Abstract
Background
The Synotis (C. B. Clarke) C. Jeffrey & Y. L. Chen is an ecologically important genus of the tribe Senecioneae, family Asteraceae. Because most species of the genus bear similar morphology, traditional morphological identification methods are very difficult to discriminate them. Therefore, it is essential to develop a reliable and effective identification method for Synotis species. In this study, the complete chloroplast (cp.) genomes of four Synotis species, S. cavaleriei (H.Lév.) C. Jeffrey & Y.L. Chen, S. duclouxii (Dunn) C. Jeffrey & Y.L. Chen, S. nagensium (C.B. Clarke) C. Jeffrey & Y.L. Chen and S. erythropappa (Bureau & Franch.) C. Jeffrey & Y. L. Chen had been sequenced using next-generation sequencing technology and reported here.
Results
These four cp. genomes exhibited a typical quadripartite structure and contained the large single-copy regions (LSC, 83,288 to 83,399 bp), the small single-copy regions (SSC, 18,262 to 18,287 bp), and the inverted repeat regions (IR, 24,837 to 24,842 bp). Each of the four cp. genomes encoded 134 genes, including 87 protein-coding genes, 37 tRNA genes, 8 rRNA genes, and 2 pseudogenes (ycf1 and rps19). The highly variable regions (trnC-GCA-petN, ccsA-psaC, trnE-UUC-rpoB, ycf1, ccsA and petN) may be used as potential molecular barcodes. The complete cp. genomes sequence of Synotis could be used as the potentially effective super-barcode to accurately identify Synotis species. Phylogenetic analysis demonstrated that the four Synotis species were clustered into a monophyletic group, and they were closed to the Senecio, Crassocephalum and Dendrosenecio in tribe Senecioneae.
Conclusions
This study will be useful for further species identification, evolution, genetic diversity and phylogenetic studies within this genus Synotis and the tribe Senecioneae.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12864-024-10663-x.
Keywords: Synotis, Chloroplast genome, Super-barcode, Comparative analysis, Phylogenetic analysis
Background
The genus Synotis (C.B. Clarke) C. Jeffrey & Y.L. Chen belongs to tribe Senecioneae of the family Asteraceae. It typically comprises subshrubs and perennial herbs. The leaves of Synotis are simple, either petiolate or sessile, with broadly ovate-cordate to narrowly oblong-lanceolate plates. Its capitula are heterogamous and radiate, always arranged in terminal or axillary [1]. It consists of approximately 60 species, mainly distributed in Bhutan, China, India, Myanmar, Nepal, Thailand, and Vietnam [1–13]. China, the diversity center of Synotis, harbors approximately 50 species, and approximately 30 species are endemic [6–9, 11–13]. The genus was separated from Senecio L. mainly because of its conspicuously caudate anthers [14]. Tang identified five series within Synotis, including ser. Synotis, ser. Erectae (C. B. Clarke) C. Jeffrey & Y. L. Chen, ser. Oliganthae (J. F. Jeffrey) C. Jeffrey & Y. L. Chen, ser. Fulvipapposae C. Jeffrey & Y. L. Chen, and ser. Hieraciifoliae M. Tang & Q. E. Yang [6]. Most species in this genus exhibit relatively similar morphological variations, making it challenging to distinguish them using traditional identification methods.
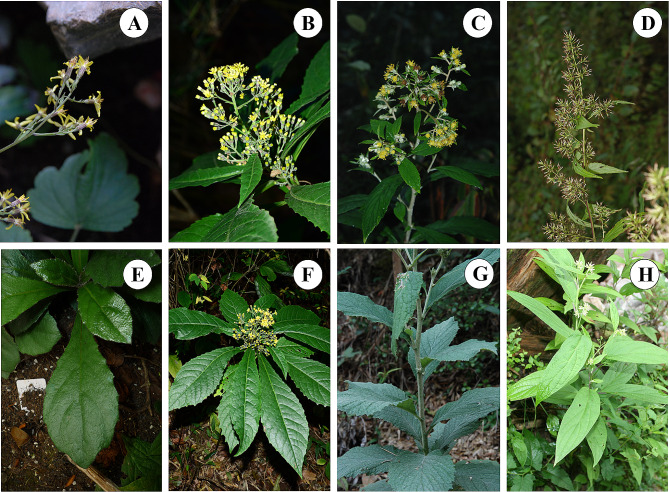
During our fieldwork in southwestern China, the materials of four species of Synotis, including S. cavaleriei (ser. Hieraciifoliae), S. duclouxii (ser. Hieraciifoliae), S. nagensium (ser. Erectae) and S. erythropappa (ser. Oliganthae) were collected. Among these species, S. nagensium is mainly distributed in China (Chongqing, Gansu, Guangdong, Guangxi, Guizhou, Hubei, Hunan, Sichuan, Xizang, Yunnan provinces), as well as in India, Nepal, Bhutan, Myanmar, Thailand and Vietnam [1, 11]. The other three species are endemic to China [1], of which S. cavaleriei is mainly distributed in Guizhou, Sichuan, and Yunnan provinces [1, 6, 8], S. duclouxii is found mainly in Sichuan and Yunnan provinces [1, 8], and S. erythropappa mainly occurs in Guizhou, Hubei, Sichuan, Xizang, and Yunnan provinces [1]. These four species often grow in mixed forests, woods, thickets and meadows [1]. They play a crucial role in ecological restoration and soil and water conservation efforts. The species of S. nagensium has disciform capitula and paniculoid thyrses, whereas S. erythropappa has broadly pyramidal compound thyrses, which two are easily distinguished from each other. However, the other two species bear the same habit, leaf and inflorescence shape, making them difficult to distinguish by traditional identification (Fig. 1).
Fig. 1.
Images of Synotis cavaleriei, S. duclouxii, S. nagensium, and S. erythropappa. (A, B,C, D) Inflorescence of plant growing in natural habitat; (E, F,G, H) morphology of leaf. ((A, E) S. cavaleriei, voucher LXF0170, SCNU, Butuo county, Liangshan prefecture, Sichuan province, China). ((B, F) S. duclouxii, voucher LXF0219, SCNU, Butuo county, Liangshan prefecture, Sichuan province, China). ((C, G) S. nagensium, voucher FZX5549, SCNU, Tongchuan District, Dazhou City, Sichuan Province, China). ((D, H) S. erythropappa, voucher FZX1038, SCNU, Puge county, Liangshan prefecture, Sichuan Province, China). All photographs by Ming Tang except B and F provides by Yi Yang
The chloroplast (cp.) is a vital organelle in green plants. It converts light energy to chemical energy through photosynthesis [15]. The chloroplast contains its own genomes [16]. In general, a typical cp. genome of the family Asteraceae contains a quadripartite architecture, with two inverted repeat regions (IRs) that separate a large single-copy region (LSC) and a small single-copy region (SSC) [17–19]. The cp. genomes generally contain 100–120 genes with lengths of 120–160 kb [15, 20, 21]. Compared with the partial cp. genes and nuclear genes, the complete cp. genomes exhibit unique characteristics, such as conserved genetics, slow evolution, maternal inheritance, and numerous mutation site information [18, 19, 22]. Consequently, the complete cp. genomes have become an efficient tool for phylogenetic studies within the family Asteraceae [23, 24] and serve as a super-barcode for distinguishing species [25–29]. The advent of high-throughput sequencing has facilitated the reporting of several cp. genomes within the Asteraceae family, including Artemisia L. [13, 18, 22, 30], Aster L. [31–36], Cavea W. W. Smith and J. Small [24], Conyza L. [26, 37], Chrysanthemum L. [38], Crepidiastrum Nakai [39], Dolomiaea DC. [40], Erigeron L. [41], Gynura Cass. [42], Heteroplexis C.C.Chang [43], Ligularia Cass. [25], Nouelia Franch. [44], Saussurea DC. [17, 45], Sinosenecio B. Nordenstam [19], Xanthium L. [46]. These studies have contributed to enhancing our understanding of the cp. genome characteristics and evolutionary relationships within the Asteraceae family.
With the rapid development of molecular technology, external transcribed spacers (ETS) [47–50], internal transcribed spacers (ITS) [47–51] and cp. fragments, such as matK-trnK-rpS16, rpS16-trnQ-psbK have been employed to determine the molecular phylogeny of Synotis [6]. However, the complete cp. genomes of genus Synotis have not yet been reported. Compared to nuclear markers and partial cp. genes, the complete cp. genomes possess highly conserved DNA sequences and many genetic information. Therefore, analyzing the complete cp. genomes may be an effective approach to solve the problem of the evolutionary relationships and taxonomic identification of species [18, 19, 22]. Meanwhile, with advances in next-generation sequencing technology, it has recently become quicker, cheaper and easier to achieve the complete plastomes than before [52–54]. Therefore, it is crucial to obtain the complete cp. genomes of Synotis for reconstructing phylogenetic relationships and species identification.
In this study, the complete cp. genomes of Synotis cavaleriei, S. duclouxii, S. nagensium, and S. erythropappa were sequenced using Illumina technology, and their gene features were characterized. The aims of this study were to: (1) assemble, annotate, and conduct a comparative analysis of the complete cp. genomes of four species of Synotis, (2) identify similarities and differences in the structural characteristics of the cp. genomes between Synotis and its related species, (3) reconstruct phylogenetic relationships among Synotis species and its related species, (4) utilize the complete cp. genomes as a super-barcode for the identification of Synotis species. These results will provide valuable insights into the DNA barcoding and phylogenetics of genus Synotis.
Results
Cp genome features of the four Synotis species
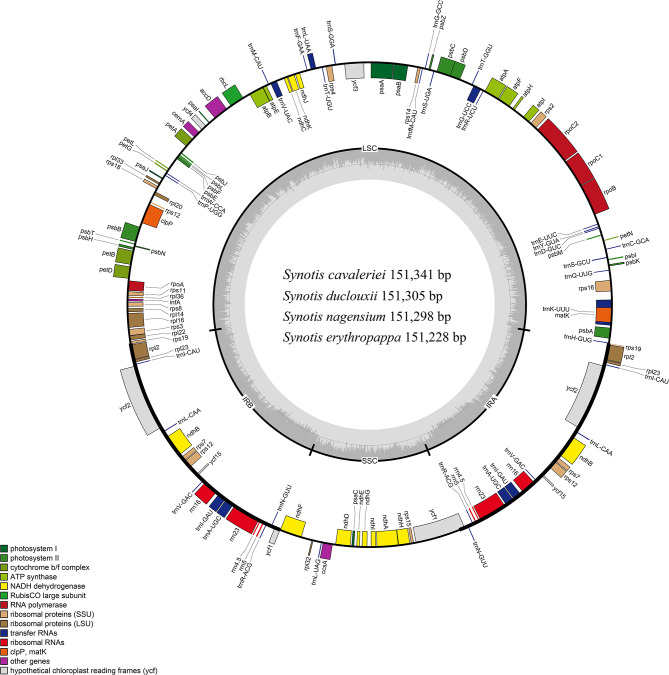
The gene map of the circular complete cp. genomes of four Synotis species is shown in Fig. 2. The GenBank numbers, GC content, gene numbers, and lengths of cp. genomes are given in Table 1. The cp. genome lengths of S. cavaleriei, S. duclouxii, S. nagensium, and S. erythropappa were 151,341 bp, 151,305 bp, 151,298 bp, and 151,228 bp, respectively (Table 1). These cp. genomes had a typical quadripartite circular structure, containing a LSC region (83,288 to 83,399 bp), an SSC region (18,262 to 18,287 bp) and two IR regions (24,837 to 24,842 bp) (Table 1; Fig. 2). The overall GC content was 37.4%, with the IR regions displaying significantly higher GC content (43.0%) compared to the LSC region (35.6%) and SSC region (30.6–30.7%).
Fig. 2.
Gene maps of the cp. genomes of Synotis species. Genes inside of the circle are transcribed clockwise and those on the outside are transcribed counter-clockwise
Table 1.
The basic cp. Genomes information of four Synotis species
| Characteristics | S. cavaleriei | S. duclouxii | S. nagensium | S. erythropappa |
|---|---|---|---|---|
| GenBank accession | OM912601 | OM912602 | OM912603 | OQ985056 |
| Genome size (bp) | 151,341 | 151,305 | 151,298 | 151,228 |
| LSC size (bp) | 83,399 | 83,334 | 83,343 | 83,288 |
| SSC size (bp) | 18,268 | 18,287 | 18,277 | 18,262 |
| IRa/IRb size (bp) | 24,837 | 24,842 | 24,839 | 24,839 |
| Number of genes | 134 | 134 | 134 | 134 |
| Protein-coding genes | 87 | 87 | 87 | 87 |
| Pseudogene | 2 | 2 | 2 | 2 |
| tRNA genes | 37 | 37 | 37 | 37 |
| rRNA genes | 8 | 8 | 8 | 8 |
| Overall GC content (%) | 37.4 | 37.4 | 37.4 | 37.4 |
| GC content in LSC (%) | 35.6 | 35.6 | 35.6 | 35.6 |
| GC content in IRa/IRb (%) | 43.0 | 43.0 | 43.0 | 43.0 |
| GC content in SSC (%) | 30.6 | 30.6 | 30.7 | 30.6 |
Each of the four cp genomes contained 134 genes, including 87 protein-coding, 37 tRNA, 8 rRNA genes, and 2 pseudogenes (ycf1 and rps19, Table 1). The cp genes of the four Synotis species can be divided into four types: named photosynthesis-related genes, self-replication-related genes, other genes and genes of unknown function. Among these genes, 7 protein-coding genes (ndhB, rpl2, rpl23, rps12, rps7, ycf15, and ycf2), 7 tRNAs (trnA-UGC, trnI-CAU, trnI-GAU, trnL-CAA, trnN-GUU, trnR-ACG, and trnV-GAC), and all 4 rRNA genes (rrn16, rrn23, rrn4.5 and rrn5) were duplicated in the IR regions (Table 1). In total, there were 19 intron-containing genes (Table 1). 16 genes (ndhA, ndhB, petB, petD, atpF, rbcL, rpl16, rpl2, rps16, rpoC1, trnA-UGC, trnG-UCC, trnI-GAU, trnK-UUU, trnL-UAA and trnV-UAC) contained only 1 intron and 3 genes (clpP, rps12 and ycf3) contained 2 introns (Table 1). The rps12 gene was trans-splicing, with the 5’ end located in the LSC region and 3’ end located in the IR regions. The majority of protein coding genes had the standard ATG start codon, but some genes had alternative start codons, such as ACG in psbL and ndhD. Four cp genome sequences of the species of Synotis were submitted to NCBI (accession number: OM912601, OM912602, OM912603, OQ985056).
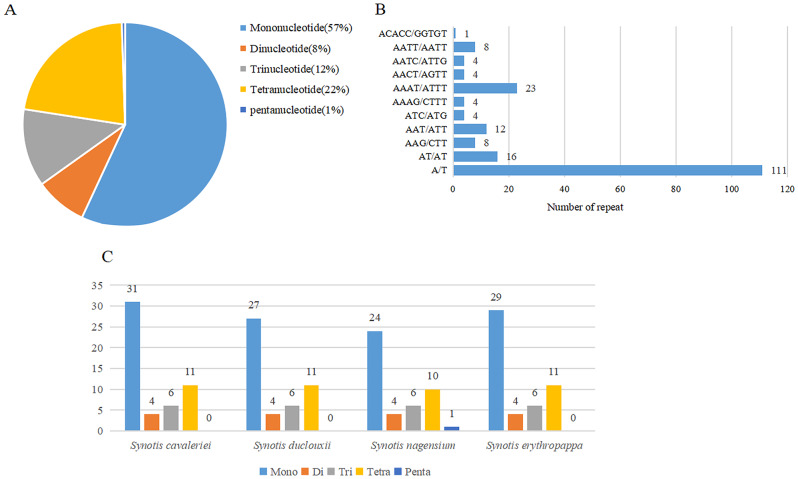
SSRs analysis
The number and types of SSRs were generally similar among the cp. genomes of four Synotis species (Fig. 3). The four sequenced plastomes contained 195 SSRs, including 111 Mononucleotide repeats (57%), 16 Dinucleotide repeats (8%), 24 Trinucleotide repeats (12%), 43 Tetranucleotide repeats (22%), and 1 Pentanucleotide repeats (1%) (Fig. 3A). The most common type of SSRs were Mononucleotide repeats composed of A/T bases (Fig. 3B). Specifically, there were 52 SSRs in S. cavaleriei, including 31 Mononucleotides, 4 Dinucleotides, 6 Trinucleotides, and 11 Tetranucleotides, 48 SSRs in S. duclouxii, including 27 Mononucleotides, 4 Dinucleotides, 6 Trinucleotides, and 11 Tetranucleotides, 45 SSRs in S. nagensium, including 24 Mononucleotides, 4 Dinucleotides, 6 Trinucleotides, 11 Tetranucleotides, and 1 Pentanucleotide, 50 SSRs in S. erythropappa, including 29 Mononucleotides, 4 Dinucleotides, 6 Trinucleotides, and 11 Tetranucleotides (Fig. 3C).
Fig. 3.
Analysis of SSRs in the four newly sequenced Synotis cp. genomes. (A) Frequencies of identified SSR types in all four plastomes, (B) Number of different SSR motifs, (C) Number of SSR repeat types
Structure comparison among the cp genomes of Synotis and its related species
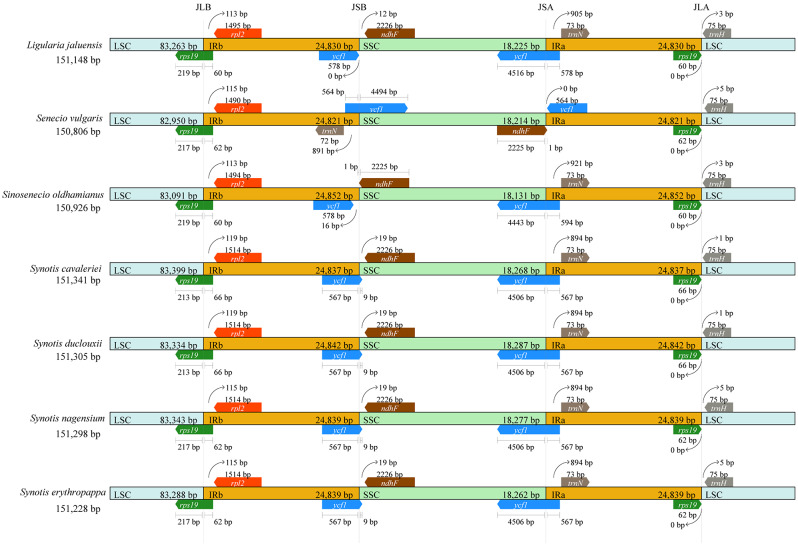
The boundaries of the IR/SC were comprehensively compared (Fig. 4) in four species of Synotis and their related species. The genes located at these boundaries included rps19, rpl2, ndhF, ycf1, and trnH genes. The length of the IR regions ranged from 24,821 to 24,852 bp in Synotis and its related species, with some degree of expansion. The cp. genomes of these species were relatively conserved, except for one major divergences in Senecio vulgaris. In the cp. genomes of Ligularia jaluensis, Sinosenecio oldhamianus, S. cavaleriei, S. duclouxii, S. nagensium and S. erythropappa, the ycf1 gene crossed the SSC/IRa boundary with the larger part located in the SSC region, and the ndhF gene located at the IRb/SSC boundary. However, in S. vulgaris, the ycf1 gene crossed the IRb/SSC boundary, with 4,494 bp located in SSC region, and the ndhF gene was near the SSC/IRa boundary. All species contained a functional copy of the rps19 gene at the LSC/IRb junction and a pseudo copy (rps19Ψ) at the IRa/LSC junction. The length of the rps19 gene was conserved at 279 bp. The rpl2 gene was present completely in the IRb region and was located away from the junction of LSC/IRb, with a length ranging from 113 to 119 bp. The trnH gene was commonly located at the LSC region, with variable gap lengths observed in the four species of Synotis and its related species.
Fig. 4.
Comparison of borders in the LSC, SSC and IR regions of the seven Senecioneae cp. genomes. Genes are denoted by colored boxes. The gaps between the genes and the boundaries are indicated by the base lengths
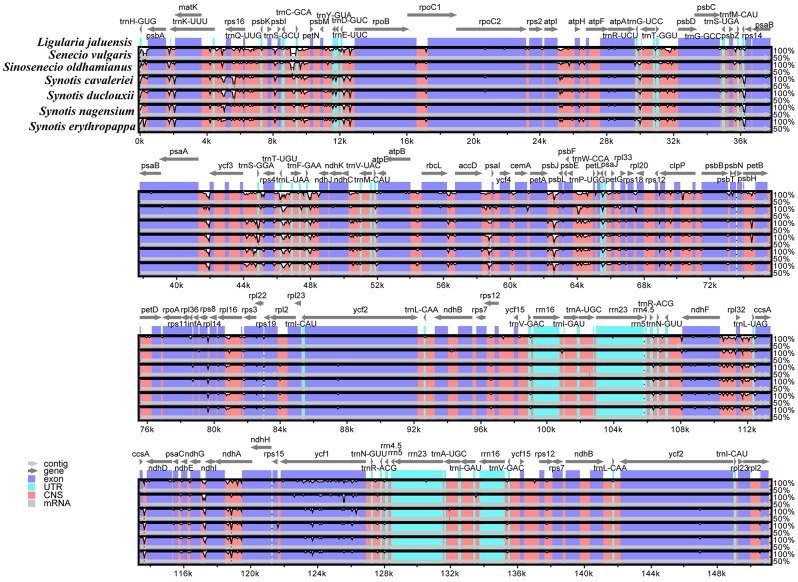
With reference to Ligularia jaluensis, the structural differences among cp. genomes of the four Synotis species and its related species were compared by mVISTA (Fig. 5). The LSC and SSC regions were more significantly divergent than the two IR regions. Additionally, the non-coding regions showed greater variability than the coding regions.
Fig. 5.
Sequence alignment of seven Senecioneae cp. genomes using the mVISTA program with Ligularia jaluensis as a reference. X-axis: the coordinates in the cp. genome, Y-axis: percent identity within 50–100%. The transcriptional direction of genes indicated by grey arrows
Codon usage patterns in Synotis cp genomes
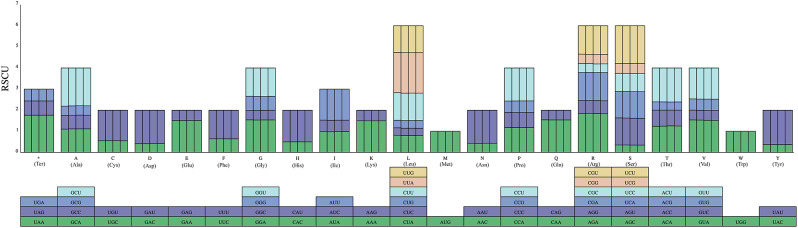
The RSCU values were calculated for the cp. genomes of Synotis cavaleriei, S. duclouxii, S. nagensium and S. erythropappa based on the protein-coding sequences (Table 2; Fig. 6). The total sequence size coding for protein genes was 77,616–78,381 bp in the cp. genomes of four species. These protein sequences encoded 22,424–22,679 codons. The cp. genomes of the four Synotis species contained 64 codons. Among these, 61 codons encoded 20 amino acids, and the other 3 were termination codons. Among these amino acids, Leucine was the most abundant amino acid in the cp. genomes of four Synotis species, whereas cysteine was the least (Table 2). The RSCU values of all codons are shown in Fig. 6. There were 32 codons with RSCU values less than 1 (RSCU < 1), which showed a lower usage frequency than expected. A total of 30 codons had RSCU values greater than 1 (RSCU > 1). Both methionine (Met) and tryptophan (Trp) exhibit no codon bias and have RSCU values of 1.
Table 2.
Codon usage and codon-anticodon recognition patterns of four Synotis species
| Amino acid | Symbol | Codon | Numbers and RSCU | |||
|---|---|---|---|---|---|---|
| S. cavaleriei | S. duclouxii | S.nagensium | S. erythropappa | |||
| * | Ter | UAA | 47/1.7625 | 47/1.7625 | 47/1.7625 | 47/1.7625 |
| * | Ter | UAG | 18/0.675 | 18/0.675 | 18/0.675 | 18/0.675 |
| * | Ter | UGA | 15/0.5625 | 15/0.5625 | 15/0.5625 | 15/0.5625 |
| A | Ala | GCA | 352/1.1043 | 362/1.1173 | 363/1.1178 | 362/1.1156 |
| A | Ala | GCC | 203/0.6369 | 207/0.6389 | 210/0.6467 | 208/0.641 |
| A | Ala | GCG | 144/0.4518 | 145/0.4475 | 145/0.4465 | 147/0.453 |
| A | Ala | GCU | 576/1.8071 | 582/1.7963 | 581/1.7891 | 581/1.7904 |
| C | Cys | UGC | 69/0.5542 | 70/0.5578 | 70/0.5578 | 69/0.552 |
| C | Cys | UGU | 180/1.4458 | 181/1.4422 | 181/1.4422 | 181/1.448 |
| D | Asp | GAC | 180/0.4114 | 182/0.4113 | 183/0.4131 | 184/0.4144 |
| D | Asp | GAU | 695/1.5886 | 703/1.5887 | 703/1.5869 | 704/1.5856 |
| E | Glu | GAA | 879/1.509 | 882/1.509 | 885/1.5128 | 883/1.512 |
| E | Glu | GAG | 286/0.491 | 287/0.491 | 285/0.4872 | 285/0.488 |
| F | Phe | UUC | 409/0.6472 | 414/0.6454 | 414/0.6444 | 414/0.6444 |
| F | Phe | UUU | 855/1.3528 | 869/1.3546 | 871/1.3556 | 871/1.3556 |
| G | Gly | GGA | 594/1.5389 | 602/1.5347 | 602/1.5377 | 601/1.5361 |
| G | Gly | GGC | 175/0.4534 | 180/0.4589 | 180/0.4598 | 181/0.4626 |
| G | Gly | GGG | 259/0.671 | 260/0.6628 | 257/0.6564 | 259/0.662 |
| G | Gly | GGU | 516/1.3368 | 527/1.3435 | 527/1.3461 | 524/1.3393 |
| H | His | CAC | 126/0.4961 | 129/0.5 | 129/0.499 | 128/0.4961 |
| H | His | CAU | 382/1.5039 | 387/1.5 | 388/1.501 | 388/1.5039 |
| I | Ile | AUA | 626/0.9812 | 628/0.9782 | 632/0.9829 | 633/0.9844 |
| I | Ile | AUC | 351/0.5502 | 351/0.5467 | 352/0.5474 | 351/0.5459 |
| I | Ile | AUU | 937/1.4687 | 947/1.4751 | 945/1.4697 | 945/1.4697 |
| K | Lys | AAA | 884/1.5021 | 889/1.5004 | 887/1.4996 | 889/1.5004 |
| K | Lys | AAG | 293/0.4979 | 296/0.4996 | 296/0.5004 | 296/0.4996 |
| L | Leu | CUA | 323/0.8095 | 324/0.8013 | 323/0.7995 | 321/0.7939 |
| L | Leu | CUC | 141/0.3534 | 140/0.3462 | 141/0.349 | 142/0.3512 |
| L | Leu | CUG | 145/0.3634 | 146/0.3611 | 147/0.3639 | 148/0.366 |
| L | Leu | CUU | 521/1.3058 | 525/1.2984 | 525/1.2995 | 524/1.296 |
| L | Leu | UUA | 761/1.9073 | 778/1.9242 | 775/1.9183 | 777/1.9217 |
| L | Leu | UUG | 503/1.2607 | 513/1.2688 | 513/1.2698 | 514/1.2712 |
| M | Met | AUG | 544/1 | 550/1 | 551/1 | 550/1 |
| N | Asn | AAC | 233/0.4283 | 234/0.4251 | 237/0.4301 | 236/0.4279 |
| N | Asn | AAU | 855/1.5717 | 867/1.5749 | 865/1.5699 | 867/1.5721 |
| P | Pro | CCA | 275/1.1752 | 278/1.1767 | 277/1.175 | 277/1.175 |
| P | Pro | CCC | 170/0.7265 | 170/0.7196 | 170/0.7211 | 169/0.7169 |
| P | Pro | CCG | 125/0.5342 | 128/0.5418 | 129/0.5472 | 130/0.5514 |
| P | Pro | CCU | 366/1.5641 | 369/1.5619 | 367/1.5567 | 367/1.5567 |
| Q | Gln | CAA | 625/1.5432 | 626/1.5381 | 626/1.5381 | 625/1.5375 |
| Q | Gln | CAG | 185/0.4568 | 188/0.4619 | 188/0.4619 | 188/0.4625 |
| R | Arg | AGA | 414/1.8455 | 417/1.8465 | 415/1.839 | 413/1.8315 |
| R | Arg | AGG | 139/0.6196 | 139/0.6155 | 139/0.616 | 139/0.6164 |
| R | Arg | CGA | 297/1.3239 | 300/1.3284 | 301/1.3338 | 301/1.3348 |
| R | Arg | CGC | 93/0.4146 | 93/0.4118 | 91/0.4032 | 93/0.4124 |
| R | Arg | CGG | 102/0.4547 | 101/0.4472 | 101/0.4476 | 101/0.4479 |
| R | Arg | CGU | 301/1.3418 | 305/1.3506 | 307/1.3604 | 306/1.357 |
| S | Ser | AGC | 98/0.3513 | 99/0.3511 | 98/0.3475 | 98/0.3477 |
| S | Ser | AGU | 358/1.2832 | 360/1.2766 | 359/1.273 | 358/1.2703 |
| S | Ser | UCA | 349/1.2509 | 354/1.2553 | 354/1.2553 | 355/1.2596 |
| S | Ser | UCC | 240/0.8602 | 243/0.8617 | 243/0.8617 | 243/0.8622 |
| S | Ser | UCG | 128/0.4588 | 130/0.461 | 132/0.4681 | 131/0.4648 |
| S | Ser | UCU | 501/1.7957 | 506/1.7943 | 506/1.7943 | 506/1.7954 |
| T | Thr | ACA | 348/1.2395 | 354/1.2443 | 357/1.2559 | 356/1.2513 |
| T | Thr | ACC | 215/0.7658 | 216/0.7592 | 213/0.7493 | 214/0.7522 |
| T | Thr | ACG | 110/0.3918 | 111/0.3902 | 109/0.3835 | 110/0.3866 |
| T | Thr | ACU | 450/1.6028 | 457/1.6063 | 458/1.6113 | 458/1.6098 |
| V | Val | GUA | 472/1.5375 | 476/1.533 | 473/1.5233 | 472/1.5189 |
| V | Val | GUC | 143/0.4658 | 144/0.4638 | 145/0.467 | 147/0.473 |
| V | Val | GUG | 164/0.5342 | 165/0.5314 | 166/0.5346 | 165/0.531 |
| V | Val | GUU | 449/1.4625 | 457/1.4718 | 458/1.475 | 459/1.4771 |
| W | Trp | UGG | 383/1 | 400/1 | 400/1 | 400/1 |
| Y | Tyr | UAC | 160/0.3778 | 160/0.3738 | 160/0.3747 | 161/0.3766 |
| Y | Tyr | UAU | 687/1.6222 | 696/1.6262 | 694/1.6253 | 694/1.6234 |
Fig. 6.
Codon content of 20 amino acids and stop codons in the protein-coding genes of the cp. genomes of the four Synotis species. Each bar represents a species, from left to right: S. cavaleriei, S. duclouxii, S. nagensium, and S. erythropappa
Nucleotide diversity analysis
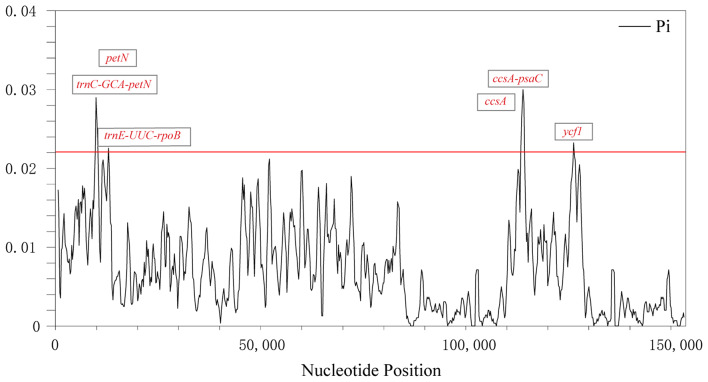
The cp. genomes contain numerous nucleotides. It can be used as the valuable DNA barcoding to resolve the phylogenetic relationships of closely related species or genera. In this study, highly variable regions were identified in Synotis and its related species using DnaSP (Fig. 7). Among the seven species, polymorphism information (Pi) values ranged from 0.02232 (petN gene) to 0.03 (ccsA-psaC region). The Pi analyses revealed that the IR regions displayed significantly lower variation compared to the LSC and SSC regions (Fig. 7). Six mutational hotspots had remarkably higher Pi values (> 0.022), including three genes (ycf1, ccsA and petN) and three intergenic regions (trnC-GCA-petN, ccsA-psaC and trnE-UUC-rpoB).
Fig. 7.
Nucleotide diversity (Pi) values among the seven Senecioneae species. X-axis: position of the midpoint of a window, Y-axis: Pi value, Pi: polymorphism information
Phylogenetic analysis
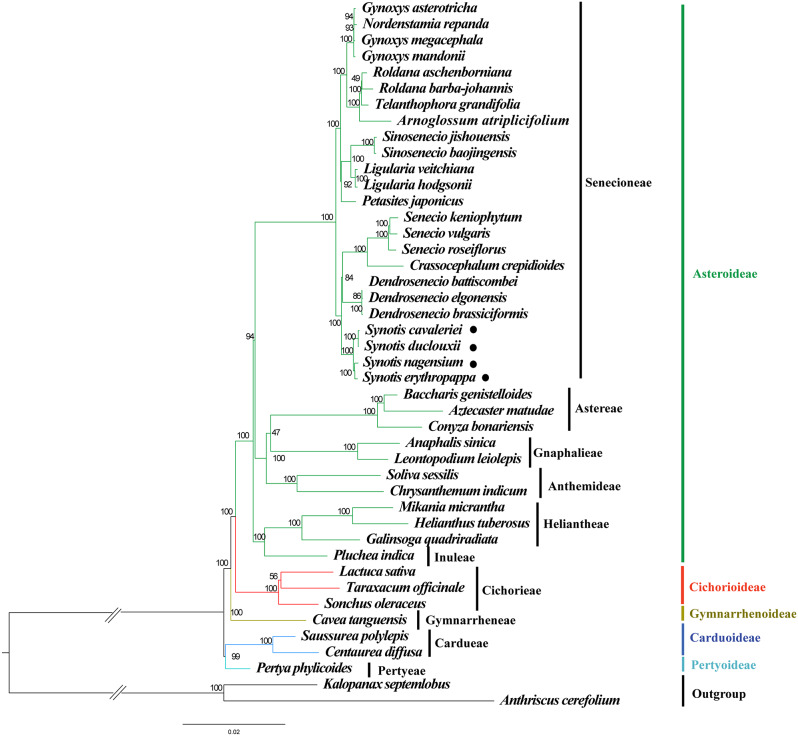
Based on the analysis of the complete cp. genome sequences, the Maximum likelihood (ML) phylogenetic tree of 42 Asteraceae species was constructed, with Anthriscus cerefolium (Apiaceae) and Kalopanax septemlobus (Araliaceae) used as outgroups (Fig. 8). In our sampling of Asteraceae, the species formed a monophyletic group and were grouped into 5 monophyletic clades, corresponding to the 5 subfamilies of Asteraceae (Asteroideae, Cichorioideae, Gymnarrhenoideae, Carduoideae and Pertyoideae). In the subfamily Asteroideae, 6 subgroups were separated, corresponding to the tribe Senecioneae, Astereae, Gnaphalieae, Anthemideae, Helenieae and Inuleae, respectively. The tribe Senecioneae contains two clades: subtribe Tussilagininae and Senecioninae. The subtribe Tussilagininae consists of 13 species in 8 genera, including Gynoxys Cass., Nordenstamia Lundin, Roldana La Llave, Telanthophora H.Rob. & Brettell, Arnoglossum Raf., Sinosenecio B.Nord., Ligularia Cass. and Petasites Mill. The subtribe Senecioninae consists of 11 species from 4 genera, including Senecio L., Crassocephalum Moench, Dendrosenecio (Hauman ex Hedberg) B.Nord. and Synotis. From the perspective of complete cp. genomes, the species of S. cavaleriei, S. duclouxii, S. nagensium and S. erythropappa were sister species, supported by a 100 bootstrap value. Among the four Synotis species, the species of S. cavaleriei clustered with S. duclouxii, and S. nagensium clustered with S. erythropappa. The genus of Synotis was phylogenetically close to Senecio, Crassocephalum and Dendrosenecio.
Fig. 8.
Phylogenetic tree was reconstructed using ML method based on complete cp. genomes of the Asteraceae. Numbers at nodes are bootstrap support values. The phylogenetic position of Synotis species is marked with black circles
Molecular age estimation
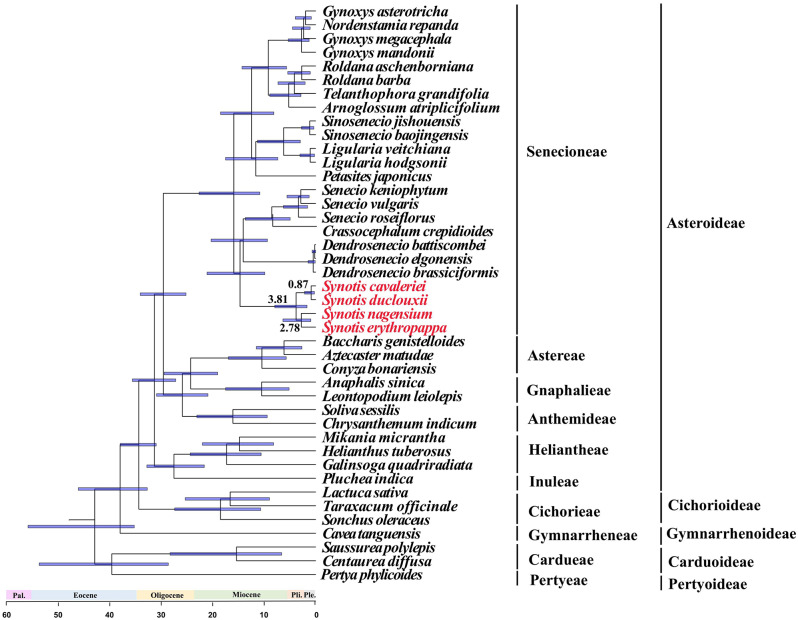
Divergence time estimates of Synotis species based on the complete cp. genomes were shown in Fig. 9. The molecular age estimation suggested that Synotis originated at 3.81 Mya. The divergence time between the S. cavaleriei and S. duclouxii was estimated at 0.87 Mya. The divergence time between the S. nagensium and S. erythropappa was estimated at 2.78 Mya.
Fig. 9.
Divergence time estimates of Synotis based on complete cp. genomes. The numbers above or below branches represent median divergence time estimates. Pal., Pli. and Ple. indicate Paleocene, Pliocene and Pleistocene, respectively. The Synotis species were marked in red
Discussion
Cp genomes structure analysis
In this study, the cp. genomes of four Synotis species were sequenced, assembled, and annotated (Fig. 2; Tables 1 and 3). This is the first report of complete cp. genomes from the genus Synotis. The complete cp. genomes also exhibited a highly conserved characteristic in terms of genomic numbers, orders, structures, GC contents and intron numbers. The length of these cp. genomes ranged from 151,228 (S. erythropappa) to 151,341 bp (S. cavaleriei). They are similar to other cp. genomes of tribe Senecioneae, such as Cremanthodium rhodocephalum [55], Ligularia veitchiana (151,253 bp) [25], Parasenecio palmatisectus (151,263 bp) [56], Sinosenecio albonervius (151,224 bp) [19], and Senecio roseiflorus (151,228 bp) [57]. Among three series within Synotis in this study, the length of the cp. genome of ser. Hieraciifoliae is the longest. The cp. genomes of four Synotis species encoded 134 genes, which is similar to the cp. genomes of Ligularia [25], Aster [33, 34], Gynura [42], and Sinosenecio [19]. Like most other species of Asteraceae, pseudogenes ycf1 and rps19 were also detected [19, 25, 30, 32, 35, 43, 56]. The ACG start codons of psbL and ndhD genes were also detected in angiosperms [38, 58–60]. The clpP, rps12, and ycf3 genes included two introns, while the other genes included one intron. All these features are consistent with the cp. genomes of most family Asteraceae [22, 25, 43, 56]. The total GC contents of the complete cp. genomes among the three series of the Synotis was 37.4% (Table 1), which is similar to previous study [19, 22, 25, 37, 55]. Among the LSC, SSC, and IR regions, the IR regions had the highest GC contents (43.0%), followed by the LSC (35.6%) and SSC regions (30.6 to 30.7%). The IR regions had the highest GC contents among the four regions, which is likely due to the presence of two copies of rRNAs (rrna4.5, rrna5, rrna23, and rrna16) within this region [22, 43].
Table 3.
The basic cp. Genomes information of four Synotis species
| Category | Gene group | Gene name |
|---|---|---|
| Photosynthesis | Subunits of photosystem I | psaA, psaB, psaC, psaI, psaJ |
| Subunits of photosystem II | psbA, psbB, psbC, psbD, psbE, psbF, psbH, psbI, psbJ, psbK, psbL, psbM, psbN, psbT, psbZ | |
| Subunits of NADH dehydrogenase | ndhA*, ndhB*(2), ndhC, ndhD, ndhE, ndhF, ndhG, ndhH, ndhI, ndhJ, ndhK | |
| Subunits of cytochrome b/f complex | petA, petB*, petD*, petG, petL, petN | |
| Subunits of ATP synthase | atpA, atpB, atpE, atpF*, atpH, atpI | |
| Large subunit of rubisco | rbcL* | |
| Self-replication | Proteins of large ribosomal subunit | rpl14, rpl16*, rpl2*(2), rpl20, rpl22, rpl23(2), rpl32, rpl33, rpl36 |
| Proteins of small ribosomal subunit | #rps19, rps11, rps12**(2), rps14, rps15, rps16*, rps18, rps19, rps2, rps3, rps4, rps7(2), rps8 | |
| Subunits of RNA polymerase | rpoA, rpoB, rpoC1*, rpoC2 | |
| Ribosomal RNAs | rrn16(2), rrn23(2), rrn4.5(2), rrn5(2) | |
| Transfer RNAs | trnA-UGC*(2), trnC-GCA, trnD-GUC, trnE-UUC, trnF-GAA, trnG-GCC, trnG-UCC*, trnH-GUG, trnI-CAU(2), trnI-GAU*(2), trnK-UUU*, trnL-CAA(2), trnL-UAA*, trnL-UAG, trnM-CAU, trnN-GUU(2), trnP-UGG, trnQ-UUG, trnR-ACG(2), trnR-UCU, trnS-GCU, trnS-GGA, trnS-UGA, trnT-GGU, trnT-UGU, trnV-GAC(2), trnV-UAC*, trnW-CCA, trnY-GUA, trnfM-CAU | |
| Other genes | Maturase | matK |
| Protease | clpP** | |
| Envelope membrane protein | cemA | |
| Acetyl-CoA carboxylase | accD | |
| c-type cytochrome synthesis gene | ccsA | |
| Translation initiation factor | infA | |
| Genes of unknown function | Conserved hypothetical chloroplast ORF | #ycf1, ycf1, ycf15(2), ycf2(2), ycf3**, ycf4 |
Notes: *: Gene with one introns, **: Gene with two introns, #: Pseudogene, (2): Gene with two copies
Repeat sequences analysis
SSRs, also called microsatellites, are widely utilized as valuable molecular markers in species identification and phylogenetic studies due to their high substitution rates [61]. In this study, a total of 195 SSRs were identified in the complete cp. genomes of four Synotis species, 111 were found to be Mononucleotide repeats, constituting the majority (57%) of all SSRs (Fig. 3A). The most common SSRs are Mononucleotide repeats composed of A/T (Fig. 3B). It is worth noting that the number of SSRs varied across different species (Fig. 3C). These findings are consistent with previous reports within the Asteraceae family, such as Artemisia [18, 38]. In this study, we analyzed the number and composition of SSRs in the cp. genomes of four Synotis species. It will serve as valuable references for further research on molecular markers and population genetics of Synotis and the tribe Senecioneae.
Genomes comparison and nucleotide diversity
Comparative analysis of the complete cp. genomes provides a pivotal reference in understanding genome evolution and evolutionary relationships within the family Asteraceae [18, 19, 22, 23, 56]. Through the comparative analysis of the IR boundary regions among Synotis and its related species, some expansion or contractions were detected, with the IR regions ranging from 24,821 to 24,852 bp (Fig. 4). Similar large-scale IR expansion had also been reported in other Asteraceae, such as Saussurea [17], Aster [35], Xanthium [46]. Furthermore, the SSC and LSC regions showed higher sequence divergence than the IR regions, and the non-coding region was more divergence than the coding region (Fig. 5), the same result was also obtained in Senecioneae [19, 44, 57]. It should be noted that mutation hotspots can serve as specific DNA barcodes for species identification [62]. In this study, six highly variable regions (Pi > 0.022) were identified, including trnC-petN, ccsA-psaC, trnE-rpoB, ycf1, ccsA and petN. These variable regions could be utilized as potential molecular barcodes for species identification and phylogenetic research in genus Synotis and tribe Senecioneae.
Phylogenetic relationship, super barcode and molecular dating analysis
In recent years, several molecular phylogenetic analyses have been conducted to resolve the phylogenetic relationships of genus Synotis. Liu et al. (2006) studied the radiation and diversification within the Ligularia-Cremanthodium-Parasenecio complex in the Qinghai-Tibetan Plateau, they used Synotis lucorum as the outgroup and inferred a phylogenetic tree of the LCP complex using ITS, ndhC-F and trnL-F [63]. Pelser et al. (2007) constructed the phylogenetic relationships for the Senecioneae using ITS data. The results showed that the species of Synotis was nested within the Cissampelopssis-Crassocephalum clade [51]. However, these studies were based on small taxon sampling and small fragments of barcoding markers. Tang (2014) reconstructed the Phylogenetic tree of the Synotis using ITS and seven cpDNA sequence regions. In his analyses, the monophyly of Synotis is strongly supported and the species of Senecio kumaonensis is also included [6]. Although some studies of phylogenetic relationships have been reported using limited nuclear and cpDNA fragments in the above studies, the research progress of genus Synotis at the complete cp. genome-scale level is relatively slow. The result indicated that all species were grouped into five monophyletic clades (Fig. 8), corresponding to the five subfamilies of Asteraceae, which was consistent with the previous study [64]. In this study, the phylogenetic trees among the three series of Synotis were constructed using complete cp. genomes for the first time. In the ML tree, the Synotis was a branch of family Asteraceae, the four Synotis species are sister groups within tribe Senecioneae and subfamily Asteroideae, and the bootstrap support values for these were 100. Our study also confirms the monophyly of Synotis. In the tribe Senecioneae, two subgroups were separated, corresponding to the subtribe Tussilagininae and Senecioninae, respectively. The genus Synotis belongs to the Senecioninae subtribe, which is consistent with the backbone of Senecioneae in previous studies [47, 51, 64].
The complete cp. genomes have been widely served as super barcode for species identification and classification [25–29]. In this study, the Senecioneae contains two clades. Within subtribe Senecioninae, a sub-clade consisting of four Synotis species was identified with high bootstrap support values (bootstrap = 100) (Fig. 8). Four Synotis species were discriminated completely based on complete cp. genome sequences. This topology shows the separation of Synotis at intra-species level, which suggested that the complete cp. genomes of Synotis might be a super barcode for species identification.
Previous studies have estimated the divergence time of the Senecioneae tribe during the Eocene period using various molecular markers. For instance, Panero and Crozier (2016) used a few cp. genomes markers [65], Han et al. (2019) utilized complete cp. genomes [42], and Mandel et al. (2019) employed nuclear phylogenomics data [66]. Huang et al. (2016) estimated the divergence time of the tribe Senecioneae during the early Miocene based on multiple nuclear genes [67]. In this study, the divergence time of the tribes Senecioneae was early Oligocene period. This study provides further support and traces the divergence time of the Senecioneae tribe.
In this study, it is an efficient attempt to utilize the complete genomes as super-barcode for the identification of genus Synotis. Our research provides a foundation for developing a reliable and efficient method for species identification of Synotis. In the future, it is necessary to further investigate and verify this assumption. In addition, Synotis is a large genus with ca. 60 species [1], and this study only analyzes the cp. genome sequences of four species. Therefore, a more comprehensive analysis of cp. genomes and a broader taxon sampling are essential in order to fully develop the super-barcode approach. The development of such a method will greatly contribute to biodiversity assessments, conservation efforts, and various fields of plant research.
Conclusions
This study reported the complete cp. genomes from four Synotis species, ranging from 151,228 (S. erythropappa) to 151,341 bp (S. cavaleriei). The structure and composition, SSRs, codon usage of the complete cp. genomes were highly conserved. The complete cp. genomes of four Synotis species encoded 134 genes, including 87 protein-coding genes, 37 tRNA genes, 8 rRNA genes, and 2 Pseudogenes (ycf1 and rps19). Phylogenetic analysis showed that four species were clustered into a monophyletic group, and they were close to the genus of Senecio, Crassocephalum and Dendrosenecio in tribe Senecioneae. The ML tree showed that the complete cp. genomes could be used as a super-barcode for the identification of Synotis species.
Materials and methods
Plant materials and DNA extraction
Fresh leaves of Synotis cavaleriei and S. duclouxii were collected from Butuo County, Liangshan Prefecture, Sichuan Province, China. Fresh leaves of S. nagensium were collected from Tongchuan District, Dazhou City, Sichuan Province, China. Fresh leaves of S. erythropappa were collected from Puge County, Liangshan Prefecture, Sichuan Province, China. (Fig. 1). These specimens were identified by Dr. Zhixi Fu at Sichuan Normal University. The voucher specimen of S. cavaleriei (LXF0170), S. duclouxii (LXF0219), S. nagensium (FZX5549) and S. erythropappa (FZX1038) were deposited in the herbarium of Sichuan Normal University (SCNU) (Contact: Prof. Dr. Zhixi Fu, fuzx2017@sicnu.edu.cn). We achieved all required permits for the protected areas from the local governments and National Park Services. This research was carried out in compliance with the relevant laws of China. Total genomic DNA was extracted from fresh leaves using the cetyltrimethylammonium bromide (CTAB) method, following the manufacturer’s instructions [68]. The Illumina Paired-End (PE) DNA Library Kit (Illumina, San Diego, CA, USA) was used to construct the DNA libraries. The genomic library was sequenced using the Illumina NovaSeq 6000 platform with a 150 bp read length (NovoGene Inc., Beijing, China).
Genomes assembly and annotation
The complete cp. genome sequences of Synotis were assembled using SPAdes software (v3.10.1) with default parameters [69]. The assembly quality was assessed using Bandage software to identify circular maps [70]. The cp. sequences were then annotated using PGA based on reference cp. genome sequences of Ligularia jaluensis (MF539931.1) [71]. The annotation results were manually inspected and adjusted using Geneious [72]. Circular maps of the cp. genomes were drawn using Organellar Genome Draw (OGDraw) (https://chlorobox.mpimp-golm.mpg.de/OGDraw.html (Accessed August 6, 2022)) [73]. Various plastome characteristics, such as the number of genes, gene length, GC contents and intron numbers were analyzed using Geneious [72]. The obtained cp. genome sequences were deposited in the NCBI database (http://www.ncbi.nlm.nih.gov/) with the GenBank accession numbers OM912601 (S. cavaleriei), OM912602 (S. duclouxii), OM912603 (S. nagensium), and OQ985056 (S. erythropappa).
Simple sequence repeats (SSRs) analysis
SSRs were analyzed using the MISA software (http://pgrc.ipkgatersleben.de/misa/ (Accessed January 3, 2023)) [74]. The minimum number of repeats for Mono-, Di-, Tri-, Tetra-, Penta- and Hexanucleotide motifs were set to 8, 5, 3, 3, 3, and 3, respectively.
Comparative analysis of cp genomes
Three cp. genomes from tribe Senecioneae were downloaded from GenBank to conduct comparative cp. genomes analysis. The species included in the analysis were Ligularia jaluensis Kom., Senecio vulgaris L. and Sinosenecio oldhamianus (Maxim.) B.Nord. The boundary differences among these genomes were visualized using mVISTA in Shuffle-LAGAN mode, with the Ligularia jaluensis as the reference (http://genome.lbl.gov/vista/mvista/submit.shtml (Accessed August 15, 2022)) [75]. The contraction and expansion of the IR boundaries among the four regions (LSC/IRa/SSC/IRb) of the cp. genome sequences were observed using the online software IRSCOPE (https://irscope.shinyapps.io/irapp/ (Accessed January 3, 2023)) [76].
Codon usage analysis
The distribution of codon usage was studied using CodonW (http://downloads.fyxm.net/ CodonW-76666.html (Accessed February 4, 2023)) with the relative synonymous codon usage (RSCU) ratio [77]. A value of RSCU > 1 indicates that the codon is used more frequently, a value of 1 indicates no codon usage preference, and a value of < 1 indicates less frequent codon usage.
Genetic divergence analysis
To identify and analyze highly variable regions of the cp. genomes sequence, the sequences of seven Senecioneae cp. genomes were aligned using the MAFFT program [78]. The nucleotide diversity (Pi) was then analyzed through the sliding window using DnaSP v5 (http://www.ub.edu/dnasp/ (Accessed January 6, 2023)) [79]. The step size was set to 200 bp with an 800 bp window length.
Phylogenetic analysis
Phylogenetic analysis was inferred based on 44 complete cp. genomes, including the 4 newly sequences of Synotis in this study, 38 cp. genomes of family Asteraceae downloaded from the NCBI database (Table S1), and Anthriscus cerefolium (Apiaceae) and Kalopanax septemlobus (Araliaceae) as outgroup. To align the sequences, the MAFFT program was used with the auto strategy [78]. The initial alignment was further manually adjusted in BioEdit to ensure accuracy [80]. The maximum-likelihood (ML) phylogenetic tree was constructed in RaxML 8.2.10 tool at CIPRES Science Gateway web (https://www.phylo.org/ (Accessed January 7, 2023)) with 1000 bootstrap replicates [81, 82].
Estimation of divergence times
The divergence times of genus Synotis were estimated using BEAST2 by lognormal relaxed clock [83]. The GTP model was chosen as a substitution model. The GTR model was chosen to generate the tree. As a calibration point, the pairwise divergence time of Asteroideae was set at 37.7 Mya [66]. A total of 10,000,000 generations were run. Tracer v.1.7 was used to check the correctness [84]. Finally, the maximum clade credibility tree was calculated in TreeAnnotator v1.8.4.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the editor and anonymous reviewers for the constructive criticism of the original manuscript. We also thank Dr. Yi Yang from Jiangxi Agricultural University provides the color images of Synotis duclouxii.
Author contributions
M.T. and Z.F. conceived and designed the research. X.L. and J.L. performed bioinformatic analyses. X.L., H.C. and J.L. carried out wet-lab experiments. T.L. and T.Q. contributed to data interpretation. X.L. and Z.F. wrote the manuscript. M.T. and Z.F. revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was financially supported by the National Natural Science Foundation of China (No. 32000158, No. 31500166, No. 31960043), the National Science & Technology Fundamental Resources Investigation Program of China (No. 2021XJKK0702), and the Foundation of Sustainable Development Research Center of Resources and Environment of Western Sichuan, Sichuan Normal University (No. 2020CXZYHJZX03). Jiangxi Provincial Key Laboratory of Conservation Biology (No. 2023SSY02081).
Data availability
All annotated chloroplast genomes have been deposited in GenBank as OM912601 (Synotis cavaleriei), OM912602 (S. duclouxii), OM912603 (S. nagensium), and OQ985056 (S. erythropappa).
Declarations
Ethics approval and consent to participate
The study was conducted the plant material that complies with relevant institutional, national, and international guidelines and legislation.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ming Tang, Email: tangming@jxau.edu.cn.
Zhixi Fu, Email: fuzx2017@sicnu.edu.cn.
References
- 1.Chen YL, Nordenstam B, Jeffrey C. Synotis (C. B. Clarke) C. Jeffrey & Y. L. Chen. – In: Wu, Z. Y. and Raven, P. H, editors, Flora of China. Vol. 20–21. Science Press; Miss. Bot. Gard. Press. 2011;pp.489–505.
- 2.Joshi S, Shrestha K, Bajracharya DM. Synotis managensis (Senecioneae: Asteraceae) – a new species from Manang, central Nepal. Pleione. 2013;7:539–43. [Google Scholar]
- 3.Tang M, Hong Y, Yang QE. Synotis baoshanensis (Asteraceae), a new species from Yunnan. China Bot Stud. 2013a;54:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang M, Wang LY, Yang QE. Synotis xinningensis (Asteraceae), a new species from Hunan. China Bot Stud. 2013b;54:e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang M, Wang LY, Yang QE. The identity of Synotis cordifolia (Asteraceae–Senecioneae). J Trop Subtrop Bot. 2013c;21:101–8. [Google Scholar]
- 6.Tang M. A Systematic Study of the Genus Synotis (Compositae-Senecioneae). PhD thesis. University of Chinese Academy of Sciences, Beijing. 2014.
- 7.Li Z, Zheng HL, Tang M. Synotis panzhouensis (Asteraceae, Senecioneae), a distinct new species with red-purple pappus from southwestern Guizhou, China. Phytokeys. 2020;166:79–86. 10.3897/phytokeys.166.58654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu YL, Zhang R, Ren C, Liu B, Zhang Y, Tang M. On the specific identity of Chinese endemic species Synotis longipes. Phytotaxa. 2020;472:269–76. 10.11646/phytotaxa.472.3.5 [DOI] [Google Scholar]
- 9.Liu YL, Zhu XX, Peng YL, Tang M. Synotis jinshajiangensis (Asteraceae: Senecioneae), a new species from northwestern Yunnan. China Phytotaxa. 2021;478:162–70. 10.11646/phytotaxa.478.1.12 [DOI] [Google Scholar]
- 10.Sennikov AN, Nuraliev MS, Kuznetsov AN, Kuznetsova SP. New national records of Asteraceae from Hoang Lien National Park, northern Vietnam. Wulfenia. 2020;27:1–9. [Google Scholar]
- 11.Tang M, Chen YS. Blumea hunanensis is a synonym of Synotis nagensium (Asteraceae: Senecioneae). Phytotaxa. 2021;487:149–56. 10.11646/phytotaxa.487.2.5 [DOI] [Google Scholar]
- 12.Zhang R, Liu YL, Tang M. Four new synonyms in Synotis (Asteraceae, Senecioneae). Phytotaxa. 2021;483:255–66. 10.11646/phytotaxa.483.3.5 [DOI] [Google Scholar]
- 13.Fan Y, Zhang R, Guo CL, Tang M. On the identity of Synotis ionodasys (Asteraceae: Senecioneae) from Yunnan, China. Phytotaxa. 2022;545:207–16. 10.11646/phytotaxa.545.2.9 [DOI] [Google Scholar]
- 14.Jeffrey C, Chen YL. Taxonomic studies on the tribe Senecioneae (Compositae) of eastern Asia. Kew Bull. 1984;39:205–446. 10.2307/4110124 [DOI] [Google Scholar]
- 15.Wicke S, Schneeweiss GM, de Pamphilis CW, Muller KF, Quandt D. The evolution of the plastid chromosome in land plants: gene content, gene order, gene function. Plant Mol Biol. 2011;76:273–97. 10.1007/s11103-011-9762-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw J, Lickey EB, Schilling EE, Small RL. Comparison of whole chloroplast genome sequences to choose noncoding regions for phylogenetic studies in angiosperms: the tortoise and the hare III. Am J Bot. 2007;94:275–88. 10.3732/ajb.94.3.275 [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Deng T, Moore MJ, Ji YH, Lin N, Zhan HJ, Meng AP, Wang HC, Sun YX, Sun H. Plastome phylogenomics of Saussurea (Asteraceae: Cardueae). BMC Plant Biol. 2019;19:290. [DOI] [PMC free article] [PubMed]
- 18.Lan Z, Shi Y, Yin Q, Gao R, Liu C, Wang W, Tian X, Liu J, NongY, Xiang L, et al. Comparative and phylogenetic analysis of complete chloroplast genomes from five Artemisia species. Front. Plant Sci. 2022;13:1049209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng JY, Zhang XS, Zhang DG, Wang Y, Deng T, Huang XH, Kuang TH, Zhou Q. Newly reported chloroplast genome of Sinosenecio albonervius Y. Liu & Q. E. Yang and comparative analyses with other Sinosenecio species. BMC Genom. 2022;23:639. [DOI] [PMC free article] [PubMed]
- 20.Daniell H, Lin CS, Yu M, Chang WJ. Chloroplast genomes: diversity, evolution, and applications in genetic engineering. Genome Biol. 2016;17:134. 10.1186/s13059-016-1004-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jansen RK, Ruhlman TA. Genomics Chloroplasts Mitochondria. 2012;35:103–26. 10.1007/978-94-007-2920-9_5 [DOI] [Google Scholar]
- 22.Shahzadi I, Mehmood F, Ali Z, Ahmed I, Mirza B. Chloroplast genome sequences of Artemisia maritima and Artemisia absinthium: comparative analyses, mutational hotspots in genus Artemisia and phylogeny in family Asteraceae. Genomics. 2020;112:1454–63. 10.1016/j.ygeno.2019.08.016 [DOI] [PubMed] [Google Scholar]
- 23.Jin GZ, Li WJ, Song F, Yang L, Wen ZB, Feng Y. Comparative analysis of complete Artemisia subgenus Seriphidium (Asteraceae: Anthemideae) chloroplast genomes: insights into structural divergence and phylogenetic relationships. BMC Plant Biol. 2023;23:136. 10.1186/s12870-023-04113-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu SH, Yang XC, Tian XY, Liu XF, Huang CP, Fu ZX. The complete chloroplast genome sequence of the monotypic and enigmatic genus Cavea (tribe Gymnarrheneae) and a comparison with other species in Asteraceae. J Genet. 2022;101:20. 10.1007/s12041-022-01360-3 [DOI] [Google Scholar]
- 25.Chen X, Zhou J, Cui Y, Wang Y, Duan B, Yao H. Identification of Ligularia herbs using the complete chloroplast genome as a super-barcode. Front Pharmacol. 2018;9:695. 10.3389/fphar.2018.00695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang AS, Wu HW, Zhu XC, Lin JM. Species identification of Conyza bonariensis assisted by chloroplast genome sequencing. Front Genet. 2018;9:374. 10.3389/fgene.2018.00374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang ZL, Zhang Y, Song MF, Guan YH, Ma XJ. Species identification of Dracaena using the complete chloroplast genome as a super-barcode. Front Pharmacol. 2019;10:1441. 10.3389/fphar.2019.01441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu L, Wu ML, Cui N, Xiang L, Li Y, Li XW, Chen SL. Plant super-barcode: a case study on genome-based identification for closely related species of Fritillaria. Chin Med. 2021;16:52. 10.1186/s13020-021-00460-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang WT, Wang XW, Shi YH, Yin QG, Gao RR, Wang MY, Xiang L, Wu L. Identification of Laportea bulbifera using the complete chloroplast genome as a potentially effective super-barcode. J Appl Genet. 2023;64:231–45. 10.1007/s13353-022-00746-4 [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Huo NX, Dong LL, Wang Y, Zhang SX, Young HA, Feng XX, Gu YQ. Complete chloroplast genome sequences of mongolia medicine Artemisia frigida and phylogenetic relationships with other plants. PLoS ONE. 2013;8:e57533. 10.1371/journal.pone.0057533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi KS, Park SJ. The complete chloroplast genome sequence of Aster Spathulifolius (Asteraceae); genomic features and relationship with Asteraceae. Gene. 2015;572:214–21. 10.1016/j.gene.2015.07.020 [DOI] [PubMed] [Google Scholar]
- 32.Park J, Shim J, Won H, Lee J. Plastid genome of Aster altaicus var. Uchiyamae Kitam., an endanger species of Korean asterids. J Species res. 2017;6:76–90. [Google Scholar]
- 33.Ou CZ, Feng YL, Hu YK, Tian XY, Fu ZX. Characterization of the complete chloroplast genome sequence of Aster hersileoides (Asteraceae, Astereae) and its phylogenetic implications. Mitochondrial DNA Part B. 2019;4:985–6. 10.1080/23802359.2019.1581109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Q, Wang XH, Yang XC, Fu LF, Gong QJ, Fu ZX. The complete chloroplast genome of Aster hypoleucus (Asteraceae: Astereae): an endemic species from China. Mitochondrial DNA Part B. 2019;4:2647–8. 10.1080/23802359.2019.1644219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tyagi S, Jung JA, Kim JS, Won SY. Comparative analysis of the complete chloroplast genome of mainland Aster Spathulifolius and other Aster species. Plants. 2020;9:568. 10.3390/plants9050568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang X, Jiang PP, Fan SJ. Characterization of the complete plastome of Aster pekinensis (Asteraceae), a perennial herb. Mitochondrial DNA Part B. 2021;6:1064–5. 10.1080/23802359.2021.1899081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng T, Liu M, Wang T, Chen H. The complete chloroplast genome sequence of medicinal plant Conyza Blinii H. Lév. Mitochondrial DNA Part B. 2017;2:50–1. 10.1080/23802359.2016.1198998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma YP, Zhao L, Zhang WJ, Zhang YH, Xing X, Duan XX, Hu J, Harris A, Liu PL, Dai SL, et al. Origins of cultivars of Chrysanthemum—evidence from the chloroplast genome and nuclear LFY gene. J Syst Evol. 2020;58:925–44. 10.1111/jse.12682 [DOI] [Google Scholar]
- 39.Do HDK, Jung J, Hyun J, Yoon SJ, Lim C, Park K, Kim JH. The newly developed single nucleotide polymorphism (SNP) markers for a potentially medicinal plant, Crepidiastrum denticulatum (Asteraceae), inferred from complete chloroplast genome data. Mol Biol Rep. 2019;46:3287–97. 10.1007/s11033-019-04789-5 [DOI] [PubMed] [Google Scholar]
- 40.Shen J, Zhang X, Landis JB, Zhang H, Deng T, Sun H, Wang H. Plastome evolution in Dolomiaea (Asteraceae, Cardueae) using phylogenomic and comparative analyses. Front Plant Sci. 2020;11:376. 10.3389/fpls.2020.00376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou JY, Li JJ, Peng SB, An XM. Characterization of the complete chloroplast genome of the invasive plant Erigeron annuus (L.) Pers. (Asterales: Asteraceae). Mitochondrial DNA Part B. 2022;7:188–90. 10.1080/23802359.2021.2018946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han TY, Li MM, Li JW, Lv H, Ren BR, Chen J, Li WL. Comparison of chloroplast genomes of Gynura species: sequence variation, genome rearrangement and divergence studies. BMC Genom. 2019;20:791. 10.1186/s12864-019-6196-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duan N, Deng LL, Zhang Y, Shi YC, Liu BB. Comparative and phylogenetic analysis based on chloroplast genome of Heteroplexis (Compositae), a protected rare genus. BMC Plant Biol. 2022;22:1–10. 10.1186/s12870-022-04000-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu XF, Han MY, Chen J, Zhang X, Chen ZY, Tang Y, Qu TM, Huang CP, Yu SH, Fu ZX. Characterization of the complete chloroplast genome sequence of Chinese endemic species of Nouelia Insignis (Hyalideae, Asteraceae) and its phylogenetic implications. Mitochondrial DNA Part B. 2022;7:600–2. 10.1080/23802359.2021.1921629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yun S, Kim SC. Comparative plastomes and phylogenetic analysis of seven Korean endemic Saussurea (Asteraceae). BMC Plant Biol. 2022;22:550. 10.1186/s12870-022-03946-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raman G, Park KT, Kim JH, Park S. Characteristics of the completed chloroplast genome sequence of Xanthium spinosum: comparative analyses, identification of mutational hotspots and phylogenetic implications. BMC Genom. 2020;21:855. 10.1186/s12864-020-07219-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pelser PB, Kennedy AH, Tepe EJ, Shidler JB, Nordenstam B, Kadereit JW, Watson LE. Patterns and causes of incongruence between plastid and nuclear senecioneae (Asteraceae) phylogenies. Am J Bot. 2010;97:856–73. 10.3732/ajb.0900287 [DOI] [PubMed] [Google Scholar]
- 48.Tang M, Ren C, Yang QE. Parasenecio Chenopodiifolius (Compositae–Senecioneae) is a Synotis and conspecific with S. otophylla based on evidence from morphology, cytology and ITS/ETS sequence data. Nord J Bot. 2014;32:824–35. 10.1111/njb.00574 [DOI] [Google Scholar]
- 49.Tong TJ, Tang M, Ren C, Yang QE. Senecio kumaonensis (Asteraceae, Senecioneae) is a Synotis based on evidence from karyology and nuclear ITS/ETS sequence data. Phytotaxa. 2017;292:35–46. 10.11646/phytotaxa.292.1.3 [DOI] [Google Scholar]
- 50.Li HM, Lazkov GA, Illarionova ID, Tong TJ, Shao YY, Ren C. Transfer of Senecio karelinioides (Asteraceae Senecioneae) to Synotis based on evidence from morphology, karyology and ITS/ETS sequence data. Nord J Bot. 2018;32:1–12. [Google Scholar]
- 51.Pelser PB, Nordenstam B, Kadereit JW, Watson LE. An ITS phylogeny of tribe Senecioneae (Asteraceae) and a new delimitation of Senecio L. Taxon. 2007;56:1077–104. 10.2307/25065905 [DOI] [Google Scholar]
- 52.Moore MJ, Dhingra A, Soltis PS, Shaw R, Farmerie WG, Folta KM, Soltis DE. Rapid and accurate pyrosequencing of angiosperm plastid genomes. BMC Plant Biol. 2006;6:17. 10.1186/1471-2229-6-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parks M, Cronn R, Liston A. Increasing phylogenetic resolution at low taxonomic levels using massively parallel sequencing of chloroplast genomes. BMC Biol. 2009;7:84. 10.1186/1741-7007-7-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruhsam M, Rai HS, Mathews S, Ross TG, Graham SW, Raubeson LA, Mei W, Thomas PI, Gardner MF, Ennos RA, et al. Does complete plastid genome sequencing improve species discrimination and phylogenetic resolution in Araucaria? Mol Ecol Resour. 2015;15:1067–78. 10.1111/1755-0998.12375 [DOI] [PubMed] [Google Scholar]
- 55.Zhong WH, Du XL, Wang XY, Cao L, Mu ZJ, Zhong GY. Comparative analyses of five complete chloroplast genomes from the endemic genus Cremanthodium (Asteraceae) in Himalayan and adjacent areas. Physiol Mol Biol Plants. 2023;29:409–20. 10.1007/s12298-023-01292-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu XF, Luo JJ, Zhang MK, Wang Q, Liu J, Wu D, Fu ZX. Phylogenomic analysis of two species of Parasenecio and comparative analysis within tribe Senecioneae (Asteraceae). Diversity. 2023;15:563. 10.3390/d15040563 [DOI] [Google Scholar]
- 57.Gichira AW, Avoga S, Li ZZ, Hu GW, Wang QF, Chen JM. Comparative genomics of 11 complete chloroplast genomes of Senecioneae (Asteraceae) species: DNA barcodes and phylogenetics. Bot Stud. 2019;60:3–17. 10.1186/s40529-019-0265-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raman G, Park S. The complete chloroplast genome sequence of Ampelopsis: gene organization, comparative analysis, and phylogenetic relationships to other angiosperms. Front Plant Sci. 2016;7:341. 10.3389/fpls.2016.00341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gichira AW, Li Z, Saina JK, Long Z, Hu G, Gituru RW, et al. The complete chloroplast genome sequence of an endemic monotypic genus Hagenia (Rosaceae): structural comparative analysis, gene content and microsatellite detection. PeerJ. 2017;5:e2846. 10.7717/peerj.2846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alzahrani DA, Yaradua SS, Albokhari EJ, Abba A. Complete chloroplast genome sequence of Barleria prionitis, comparative chloroplast genomics and phylogenetic relationships among Acanthoideae. BMC Genom. 2020;21:393. 10.1186/s12864-020-06798-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Upadhyay A, Kadam US, Chacko P, Karibasappa GS. Microsatellite and RAPD analysis of grape (Vitis spp.) accessions and identification of duplicates/misnomers in germplasm collection. Indian J Hortic. 2010;67:8–15. [Google Scholar]
- 62.Li HL, Xiao WJ, Tong T, Li YL, Zhang M, Lin XX, et al. The specific DNA barcodes based on chloroplast genes for species identification of Orchidaceae plants. Sci Rep. 2021;11:1424. 10.1038/s41598-021-81087-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu J, Wang Y, Wang A, Hideaki O, Abbott R. Radiation and diversification within the Ligularia-Cremanthodium-Parasenecio complex (Asteraceae) triggered by uplift of the Qinghai-Tibetan plateau. Mol Phylogenet Evol. 2006;38:31–49. 10.1016/j.ympev.2005.09.010 [DOI] [PubMed] [Google Scholar]
- 64.Fu ZX, Jiao BH, Nie B, Zhang GJ, Gao TG. A comprehensive generic-level phylogeny of the sunflower family: implications for the systematics of Chinese Asteraceae. J Syst Evol. 2016;54:416–37. 10.1111/jse.12216 [DOI] [Google Scholar]
- 65.Panero JL, Crozier BS. Macroevolutionary dynamics in the early diversification of Asteraceae. Mol Phylogenet Eevol. 2016;99:116–32. 10.1016/j.ympev.2016.03.007 [DOI] [PubMed] [Google Scholar]
- 66.Mandel JR, Dikow RB, Siniscalchi CM, Thapa R, Watson LE, Funk VA. A fully resolved backbone phylogeny reveals numerous dispersals and explosive diversifications throughout the history of Asteraceae. Proc Natl Acad Sci. 2019;116:14083–8. 10.1073/pnas.1903871116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang CH, Zhang CF, Liu M, Hu Y, Gao TG, Qi J, Ma H. Multiple polyploidization events across Asteraceae with two nested events in the early history revealed by nuclear phylogenomics. Mol Biol Evol. 2016;33:2820–35. 10.1093/molbev/msw157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Allen G, Flores-Vergara M, Krasynanski S, Kumar S, Thompson WF. A modified protocol for rapid DNA isolation from plant tissues using cetyltrimethylammonium bromide. Nat Protoc. 2006;1:2320–5. 10.1038/nprot.2006.384 [DOI] [PubMed] [Google Scholar]
- 69.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19:455–77. 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wick RR, Schultz MB, Zobel J, Holt KE, Bandage. Interactive visualisation of de novo genome assemblies. Bioinform. 2015;31:3350–2. 10.1093/bioinformatics/btv383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qu XJ, Moore MJ, Li DZ, Yi TS. PGA: A software package for rapid, accurate, and flexible batch annotation of plastomes. Plant Methods. 2019;15:50. 10.1186/s13007-019-0435-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinform. 2012;28:1647–9. 10.1093/bioinformatics/bts199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Greiner S, Lehwark P, Bock R. Organellar Genome DRAW (OGDRAW) version 1.3.1: expanded toolkit for the graphical visualization of organellar genomes. Nucleic Acids Res. 2019;47:59–64. 10.1093/nar/gkz238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beier S, Thiel T, Munch T, Scholz U, Mascher M. MISA-web: a web server for microsatellite prediction. Bioinform. 2017;33:2583–5. 10.1093/bioinformatics/btx198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Frazer KA, Pachter L, Poliakov A, Rubin EM, Dubchak I. VISTA: computational tools for comparative genomics. Nucleic Acids Res. 2004;32:273–9. 10.1093/nar/gkh458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Amiryousefi A, Hyvönen J, Poczai P, IRscope. An online program to visualize the junction sites of chloroplast genomes. Bioinform. 2018;34:3030–1. 10.1093/bioinformatics/bty220 [DOI] [PubMed] [Google Scholar]
- 77.Sharp PM, Li WH. The codon adaptation index-a measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res. 1987;15:1281–95. 10.1093/nar/15.3.1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Katoh K, Rozewicki J, Yamada KD. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 2019;20:1160–6. 10.1093/bib/bbx108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinform. 2009;25:1451–2. 10.1093/bioinformatics/btp187 [DOI] [PubMed] [Google Scholar]
- 80.Hall TA, BioEdit. A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic acids symp. ser. 1999;41:95–98.
- 81.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinform. 2014;30:1312–3. 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miller MA, Pfeiffer W, Schwartz T. November. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 2010;pp. 1–8.
- 83.Bouckaert R, Heled J, Denise K, Vaughan T, Wu CH, Xie D, Suchard MA, Rambaut A, Drummond AJ, et al. BEAST2: a software platform for bayesian evolutionary analysis. PLoS Comput Biol. 2014;10:e1003537. 10.1371/journal.pcbi.1003537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA. Posterior summarization in bayesian phylogenetics using Tracer 1.7. Syst Biol. 2018;67:901–4. 10.1093/sysbio/syy032 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All annotated chloroplast genomes have been deposited in GenBank as OM912601 (Synotis cavaleriei), OM912602 (S. duclouxii), OM912603 (S. nagensium), and OQ985056 (S. erythropappa).