Abstract
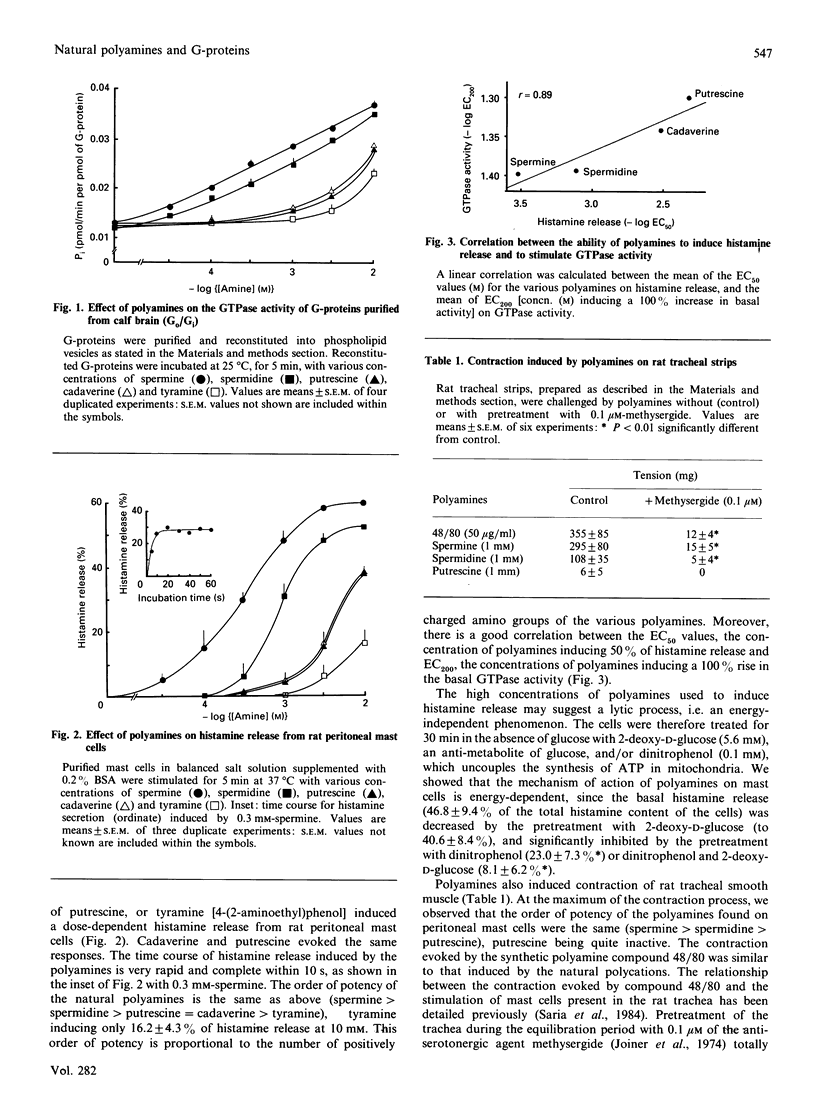
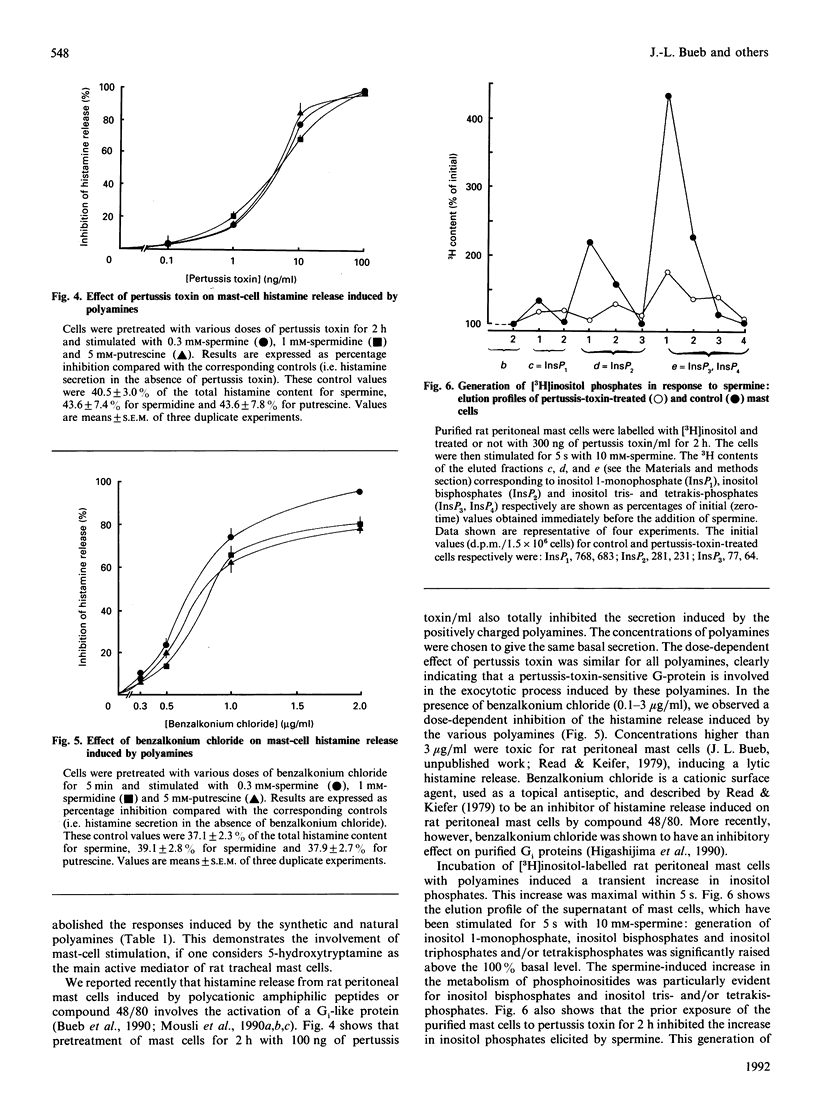
The natural polyamines spermine and spermidine, the biosynthetic precursor putrescine and their analogues cadaverine and tyramine stimulate the GTPase activity of purified GTP-binding proteins (Go/Gi) from calf brain reconstituted into phospholipid vesicles. The order of potency was spermine greater than spermidine greater than putrescine = cadaverine greater than tyramine. The physiological relevance of this observation was assessed, showing the same order of potency of polyamines in the stimulation of peritoneal and tracheal rat mast cells. The activation of rat mast cells by polyamines was inhibited by benzalkonium chloride or by a 2 h pretreatment of the cells with pertussis toxin. The increase in inositol phosphates evoked by polyamines was also inhibited by pertussis toxin. Therefore we propose that intracellular polyamines might control the basal level of second messengers and modulate extracellular signals transduced through G-protein-coupled receptors.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aridor M., Traub L. M., Sagi-Eisenberg R. Exocytosis in mast cells by basic secretagogues: evidence for direct activation of GTP-binding proteins. J Cell Biol. 1990 Sep;111(3):909–917. doi: 10.1083/jcb.111.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowlin T. L., Hoeper B. J., Rosenberger A. L., Davis G. F., Sunkara P. S. Effects of three irreversible inhibitors of ornithine decarboxylase on macrophage-mediated tumoricidal activity and antitumor activity in B16F1 tumor-bearing mice. Cancer Res. 1990 Aug 1;50(15):4510–4514. [PubMed] [Google Scholar]
- Brabet P., Pantaloni C., Rouot B., Toutant M., Garcia-Sainz A., Bockaert J., Homburger V. Multiple species and isoforms of Bordetella pertussis toxin substrates. Biochem Biophys Res Commun. 1988 May 16;152(3):1185–1192. doi: 10.1016/s0006-291x(88)80410-8. [DOI] [PubMed] [Google Scholar]
- Brandt D. R., Asano T., Pedersen S. E., Ross E. M. Reconstitution of catecholamine-stimulated guanosinetriphosphatase activity. Biochemistry. 1983 Sep 13;22(19):4357–4362. doi: 10.1021/bi00288a002. [DOI] [PubMed] [Google Scholar]
- Bueb J. L., Mousli M., Bronner C., Rouot B., Landry Y. Activation of Gi-like proteins, a receptor-independent effect of kinins in mast cells. Mol Pharmacol. 1990 Dec;38(6):816–822. [PubMed] [Google Scholar]
- Charlton J. A., Baylis P. H. Stimulation of Na+/K(+)-ATPase activity by polyamines in the rat renal medullary cells of the thick ascending limb of Henle's loop. J Endocrinol. 1990 Dec;127(3):377–382. doi: 10.1677/joe.0.1270377. [DOI] [PubMed] [Google Scholar]
- Clô C., Caldarera C. M., Tantini B., Benalal D., Bachrach U. Polyamines and cellular adenosine 3' :5'-cyclic monophosphate. Biochem J. 1979 Sep 15;182(3):641–649. doi: 10.1042/bj1820641a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochet C., Chambaz E. M. Polyamine-mediated protein phosphorylations: a possible target for intracellular polyamine action. Mol Cell Endocrinol. 1983 Jun;30(3):247–266. doi: 10.1016/0303-7207(83)90062-x. [DOI] [PubMed] [Google Scholar]
- Dempsey R. J., Maley B. E., Cowen D., Olson J. W. Ornithine decarboxylase activity and immunohistochemical location in postischemic brain. J Cereb Blood Flow Metab. 1988 Dec;8(6):843–847. doi: 10.1038/jcbfm.1988.141. [DOI] [PubMed] [Google Scholar]
- Fan C. C., Koenig H. The role of polyamines in beta-adrenergic stimulation of calcium influx and membrane transport in rat heart. J Mol Cell Cardiol. 1988 Sep;20(9):789–799. doi: 10.1016/s0022-2828(88)80004-x. [DOI] [PubMed] [Google Scholar]
- Frossard N., Muller F. Epithelial modulation of tracheal smooth muscle response to antigenic stimulation. J Appl Physiol (1985) 1986 Oct;61(4):1449–1456. doi: 10.1152/jappl.1986.61.4.1449. [DOI] [PubMed] [Google Scholar]
- Higashijima T., Burnier J., Ross E. M. Regulation of Gi and Go by mastoparan, related amphiphilic peptides, and hydrophobic amines. Mechanism and structural determinants of activity. J Biol Chem. 1990 Aug 25;265(24):14176–14186. [PubMed] [Google Scholar]
- Higashijima T., Ferguson K. M., Smigel M. D., Gilman A. G. The effect of GTP and Mg2+ on the GTPase activity and the fluorescent properties of Go. J Biol Chem. 1987 Jan 15;262(2):757–761. [PubMed] [Google Scholar]
- Higashijima T., Uzu S., Nakajima T., Ross E. M. Mastoparan, a peptide toxin from wasp venom, mimics receptors by activating GTP-binding regulatory proteins (G proteins). J Biol Chem. 1988 May 15;263(14):6491–6494. [PubMed] [Google Scholar]
- Homburger V., Brabet P., Audigier Y., Pantaloni C., Bockaert J., Rouot B. Immunological localization of the GTP-binding protein Go in different tissues of vertebrates and invertebrates. Mol Pharmacol. 1987 Apr;31(4):313–319. [PubMed] [Google Scholar]
- Joiner P. D., Wall M., Davis L. B., Hahn F. Role of amines in anaphylactic contraction of guinea pig isolated smooth muscle. J Allergy Clin Immunol. 1974 May;53(5):261–270. doi: 10.1016/0091-6749(74)90104-3. [DOI] [PubMed] [Google Scholar]
- Khan N. A., Quemener V., Moulinoux P. Inhibition of adenylate cyclase activity by polyamines in human erythrocyte plasma membranes. Life Sci. 1990;46(1):43–47. doi: 10.1016/0024-3205(90)90055-v. [DOI] [PubMed] [Google Scholar]
- Koenig H., Goldstone A., Lu C. Y. Polyamines regulate calcium fluxes in a rapid plasma membrane response. Nature. 1983 Oct 6;305(5934):530–534. doi: 10.1038/305530a0. [DOI] [PubMed] [Google Scholar]
- Löser C., Fölsch U. R., Paprotny C., Creutzfeldt W. Polyamines in human gastric and esophageal cancer. Scand J Gastroenterol. 1989 Dec;24(10):1193–1199. doi: 10.3109/00365528909090786. [DOI] [PubMed] [Google Scholar]
- McCormack J. G. Effects of spermine on mitochondrial Ca2+ transport and the ranges of extramitochondrial Ca2+ to which the matrix Ca2+-sensitive dehydrogenases respond. Biochem J. 1989 Nov 15;264(1):167–174. doi: 10.1042/bj2640167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousli M., Bronner C., Bockaert J., Rouot B., Landry Y. Interaction of substance P, compound 48/80 and mastoparan with the alpha-subunit C-terminus of G protein. Immunol Lett. 1990 Sep;25(4):355–357. doi: 10.1016/0165-2478(90)90207-7. [DOI] [PubMed] [Google Scholar]
- Mousli M., Bronner C., Bueb J. L., Tschirhart E., Gies J. P., Landry Y. Activation of rat peritoneal mast cells by substance P and mastoparan. J Pharmacol Exp Ther. 1989 Jul;250(1):329–335. [PubMed] [Google Scholar]
- Mousli M., Bronner C., Landry Y., Bockaert J., Rouot B. Direct activation of GTP-binding regulatory proteins (G-proteins) by substance P and compound 48/80. FEBS Lett. 1990 Jan 1;259(2):260–262. doi: 10.1016/0014-5793(90)80023-c. [DOI] [PubMed] [Google Scholar]
- Mousli M., Bueb J. L., Bronner C., Rouot B., Landry Y. G protein activation: a receptor-independent mode of action for cationic amphiphilic neuropeptides and venom peptides. Trends Pharmacol Sci. 1990 Sep;11(9):358–362. doi: 10.1016/0165-6147(90)90179-c. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Ui M. Simultaneous inhibitions of inositol phospholipid breakdown, arachidonic acid release, and histamine secretion in mast cells by islet-activating protein, pertussis toxin. A possible involvement of the toxin-specific substrate in the Ca2+-mobilizing receptor-mediated biosignaling system. J Biol Chem. 1985 Mar 25;260(6):3584–3593. [PubMed] [Google Scholar]
- Paschen W., Röhn G., Meese C. O., Djuricic B., Schmidt-Kastner R. Polyamine metabolism in reversible cerebral ischemia: effect of alpha-difluoromethylornithine. Brain Res. 1988 Jun 21;453(1-2):9–16. doi: 10.1016/0006-8993(88)90138-2. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., McCann P. P. Polyamine metabolism and function. Am J Physiol. 1982 Nov;243(5):C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- Read G. W., Kiefer E. F. Benzalkonium chloride: selective inhibitor of histamine release induced by compound 48/80 and other polyamines. J Pharmacol Exp Ther. 1979 Dec;211(3):711–715. [PubMed] [Google Scholar]
- SHORE P. A., BURKHALTER A., COHN V. H., Jr A method for the fluorometric assay of histamine in tissues. J Pharmacol Exp Ther. 1959 Nov;127:182–186. [PubMed] [Google Scholar]
- Sacaan A. I., Johnson K. M. Spermine enhances binding to the glycine site associated with the N-methyl-D-aspartate receptor complex. Mol Pharmacol. 1989 Dec;36(6):836–839. [PubMed] [Google Scholar]
- Saria A., Hua X., Skofitsch G., Lundberg J. M. Inhibition of compound 48/80--induced vascular protein leakage by pretreatment with capsaicin and a substance P antagonist. Naunyn Schmiedebergs Arch Pharmacol. 1984 Nov;328(1):9–15. doi: 10.1007/BF00496097. [DOI] [PubMed] [Google Scholar]
- Schuber F. Influence of polyamines on membrane functions. Biochem J. 1989 May 15;260(1):1–10. doi: 10.1042/bj2600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler N. Polyamine metabolism. Digestion. 1990;46 (Suppl 2):319–330. doi: 10.1159/000200405. [DOI] [PubMed] [Google Scholar]
- Sternweis P. C., Robishaw J. D. Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. J Biol Chem. 1984 Nov 25;259(22):13806–13813. [PubMed] [Google Scholar]
- Sternweis P. C. The purified alpha subunits of Go and Gi from bovine brain require beta gamma for association with phospholipid vesicles. J Biol Chem. 1986 Jan 15;261(2):631–637. [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines in microorganisms. Microbiol Rev. 1985 Mar;49(1):81–99. doi: 10.1128/mr.49.1.81-99.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Tomita U., Inanobe A., Kobayashi I., Takahashi K., Ui M., Katada T. Direct interactions of mastoparan and compound 48/80 with GTP-binding proteins. J Biochem. 1991 Jan;109(1):184–189. doi: 10.1093/oxfordjournals.jbchem.a123342. [DOI] [PubMed] [Google Scholar]
- Walz F. G., Jr, Kitareewan S. Spermine stabilization of folded ribonuclease T1. J Biol Chem. 1990 May 5;265(13):7127–7137. [PubMed] [Google Scholar]
- Wang J. Y., Johnson L. R. Luminal polyamines stimulate repair of gastric mucosal stress ulcers. Am J Physiol. 1990 Oct;259(4 Pt 1):G584–G592. doi: 10.1152/ajpgi.1990.259.4.G584. [DOI] [PubMed] [Google Scholar]
- Welsh N. A role for polyamines in glucose-stimulated insulin-gene expression. Biochem J. 1990 Oct 15;271(2):393–397. doi: 10.1042/bj2710393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K., Romano C., Dichter M. A., Molinoff P. B. Modulation of the NMDA receptor by polyamines. Life Sci. 1991;48(6):469–498. doi: 10.1016/0024-3205(91)90463-l. [DOI] [PubMed] [Google Scholar]


