 10 mm, LVEF (≥50%), diabetes, eGFR (<60 mL/min/1.73 m2), hyperlipidemia, hypertension, prior stroke or TIA, BMI, per 5, current or former smoker, RVD (>3.0 mm), bifurcation, lesion location (LAD, vs non-LAD).
BMI, body mass index; CI, confidence interval; eGFR, estimated glomerular filtration rate; LAD, left anterior descending; LVEF, left ventricular ejection fraction; MACE, major adverse cardiovascular events; MI, myocardial infarction; OR, odds ratio; RVD, reference vessel diameter; TIA, transient ischemic attack; TVR, target vessel revascularization.
Discussion
The major findings from the Disrupt CAD III study at 1-year follow-up, the largest and longest clinical follow-up to date of patients with severely calcified lesions treated with coronary IVL prior to DES implantation, are as follows: First, lesion preparation with IVL prior to stent placement in severely calcified coronary lesions resulted in favorable 1-year rates of MACE, TLF, ID-TLR, ID-TVR, and ST. Second, MACE and ID-TVR rates were similar in most of the subgroups analyzed. Lesions with length ≥25 mm had higher 1-year rates of MACE than shorter lesions, principally due to increased rates of NQWMI. Finally, bifurcation lesions, prior MI, and history of smoking were independent predictors of MACE at 1 year.
Percutaneous treatment of severely calcified coronary lesions remains a challenge and may be associated with early complications (perforation, dissection) as well as inadequate lesion preparation leading to stent underexpansion and suboptimal MSAs9,22 which are powerful predictors of long-term adverse clinical outcomes including ST, angiographic, and clinical restenosis.9,23, 24, 25 As a result, prior studies have demonstrated that adverse clinical events accrue over time following treatment with either first- or second-generation DES implantation in moderately or severely calcified lesions.7,9 In the largest and longest (5-year follow-up) patient-level meta-analysis to date evaluating the impact of target lesion calcification on clinical outcomes following DES implantation, severe lesion calcification was associated with a 44% relative increase in cardiac death, a 23% relative increase in target vessel MI, and a 21% increase in TLF compared with noncalcified lesions.7 In the Disrupt CAD III study, effective target lesion calcium modification by IVL pretreatment was demonstrated in the 100-patient OCT substudy.14 In that study, the MSA and % stent expansion at the site of maximum lesion calcification were 6.5 ± 2.1 mm2 and 102 ± 29%, respectively. These results were achieved despite the fact that 100% of cases were confirmed to have severe calcification by core lab assessment with an average calcium angle and thickness of 293 ± 77° and 0.96 ± 0.25 mm, respectively. Moreover, low rates of serious angiographic complications were observed (flow-limiting dissection [0.3%], perforation [0.3%], slow flow/no-reflow [0.0%]) reflecting the favorable safety profile and relative ease of use of IVL in these complex lesions. The large MSA and % stent expansion measures translated into acceptably low 1-year rates of ID-TLR (4.3%) and ST (1.1%), with only one ST event beyond 30 days.
These observations confirm and extend the observed benefit of IVL in the Disrupt CAD III 30-day primary endpoint report which utilized objective performance goals derived from the ORBIT II trial for US regulatory approval of orbital atherectomy.19 In this regard, although cross-study comparisons are less than definitive, the data from the Disrupt CAD III and ORBIT II studies may be evaluated given their similar trial designs, study populations, endpoints, and endpoint definitions. MACE at 30 days in Disrupt CAD III and ORBIT II were observed in 7.8% and 10.4% of patients, respectively, and in both studies was largely driven by periprocedural NQWMI (5.7% and 8.6%, respectively) with cardiac death at 30 days in 0.5% and 0.2% of patients, respectively. MACE at 1 year in Disrupt CAD III and ORBIT II occurred in 13.8% and 16.9% of patients, respectively, largely driven by NQWMI (9.2% versus 9.7%, respectively) with cardiac death in 1.1% and 3.2% of patients, respectively. Two small “real-world” registries that included patients with acute coronary syndromes and IVL treatment of in-stent restenosis have also reported favorable safety and effectiveness outcomes of coronary IVL with low rates of adverse clinical events at 1 year.26,27 Thus, the relative 1-year benefits of IVL were sustained in both indirect comparisons with orbital atherectomy28,29 and with historical cohorts of patients with moderate or severe target lesion calcification undergoing PCI with other lesion preparation techniques.6,30,31 Nonetheless, adequately powered randomized trials are required to further evaluate the relative safety and effectiveness of different types of calcium-modifying therapies for calcified lesions.
IVL has demonstrated safe and predictable modification of severely calcified plaque14,32, 33, 34, 35, 36 with a short learning curve as the IVL device is inherently similar to standard balloon-based PCI. In the Disrupt CAD III study, freedom from 30-day MACE, procedural success, device crossing success, and outcomes were similar between roll-in procedures (first case for each site) and procedures included in the pivotal analysis despite the severe calcification of all target lesions.14 This is in contrast to the steep learning curve that has been described with atherectomy for which high operator volume is an important factor determining favorable outcomes.37, 38, 39 The safety and effectiveness of coronary IVL as an adjunct to stenting has been observed by others in smaller, initial “real-world” clinical experiences with limited 1-year follow-up.26,27 Additionally, although aggressive dilatation using NC balloons,40 scoring or cutting balloons, or calcium ablation by atherectomy41 have been shown to improve luminal gain and stent expansion in calcified coronary lesions, procedural complications including perforation, severe dissection, and distal embolization remain a concern.19,42,43 The differential, nonablative mechanism of IVL action that modifies calcium in a circumferential, transmural fashion at low balloon inflation pressures without either wire bias or significant thermal energy generation has theoretic appeal and may reduce early and late adverse events.17 IVL may also provide an advantage in the treatment of calcified bifurcation lesions as side branch protection using a guidewire may be performed without a risk of wire entrapment or severing as may occur with rotational or orbital atherectomy.44,45 This may be of particular importance for the treatment of calcified left main lesions where simultaneous access to branch vessels may mitigate the risk of acute vessel closure.46
Although Disrupt CAD III and other studies have demonstrated that IVL is safe and effective for treatment of severely calcified coronary lesions, a single calcific plaque modification technique may not be sufficient in all cases. Indeed, successful adjunctive use of atherectomy to facilitate IVL catheter crossing in tight calcified lesions and IVL use to spot-treat undilatable lesions after suboptimal PCI (with balloon angioplasty or atherectomy) have been reported.47, 48, 49, 50 Thus, the complementary roles of IVL and other lesion preparation devices in the overall treatment algorithm of calcified coronary lesions continue to be refined.51,52
Several potential limitations of the present analysis deserve mention. First, Disrupt CAD III is a single-arm study without a randomized comparator or concurrent control arm; as such, comparisons with ORBIT II or other trials should be considered hypothesis generating. Furthermore, randomized studies would be required to compare the impact of IVL treatment versus other calcium-modifying technologies on longer term outcomes. Second, multiple angiographic and patient demographic subsets were excluded per protocol which limits broader generalization of the observations to a “real-world,” all-comers population. These groups include biomarker-positive acute coronary syndromes, severe renal insufficiency, extreme target vessel tortuosity, or unprotected left main, ostial, and saphenous vein bypass graft target lesions. Nevertheless, this study represents the largest clinical trial experience with coronary IVL in patients with severe lesion calcification who are often excluded from participation in most clinical trials. Similarly, patients with moderately calcified lesions were not included in the present study. The relative safety and effectiveness of IVL has not been examined in such lesions. Finally, the relationship between the intravascular imaging findings from the Disrupt III OCT substudy and 1-year clinical outcomes has not yet been analyzed. A larger pooled analysis from the Disrupt CAD study is ongoing and will be better powered to assess these relationships.
Conclusions
The 1-year follow-up from the large-scale single-arm Disrupt CAD III study has demonstrated acceptably low rates of adverse clinical events, particularly ID-TLR and ST, with only a single isolated ST event beyond 30 days. This experience suggests that the beneficial impact of IVL on lesion calcium modification and subsequent stent expansion is sustained to at least 1 year. Further study is required to determine if IVL can effectively reduce the longer term (beyond 1 year) annualized incidence of adverse stent device–related events in patients with severe target lesion calcification.
Acknowledgments
Declaration of competing interest
Dr. Kereiakes is a consultant for SINO Medical Sciences Technologies, Inc, CeleCor, HLT, Foldax, Elixir Medical, Svelte Medical Systems, Inc, Caliber Therapeutics/Orchestra Biomed, and Shockwave Medical and is a stockholder in Ablative Solutions, Inc. Dr. Hill reports fees and grant support from Abbott Vascular, Boston Scientific, Abiomed, and Shockwave Medical and is a stockholder in Shockwave Medical. Dr. Shlofmitz is a speaker for Shockwave Medical, Inc. Dr. Klein reports no relationships with industry. Dr. Riley reports honoraria from Boston Scientific, Asahi Intecc, and Medtronic. Dr. Price reports personal fees from Abbott Vascular, Boston Scientific, Medtronic, Shockwave Medical, and W.L. Gore. Dr. Herrmann reports research funding from Abbott, Boston Scientific, Medtronic, and Shockwave Medical and is a consultant for Abbott, Medtronic, and Shockwave. Dr. Bachinsky is a consultant and speaker's bureau for and receives research grant support from Abbott Vascular, Boston Scientific, BD Bard Vascular, Medtronic, and Shockwave Medical. Dr. Waksman is on the Advisory Board of Amgen, Boston Scientific, Cardioset, Cardiovascular Systems Inc, Medtronic, Philips, and Pi-Cardia Ltd; is a consultant for Amgen, Biotronik, Boston Scientific, Cardioset, Cardiovascular Systems Inc, Medtronic, Philips, and Pi-Cardia Ltd.; has received grant support from AstraZeneca, Biotronik, Boston Scientific, and Chiesi; is a speaker for AstraZeneca and Chiesi; and is a stockholder in MedAlliance; Dr. Stone has received speaker honoraria from Cook and Infraredx; has served as a consultant to Valfix, TherOx, Robocath, HeartFlow, Ablative Solutions, Vectorious, Miracor, Neovasc, Abiomed, Ancora, Elucid Bio, Occlutech, CorFlow, Apollo Therapeutics, Impulse Dynamics, Reva, Vascular Dynamics, Shockwave, V-Wave, Cardiomech, and Gore; and has equity/options from Ancora, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, Valfix, and MedFocus family of funds.
Funding sources
This work was supported by Shockwave Medical, Inc.
Peer review statement
Given his role as Deputy Editor, Dean J. Kereiakes had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Alexandra Lansky.
Footnotes
To access the supplementary material accompanying this article, visit the online version of the Journal of the Society for Cardiovascular Angiography & Interventions at https://doi.org/10.1016/j.jscai.2021.100001.
Supplementary material
References
- 1.Allison M.A., Criqui M.H., Wright C.M. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24(2):331–336. doi: 10.1161/01.ATV.0000110786.02097.0c. [DOI] [PubMed] [Google Scholar]
- 2.Chen N.X., Moe S.M. Vascular calcification: pathophysiology and risk factors. Curr Hypertens Rep. 2012;14(3):228–237. doi: 10.1007/s11906-012-0265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madhavan M.V., Tarigopula M., Mintz G.S., Maehara A., Stone G.W., Généreux P. Coronary artery calcification: pathogenesis and prognostic implications. J Am Coll Cardiol. 2014;63(17):1703. doi: 10.1016/j.jacc.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Vliegenthart R., Oudkerk M., Hofman A., et al. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation. 2005;112(4):572–577. doi: 10.1161/CIRCULATIONAHA.104.488916. [DOI] [PubMed] [Google Scholar]
- 5.Vavuranakis M., Toutouzas K., Stefanadis C., Chrisohou C., Markou D., Toutouzas P. Stent deployment in calcified lesions: can we overcome calcific restraint with high-pressure balloon inflations? Catheter Cardiovasc Interv. 2001;52(2):164–172. doi: 10.1002/1522-726x(200102)52:2<164::aid-ccd1041>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 6.Genereux P., Madhavan M.V., Mintz G.S., et al. Ischemic outcomes after coronary intervention of calcified vessels in acute coronary syndromes. Pooled analysis from the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) and ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) TRIALS. J Am Coll Cardiol. 2014;63(18):1845–1854. doi: 10.1016/j.jacc.2014.01.034. [DOI] [PubMed] [Google Scholar]
- 7.Guedeney P., Claessen B.E., Mehran R., et al. Coronary calcification and long-term outcomes according to drug-eluting stent generation. JACC Cardiovasc Interv. 2020;13(12):1417–1428. doi: 10.1016/j.jcin.2020.03.053. [DOI] [PubMed] [Google Scholar]
- 8.Fujino A., Mintz G.S., Matsumura M., et al. A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. 2018;13(18):e2182–e2189. doi: 10.4244/EIJ-D-17-00962. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi Y., Okura H., Kume T., et al. Impact of target lesion coronary calcification on stent expansion. Circ J. 2014;78(9):2209–2214. doi: 10.1253/circj.CJ-14-0108. [DOI] [PubMed] [Google Scholar]
- 10.di Mario C., Koskinas K.C., Raber L. Clinical benefit of IVUS guidance for coronary stenting: the ULTIMATE step toward definitive evidence? J Am Coll Cardiol. 2018;72(24):3138–3141. doi: 10.1016/j.jacc.2018.10.029. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J., Gao X., Kan J., et al. Intravascular ultrasound versus angiography-guided drug-eluting stent implantation: the ULTIMATE trial. J Am Coll Cardiol. 2018;72(24):3126–3137. doi: 10.1016/j.jacc.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Choi K.H., Song Y.B., Lee J.M., et al. Impact of intravascular ultrasound-guided percutaneous coronary intervention on long-term clinical outcomes in patients undergoing complex procedures. JACC Cardiovasc Interv. 2019;12(7):607–620. doi: 10.1016/j.jcin.2019.01.227. [DOI] [PubMed] [Google Scholar]
- 13.Hong S.J., Mintz G.S., Ahn C.M., et al. Effect of intravascular ultrasound-guided drug-eluting stent implantation: 5-year follow-up of the IVUS-XPL randomized trial. JACC Cardiovasc Interv. 2020;13(1):62–71. doi: 10.1016/j.jcin.2019.09.033. [DOI] [PubMed] [Google Scholar]
- 14.Hill J.M., Kereiakes D.J., Shlofmitz R.A., et al. Intravascular lithotripsy for treatment of severely calcified coronary artery disease: the Disrupt CAD III study. J Am Coll Cardiol. 2020;76(22):2635–2646. doi: 10.1016/j.jacc.2020.09.603. [DOI] [PubMed] [Google Scholar]
- 15.Kereiakes D.J., Hill J.M., Ben-Yehuda O., Maehara A., Alexander B., Stone G.W. Evaluation of safety and efficacy of coronary intravascular lithotripsy for treatment of severely calcified coronary stenoses: design and rationale for the Disrupt CAD III trial. Am Heart J. 2020;225:10–18. doi: 10.1016/j.ahj.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Karimi Galougahi K., Patel S., Shlofmitz R.A., et al. Calcific plaque modification by acoustic shockwaves: intravascular lithotripsy in coronary interventions. Circ Cardiovasc Interv. 2020;114(1):e0093540. doi: 10.1161/CIRCINTERVENTIONS.120.009354. [DOI] [PubMed] [Google Scholar]
- 17.Kereiakes D.J., Virmani R., Hokama J.Y., et al. Principles of intravascular lithotripsy for calcific plaque modification. JACC Cardiovasc Interv. 2021;14(12):1275–1292. doi: 10.1016/j.jcin.2021.03.036. [DOI] [PubMed] [Google Scholar]
- 18.Valgimigli M., Bueno H., Byrne R.A., et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2018;39(3):213–260. doi: 10.1093/eurheartj/ehx419. [DOI] [PubMed] [Google Scholar]
- 19.Chambers J.W., Feldman R.L., Himmelstein S.I., et al. Pivotal trial to evaluate the safety and efficacy of the orbital atherectomy system in treating de novo, severely calcified coronary lesions (ORBIT II) JACC Cardiovasc Interv. 2014;7(5):510–518. doi: 10.1016/j.jcin.2014.01.158. [DOI] [PubMed] [Google Scholar]
- 20.Thygesen K., Alpert J.S., Jaffe A.S., et al. Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018;72(18):2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 21.Moussa I.D., Klein L.W., Shah B., et al. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: an expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI) J Am Coll Cardiol. 2013;62(17):1563–1570. doi: 10.1016/j.jacc.2013.08.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mori S., Yasuda S., Kataoka Y., Morii I., Kawamura A., Miyazaki S. Significant association of coronary artery calcification in stent delivery route with restenosis after sirolimus-eluting stent implantation. Circ J. 2009;73(10):1856–1863. doi: 10.1253/circj.cj-09-0080. [DOI] [PubMed] [Google Scholar]
- 23.Fujimura T., Matsumura M., Witzenbichler B., et al. Stent expansion indexes to predict clinical outcomes: an IVUS substudy from ADAPT-DES. JACC Cardiovasc Interv. 2021;14(15):1639–1650. doi: 10.1016/j.jcin.2021.05.019. [DOI] [PubMed] [Google Scholar]
- 24.Soeda T., Uemura S., Park S.J., et al. Incidence and clinical significance of poststent optical coherence tomography findings: one-year follow-up study from a multicenter registry. Circulation. 2015;132(11):1020–1029. doi: 10.1161/CIRCULATIONAHA.114.014704. [DOI] [PubMed] [Google Scholar]
- 25.Prati F., Romagnoli E., Burzotta F., et al. Clinical impact of OCT findings during PCI: the CLI-OPCI II study. JACC Cardiovasc Imaging. 2015;8(11):1297–1305. doi: 10.1016/j.jcmg.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 26.Wong B., El-Jack S., Newcombe R., Glenie T., Armstrong G., Khan A. Calcified coronary lesions treated with intravascular lithotripsy: one-year outcomes. J Invasive Cardiol. 2020;32(7):E200–E201. [PubMed] [Google Scholar]
- 27.Wiens E.J., Sklar J.C., Wei Y.H., Aleem Q., Minhas K. Real-world outcomes in treatment of highly calcified coronary lesions with intravascular shockwave lithotripsy. Indian Heart J. 2021;73(5):653–655. doi: 10.1016/j.ihj.2021.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Genereux P., Lee A.C., Kim C.Y., et al. Orbital atherectomy for treating de novo severely calcified coronary narrowing (1-year results from the pivotal ORBIT II trial) Am J Cardiol. 2015;115(12):1685–1690. doi: 10.1016/j.amjcard.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Lee M., Genereux P., Shlofmitz R., et al. Orbital atherectomy for treating de novo, severely calcified coronary lesions: 3-year results of the pivotal ORBIT II trial. Cardiovasc Revasc Med. 2017;18(4):261–264. doi: 10.1016/j.carrev.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Copeland-Halperin R.S., Baber U., Aquino M., et al. Prevalence, correlates, and impact of coronary calcification on adverse events following PCI with newer-generation DES: findings from a large multiethnic registry. Catheter Cardiovasc Interv. 2018;91(5):859–866. doi: 10.1002/ccd.27204. [DOI] [PubMed] [Google Scholar]
- 31.Moussa I., Ellis S.G., Jones M., et al. Impact of coronary culprit lesion calcium in patients undergoing paclitaxel-eluting stent implantation (a TAXUS-IV sub study) Am J Cardiol. 2005;96(9):1242–1247. doi: 10.1016/j.amjcard.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 32.Ali Z.A., Brinton T.J., Hill J.M., et al. Optical coherence tomography characterization of coronary lithoplasty for treatment of calcified lesions: first description. JACC Cardiovasc Imaging. 2017;10(8):897–906. doi: 10.1016/j.jcmg.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 33.Brinton T.J., Ali Z.A., Hill J.M., et al. Feasibility of shockwave coronary intravascular lithotripsy for the treatment of calcified coronary stenoses: first description. Circulation. 2019;139(6):834–836. doi: 10.1161/CIRCULATIONAHA.118.036531. [DOI] [PubMed] [Google Scholar]
- 34.Ali Z.A., McEntegart M., Hill J.M., Spratt J.C. Intravascular lithotripsy for treatment of stent underexpansion secondary to severe coronary calcification. Eur Heart J. 2020;41(3):485–486. doi: 10.1093/eurheartj/ehy747. [DOI] [PubMed] [Google Scholar]
- 35.Saito S., Yamazaki S., Takahashi A., et al. Intravascular lithotripsy for vessel preparation in severely calcified coronary arteries prior to stent placement ― primary outcomes from the Japanese Disrupt CAD IV study. Circ J. 2021;85(6):826–833. doi: 10.1253/circj.CJ-20-1174. [DOI] [PubMed] [Google Scholar]
- 36.Kereiakes D.J., Di Mario C., Riley R.F., et al. Intravascular lithotripsy for treatment of calcified coronary lesions: patient-level pooled analysis of the Disrupt CAD studies. JACC Cardiovasc Interv. 2021;14(12):1337–1348. doi: 10.1016/j.jcin.2021.04.015. [DOI] [PubMed] [Google Scholar]
- 37.Kinnaird T., Gallagher S., Sharp A., et al. Operator volumes and in-hospital outcomes: an analysis of 7,740 rotational atherectomy procedures from the BCIS National Database. JACC Cardiovasc Interv. 2021;14(13):1423–1430. doi: 10.1016/j.jcin.2021.04.034. [DOI] [PubMed] [Google Scholar]
- 38.Sakakura K., Inohara T., Kohsaka S., et al. Incidence and determinants of complications in rotational atherectomy: insights from the National clinical data (J-PCI Registry) Circ Cardiovasc Interv. 2016;9(11) doi: 10.1161/CIRCINTERVENTIONS.116.004278. [DOI] [PubMed] [Google Scholar]
- 39.Beohar N., Kaltenbach L.A., Wojdyla D., et al. Trends in usage and clinical outcomes of coronary atherectomy: a report from the national cardiovascular data registry CathPCI registry. Circ Cardiovasc Interv. 2020;13(2):e008239. doi: 10.1161/CIRCINTERVENTIONS.119.008239. [DOI] [PubMed] [Google Scholar]
- 40.Secco G.G., Buettner A., Parisi R., et al. Clinical experience with very high-pressure dilatation for resistant coronary lesions. Cardiovasc Revasc Med. 2019;20(12):1083–1087. doi: 10.1016/j.carrev.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 41.Hemetsberger R., Gori T., Toelg R., et al. Optical coherence tomography assessment in patients treated with rotational atherectomy versus modified balloons: PREPARE-CALC OCT. Circ Cardiovasc Interv. 2021;14(3):e009819. doi: 10.1161/CIRCINTERVENTIONS.120.009819. [DOI] [PubMed] [Google Scholar]
- 42.Abdel-Wahab M., Richardt G., Joachim Buttner H., et al. High-speed rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: the randomized ROTAXUS (Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease) trial. JACC Cardiovasc Interv. 2013;6(1):10–19. doi: 10.1016/j.jcin.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 43.Abdel-Wahab M., Toelg R., Byrne R.A., et al. High-speed rotational atherectomy versus modified balloons prior to drug-eluting stent implantation in severely calcified coronary lesions. Circ Cardiovasc Interv. 2018;11(10):e007415. doi: 10.1161/CIRCINTERVENTIONS.118.007415. [DOI] [PubMed] [Google Scholar]
- 44.del Olmo V.V., Rodríguez-Leor O., Redondo A., et al. Intracoronary lithotripsy in a high-risk real-world population. First experience in severely calcified, complex coronary lesions. REC Interv Cardiol. 2020;2:76–81. doi: 10.24875/recice.M19000083. [DOI] [Google Scholar]
- 45.Shlofmitz E., Martinsen B.J., Lee M., et al. Orbital atherectomy for the treatment of severely calcified coronary lesions: evidence, technique, and best practices. Expert Rev Med Devices. 2017;14(11):867–879. doi: 10.1080/17434440.2017.1384695. [DOI] [PubMed] [Google Scholar]
- 46.Yeoh J., Hill J. Intracoronary lithotripsy for the treatment of calcified plaque. Interv Cardiol Clin. 2019;8(4):411–424. doi: 10.1016/j.iccl.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 47.Gonzalvez-Garcia A., Jimenez-Valero S., Galeote G., Moreno R., Lopez de Sa E., Jurado-Roman A. RotaTripsy: combination of rotational atherectomy and intravascular lithotripsy in heavily calcified coronary lesions: a case series. Cardiovasc Revasc Med. 2021 doi: 10.1016/j.carrev.2021.04.011. [DOI] [PubMed] [Google Scholar]
- 48.Kaur N., Mehrotra S., Pruthvi C.R. Intravascular lithoplasty device failure in highly calcified coronary vessel – a case report. Asian J Cardiol Res. 2021;4(1):8–13. [Google Scholar]
- 49.Venuti G., D’Agosta G., Tamburino C., La Manna A. Coronary lithotripsy for failed rotational atherectomy, cutting balloon, scoring balloon, and ultra-high-pressure non-compliant balloon. Catheter Cardiovasc Interv. 2019;94(3):E111–E115. doi: 10.1002/ccd.28287. [DOI] [PubMed] [Google Scholar]
- 50.Aznaouridis K., Bonou M., Masoura C., Kapelios C., Tousoulis D., Barbetseas J. Rotatripsy: a hybrid “drill and Disrupt” approach for treating heavily calcified coronary lesions. J Invasive Cardiol. 2020;32(6):E175. [PubMed] [Google Scholar]
- 51.Sorini Dini C., Nardi G., Ristalli F., Mattesini A., Hamiti B., Di Mario C. Contemporary approach to heavily calcified coronary lesions. Interv Cardiol. 2019;14(3):154–163. doi: 10.15420/icr.2019.19.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riley R.F., Henry T.D., Mahmud E., et al. SCAI position statement on optimal percutaneous coronary interventional therapy for complex coronary artery disease. Catheter Cardiovasc Interv. 2020;96(2):346–362. doi: 10.1002/ccd.28994. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.