Abstract
Stent failure remains the major drawback to the use of coronary stents as a revascularization strategy. Recent advances in imaging have substantially improved our understanding of the mechanisms underlying these occurrences, which have in common numerous clinical risk factors and mechanical elements at the time of stent implantation. In-stent restenosis remains a common clinical problem despite numerous improvements in-stent design and polymer coatings over the past 2 decades. It generates significant health care cost and is associated with an increased risk of death and rehospitalization. Stent thrombosis causes abrupt closure of the stented artery and therefore carries a high risk of myocardial infarction and death. This Society for Cardiovascular Angiography & Interventions (SCAI) Expert Consensus Statement suggests updated practical algorithmic approaches to in-stent restenosis and stent thrombosis. A pragmatic outline of assessment and management of patients presenting with stent failure is presented. A new SCAI classification that is time-sensitive with mechanistic implications of in-stent restenosis is proposed. Emphasis is placed on frequent use of intracoronary imaging and assessment of timing to determine the precise etiology because that information is crucial to guide selection of the best treatment option. SCAI recommends image-guided coronary stenting at the time of initial implantation to minimize the occurrence of stent failure. When in-stent restenosis and stent thrombosis are encountered, imaging should be strongly considered to optimize the subsequent approach.
Keywords: coronary stenting, in-stent restenosis, major adverse cardiovascular events, stent thrombosis, target vessel failure
Introduction
Coronary stenting has transformed revascularization strategy, producing excellent procedural and clinical outcomes in myriad clinical settings. Despite proven short and long-term benefits, in-stent restenosis (ISR) and stent thrombosis (ST) continue to be limitations. There remains no definitive management approach for either condition despite a greater understanding of the underlying mechanisms ensuing from advances in intracoronary imaging.
In this Society for Cardiovascular Angiography & Interventions (SCAI) Expert Consensus Statement, practical algorithmic approaches to ISR and ST are offered. A pragmatic outline of assessment and management of patients presenting with stent failure is presented. A new SCAI classification that is time-sensitive with mechanistic implications of ISR is proposed. Emphasis is placed on frequent use of intracoronary imaging and assessment of timing to determine the precise etiology, as that information is crucial to guide selection of the best treatment option.
Methodology
This statement has been developed according to SCAI Publications Committee policies for writing group composition, disclosure and management of relationships with industry, internal and external review, and organizational approval. Detailed author disclosures are included as Supplemental Table 1. The work of the writing committee was supported exclusively by SCAI, a nonprofit medical specialty society, without commercial support. Writing group members contributed to this effort on a volunteer basis and did not receive payment from SCAI. Group members in each section performed literature searches, and the section leads in collaboration authored initial section drafts with other members of the writing group. The draft manuscript was peer reviewed in February 2023 and the document was revised to address pertinent comments. The writing group unanimously approved the final version of the document. The SCAI Publications Committee and Executive Committee endorsed the document as official society guidance in March 2023. SCAI statements are primarily intended to help clinicians make decisions about treatment alternatives. Clinicians also must consider the clinical presentation, setting, and preferences of individual patients to make judgments about the optimal approach.
In-stent restenosis
ISR remains a common clinical problem despite numerous improvements in-stent design and polymer coatings over the past 2 decades. ISR generates significant health care cost and is associated with an increased risk of death and rehospitalization. The incidence of ISR is 10%; 25% of ISR cases present with acute myocardial infarction (MI) with a 30-day mortality rate of 10% to 25%.1, 2, 3, 4
Risk factors
Clinical. The incidence of ISR varies depending on individual patient, angiographic and procedural characteristics as listed in Table 1.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 Second-generation drug-eluting stents (DES) have a 5.7% ISR rate in patients without diabetes, and 8.7% rate in those with diabetes.5 Beyond 1 year, there is a gradual increase in major adverse cardiovascular events (MACE); the 5-year ISR rate is 9% to 12% in noncomplex lesions.6
Table 1.
| Patient factors | Angiographic factors | Procedural factors |
|---|---|---|
|
|
|
ACS, acute coronary syndrome; CAD, coronary artery disease; ISR, in-stent restenosis.
Recurrent ISR is not unusual in contemporary practice. The failure to appreciate and address the original mechanism of ISR underlies refractory cases of recurrence. As in first ISR, the use of intracoronary imaging may provide insights into the underlying mechanisms. Recurrent ISR occurs in approximately 20% of all ISR cases.7,8 Recurrence is independently predicted by the number of stents placed at the location.9,10 The 1-year MACE (43.1%) and target lesion revascularization (41.2%) rates were significantly higher in the ≥3 stent layer group than in the 1-stent-layer and 2-stent-layer groups. Importantly, on multivariable analysis, the number of metallic layers and hemodialysis requirement were identified as independent predictors of MACE. A third layer of metal is almost always associated with underexpansion and should be avoided.
Pathogenesis and contributory factors
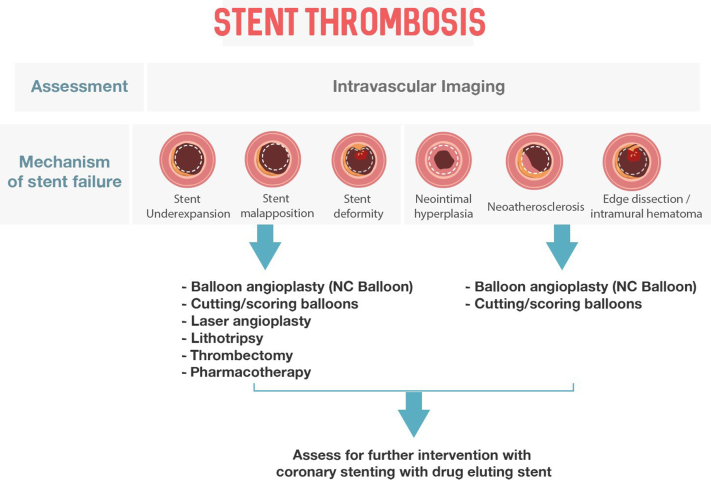
The preferred treatment strategy depends on a precise diagnosis and understanding of the cause. Consequently, identifying the mechanism in each case using intracoronary imaging and optimizing the interventional result are critical steps (Table 1).
Biologic factors
The primary biologic mechanism of ISR is neointimal tissue proliferation or hyperplasia, an exaggerated homeostatic healing response to arterial wall damage sustained during stent implantation.1 The distribution of neointimal tissue proliferation may be focal or diffuse along the length of the stent. Causative factors are local inflammation resulting from mechanical disruption of the intima/media leading to aggressive neointimal hyperplasia/proliferation that consists of smooth muscle cells and extracellular matrix. Hypersensitivity reactions to the metal and/or the polymer of early-generation DES are also recognized mechanisms of neointimal hyperplasia.1
Neoatherosclerosis is an increasingly recognized mechanism of stent failure seen with current generation DES. It is characterized by accumulation of lipid-laden foamy macrophages sometimes with necrotic core formation within stented segments.16 Injury to the vessel by balloon inflation and stent deployment stimulates neointima formation. The subsequent intimal and medial damage leads to proliferation and migration of vascular smooth muscle cells, macrophages, and extracellular matrix formation. These activate the coagulation cascade and an inflammatory response. This combination of events, along with elution of antiproliferative drug, inhibits endothelialization. The lack of endothelium allows incorporation of low-density lipoprotein into the artery wall early after DES implantation. At later stages, the healed in-stent neointima is prone to atherosclerosis development.
Additional mechanisms of ISR include elastic recoil and relocation/subluxation of axially transmitted plaque (tissue intrusion) (especially early) and reorganization of thrombus, neointima formation, and remodeling (especially late).6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16
Mechanical factors
The primary mechanical cause of ISR is underexpansion. This may result from stent undersizing, low deployment pressures, or underlying calcified lesions. Other mechanical causes include stent recoil, longitudinal stent deformation, stent fracture, crushed stents, dislocated stents, and geographic miss. Geographic miss results from incorrect placement of the stent so that it does not fully cover the diseased segment. Stent fracture may be seen at hinge points in the coronary artery and after stenting a calcified nodule. Other findings, such as early-stent malapposition, tissue prolapse, and asymmetry/eccentricity have little or no prognostic value. Stent underexpansion may occur as a result of undersizing, low deployment pressures, or heavily calcified lesions.9, 10, 11, 12, 13
Some interventional cardiologists favor routine poststent placement dilation with high-pressure balloons; while this can be an effective strategy, it can also lead to edge dissections. Instead, postprocedural imaging might be a more effective use of time and effort.
Definition and classification
In-stent restenosis is established angiographically as a binary event, defined as recurrent diameter stenosis at the stent segment >50% of the vessel diameter.16 Additional criteria for clinically relevant ISR include: recurrent angina, objective signs of ischemia, or abnormal fractional flow reserve.17, 18, 19
Morphologic patterns
Coronary angiography remains the standard diagnostic method to determine ISR severity and morphologic pattern:
Mehran
The Mehran System19 classifies restenotic lesions on the basis of morphology and extent of disease, with 4 subclasses based on location within the stented segment. Lesions were classified as focal (class I), diffuse intrastent (class II), diffuse proliferative (class III), and total occlusion (class IV). This schema was highly relevant to bare metal stenting (BMS), but its applicability to DES ISR is uncertain.
Waksman
The Waksman ISR Classification20 is based on mechanistic considerations informed by intracoronary imaging. There are 5 groups of DES ISR identified: mechanical (type I; underexpansion I A, stent fracture I B), biologic (type II; intimal hyperplasia II A, neoatherosclerosis noncalcified II B, neoatherosclerosis calcified II C), mixed pattern (type III), chronic total occlusions (type IV), and lesions previously treated with >2 stents (type V).
Intravascular ultrasound- and optical coherence tomography-based classifications
Kang et al21 has proposed an intravascular ultrasound (IVUS)-based classification that incorporates length of the restenosis as well as minimal luminal area. Gonzalo et al22 and Ali et al23 have proposed optical coherence tomography (OCT) classifications that rely on both quantitative and qualitative parameters.
Timing
Table 2 is the proposed new SCAI classification incorporating the cause of ISR based on time from implantation. SCAI recommends that early (<30 days), late (30 days to 1 year), and very late (>1 year) timing categories be adopted for all future diagnostic and therapeutic studies. By integrating mechanistic etiology with timing, this classification will be useful to determine best treatment options.
Table 2.
SCAI classification of in-stent restenosis: a system based on time interval and causative factor
| Classification | Time interval | Morphologic substrates |
|---|---|---|
| Early | <30 d |
|
| Late | 30 d to 1 y |
|
| Very late | >1 y |
|
BMS, bare metal stent; ISR, in-stent restenosis.
Imaging adjuncts to diagnosis
SCAI strongly recommends routine evaluation by intravascular imaging to determine the cause of ISR, to inform therapeutic strategy, and to confirm effective treatment after percutaneous coronary intervention (PCI).24, 25, 26, 27, 28, 29 Identifying the mechanism of stent failure is paramount because the causative factors will influence the selection of treatment and devices to manage the ISR, ultimately impacting the durability of the repeat revascularization. Despite being the primary means of assessing ISR in clinical practice, angiography alone is usually inadequate because of limited resolution and inherent deficiency in quantifying vessel size, stent size, stent expansion, number of stent layers, in-stent calcific neoatherosclerosis, and extrastent calcific disease. Identifying the mechanism of ISR depends on visualizing the stent and its relation to the arterial wall, rather than the lumen itself.
In contrast to angiography, IVUS and OCT provide detailed assessment of the native artery and stented segment (Figure 1A, B). Recent intravascular imaging studies demonstrate that suboptimal stent deployment is common—occurring in 31% to 58% of patients—and that suboptimal stent deployment confers an increased risk of adverse events.30, 31, 32, 33 The relative advantages of IVUS and OCT are summarized in Table 3.34,35
Figure 1.
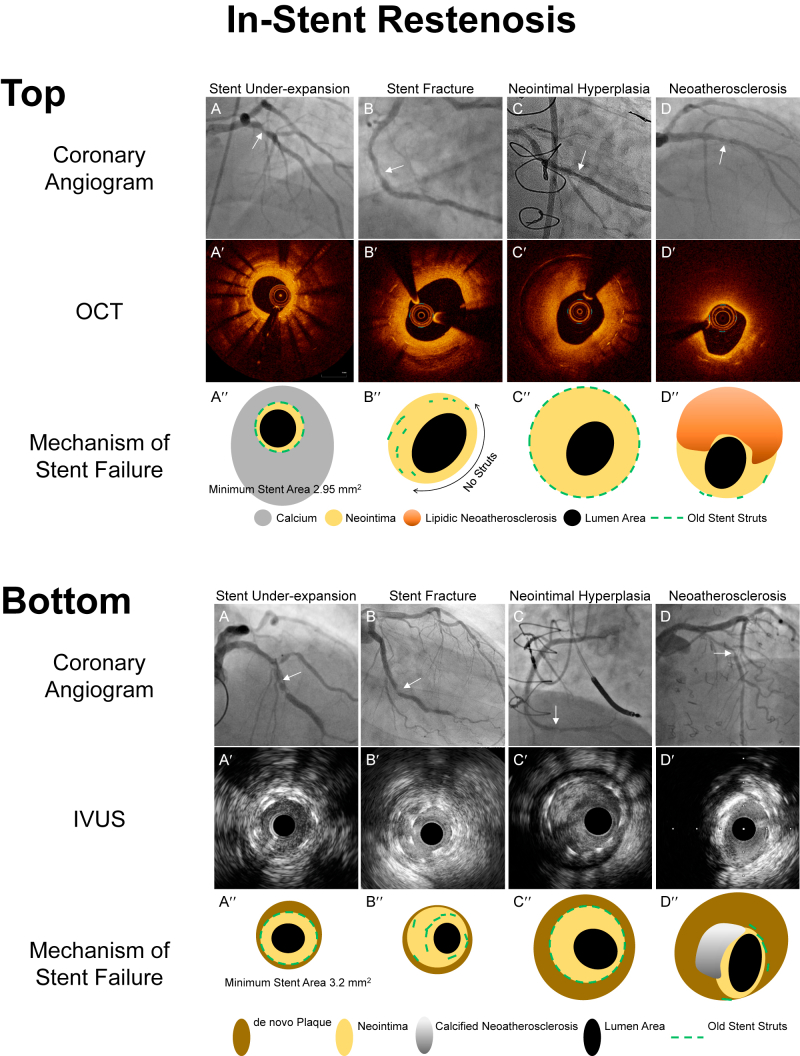
Mechanisms of in-stent restenosis evaluated by intravascular imaging. A′-D′ are optical coherence tomography (OCT) or intravascular ultrasound (IVUS) images corresponding to the in-stent restenosis seen in the angiographic images (A-D, white arrows). A″-D″ are representative diagrams provided to clarify the intracoronary images, A′-D′. Top. Mechanisms of in-stent restenosis evaluated by OCT. (A) A patient experienced recurrent in-stent restenosis (ISR), and OCT visualized a severely underexpanded stent because of circumferential thick calcium behind stent with only a minimum amount of neointimal hyperplasia. (B) This patient was treated with a single drug-eluting stent. At the time of ISR, the OCT image showed a lack of stent struts over half of the arterial circumference (double headed arrow) while stents struts were overlapped at 7 to 9 o’clock. These are typical features of stent fracture. (C) Excess amount of neointimal hyperplasia within a well-expanded stent. (D) Lipidic neointima (strong signal attenuation) within the stent struts indicating neoatherosclerosis. Bottom. Mechanisms of in-stent restenosis evaluated by IVUS (A) IVUS visualized an underexpanded stent with a minimum amount of neointimal hyperplasia. By looking at the adjacent segment, the cause of underexpansion was a small vessel with a myocardial bridge. (B) IVUS delineates overlapped struts within a single stent at 7 to 10 o’clock indicating stent fracture. (C) Excess amount of neointimal hyperplasia within a well-expanded old stent. (D) Calcified plaque (superficial hyperintensity with acoustic shadow from 8 to 12 o’clock) within the stent indicates neoatherosclerosis.
Table 3.
| IVUS | OCT | |
|---|---|---|
| Assessing lesion severity in left main disease | +++ | + |
| Assessing de novo lesion characteristics | ||
| Thin cap fibroatheroma | − | +++ |
| Thrombus | + | +++ |
| Plaque rupture | ++ | +++ |
| Calcified nodule | + | +++ |
| Dissection | ++ | +++ |
| Positive remodeling | +++ | + |
| Plaque burden | +++ | + |
| Aorto-ostial disease | +++ | − |
| Stent optimization | ||
| Expansion | ++ | +++ |
| Apposition | ++ | +++ |
| Stent failure | ||
| Neointimal hyperplasia | + | ++ |
| Underexpansion | ++ | +++ |
| Malapposition | ++ | +++ |
| Renal impairment | +++ | + |
+++ Excellent; ++ Good; + Poor; − Not advised
IVUS, intravascular ultrasound; OCT, optical coherence tomography.
Suboptimal minimal stent area (MSA) is a major predictor of stent failure, and an IVUS optimized MSA of >5.0 mm2 or OCT optimized MSA of >4.5 mm2 are optimal goals. Another useful criterion is to achieve a target MSA >90% of the closest proximal or distal reference segment. In addition, intraluminal diagnostic imaging should be performed to ensure that there are no inflow or outflow obstructions within 5 mm of the proximal or distal stent edge. In particular, any major edge dissections (defined as >60°, >3 mm in length, or penetrating the media) should be stented.33, 34, 35
Physiologic assessment
Patients with ISR of intermediate range severity on coronary angiography present a clinical challenge because of potential short and long-term complications, and it is recommended that objective evidence of myocardial ischemia is demonstrated prior to proceeding with repeat intervention. Even though there are no randomized clinical trials (RCTs) assessing coronary physiology to guide management of ISR, there are several retrospective observational trials that suggest that it may assist in clinical decision-making.36,37 Deferral of coronary revascularization in patients with ISR and fractional flow reserve >0.80 was associated with similar outcomes over 36 months to patients with de novo coronary stenosis.37 Further studies may define the value of coronary physiology assessment in developing decision strategy before and after intervention.
Proposed treatment strategies
A summary of existing RCTs and registries,20,38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51 including clinical situations in which particular treatment modalities have been shown to be advantageous, are presented in Table 4.52, 53, 54, 55 The most common treatment approach for the first episode of ISR is to implant a second DES, based on the rationale that DES therapy has superior efficacy over balloon angioplasty alone. However, this is not always necessary and may not be the best solution, particularly when the reference vessel and the resultant minimal lumen area are small.20 If the underlying etiology is not directly addressed and corrected, there is a high likelihood of recurrent ISR, and the rate of ISR in second layer DES is high: 12% to 16% at 12 months and 33% at 3 to 5 years.56, 57, 58
Table 4.
Summary of in-stent restenosis and stent thrombosis management strategies
| Modalities of treatment |
When to consider |
Other considerations |
|---|---|---|
| In-stent restenosis1,2,20,38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51 | ||
| Balloon angioplasty |
|
|
| Repeat DES |
|
|
| Cutting and scoring balloons |
|
|
| Atheroablation |
|
|
| DCB |
|
|
| Vascular brachytherapy |
|
|
| Intravascular lithotripsy |
|
|
| Stent thrombosis52, 53, 54, 55 | ||
|---|---|---|
| Balloon angioplasty |
|
|
| Repeat DES |
|
|
| Aspiration thrombectomy |
|
|
| Pharmacologic therapies |
|
|
DAPT, dual antiplatelet therapy; DCB, drug-coated balloon; DES, drug-eluting stent; ISR, in-stent restenosis; MLD, minimal lumen diameter; PCI, percutaneous coronary intervention; PTA, percutaneous transluminal angioplasty; STEMI, ST-elevation myocardial infarction; TLR, target lesion revascularization.
Avoid when there are already 2 layers of stent
General strategic approach
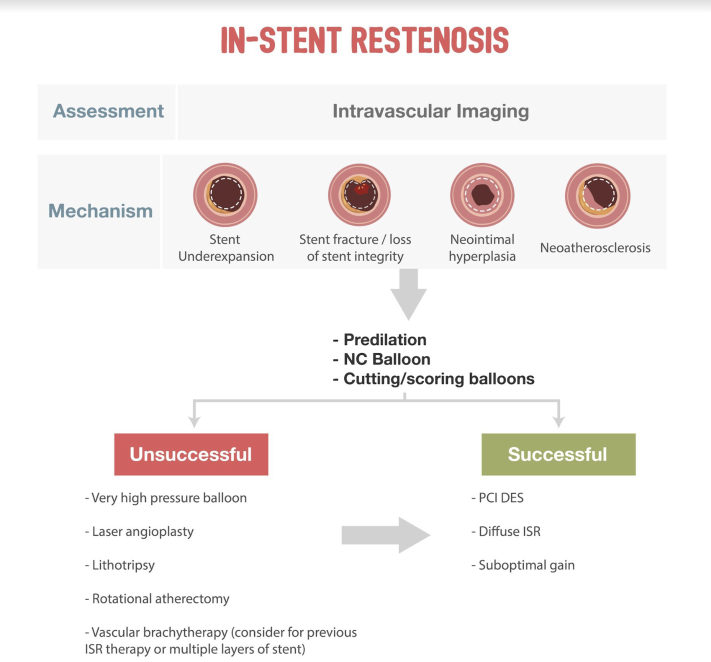
The critical principle is to obtain the largest acute lumen gain as possible by maximizing the immediate postprocedural minimal luminal area. To operationalize this concept, a complete diagnostic evaluation of the cause of ISR must be pursued.59 An algorithmic approach is provided in Figure 2. Repeat PCI should be routinely performed following intracoronary imaging assessment. The mechanism of the initial ISR should be determined, with correction of any underlying mechanical factors with image guidance to ensure optimal sizing and expansion. A second stent should be image-guided to ensure correct stent expansion to ensure appropriate stent expansion.
Figure 2.
SCAI algorithmic approach to in-stent restenosis. DES, drug-eluting stents; ISR, in-stent restenosis; PCI, percutaneous coronary intervention.
Besides repeat DES, a number of adjunct treatments exist that may be highly effective.58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69 If there is significant underexpansion, it is critical to increase expansion by applying high-pressure balloons. If there is additional hyperplasia, perhaps preparation with scoring/cutting balloons, rotational atherectomy (RA), orbital atherectomy (OAS), drug-coated balloons (DCBs), vascular brachytherapy (VBT), excimer laser coronary angioplasty (ELCA), or intravascular lithotripsy (IVL) may be useful.
When ISR is predominantly because of neointimal hyperplasia, treatment is dependent on the pattern of ISR. For focal ISR, a high-pressure or scoring/cutting balloon may be sufficient; ELCA or atherectomy may be beneficial in selected cases. For diffuse ISR, atherectomy or scoring/cutting balloon angioplasty followed by repeat DES implantation is typically advised.
If stent underexpansion is not because of calcification, atheroablation should be used only if significant neointimal hyperplasia is also present. RA, OAS, and ELCA may debulk neointima hyperplasia, although mechanistic evaluations fail to demonstrate this effect. In cases where intravascular imaging identifies an arc of calcium >270° or >0.67 mm in thickness, atherectomy vessel preparation should be considered to optimize lesion and stent expansion.60, 61, 62, 63, 64, 65, 66, 67, 68, 69
If stent underexpansion is due to significant peri-stent calcium (>90°), RA, OAS, and ELCA may be employed to improve stent underexpansion by disrupting the calcified plaque behind the stent. IVL may also be useful. These techniques are associated with calcium modification and/or fracture, and when followed by high-pressure inflations may reduce stent underexpansion. However, if unsuccessful, coronary artery bypass grafting may be necessary (see Figure 3).
Figure 3.
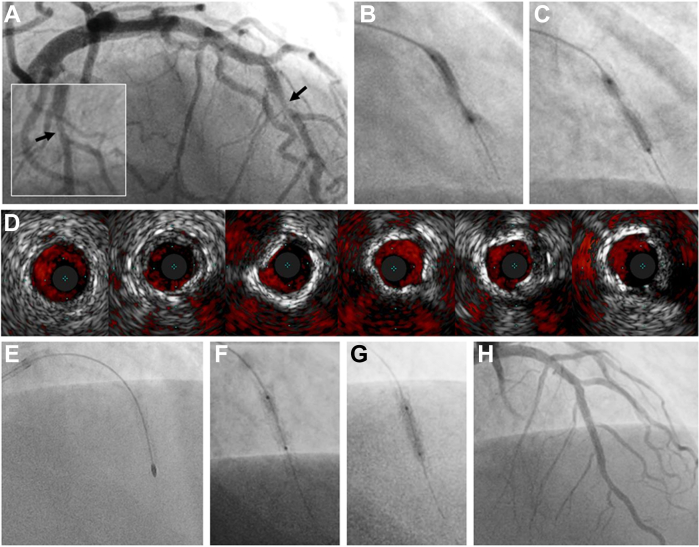
Stepwise, imaging-guided treatment of a calcified ISR lesion. In-stent restenosis of a first-generation drug-eluting stent (Taxus, Boston Scientific) in the mid LAD, implanted 18 years prior and confirmed patent several years afterward. Intrastent (type II) restenosis (A, inset with orthogonal view) was noted on angiography with multiple unsuccessful attempts made at dilatation (B, C) using noncompliant balloons (24-26 atm). Subsequent IVUS imaging (D, distal to proximal) reveals both calcified and noncalcified tissue within an appropriately sized stent, likely representing neoatherosclerosis. Despite successful rotational atherectomy (E: 1.5 mm burr, 160-170,000 rpm × 8 passes), non-compliant balloon dilation still revealed a focal waist (F). Complete expansion of a 2.5 × 12 mm Shockwave C2 IVL balloon was achieved after 80 pulses at 6 atm (G) and confirmed with IVUS. A 2.75 × 26 mm drug-eluting stent was implanted at 20 atm with excellent angiographic (G) and IVUS results. IVL, intravascular lithotripsy; IVUS, intravascular ultrasound; LAD, left anterior descending artery.
Balloon angioplasty
Balloon angioplasty should be the initial step in focal lesions or if short dual antiplatelet therapy (DAPT) duration is required. In the setting of stent underexpansion, high-pressure noncompliant balloon inflations are the preferred strategy.
Super high-pressure balloons
Double layer, noncompliant coronary balloons (OPN NC, SIS Medical) capable of inflation pressures ranging from 35 to 55 atm have recently become available in the United States. This class of percutaneous transluminal coronary angioplasty balloon has performed favorably in severely calcified de novo lesions and may be a consideration in ISR secondary to an underexpanded stent.
Repeat DES
In general, repeat DES implantation has historically shown superior results compared with balloon angioplasty alone. However, this approach should only be undertaken once appropriate sizing and expansion of the original stent has been assured using intravascular imaging. A second stent may not be necessary if the original stent was underdeployed and can be corrected.
If focal edge restenosis, stent gap, or stent fracture is identified, conventional or high-pressure balloon dilation at the site of the mechanical complication should be the initial treatment. This should then be followed by repeat DES implantation when the ISR is focal. Repeat DES to cover the entire diseased segment can be performed when the ISR is diffuse or proliferative, but care should be taken to minimize the stent coverage as much as possible.44, 45, 46, 47, 48, 49, 50, 51, 52 There is no definitive evidence regarding which type of DES should be used to treat ISR of a previously implanted DES, and there is no consensus on whether a different stent type or drug should be used when an additional DES is implanted. However, the RIBS III trial38 assessed the impact of selecting a different DES for treatment of ISR and demonstrated better angiographic and clinical outcomes at 9-month follow-up in the cohort that received a different DES than the first implanted stent.
Cutting and scoring balloons
The use of balloons incorporating cutting or scoring elements has been shown in very small series to result in better acute angiographic outcomes in ISR compared with BA.58,59 Small IVUS-guided studies suggest that the use of cutting compared with traditional balloons is associated with larger lumen gain, lower lumen loss, and preserved angiographic result at follow-up.45,69 However, a randomized study comparing standard balloons vs cutting balloons for the treatment of ISR failed to demonstrate superiority of the cutting balloon in terms of recurrent ISR and MACE.58
Atheroablation
Despite the appealing concept of atheroablation, clinical trials for the treatment of ISR have been negative.62, 63, 64 The utilization of RA when IVUS or OCT confirms the presence of calcium within neoatherosclerotic plaques is reasonable, but no controlled trials exist. RA might also have value in lesions refractory to high-pressure balloon angioplasty. However, the use of mechanical atheroablative technologies within stents poses risks of device entrapment, and some suggest reserving mechanical atherectomy for bailout use. Excimer laser has similarly shown no special benefits.61,64,67
DCBs
Preliminary clinical studies using sirolimus-based DCBs showed promising preliminary results; however, randomized trials are needed to demonstrate efficacy with DES in ISR. Most data are with paclitaxel DCBs. Several trials and meta-analyses have demonstrated similar outcomes of DCB compared with DES in the management of ISR44,45,70,71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83 and are summarized in Table 5. Although coronary DCBs are not available in the United States, the European Society of Cardiology/European Association for Cardio-Thoracic Surgery Guidelines65 give DCBs a class I indication for the treatment of ISR. DCB outcomes may be optimized with inflation time >60 seconds and a balloon: artery ratio >0.91. Neointimal modification with RA improves acute luminal improvement over DCB therapy alone, with lower late lumen loss; however, there are similar clinical outcomes at 6 months.50
Table 5.
Selected Trials Evaluating Management Strategies for In-stent Restenosis
| Trial, publication year | Investigation Time |
No. of lesions centers, region | Design | Drug-coated balloon, carrier agent, commercial name | Control device | Restenotic stent | Endpoint(s) | Follow-up (mo) | Principal findings | P-value |
|---|---|---|---|---|---|---|---|---|---|---|
| PCB vs Uncoated balloon | ||||||||||
| PACCOCATH ISR I&II, 201275 | Dec 2003 - Dec 2005 | 54/54 Multicenter, Germany |
Core lab, CEC | Paclitaxel, iopromide 3 μg/mm2, PACCOCATH | Uncoated balloon | BMS, DES | LLL (mm) | 6 | 0.11 ± 0.44 vs 0.80 ± 0.79 | .001 |
| TLR (%) | 12/60 | 4 vs 37 / 9 vs 39 | .001/.004 | |||||||
| MACE (%) | 12/60 | 9 vs 44 / 28 vs 59 | .001/.009 | |||||||
| Habara et al, 201186 | Sep 2008 - Nov 2009 | 25/25 1, Japan |
— | Paclitaxel, iopromide 3 μg/mm2, SeQuent Please | Uncoated balloon | SES | LLL (mm) | 6 | 0.18 ± 0.45 vs 0.72 ± 0.55 | <.01 |
| TLR (%) | 6 | 4 vs 42 | <.01 | |||||||
| MACE (%) | 6 | 4 vs 40 | <.01 | |||||||
| PEPCAD-DES, 201272,76 | Nov 2009 - Apr 2011 | 72/38 Multicenter, Germany |
Core lab | Paclitaxel, iopromide 3 μg/mm2, SeQuent Please | Uncoated balloon | DES | LLL (mm) | 6 | 0.43 ± 0.61 vs. 1.03 ± 0.77 | <.01 |
| TLR (%) | 6/36 | 15 vs 37 / 19 vs 37 | <.01/<.01 | |||||||
| MACE (%) | 6/36 | 17 vs 50.0 / 21 vs 53 | <.01/<.01 | |||||||
| PCB vs DES | ||||||||||
| PEPCAD II, 200970,77 | Jan 2006 - Dec 2006 | 66/65 10, Germany |
Core lab, CEC | Paclitaxel, iopromide 3 μg/mm2, SeQuent Please | PES, durable polymer, stainless steel (132 μm) | BMS | LLL (mm) | 6 | 0.17 ± 0.42 vs 0.38 ± 0.6 | .03 |
| TLR (%) | 12 | 6 vs 15 | .15 | |||||||
| MACE (%) | 12/36 | 9 vs 22 / 35 vs 42 | .08/– | |||||||
| SEDUCE, 201474 | Jun 2009 - Oct 2011 | 24/25 2, Belgium |
Core lab, CEC | Paclitaxel, iopromide 3 μg/mm2, SeQuent Please | EES, durable polymer, CoCr (81 μm) | BMS | LLL (mm) | 9 | 0.28 vs 0.07 | .1 |
| TLR (%) | 12 | 4.2 vs 8 | .576 | |||||||
| RIBS V, 201473 | Jan 2010 - Jan 2012 | 95/94 25, Spain |
Core lab, CEC | Paclitaxel, iopromide 3 μg/mm2, SeQuent Please | EES, durable polymer, CoCr (81 μm) | BMS | LLL (mm) | 6 to 9 | 0.14 ± 0.5 vs 0.04 ± 0.5 | .14 |
| TLR (%) | 12/36 | 6 vs 1 / 8 vs. 2 | .09/.04 | |||||||
| MACE (%) | 12/36 | 8 vs 6 / 12 vs 10 | .60/.64 | |||||||
| TIS, 201678 | Jan 2012 - Aug 2014 | 74/74 1, Czech Rep. |
Core lab, CEC | Paclitaxel, iopromide 3 μg/mm2, SeQuent Please | EES, durable polymer, CoCr (81 μm) | BMS | LLL (mm) | 12 | 0.02 vs 0.19 | <.01 |
| TVR (%) | 12 | 7.4 vs 16.2 | .110 | |||||||
| MACE (%) | 12 | 10.3 vs 19.1 | .213 | |||||||
| ISAR-DESIRE3, 201371,79 | Aug 2009 - Oct 2011 | 137/131/134 3, Germany |
Core lab, CEC | Paclitaxel, iopromide 3 μg/mm2, SeQuent Please |
|
DES | ISR diameter (%) | 6 to 8 | 38% vs 37.4% vs 54.1% | <.01a |
| TLR (%) | 12/36 | 22 vs 14 vs 44 / 33 vs 24 vs 51 | .09/.11b | |||||||
| MACE (%) | 12/36 | 24 vs 19 vs 46 / 38 vs 38 vs 56 | .5/.91b | |||||||
| PEPCAD China ISR, 201480 | Mar 2011 - Apr 2012 | 113/108 17, China |
Core lab, CEC | Paclitaxel, iopromide 3 μg/mm2, SeQuent Please | PES, durable polymer, stainless steel (132 μm) | DES | LLL (mm) | 9 | 0.46 ± 0.51 vs 0.55 ± 0.61 | .0005a |
| TLR (%) | 12/24 | 15.6 vs 12.3 / 15.9 vs 13.7 | .48/.66 | |||||||
| TLF (%) | 12/24 | 16.5 vs 16 / 16.8 vs 18.6 | .92/.73 | |||||||
| RIBS IV, 201844 | Jan 2010 - Aug 2013 | 154/155 23, Spain |
Core lab, CEC | Paclitaxel, iopromide 3 μg/mm2, SeQuent Please | EES, durable polymer, CoCr (81 μm) | DES | Binary restenosis | 6 to 9 | 19% vs 11% | .27 |
| TLR (%) | 12 | 16.2 vs 21.8 | .26 | |||||||
| MACE (%) | 12 | 18.4 vs 23.3 | .35 | |||||||
| RESTORE, 201881 | Apr 2013 - Oct 2016 | 86/86 10, South Korea |
Core lab, CEC | Paclitaxel, iopromide 3 μg/mm2 | EES, durable polymer, CoCr (81 μm) | DES | LLL (mm) | 9 | 0.15 ± 0.49 vs 0.19 ± 0.41 | .54 |
| TLR (%) | 12 | 7 vs 5 | .51 | |||||||
| MACE (%) | 12 | 6 vs 1 | .10 | |||||||
| DARE, 201847 | May 2010 - Jun 2015 | 137/141 8, Netherlands |
Core lab, CEC | Paclitaxel, iopromide 3 μg/mm2, SeQuent Please | EES, durable polymer, CoCr (81 μm) | BMS, DES | MLD (mm) | 6 | 1.71 ± 0.51 vs 1.74 ± 0.61 | <.01a |
| TVR (%) | 12 | 7.1 vs 8.8 | .65 | |||||||
| MACE (%) | 12 | 10.9 vs 9.2 | .66 | |||||||
| BIOLUX-RCT, 201882 | Aug 2012 - Jan 2015 | 163/80 14, Germany, Latvia |
Core lab, CEC | Paclitaxel, BTHC 3 μg/mm2, Pantera Lux | DES, bioresorbable polymer, CoCr (60–80 μm) | BMS, DES | LLL (mm) | 6 | 0.03 ± 0.40 vs 0.20 ± 0.70 | .40 |
| TLR (%) | 12 | 12.5 vs 10.1 | .82 | |||||||
| TLF (%) | 12 | 16.9 vs 14.2 | .65 | |||||||
| DAEDALUS, 202083 | Pooled analysis of 10 RCTc | Core lab, CEC | Paclitaxel, iopromide/BTHC 3 μg/mm2 | DES | BMS, DES | TLR (%) | 36 | 16 vs 12, HR 1.27 (0.90-1.79) | .17 | |
| Safety endpointd | 36 | 9 vs 11, HR 0.79 (0.58-1.10) | .16 | |||||||
| SCB vs PCB | ||||||||||
| FIM LIMUS DCB, 201984 | Dec 2015 - Jan 2017 | 25/25 5, Malaysia |
Core lab, CEC | Sirolimus, crystalline coating 4 μg/mm2, SeQuent SCB | Paclitaxel, iopromide 3 μg/mm2, SeQuent Please | DES | LLL (mm) | 6 | 0.21 ± 0.54 vs 0.17 ± 0.55 | .794 |
| TLR (%) | 12 | 16 vs 12 | >.99 | |||||||
| MACE (%) | 12 | 16 vs 12 | >.99 | |||||||
| Scheller et al. 202285 | Dec 2015 - Feb 2020 | 50/51 10, Malaysia, Germany, Switzerland |
Core lab, CEC | Sirolimus, crystalline coating 4 μg/mm2, SeQuent SCB | Paclitaxel, iopromide 3 μg/mm2, SeQuent Please | DES | LLL (mm) | 6 | 0.25 ± 0.57 vs 0.26 ± 0.60 | <.35a |
| TLR (%) | 12 | 16 vs 10 | .39 | |||||||
| MACE (%) | 12 | 18 vs 14 | .60 |
BMS, bare metal stent; BTHC, butyryl-tri-hexyl citrate; CEC, clinical events committee; CoCr, cobalt-chromium; DES, drug-eluting stent; EES, everolimus-eluting stent; ISR, in-stent restenosis; LLL, late lumen loss; MACE, major adverse cardiovascular events; MLD, minimal lumen diameter; PCB, paclitaxel-cboated balloon; PES, paclitaxel-eluting stent; SCB, sirolimus-coated balloon; TLF, target lesion failure; TLR, target lesion revascularization; TVR, target vessel revascularization;
Non-inferiority.
PCB vs PES.
PEPCAD II, ISAR-DESIRE 3, PEPCAD China ISR, RIBS V, SEDUCE, RIBS IV, TIS, DARE, RESTORE, BIOLUX-RCT.
All-cause death, myocardial infarction, or target lesion thrombosis.
VBT
Vascular brachytherapy inhibits neointimal formation within the stent by delivering radioactive Strontium-90 β-radiation locally, decreasing proliferation. This treatment modality was commonly employed for bare metal ISR 2 decades ago, with minimal long-term benefit demonstrated. The use of VBT has undergone resurgence in use for DES ISR.86, 84, 87 The more overlapping stent layers there are, the less effective brachytherapy is for ISR. With 3 or more stent layers, the 3-year target lesion failure rate exceeds 50%. Accordingly, many centers consider recurrent ISR after failure of 2 DES layers to be an indication for intraventricular block.
IVL
Intravascular lithotripsy is a promising new approach in calcified lesions and has been evaluated in nonrandomized series as a treatment for highly calcified neoatherosclerosis causing ISR.45,51,88 The mechanism of action is emission of an electrical charge from a pair of lithotripters resulting in generation and collapse of vapor bubbles within a pressurized, fluid-filled semicompliant balloon. This results in acoustic shockwaves that exert an instantaneous field force of up to 50 atm creating fractures within intimal and medial calcium, facilitating subsequent stent expansion as assessed by IVUS and OCT. Whether this device provides a long-term benefit, alone or in combination, in this difficult morphologic subset will require RCTs. IVL has also been combined with VBT.50
Management of recurrent ISR
Patients with recurrent ISR are refractory to usual treatment modalities.60 The rates of repeat revascularization have been reported to exceed 50% within 2 years. For this reason, new approaches are often tried in these cases, but typically published results are anecdotal reports and uncontrolled series, rather than controlled trials.
Drug-coated balloons may have a beneficial effect in recurrent ISR, but more investigation is needed. Adverse events are significantly higher in patients treated with DCBs with >2 stent layers versus no significant differences in patients with 1 or 2 prior stents. Atheroablative modalities are often employed, but published reports are anecdotal. Multilayer (>2 stents) underexpanded ISR is particularly recalcitrant to standard treatment. VBT is often reserved for refractory ISR and might be considered when multiple layers of stent are present.50,84,87
Surgical revascularization should be considered in discussion with the patient after several interventional procedures have failed. Although no studies have supported a specific approach as to when repeat procedures should be avoided, Table 6 lists key considerations to assist in deciding which patients might be better treated with coronary artery bypass graft surgery or optimal medical therapy. A heart team approach may be advisable to consider all of the relevant factors in an individual case.
Table 6.
Considerations for CABG in refractory/recurrent in-stent restenosis
|
|
|
|
|
|
|
CABG, coronary artery bypass grafting; CAD, coronary artery disease; LAD, left anterior descending coronary artery; LM, left main coronary artery; LV, left ventricular.
Stent thrombosis
ST is an acute or subacute thrombotic occlusion that usually presents as an acute MI or acute coronary syndrome and is associated with high rates of morbidity and mortality. These thrombi can be notoriously difficult to treat with traditional interventional techniques because they tend to be large, friable, and adherent.89
Incidence and clinical presentation
The overall incidence of ST is 0.5% to 1.0% in the first year and 0.2% to 0.6% in every subsequent year.90, 91, 92 The rate is lower for elective stent placement (0.3%-0.5%) but higher in acute coronary syndrome (3.4%) and MI. In contemporary practice, the observed mortality rate (∼30%) is high, although recent clinical trials and studies requiring autopsy confirmation suggest a better survival, with an average rate of <10%.90, 91, 92 The ST rate is higher in ST-elevation myocardial infarction presentations treated with primary stenting.93, 94, 95
Stent thrombosis causes abrupt closure of the stented artery, and therefore carries a high risk of MI and death. As with any acute vessel closure, the clinical ramifications of ST are influenced by the amount of threatened myocardium, the degree of myocardial viability, the presence and adequacy of collaterals, and the speed and success of revascularization. Approximately 20% of patients with a first ST experience a recurrent ST episode within 2 years.92
Classification
Stent thrombosis is classified by the Academic Research Consortium criteria96 based on the presenting clinical scenario and timing after initial stent placement. Timing is classified as acute, subacute, early, late, and very late. ST occurring within 24 hours is acute; 24 hours to 30 days is subacute; from 30 days to 1 year is late; and >1 year is defined as very late. Early ST is defined as occurring within 30 days (ie, acute plus subacute). The clinical presentation defines whether the likelihood of ST is definite, probable, or possible. This categorization accurately portrays ST and is important in investigating its pathophysiologic associations.97
Pathogenesis
Numerous clinical and technical risk factors have been associated with ST, as summarized in Table 7.98, 99, 100, 101, 102, 103, 104, 105 Prevention of ST is dependent on optimal stent implantation and the duration and compliance with DAPT. Premature or patient-initiated termination of DAPT, sometimes because of bleeding or perioperative concerns, is responsible for most cases of ST.100,101,106, 107, 108, 109, 110, 111, 112 Congenital or acquired hyporesponder DAPT status seen with clopidogrel is uncommon with prasugrel or ticagrelor.107,108 Prior generation stents were susceptible to ST with discontinuation of DAPT out to 5 years and longer in anecdotal cases. With the newest generation of stents, the duration of treatment can be decreased safely to 3 months113 or 1 month.114
Table 7.
| Clinical risk factors | Procedure related | Lesion related | Stent related | Antiplatelet related |
|---|---|---|---|---|
|
|
|
|
|
ACS, acute coronary syndrome; NSTEMI, non-ST-elevation myocardial infarction; STEMI, ST-elevation myocardial infarction
Circumstances that abet stasis and turbulence, such as underexpanded stents, stents in small vessels, or long lesions, are associated with ST.115, 116, 117, 118, 119, 120, 121 These common mechanical etiologies of ST are associated with stent underexpansion in both early and late ST. A second concern is injury or endothelial disruption caused by edge dissection. The delayed healing and endothelial in-growth observed with early-generation DES are now less common. Early, late, or very late ST can be associated with a calcified nodule, perhaps because of consequent underexpansion.
Neointima containing smooth muscle cells develops beginning 2 weeks after BMS.106,121 However, DES, depending on the antiproliferative drug and generation, demonstrate delayed endothelial maturation as compared with BMS.107 When there is an intense neointimal growth reaction to the stent, which is an exaggerated response to arterial healing that peaks at 30 days to 6 months after implantation, vascular narrowing, which can precipitate ST, may occur. Neointimal thickness at stent strut sites is increased when there has been damage to the tunica media. Drug elution and coating polymers may delay this time frame.98,103,105,121,122
Correlates of timing of ST and mechanism
The incidence of Academic Research Consortium definite or probable ST within 2 years is 4.4% and is distributed among the acute, subacute, late, and very late time periods. Each time frame is associated with somewhat different mechanistic circumstances.99, 100, 101, 102, 103,105,121, 122, 123, 124
Early ST
The strongest predictor of early ST is premature discontinuation of DAPT in the first 30 days following stent implantation.122 Mechanical predictors of early ST are similar to those associated with ISR—underexpansion and inflow-outflow problems, including geographic miss, significant residual dissections, and especially occult intramural hematomas—especially at the distal stent edge. Early-stent occlusion is usually the consequence of platelet-rich thrombi forming on an inadequate procedural result. The adequacy of stent expansion and the presence of an occult intimal dissection should be specifically evaluated when the etiology is unclear.
Acute ST
The incidence is 0.2% to 0.6%.
Subacute ST
The incidence is 1.0% to 1.3%.
Late ST
The incidence is 0.4% to 0.6%. The unifying morphologic finding is impaired neointimal healing, defined as delayed development of an endothelialized layer of smooth muscle cells and extracellular matrix that completely covers the stent.123
Several morphologic substrates have been associated with late ST. Stenting across major arterial side branches is well recognized. Overlapping and bifurcation stenting125 are prone to underexpansion and malapposition; careful technique and close attention to adjunct imaging are essential. Stent struts that are not apposed to the vessel wall, which can be because of malapposition or late remodeling, produce increased blood flow turbulence and low-flow foci at the margins of the stent. Low-flow velocity increases fibrin and platelet deposition. Intimal dissection and plaque disruption in the arterial segments adjacent to stents can progress and cause flow obstruction. Stenting of lipid-rich plaques with plaque prolapse also increases risk of ST. Lipid-rich plaques with significant necrosis are prone to stent struts penetrating deeply into the lipid core, thus losing contact with the vessel wall. Also, stents placed in a stenosis with large lipid core might delay the development of a fully endothelialized neointima because of scarcity of migrating and proliferating smooth muscle cells in proximity to the stent. Finally, diffuse ISR with thrombosis can occur because of overproduction of intimal hyperplasia and neoatherosclerosis.103,118, 119, 120, 121
Residual stent edge dissection has been associated with target lesion revascularization.101,123,124 Edge dissections are defined as being major by OCT when they extend in an arc of >60° and are >3 mm in length. Intimal dissection and plaque disruption in the arterial segments adjacent to stents can progress and cause flow obstruction.
Very late ST
The incidence has been reported between 0.4% to 0.8%. Very late ST (VLST) occurs 1 to 5 years after stent implantation at a rate of 2% per year. The most commonly identified causes of VLST are malapposition, uncovered struts, neoatherosclerosis, and stent underexpansion. The degree and extent of malapposition and uncovered stent struts are the most important correlates of thrombus formation in VLST.101,124 Intravascular imaging can identify suboptimal stent deployment.
Diagnostic imaging modalities
Intravascular imaging is crucial to developing rational individual case strategy because the causative mechanism is highly influential in selecting treatment strategy to manage the thrombus and prevent its recurrence. Although no imaging modality is rated in the class I category by existing guidelines because of an absence of randomized trials, angiography alone is clearly inadequate because of its intrinsic inadequacy to assess stent expansion, neoatherosclerosis, calcification, and remodeling. IVUS and OCT provide detailed assessment of the stented segment and accurately identify the underlying mechanisms. For these reasons, SCAI recommends that imaging be strongly considered when the etiology of ST is uncertain from clinical and angiographic information.
Angiographic factors
The extent of coronary artery disease is an independent predictor of ST.105,122 The Dutch Stent Thrombosis Registry included 21,009 consecutive patients and showed that multivessel disease, long lesions, and multiple lesions (total stent length) are independent determinants of ST.117 The volume of thrombus burden is important in appraising the risk of distal embolization, as is the degree of residual flow, presence of collaterals, and status of the microvasculature.53, 54, 55 The size of the jeopardized myocardial segment and the residual left ventricular function are also critical factors.
Intravascular imaging
There is substantial data suggesting that intravascular imaging improves outcomes (see Figures 4A, B and 5). IVUS has been considered the standard imaging modality for ST.101,102,122 OCT, which has 10-fold higher axial resolution, has more recently shown great promise.115,119,126,127 Although the absence of a multicenter, controlled trial limits a formal level of indication,24 these modalities appear to be useful in optimal lesion preparation strategies.
Figure 4.
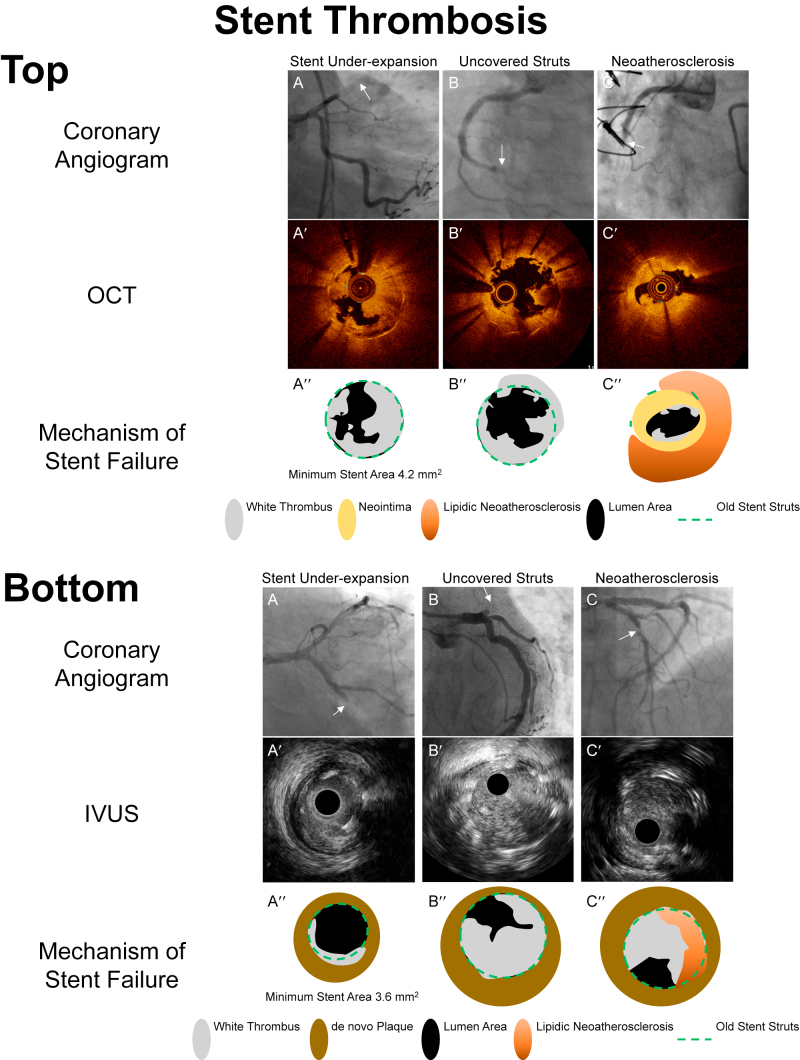
Mechanisms of stent thrombosis evaluated by intravascular imaging. A′-C′ are optical coherence tomography (OCT) or intravascular ultrasound (IVUS) images corresponding to stent thrombosis seen in the angiographic images A-C (white arrows). A″-C″ are representative diagrams provided to clarify the intracoronary images A′-C′. Top. Mechanisms of stent thrombosis evaluated by OCT (A) Subacute stent thrombosis in which OCT showed a severely underexpanded stent occupied by white thrombus in the mid left anterior descending artery. (B) Very late stent thrombosis occurred 2 hours after noncardiac surgery and after discontinuation of antiplatelet therapy. OCT showed uncovered stent struts occupied by white thrombus. (C) Lipidic plaque (strong signal attenuation) within stent struts indicating neoatherosclerosis resulting in plaque rupture with thrombus. Bottom. Mechanisms of stent thrombosis evaluated by IVUS (A) Subacute stent thrombosis in which IVUS showed a severely underexpanded stent and thrombus in the mid left circumflex artery. (B) Very late stent thrombosis in which IVUS showed a well-expanded stent occupied by thrombus. Because there is no neointimal hyperplasia, it was speculated that uncovered stent struts were the cause of stent thrombosis. (C) Lipidic plaque (strong signal attenuation) appeared within the stent struts indicating neoatherosclerosis resulting in plaque rupture with thrombus.
Figure 5.
Early restenosis due to re-protrusion of a calcified nodule. This patient underwent percutaneous coronary intervention to treat lesions in the distal and mid right coronary artery. Optical coherence tomography (OCT) showed an eruptive calcified nodule (white arrows) in both lesions. A calcified nodule is characterized by an accumulation of small calcium fragments typically with strong signal attenuation due to accompanying and overlying fibrin. The patient came back for staged procedure of LAD (left anterior descending artery) 6 weeks later. OCT showed reprotruding calcified nodules within the stent.
There are several well-defined imaging criteria to optimize stenting (Table 8).115, 116, 117, 118, 119, 120 IVUS can be of great value during stent placement to assess stent sizing, expansion, and apposition. Several studies suggest that IVUS-guided stent placement reduces ST, restenosis, and repeat revascularization.101,102,116,118,128, 129, 130 Stent undersizing occurs frequently when visual estimation alone is used for size selection, and this is a major contributor to the risk of ST (and ISR). The incidence of ST is significantly reduced when IVUS is used to guide stent placement and optimize expansion.128 In the Assessment of Dual Antiplatelet Therapy With Drug-Eluting Stents (ADAPT-DES) study,129 at 1 year, there was a significant reduction in definite/probable ST (0.52% vs 1.04%, P =.01) and MI (2.5% vs 3.7%, P =.002)128 when IVUS guidance was employed.
Table 8.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Optical coherence tomography may be superior to IVUS in assessing the cause of ST. In particular, uncovered struts and underexpansion are seen in acute and subacute ST, and neoatherosclerosis, uncovered struts, and malapposition are seen in late and very late ST.126,131,132 OCT has been shown to be highly effective in determining the underlying cause of VLST in >98% of cases, and multiple mechanisms are usually present (55%). OCT demonstrates malapposition, neoatherosclerosis, uncovered struts, and stent underexpansion underlying ST.127 OCT identified adverse features requiring further intervention in 35% of cases, with significantly lower risk of death and MI at 1 year in the CLI-OPCI trial.97
In ULTIMATE,130 IVUS-guided PCI was associated with important reductions in target vessel failure (cardiac death, MI, or target vessel revascularization). At 3 years, target vessel failure occurred in 47 patients (6.6%) in the IVUS-guided group and in 76 patients (10.7%) in the angiography-guided group (P = .01), driven mainly by the decrease in clinically driven target vessel revascularization (4.5% vs 6.9%; P = .05). Both OPINION133 and ILUMIEN III131 compared IVUS and OCT and concluded that these methods have similar treatment outcomes.
Mechanical and pharmacologic treatment of ST
Balloon inflation, aspiration thrombectomy and pharmacologic treatment with anticoagulants and antiplatelet drugs are the mainstays of treatment (Table 4).52, 53, 54, 55 Emergent PCI is indicated when the presentation is acute, although optimal reperfusion is achieved in only 2/3.89 The SCAI recommended algorithm to organize these approaches is provided in Figure 6.
Figure 6.
SCAI algorithmic approach to stent thrombosis.
Following diagnostic angiography, most ST occlusions can be treated initially with balloon angioplasty alone, sometimes with adjunctive thrombus aspiration when the clot burden is large. Additional stent implantation should ordinarily be limited to significant residual dissections, especially if recent DAPT has been discontinued. High-pressure inflations with noncompliant balloons to assure stent apposition may be necessary in some cases. At this time, no particular stent design or polymer coating has been shown to prevent ST more than others.
Glycoprotein IIb/IIIa antagonists should be considered to improve microvascular reperfusion because of distal embolization, and prolonged infusions up to 72 hours have been successful in anecdotal cases. Prolonged anticoagulation and antiplatelet therapy may be beneficial when residual thrombus is detected following intervention. Compliance and drug resistance should be evaluated in detail. More potent antiplatelet therapy should be considered, including aspirin, prasugrel, or ticagrelor.107,108,134 If platelet aggregation studies are available and reveal insufficient (<50%) inhibition of platelet aggregation with standard DAPT, the sustained administration of 150 mg/d clopidogrel may be considered. Long-term non-vitamin K oral anticoagulants or warfarin are rarely necessary but may be considered for selected cases of recurrent ST. Although mechanical and extraction thrombectomy have been employed anecdotally, there are no large-scale studies to evaluate their benefit or subgroups of most/least promise.135
After flow is re-established and the thrombus is no longer angiographically visible, angiographic and clinical circumstances that reflect distal embolization should be sought and treated. Once the patient is stabilized, the etiology of the stent closure should be pursued. If DAPT has not been interrupted, the stent should be assessed with either IVUS or OCT to determine the adequacy of stent apposition, expansion, and the presence of edge dissections or intramural hematomas. Optimization of stent deployment with appropriate high-pressure balloon expansion should be performed. Additional stent implantation should be avoided if possible because the probability of recurrent ST increases proportionately to the stent length; however, treatment of edge dissections and progression of disease with additional stents are imperative to prevent repeat ST.
Conclusion
Stent failure remains the major drawback to the use of coronary stents as a revascularization strategy. Recent advances in imaging have substantially improved our understanding of the mechanisms underlying both ISR and ST, which have in common numerous clinical risk factors and mechanical elements at the time of stent implantation. SCAI recommends image-guided PCI at the time of initial stent implantation to minimize the occurrence of ISR and ST. When ISR or ST is encountered, imaging should be strongly considered to optimize the subsequent approach to these challenging cases.
Peer review statement
Given his role as associate editor, Sandeep Nathan had no involvement in the peer review of this article and has no access to information regarding its peer review.
Declaration of competing interest
Ziad Ali served on the advisory council and received honoraria and consulting fees from Boston Scientific and Abbott Laboratories. Roxana Mehran is a primary investigator for Abbott. Gary Mintz received honoraria from Boston Scientific. Amir Lotfi, Lloyd Klein, John Messenger, Sunil Rao, Karim Al-Azizi, Yader Sandoval, Sandeep Nathan, Jennifer Rymer, and Akiko Maehara reported no financial interests.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
To access the supplementary material accompanying this article, visit the online version of the Journal of the Society for Cardiovascular Angiography & Interventions at 10.1016/j.jscai.2023.100971.
Supplementary material
References
- 1.Dangas G.D., Claessen B.E., Caixeta A., Sanidas E.A., Mintz G.S., Mehran R. In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol. 2010;56(23):1897–1907. doi: 10.1016/j.jacc.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 2.Moussa I.D., Mohananey D., Saucedo J., et al. Trends and outcomes of restenosis after coronary stent implantation in the United States. J Am Coll Cardiol. 2020;76(13):1521–1531. doi: 10.1016/j.jacc.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Palmerini T., Della Riva D., Biondi-Zoccai G., et al. Mortality following nonemergent, uncomplicated target lesion revascularization after percutaneous coronary intervention: an individual patient data pooled analysis of 21 randomized trials and 32,524 patients. J Am Coll Cardiol Intv. 2018;11(9):892–902. doi: 10.1016/j.jcin.2018.01.277. [DOI] [PubMed] [Google Scholar]
- 4.Nakatsuma K., Shiomi H., Natsuaki M., et al. Second-generation versus first-generation drug-eluting stents in patients with and without diabetes mellitus: pooled analysis from the RESET and NEXT trials. Cardiovasc Interv Ther. 2018;33(2):125–134. doi: 10.1007/s12928-017-0458-9. [DOI] [PubMed] [Google Scholar]
- 5.Iqbal J., Serruys P.W., Silber S., et al. Comparison of zotarolimus- and everolimus-eluting coronary stents: final 5-year report of the RESOLUTE all-comers trial. Circ Cardiovasc Interv. 2015;8(6) doi: 10.1161/CIRCINTERVENTIONS.114.002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piraino D., Cimino G., Buccheri D., Dendramis G., Andolina G., Cortese B. Recurrent in-stent restenosis, certainty of its origin, uncertainty about treatment. Int J Cardiol. 2017;230:91–96. doi: 10.1016/j.ijcard.2016.12.073. [DOI] [PubMed] [Google Scholar]
- 7.Cassese S., Xu B., Habara S., et al. Incidence and predictors of reCurrent restenosis after drug-coated balloon angioplasty for restenosis of a drUg-eluting stent: the ICARUS cooperation. Rev Esp Cardiol (Engl Ed) 2018;71(8):620–627. doi: 10.1016/j.rec.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Song L., Mintz G.S., Yin D., et al. Characteristics of early versus late in-stent restenosis in second-generation drug-eluting stents: an optical coherence tomography study. EuroIntervention. 2017;13(3):294–302. doi: 10.4244/EIJ-D-16-00787. [DOI] [PubMed] [Google Scholar]
- 9.Yabushita H., Kawamoto H., Fujino Y., et al. Clinical outcomes of drug-eluting balloon for in-stent restenosis based on the number of metallic layers. Circ Cardiovasc Interv. 2018;11(8) doi: 10.1161/CIRCINTERVENTIONS.117.005935. [DOI] [PubMed] [Google Scholar]
- 10.Alfonso F., Byrne R.A., Rivero F., Kastrati A. Current treatment of in-stent restenosis. J Am Coll Cardiol. 2014;63(24):2659–2673. doi: 10.1016/j.jacc.2014.02.545. [DOI] [PubMed] [Google Scholar]
- 11.Nakazawa G., Otsuka F., Nakano M., et al. The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. J Am Coll Cardiol. 2011;57(11):1314–1322. doi: 10.1016/j.jacc.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bossi I., Klersy C., Black A.J., et al. In-stent restenosis: long-term outcome and predictors of subsequent target lesion revascularization after repeat balloon angioplasty. J Am Coll Cardiol. 2000;35(6):1569–1576. doi: 10.1016/s0735-1097(00)00584-2. [DOI] [PubMed] [Google Scholar]
- 13.Zahn R., Hamm C.W., Schneider S., et al. Incidence and predictors of target vessel revascularization and clinical event rates of the sirolimus-eluting coronary stent (results from the prospective multicenter German Cypher Stent Registry) Am J Cardiol. 2005;95(11):1302–1308. doi: 10.1016/j.amjcard.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 14.Costa M.A., Angiolillo D.J., Tannenbaum M., et al. Impact of stent deployment procedural factors on long-term effectiveness and safety of sirolimus-eluting stents (final results of the multicenter prospective STLLR trial) Am J Cardiol. 2008;101(12):1704–1711. doi: 10.1016/j.amjcard.2008.02.053. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi Y., De Gregorio J., Kobayashi N., et al. Stented segment length as an independent predictor of restenosis. J Am Coll Cardiol. 1999;34(3):651–659. doi: 10.1016/s0735-1097(99)00303-4. [DOI] [PubMed] [Google Scholar]
- 16.Nusca A., Viscusi M.M., Piccirillo F., et al. In stent neo-atherosclerosis: pathophysiology, clinical implications, prevention, and therapeutic approaches. Life (Basel) 2022;12(3):393. doi: 10.3390/life12030393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang S., Houghton P.J. Mechanisms of resistance to rapamycins. Drug Resist Update. 2001;4(6):378–391. doi: 10.1054/drup.2002.0227. [DOI] [PubMed] [Google Scholar]
- 18.Nebeker J.R., Virmani R., Bennett C.L., et al. Hypersensitivity cases associated with drug-eluting coronary stents: a review of available cases from the Research on Adverse Drug Events and Reports (RADAR) project. J Am Coll Cardiol. 2006;47(1):175–181. doi: 10.1016/j.jacc.2005.07.071. [DOI] [PubMed] [Google Scholar]
- 19.Mehran R., Dangas G., Abizaid A.S., et al. Angiographic patterns of in-stent restenosis: classification and implications for long-term outcome. Circulation. 1999;100(18):1872–1878. doi: 10.1161/01.cir.100.18.1872. [DOI] [PubMed] [Google Scholar]
- 20.Shlofmitz E., Iantorno M., Waksman R. Restenosis of drug-eluting stents: a new classification system based on disease mechanism to guide treatment and state-of-the-art review. Circ Cardiovasc Interv. 2019;12(8) doi: 10.1161/CIRCINTERVENTIONS.118.007023. [DOI] [PubMed] [Google Scholar]
- 21.Kang S.J., Mintz G.S., Park D.W., et al. Tissue characterization of in-stent neointima using intravascular ultrasound radiofrequency data analysis. Am J Cardiol. 2010;106(11):1561–1565. doi: 10.1016/j.amjcard.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalo N., Serruys P.W., Okamura T., et al. Optical coherence tomography patterns of stent restenosis. Am Heart J. 2009;158(2):284–293. doi: 10.1016/j.ahj.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Ali Z.A., Roleder T., Narula J., et al. Increased thin-cap neoatheroma and periprocedural myocardial infarction in drug-eluting stent restenosis: multimodality intravascular imaging of drug-eluting and bare-metal stents. Circ Cardiovasc Interv. 2013;6(5):507–517. doi: 10.1161/CIRCINTERVENTIONS.112.000248. [DOI] [PubMed] [Google Scholar]
- 24.Lotfi A., Davies J.E., Fearon W.F., Grines C.L., Kern M.J., Klein L.W. Focused update of expert consensus statement: use of invasive assessments of coronary physiology and structure: a position statement of the Society of Cardiac Angiography and Interventions. Catheter Cardiovasc Interv. 2018;92(2):336–347. doi: 10.1002/ccd.27672. [DOI] [PubMed] [Google Scholar]
- 25.Levine G.N., Bates E.R., Blankenship J.C., et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. 2013;82(4):E266–E355. doi: 10.1002/ccd.23390. [DOI] [PubMed] [Google Scholar]
- 26.Kang S.J., Mintz G.S., Park D.W., et al. Mechanisms of in-stent restenosis after drug-eluting stent implantation: intravascular ultrasound analysis. Circ Cardiovasc Interv. 2011;4(1):9–14. doi: 10.1161/CIRCINTERVENTIONS.110.940320. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J., Gao X., Kan J., et al. Intravascular ultrasound versus angiography-guided drug-eluting stent implantation: the ULTIMATE Trial. J Am Coll Cardiol. 2018;72(24):3126–3137. doi: 10.1016/j.jacc.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 28.Jensen L.O., Vikman S., Antonsen L., et al. Intravascular ultrasound assessment of minimum lumen area and intimal hyperplasia in in-stent restenosis after drug-eluting or bare-metal stent implantation. The Nordic Intravascular Ultrasound Study (NIVUS) Cardiovasc Revasc Med. 2017;18(8):577–582. doi: 10.1016/j.carrev.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 29.Prati F., Romagnoli E., Burzotta F., et al. Clinical impact of OCT findings during PCI: the CLI-OPCI II Study. J Am Coll Cardiol Img. 2015;8(11):1297–1305. doi: 10.1016/j.jcmg.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Alfonso F., Cuesta J. The therapeutic dilemma of recurrent in-stent restenosis. Circ Cardiovasc Interv. 2018;11(8) doi: 10.1161/CIRCINTERVENTIONS.118.007109. [DOI] [PubMed] [Google Scholar]
- 31.Alfonso F., Scheller B. Management of recurrent in-stent restenosis: onion skin full metal jacket? EuroIntervention. 2013;9(7):781–785. doi: 10.4244/EIJV9I7A129. [DOI] [PubMed] [Google Scholar]
- 32.Alfonso F., García J., Pérez-Vizcayno M.J., et al. New stent implantation for recurrences after stenting for in-stent restenosis: implications of a third metal layer in human coronary arteries. J Am Coll Cardiol. 2009;54(11):1036–1038. doi: 10.1016/j.jacc.2009.04.082. [DOI] [PubMed] [Google Scholar]
- 33.Maejima N., Hibi K., Saka K., et al. Relationship between thickness of calcium on optical coherence tomography and crack formation after balloon dilatation in calcified plaque requiring rotational atherectomy. Circ J. 2016;80(6):1413–1419. doi: 10.1253/circj.CJ-15-1059. [DOI] [PubMed] [Google Scholar]
- 34.Maehara A., Matsumura M., Ali Z.A., Mintz G.S., Stone G.W. IVUS-guided versus OCT-guided coronary stent implantation: a critical appraisal. J Am Coll Cardiol Img. 2017;10(12):1487–1503. doi: 10.1016/j.jcmg.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 35.Maehara A., Ben-Yehuda O., Ali Z., et al. Comparison of stent expansion guided by optical coherence tomography versus intravascular ultrasound: the ILUMIEN II study (observational study of optical coherence tomography [OCT] in patients undergoing fractional flow reserve [FFR] and percutaneous coronary intervention) J Am Coll Cardiol Intv. 2015;8:1704–1714. doi: 10.1016/j.jcin.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 36.Nam C.W., Rha S.W., Koo B.K., et al. Usefulness of coronary pressure measurement for functional evaluation of drug-eluting stent restenosis. Am J Cardiol. 2011;107(12):1783–1786. doi: 10.1016/j.amjcard.2011.02.328. [DOI] [PubMed] [Google Scholar]
- 37.McInerney A., Travieso Gonzalez A., Castro Mejía A., et al. Long-term outcomes after deferral of revascularization of in-stent restenosis using fractional flow reserve. Catheter Cardiovasc Interv. 2022;99(3):723–729. doi: 10.1002/ccd.29823. [DOI] [PubMed] [Google Scholar]
- 38.Alfonso F., Perez-Vizcayno M.J., Hernandez R., et al. Investigators R.-I. A randomized comparison of sirolimus-eluting stent with balloon angioplasty in patients with in-stent restenosis: results of the Restenosis Intrastent: Balloon Angioplasty Versus Elective Sirolimus-Eluting Stenting (RIBS-II) trial. J Am Coll Cardiol. 2006;47(11):2152–2160. doi: 10.1016/j.jacc.2005.10.078. [DOI] [PubMed] [Google Scholar]
- 39.Kastrati A., Mehilli J., von Beckerath N., et al. Sirolimus-eluting stent or paclitaxel-eluting stent vs balloon angioplasty for prevention of recurrences in patients with coronary in-stent restenosis: a randomized controlled trial. JAMA. 2005;293(2):165–171. doi: 10.1001/jama.293.2.165. [DOI] [PubMed] [Google Scholar]
- 40.Mehilli J., Byrne R.A., Tiroch K., et al. Randomized trial of paclitaxel- versus sirolimus-eluting stents for treatment of coronary restenosis in sirolimus-eluting stents: the ISAR-DESIRE 2 (intracoronary stenting and angiographic results: drug eluting stents for in-stent restenosis 2) study. J Am Coll Cardiol. 2010;55(24):2710–2716. doi: 10.1016/j.jacc.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 41.Kokkinidis D.G., Prouse A.F., Avner S.J., Lee J.M., Waldo S.W., Armstrong E.J. Second-generation drug-eluting stents versus drug-coated balloons for the treatment of coronary in-stent restenosis: A systematic review and meta-analysis. Catheter Cardiovasc Interv. 2018;92(2):285–299. doi: 10.1002/ccd.27359. [DOI] [PubMed] [Google Scholar]
- 42.Navarese E.P., Austin D., Gurbel P.A., et al. Drug-coated balloons in treatment of in-stent restenosis: a meta-analysis of randomised controlled trials. Clin Res Cardiol. 2013;102(4):279–287. doi: 10.1007/s00392-012-0532-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goel S.S., Dilip Gajulapalli R., Athappan G., et al. Management of drug eluting stent in-stent restenosis: a systematic review and meta-analysis. Catheter Cardiovasc Interv. 2016;87(6):1080–1091. doi: 10.1002/ccd.26151. [DOI] [PubMed] [Google Scholar]
- 44.Alfonso F., Pérez-Vizcayno M.J., Cárdenas A., et al. A prospective randomized trial of drug-eluting balloons versus everolimus-eluting stents in patients with in-stent restenosis of drug-eluting stents: the RIBS IV randomized clinical trial. J Am Coll Cardiol. 2015;66(1):23–33. doi: 10.1016/j.jacc.2015.04.063. [DOI] [PubMed] [Google Scholar]
- 45.Kufner S., Joner M., Schneider S., et al. Neointimal modification with scoring balloon and efficacy of drug-coated balloon therapy in patients with restenosis in drug-eluting coronary stents: a randomized controlled trial. J Am Coll Cardiol Intv. 2017 10;10(13):1332–1340. doi: 10.1016/j.jcin.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 46.Vom Dahl J., Dietz U., Haager P.K., et al. Rotational atherectomy does not reduce recurrent in-stent restenosis: results of the angioplasty versus rotational atherectomy for treatment of diffuse in-stent restenosis trial (ARTIST) Circulation. 2002;105(5):583–588. doi: 10.1161/hc0502.103347. [DOI] [PubMed] [Google Scholar]
- 47.Baan J., Jr., Claessen B.E., Dijk K.B., et al. A randomized comparison of paclitaxel-eluting balloon versus everolimus-eluting stent for the treatment of any in-stent restenosis: the DARE trial. J Am Coll Cardiol Intv. 2018;11(3):275–283. doi: 10.1016/j.jcin.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 48.Megaly M., Glogoza M., Xenogiannis I., et al. Outcomes of intravascular brachytherapy for recurrent drug-eluting in-stent restenosis. Catheter Cardiovasc Interv. 2021;97(1):32–38. doi: 10.1002/ccd.28716. [DOI] [PubMed] [Google Scholar]
- 49.Varghese M.J., Bhatheja S., Baber U., et al. Intravascular brachytherapy for the management of repeated multimetal-layered drug-eluting coronary stent restenosis. Circ Cardiovasc Interv. 2018;11(10) doi: 10.1161/CIRCINTERVENTIONS.118.006832. [DOI] [PubMed] [Google Scholar]
- 50.Chen G., Zrenner B., Pyxaras S.A. Combined rotational atherectomy and intravascular lithotripsy for the treatment of severely calcified in-stent neoatherosclerosis: a mini-review. Cardiovasc Revasc Med. 2019;20(9):819–821. doi: 10.1016/j.carrev.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 51.Tovar Forero M.N., Sardella G., Salvi N., et al. Coronary lithotripsy for the treatment of underexpanded stents: the international and multicentre CRUNCH registry. EuroIntervention. 2022;18(7):574–581. doi: 10.4244/EIJ-D-21-00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mahmoud K.D., Vlaar P.J., van den Heuvel A.F., Hillege H.L., Zijlstra F., de Smet B.J. Usefulness of thrombus aspiration for the treatment of coronary stent thrombosis. Am J Cardiol. 2011;108(12):1721–1727. doi: 10.1016/j.amjcard.2011.07.044. [DOI] [PubMed] [Google Scholar]
- 53.Reeves R.R., Patel M., Armstrong E.J., et al. Angiographic characteristics of definite stent thrombosis: role of thrombus grade, collaterals, epicardial coronary flow, and myocardial perfusion. Catheter Cardiovasc Interv. 2015;85(1):13–22. doi: 10.1002/ccd.25519. [DOI] [PubMed] [Google Scholar]
- 54.Armstrong E.J., Feldman D.N., Wang T.Y., et al. Clinical presentation, management, and outcomes of angiographically documented early, late, and very late stent thrombosis. J Am Coll Cardiol Intv. 2012;5(2):131–140. doi: 10.1016/j.jcin.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 55.Claessen B.E., Henriques J.P., Jaffer F.A., Mehran R., Piek J.J., Dangas G.D. Stent thrombosis: a clinical perspective. J Am Coll Cardiol Intv. 2014;7(10):1081–1092. doi: 10.1016/j.jcin.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 56.Goto K., Zhao Z., Matsumura M., et al. Mechanisms and patterns of intravascular ultrasound in-stent restenosis among bare metal stents and first- and second-generation drug-eluting stents. Am J Cardiol. 2015;116(9):1351–1357. doi: 10.1016/j.amjcard.2015.07.058. [DOI] [PubMed] [Google Scholar]
- 57.Albiero R., Silber S., Di Mario C., et al. Cutting balloon versus conventional balloon angioplasty for the treatment of in-stent restenosis: results of the restenosis cutting balloon evaluation trial (RESCUT) J Am Coll Cardiol. 2004;43(6):943–949. doi: 10.1016/j.jacc.2003.09.054. [DOI] [PubMed] [Google Scholar]
- 58.Park S.J., Kim K.H., Oh I.Y., et al. Comparison of plain balloon and cutting balloon angioplasty for the treatment of restenosis with drug-eluting stents vs bare metal stents. Circ J. 2010;74(9):1837–1845. doi: 10.1253/circj.cj-09-1041. [DOI] [PubMed] [Google Scholar]
- 59.Claessen B.E., Kini A.S., Mehran R., Sharma S.K., Dangas G.D. Coronary in-stent restenosis: an algorithmic approach to the treatment of coronary in-stent restenosis. J Invasive Cardiol. 2019;(suppl):9–12. [Google Scholar]
- 60.Theodoropoulos K., Mennuni M.G., Dangas G.D., et al. Resistant in-stent restenosis in the drug eluting stent era. Catheter Cardiovasc Interv. 2016;88(5):777–785. doi: 10.1002/ccd.26559. [DOI] [PubMed] [Google Scholar]
- 61.Lee T., Shlofmitz R.A., Song L., et al. The effectiveness of excimer laser angioplasty to treat coronary in-stent restenosis with peri-stent calcium as assessed by optical coherence tomography. EuroIntervention. 2019;15(3):e279–e288. doi: 10.4244/EIJ-D-18-00139. [DOI] [PubMed] [Google Scholar]
- 62.Sharma S.K., Kini A., Mehran R., Lansky A., Kobayashi Y., Marmur J.D. Randomized trial of rotational atherectomy versus balloon angioplasty for diffuse in-stent restenosis (ROSTER) Am Heart J. 2004;147(1):16–22. doi: 10.1016/j.ahj.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 63.Ferri L.A., Jabbour R.J., Giannini F., et al. Safety and efficacy of rotational atherectomy for the treatment of undilatable underexpanded stents implanted in calcific lesions. Catheter Cardiovasc Interv. 2017;90(2):E19–E24. doi: 10.1002/ccd.26836. [DOI] [PubMed] [Google Scholar]
- 64.Latib A., Takagi K., Chizzola G., et al. Excimer laser lesion modification to expand non-dilatable stents: the ELLEMENT registry. Cardiovasc Revasc Med. 2014;15(1):8–12. doi: 10.1016/j.carrev.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 65.Neumann F.J., Sousa-Uva M., Ahlsson A., et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165. doi: 10.1093/eurheartj/ehy855. [DOI] [PubMed] [Google Scholar]
- 66.Rhee T.M., Lee J.M., Shin E.S., et al. Impact of optimized procedure-related factors in drug-eluting balloon angioplasty for treatment of in-stent restenosis. J Am Coll Cardiol Intv. 2018;11(10):969–978. doi: 10.1016/j.jcin.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 67.Ashikaga T., Yoshikawa S., Isobe M. The effectiveness of excimer laser coronary atherectomy with contrast medium for underexpanded stent: the findings of optical frequency domain imaging. Catheter Cardiovasc Interv. 2015;86(5):946–949. doi: 10.1002/ccd.25915. [DOI] [PubMed] [Google Scholar]
- 68.Rheude T., Fitzgerald S., Allali A., et al. Rotational atherectomy or balloon-based techniques to prepare severely calcified coronary lesions. J Am Coll Cardiol Intv. 2022;15(18):1864–1874. doi: 10.1016/j.jcin.2022.07.034. [DOI] [PubMed] [Google Scholar]
- 69.Muramatsu T., Tsukahara R., Ho M., et al. Efficacy of cutting balloon angioplasty for in-stent restenosis: an intravascular ultrasound evaluation. J Invasive Cardiol. 2001;13(6):439–444. [PubMed] [Google Scholar]
- 70.Unverdorben M., Vallbracht C., Cremers B., et al. Paclitaxel-coated balloon catheter versus paclitaxel-coated stent for the treatment of coronary in-stent restenosis: the three-year results of the PEPCAD II ISR study. EuroIntervention. 2015;11(8):926–934. doi: 10.4244/EIJY14M08_12. [DOI] [PubMed] [Google Scholar]
- 71.Alfonso F., Pérez-Vizcayno M.J., Cárdenas A., et al. A randomized comparison of drug-eluting balloon versus everolimus-eluting stent in patients with bare-metal stent-in-stent restenosis: the RIBS V clinical trial (restenosis intra-stent of bare metal stents: paclitaxel-eluting balloon vs. everolimus-eluting stent) J Am Coll Cardiol. 2014;63(14):1378–1386. doi: 10.1016/j.jacc.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 72.Adriaenssens T., Dens J., Ughi G., et al. Optical coherence tomography study of healing characteristics of paclitaxel-eluting balloons vs. everolimus-eluting stents for in-stent restenosis: the SEDUCE (Safety and Efficacy of a Drug elUting balloon in Coronary artery rEstenosis) randomised clinical trial. EuroIntervention. 2014;10(4):439–448. doi: 10.4244/EIJV10I4A77. [DOI] [PubMed] [Google Scholar]
- 73.Scheller B., Clever Y.P., Kelsch B., et al. Long-term follow-up after treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. J Am Coll Cardiol Intv. 2012;5(3):323–330. doi: 10.1016/j.jcin.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 74.Unverdorben M., Vallbracht C., Cremers B., et al. Paclitaxel-coated balloon catheter versus paclitaxel-coated stent for the treatment of coronary in-stent restenosis. Circulation. 2009;119(23):2986–2994. doi: 10.1161/CIRCULATIONAHA.108.839282. [DOI] [PubMed] [Google Scholar]
- 75.Pleva L., Kukla P., Kusnierova P., Zapletalova J., Hlinomaz O. Comparison of the efficacy of paclitaxel-eluting balloon catheters and everolimus-eluting stents in the treatment of coronary in-stent restenosis: the treatment of in-stent restenosis study. Circ Cardiovasc Interv. 2016;9(4) doi: 10.1161/CIRCINTERVENTIONS.115.003316. [DOI] [PubMed] [Google Scholar]
- 76.Byrne R.A., Neumann F.J., Mehilli J., et al. Paclitaxel-eluting balloons, paclitaxel-eluting stents, and balloon angioplasty in patients with restenosis after implantation of a drug-eluting stent (ISAR-DESIRE 3): a randomised, open-label trial. Lancet. 2013;381(9865):461–467. doi: 10.1016/S0140-6736(12)61964-3. [DOI] [PubMed] [Google Scholar]
- 77.Xu B., Qian J., Ge J., et al. Two-year results and subgroup analyses of the PEPCAD China in-stent restenosis trial: a prospective, multicenter, randomized trial for the treatment of drug-eluting stent in-stent restenosis. Catherer Cardiovasc Interv. 2016;87(suppl 1):624–629. doi: 10.1002/ccd.26401. [DOI] [PubMed] [Google Scholar]
- 78.Wong Y.T.A., Kang D.Y., Lee J.B., et al. Comparison of drug-eluting stents and drug-coated balloon for the treatment of drug-eluting coronary stent restenosis: a randomized RESTORE Trial. Am Heart J. 2018;197:35–42. doi: 10.1016/j.ahj.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 79.Jensen C.J., Richardt G., Tölg R., et al. Angiographic and clinical performance of a paclitaxel-coated balloon compared to a second-generation sirolimus-eluting stent in patients with in-stent restenosis: the BIOLUX randomised controlled trial. EuroIntervention. 2018;14(10):1096–1103. doi: 10.4244/EIJ-D-17-01079. [DOI] [PubMed] [Google Scholar]
- 80.Giacoppo D., Alfonso F., Xu B., et al. Paclitaxel-coated balloon angioplasty vs. drug-eluting stenting for the treatment of coronary in-stent restenosis: a comprehensive, collaborative, individual patient data meta-analysis of 10 randomized clinical trials (DAEDALUS study) Eur Heart J. 2020;41(38):3715–3728. doi: 10.1093/eurheartj/ehz594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ali R.M., Abdul Kader M.A.S.K., Wan Ahmad W.A., et al. Treatment of coronary drug-eluting stent restenosis by a sirolimus- or paclitaxel-coated balloon. J Am Coll Cardiol Intv. 2019;12(6):558–566. doi: 10.1016/j.jcin.2018.11.040. [DOI] [PubMed] [Google Scholar]
- 82.Scheller B., Mangner N., Abdul Kader M.A.S.K., et al. Combined analysis of two parallel randomized trials of sirolimus-coated and paclitaxel-coated balloons in coronary in-stent restenosis lesions. Circ Cardiovasc Interv. 2022;15(9) doi: 10.1161/CIRCINTERVENTIONS.122.012305. [DOI] [PubMed] [Google Scholar]
- 83.Habara S., Mitsudo K., Kadota K., et al. Effectiveness of paclitaxel-eluting balloon catheter in patients with sirolimus-eluting stent restenosis. J Am Coll Cardiol Intv. 2011;4(2):149–154. doi: 10.1016/j.jcin.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 84.Ho E., Denby K., Cheria S., et al. Intracoronary brachytherapy for drug-eluting stent restenosis: outcomes and clinical correlates. JSCAI. 2023;2(1) [Google Scholar]
- 85.Rittger H., Brachmann J., Sinha A.M., et al. A randomized, multicenter, single-blinded trial comparing paclitaxel-coated balloon angioplasty with plain balloon angioplasty in drug-eluting stent restenosis: the PEPCAD-DES Study. J Am Coll Cardiol. 2012;59(15):1377–1382. doi: 10.1016/j.jacc.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 86.Nikolakopoulos I., Vemmou E., Xenogiannis I., Brilakis E.S. Combined use of intravascular lithotripsy and brachytherapy: a new approach for the treatment of recurrent coronary in-stent restenosis. Catheter Cardiovasc Interv. 2021;97(7):1402–1406. doi: 10.1002/ccd.29332. [DOI] [PubMed] [Google Scholar]
- 87.Megaly M., Glogoza M., Xenogiannis I., et al. Coronary intravascular brachytherapy for recurrent coronary drug-eluting stent in-stent restenosis: a systematic review and meta-analysis. Cardiovasc Revasc Med. 2021;23:28–35. doi: 10.1016/j.carrev.2020.08.035. [DOI] [PubMed] [Google Scholar]
- 88.Wańha W., Tomaniak M., Wańczura P., et al. Intravascular lithotripsy for the treatment of stent underexpansion: the multicenter IVL-DRAGON registry. J Clin Med. 2022;11(7):1779–1785. doi: 10.3390/jcm11071779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Holmes D.R., Jr., Kereiakes D.J., Garg S., et al. Stent thrombosis. J Am Coll Cardiol. 2010;56(17):1357–1365. doi: 10.1016/j.jacc.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 90.Wiviott S.D., Braunwald E., McCabe C.H., et al. Intensive oral antiplatelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary syndromes treated with percutaneous coronary intervention and stenting in the TRITON-TIMI 38 trial: a subanalysis of a randomised trial. Lancet. 2008;371(9621):1353–1363. doi: 10.1016/S0140-6736(08)60422-5. [DOI] [PubMed] [Google Scholar]
- 91.Mauri L., Hsieh W.H., Massaro J.M., Ho K.K., D’Agostino R., Cutlip D.E. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007;356(10):1020–1029. doi: 10.1056/NEJMoa067731. [DOI] [PubMed] [Google Scholar]
- 92.Kirtane A.J., Stone G.W. How to minimize stent thrombosis. Circulation. 2011;124(11):1283–1287. doi: 10.1161/CIRCULATIONAHA.110.976829. [DOI] [PubMed] [Google Scholar]
- 93.Dangas G.D., Caixeta A., Mehran R., et al. Frequency and predictors of stent thrombosis after percutaneous coronary intervention in acute myocardial infarction. Circulation. 2011;123(16):1745–1756. doi: 10.1161/CIRCULATIONAHA.110.981688. [DOI] [PubMed] [Google Scholar]
- 94.Dangas G.D., Claessen B.E., Mehran R., Xu K., Stone G.W. Stent thrombosis after primary angioplasty for STEMI in relation to non-adherence to dual antiplatelet therapy over time: results of the HORIZONS-AMI trial. EuroIntervention. 2013;8(9):1033–1039. doi: 10.4244/EIJV8I9A159. [DOI] [PubMed] [Google Scholar]
- 95.Sabaté M., Brugaletta S., Cequier A., et al. Clinical outcomes in patients with ST-segment elevation myocardial infarction treated with everolimus-eluting stents versus bare-metal stents (EXAMINATION): 5-year results of a randomised trial. Lancet. 2016;387(10016):357–366. doi: 10.1016/S0140-6736(15)00548-6. [DOI] [PubMed] [Google Scholar]
- 96.Cutlip D.E., Windecker S., Mehran R., et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115(17):2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 97.Cutlip D.E., Nakazawa G., Krucoff M.W., et al. Autopsy validation study of the academic research consortium stent thrombosis definition. J Am Coll Cardiol Intv. 2011;4(5):554–559. doi: 10.1016/j.jcin.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 98.Grewe P.H., Deneke T., Machraoui A., Barmeyer J., Müller K.M. Acute and chronic tissue response to coronary stent implantation: pathologic findings in human specimen. J Am Coll Cardiol. 2000;35(1):157–163. doi: 10.1016/s0735-1097(99)00486-6. [DOI] [PubMed] [Google Scholar]
- 99.Iakovou I., Schmidt T., Bonizzoni E., et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293(17):2126–2130. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- 100.Généreux P., Rutledge D.R., Palmerini T., et al. Stent thrombosis and dual antiplatelet therapy interruption with everolimus-eluting stents: insights from the Xience V Coronary Stent System trials. Circ Cardiovasc Interv. 2015;8(5) doi: 10.1161/CIRCINTERVENTIONS.114.001362. [DOI] [PubMed] [Google Scholar]
- 101.Uren N.G., Schwarzacher S.P., Metz J.A., et al. Predictors and outcomes of stent thrombosis: an intravascular ultrasound registry. Eur Heart J. 2002;23(2):124–132. doi: 10.1053/euhj.2001.2707. [DOI] [PubMed] [Google Scholar]
- 102.Alfonso F., Suárez A., Angiolillo D.J., et al. Findings of intravascular ultrasound during acute stent thrombosis. Heart. 2004;90(12):1455–1459. doi: 10.1136/hrt.2003.026047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Joner M., Nakazawa G., Finn A.V., et al. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J Am Coll Cardiol. 2008;52(5):333–342. doi: 10.1016/j.jacc.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 104.Généreux P., Stone G.W., Harrington R.A., et al. Impact of intraprocedural stent thrombosis during percutaneous coronary intervention: insights from the CHAMPION PHOENIX Trial (clinical trial comparing cangrelor to clopidogrel standard of care therapy in subjects who require percutaneous coronary intervention) J Am Coll Cardiol. 2014;63(7):619–629. doi: 10.1016/j.jacc.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 105.Aoki J., Lansky A.J., Mehran R., et al. Early stent thrombosis in patients with acute coronary syndromes treated with drug-eluting and bare metal stents: the Acute Catheterization and Urgent Intervention Triage Strategy trial. Circulation. 2009;119(5):687–698. doi: 10.1161/CIRCULATIONAHA.108.804203. [DOI] [PubMed] [Google Scholar]
- 106.Husted S., Boersma E. Case study: ticagrelor in PLATO and prasugrel in TRITON-TIMI 38 and TRILOGY-ACS trials in patients with acute coronary syndromes. Am J Ther. 2016;23(6):e1876–e1889. doi: 10.1097/MJT.0000000000000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gurbel P.A., Bliden K.P., Guyer K., et al. Platelet reactivity in patients and recurrent events post-stenting: results of the PREPARE POST-STENTING study. J Am Coll Cardiol. 2005;46(10):1820–1826. doi: 10.1016/j.jacc.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 108.Gurbel P.A., Bliden K.P., Samara W., et al. Clopidogrel effect on platelet reactivity in patients with stent thrombosis: results of the CREST study. J Am Coll Cardiol. 2005;46(10):1827–1832. doi: 10.1016/j.jacc.2005.07.056. [DOI] [PubMed] [Google Scholar]
- 109.Wenaweser P., Dörffler-Melly J., Imboden K., et al. Stent thrombosis is associated with an impaired response to antiplatelet therapy. J Am Coll Cardiol. 2005;45(11):1748–1752. doi: 10.1016/j.jacc.2005.01.058. [DOI] [PubMed] [Google Scholar]
- 110.Pfisterer M., Brunner-La Rocca H.P., Buser P.T., et al. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J Am Coll Cardiol. 2006;48(12):2584–2591. doi: 10.1016/j.jacc.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 111.Eisenstein E.L., Anstrom K.J., Kong D.F., et al. Clopidogrel use and long-term clinical outcomes after drug-eluting stent implantation. JAMA. 2007;297(2):159–168. doi: 10.1001/jama.297.2.joc60179. [DOI] [PubMed] [Google Scholar]
- 112.Schulz S., Schuster T., Mehilli J., et al. Stent thrombosis after drug-eluting stent implantation: incidence, timing, and relation to discontinuation of clopidogrel therapy over a 4-year period. Eur Heart J. 2009;30(22):2714–2721. doi: 10.1093/eurheartj/ehp275. [DOI] [PubMed] [Google Scholar]
- 113.Mehran R., Baber U., Sharma S.K., et al. Ticagrelor with or without aspirin in high-risk patients after PCI. N Engl J Med. 2019;381(21):2032–2042. doi: 10.1056/NEJMoa1908419. [DOI] [PubMed] [Google Scholar]
- 114.Kandzari D.E., Kirtane A.J., Windecker S., et al. One-month dual antiplatelet therapy following percutaneous coronary intervention with zotarolimus-eluting stents in high-bleeding-risk patients. Circ Cardiovasc Interv. 2020;13(11) doi: 10.1161/CIRCINTERVENTIONS.120.009565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gomez-Lara J., Salvatella N., Gonzalo N., et al. IVUS-guided treatment strategies for definite late and very late stent thrombosis. EuroIntervention. 2016;12(11):e1355–e1365. doi: 10.4244/EIJY15M12_08. [DOI] [PubMed] [Google Scholar]
- 116.Cook S., Ladich E., Nakazawa G., et al. Correlation of intravascular ultrasound findings with histopathological analysis of thrombus aspirates in patients with very late drug-eluting stent thrombosis. Circulation. 2009;120(5):391–399. doi: 10.1161/CIRCULATIONAHA.109.854398. [DOI] [PubMed] [Google Scholar]
- 117.Van Werkum J.W., Heestermans A.A., Zomer A.C., et al. Predictors of coronary stent thrombosis: the Dutch Stent Thrombosis Registry. J Am Coll Cardiol. 2009;53(16):1399–1409. doi: 10.1016/j.jacc.2008.12.055. [DOI] [PubMed] [Google Scholar]
- 118.Pesarini G., Dandale R., Rigamonti A., et al. Late and very late coronary stent thrombosis: intravascular ultrasound findings and associations with antiplatelet therapy. Catheter Cardiovasc Interv. 2013;82(7):1056–1065. doi: 10.1002/ccd.24938. [DOI] [PubMed] [Google Scholar]
- 119.Kang S.J., Lee C.W., Song H., et al. OCT analysis in patients with very late stent thrombosis. J Am Coll Cardiol Img. 2013;6(6):695–703. doi: 10.1016/j.jcmg.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 120.Murata A., Wallace-Bradley D., Tellez A., et al. Accuracy of optical coherence tomography in the evaluation of neointimal coverage after stent implantation. J Am Coll Cardiol Img. 2010;3(1):76–84. doi: 10.1016/j.jcmg.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 121.Farb A., Sangiorgi G., Carter A.J., et al. Pathology of acute and chronic coronary stenting in humans. Circulation. 1999;99(1):44–52. doi: 10.1161/01.cir.99.1.44. [DOI] [PubMed] [Google Scholar]
- 122.Cheneau E., Leborgne L., Mintz G.S., et al. Predictors of subacute stent thrombosis: results of a systematic intravascular ultrasound study. Circulation. 2003;108(1):43–47. doi: 10.1161/01.CIR.0000078636.71728.40. [DOI] [PubMed] [Google Scholar]
- 123.Windecker S., Meier B. Late coronary stent thrombosis. Circulation. 2007;116(17):1952–1965. doi: 10.1161/CIRCULATIONAHA.106.683995. [DOI] [PubMed] [Google Scholar]
- 124.Madhavan M.V., Kirtane A.J., Redfors B., et al. Stent-related adverse events >1 year after percutaneous coronary intervention. J Am Coll Cardiol. 2020;75(6):590–604. doi: 10.1016/j.jacc.2019.11.058. [DOI] [PubMed] [Google Scholar]
- 125.Franchin L., Kang J., De Filippo O., et al. Incidence and predictors of stent thrombosis in patients treated with stents for coronary bifurcation narrowing (from the BIFURCAT registry) Am J Cardiol. 2021;156:24–31. doi: 10.1016/j.amjcard.2021.06.031. [DOI] [PubMed] [Google Scholar]
- 126.Prati F., Di Vito L., Biondi-Zocca G., et al. Angiography alone versus angiography plus optical coherence tomography to guide decision-making during percutaneous coronary intervention: the Centro per la Lotta contro l’Infarto-Optimisation of Percutaneous Coronary Intervention (CLI-OPCI) study. EuroIntervention. 2012;8(7):823–829. doi: 10.4244/EIJV8I7A125. [DOI] [PubMed] [Google Scholar]
- 127.Taniwaki M., Radu M.D., Zaugg S., et al. Mechanisms of very late drug-eluting stent thrombosis assessed by optical coherence tomography. Circulation. 2016;133(7):650–660. doi: 10.1161/CIRCULATIONAHA.115.019071. [DOI] [PubMed] [Google Scholar]
- 128.Zhang Y., Farooq V., Garcia-Garcia H.M., et al. Comparison of intravascular ultrasound versus angiography-guided drug-eluting stent implantation: a meta-analysis of one randomised trial and ten observational studies involving 19,619 patients. EuroIntervention. 2012;8(7):855–865. doi: 10.4244/EIJV8I7A129. [DOI] [PubMed] [Google Scholar]
- 129.Witzenbichler B., Maehara A., Weisz G., et al. TCT-21 Use of IVUS reduces stent thrombosis: results from the prospective, multicenter ADAPT-DES study. J Am Coll Cardiol. 2012;60(17):B6. (suppl B):B6. [Google Scholar]
- 130.Gao X.F., Ge Z., Kong X.Q., et al. 3-year outcomes of the ULTIMATE trial comparing intravascular ultrasound versus angiography-guided drug-eluting stent implantation. J Am Coll Cardiol Intv. 2021;14(3):247–257. doi: 10.1016/j.jcin.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 131.Ali Z.A., Maehara A., Généreux P., et al. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomised controlled trial. Lancet. 2016;388(10060):2618–2628. doi: 10.1016/S0140-6736(16)31922-5. [DOI] [PubMed] [Google Scholar]
- 132.Adriaenssens T., Joner M., Godschalk T.C., et al. Optical coherence tomography findings in patients with coronary stent thrombosis: a report of the PRESTIGE consortium (prevention of late stent thrombosis by an interdisciplinary global European effort) Circulation. 2017;136(11):1007–1021. doi: 10.1161/CIRCULATIONAHA.117.026788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Otake H., Kubo T., Takahasi H., et al. Optical frequency domain imaging versus intravascular ultrasound in percutaneous coronary intervention (OPINION trial): results from the OPINION imaging study. J Am Coll Cardiol Img. 2018;11(1):111–123. doi: 10.1016/j.jcmg.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 134.Capodanno D., Alfonso F., Levine G.N., Valgimigli M., Angiolillo D.J. ACC/AHA versus ESC guidelines on dual antiplatelet therapy: JACC guideline comparison. J Am Coll Cardiol. 2018;72(23 Pt A):2915–2931. doi: 10.1016/j.jacc.2018.09.057. [DOI] [PubMed] [Google Scholar]
- 135.Jolly S.S., James S., Džavík V., et al. Thrombus aspiration in ST-segment–elevation myocardial infarction: an individual patient meta-analysis: Thrombectomy Trialists Collaboration. Circulation. 2017;135(2):143–152. doi: 10.1161/CIRCULATIONAHA.116.025371. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.