Abstract
Background
Triple-negative breast cancer (TNBC) has a poor prognosis compared with other breast cancer subtypes. This systematic review and meta-analysis examines whether known risk factors for breast cancer are also associated with TNBC in adult women.
Methods
EMBASE, Medline, SCOPUS, and gray literature were queried with no limit on the date or language of publication. The exposures of interest included parity, breastfeeding, duration of breastfeeding, age at menarche, age at first live birth, oral contraceptive (OC) use, duration of OC use, use of menopausal hormone therapy (MHT), family history, body mass index (BMI), alcohol use, smoking, and breast density. The main outcome of interest was TNBC. Study quality was determined using the Newcastle-Ottawa scale for case control studies and cohort studies. We estimated weighted odds ratios from random effects models to study the exposure–outcome associations. Protocol was registered under the number: PROSPERO 2021 CRD42021254594.
Results
Thirty-three studies were included. Family history, longer duration of OC use, and higher breast density were significantly associated with increased risk for TNBC, whereas later age at menarche, later age at first birth, and breastfeeding were protective against TNBC. Parity, MHT, alcohol, smoking, and BMI were not significantly associated with TNBC overall, but higher parity was associated with higher risk among Black women.
Conclusion
Our findings highlight that TNBC has a distinct risk factor profile compared with overall breast cancer. This can be the foundational work in identification of actionable TNBC risk factors to improve prevention and early detection of these poor prognosis breast tumors.
Breast cancer is a heterogeneous disease with distinct molecular subtypes (1). The basal-like subtype, which is marked by expression of genes usually found in basal cells of the normal breast, is associated with poor prognosis (2). Immunohistochemistry is typically used to measure expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) as a proxy for molecular subtype. Cancers that do not express ER, PR, or HER2 are known as triple-negative breast cancers (TNBC), which to a large extent overlap with the basal-like molecular subtype. TNBCs are more aggressive, are less likely to be detected by mammography screening than other breast cancers (3), and do not respond to therapies targeting the estrogen or HER2 pathways, making TNBCs more deadly than hormone receptor positive subtypes (4). The 5-year survival for TNBC is just over 75%, compared with 95% for ER/PR+HER2- tumors (5,6). Additionally, TNBCs have been found to have a higher prevalence among African ancestry populations (7). Given the stark differences in prognosis between subtypes, it is crucial to understand the unique risk profile for TNBC in order to aid prevention and screening efforts.
Prior studies have shown that breast cancer subtypes have unique etiologies (8). A systematic review found that most established breast cancer risk factors were associated with ER/PR+HER2- breast cancer; associations with TNBC were less consistent, partly because of fewer studies and smaller samples sizes among TNBCs (8). However, there were too few studies at the time to perform a meta-analysis. Few meta-analyses have been conducted that solely evaluate the risk factors for TNBC. Prior meta-analyses mainly have assessed reproductive and lifestyle risk factors (9-13). Only one meta-analysis, to our knowledge, has reviewed the association of breast density with ER, PR, and HER2 status; however, receptor status was analyzed individually rather than grouped into subtypes (14).
This systematic review and meta-analysis examined the associations of known breast cancer risk factors, including age, age at menarche, age at first live birth, parity, breastfeeding and breastfeeding duration, family history of breast cancer, oral contraceptive (OC) use and duration of use, age at menopause, use of menopausal hormone therapy (MHT), breast density, prior biopsy of benign breast disease, body mass index (BMI), and alcohol consumption with TNBCs in adult women.
Methods
Our study methodology was developed a priori and was registered at PROSPERO register for systematic review protocols: registration number PROSPERO 2021 CRD42021254594 (15). All methods and findings have been reported as per PRISMA guidelines; the PRISMA checklist is present in Supplementary Table 1 (available online).
Search strategy
Keywords that included alternative terms for exposures and outcome were drafted and reviewed by all members of the team. The search queries for specific databases were drafted by BN and reviewed by the team. We executed searches on Medline, EMBASE, and SCOPUS with no restriction on date or language of publication. A gray literature search was conducted on Google Scholar and reference lists of included studies. The detailed search queries used for each database are provided in Supplementary Figure 1 (available online). The search was initially conducted on May 26, 2021, and was repeated on May 29, 2022, and November 22, 2022.
Study selection
Studies were screened using the web-based application Rayyan (16). Four reviewers performed the screening (NK, SE, SB, and CF) in a blinded fashion. Each reviewer checked 20% of each reviewer’s work. All conflicts pertaining to decision on exclusion/inclusion of studies were resolved in a group discussion and voting. Screening was performed in 3 stages: 1) titles + abstract screen, 2) full-text screen, and 3) screening during data extraction stage where studies were excluded if data were not available from the authors.
Study inclusion and exclusion criteria
The outcome of interest was TNBC, and it was defined as invasive breast cancer tumors that are ER-, PR-, and HER2- as assessed by immunohistochemistry, fluorescent in situ hybridization, or genomic profiling. Studies based on biological males or participants with unilateral or bilateral mastectomy were excluded. To ensure minimum study quality and assess temporality, we restricted to cohort and case-control designs. Studies were included if they compared occurrence of TNBC with no cancer and excluded if the comparison group were not cancer-free.
Exposures
All studies that reported association between TNBC and any of the following exposures were included: age, age at menarche, age at first live birth, parity, breastfeeding, duration of breastfeeding, family history of breast cancer, OC use, duration of OC use, use of MHT, breast density, BMI, alcohol consumption, and smoking. Family history was defined as a prior diagnosis of breast cancer in a first-degree relative. For binary exposures (breastfeeding, OC use, MHT use, family history, alcohol, smoking), the comparison groups consisted of participants who did not have the exposure. For ordinal or continuous exposures (BMI, age at menarche, age at first live birth, duration of breastfeeding, duration of OC use, breast density), we considered the exposed category to be the one with highest degree of exposure, and the comparator category to be the one with lowest degree of exposure. For example, in the case of the continuous exposure, duration of breastfeeding, the exposed group had participants with highest duration of breastfeeding (≥12 months), and the comparison group had participants with least duration of breastfeeding (0 months).
Data extraction and management
Five reviewers (NK, SE, SB, CF, and SAN) independently performed data extraction using a tabular template (Supplementary Figure 2, available online). This template captured information on authors, year of publication, study setting, study design, exposures, and outcome. For all ordinal and continuous exposures, we reconciled the level of exposure for exposed and comparison group for all studies at the extraction stage to enable pooling and meta-analysis. Whenever data were not available for exposures of interest in the desired format in the published material, we reached out to the corresponding authors to share the requisite data.
Risk of bias
SE and CF independently assessed the study quality using the Newcastle Ottawa Quality Assessment Scale for case control studies and for cohort studies (17). Assessment entailed rating the studies on the basis of the following 3 elements for case control studies: participant selection procedures (case definition, representativeness of cases, cohort selection, and definition), comparability of cases and controls, and ascertainment of exposure (method of measurement, similarity of measurement method for cases and controls, nonresponse rate). For cohort studies, in addition to the previously described criteria for selection and comparability, the third component was ascertainment of outcome. This was assessed on the basis of the measurement method, length of follow-up, and lost to follow-up. Maximum possible score for selection component was 4, and comparability component could receive maximum score of 2. The third component of ascertainment of exposure/outcome could receive a maximum score of 3. The scores of all 3 components were converted into percentages for interpretation. An example of assessment of risk of bias is provided in Supplementary Figure 3 (available online).
Statistical analysis
The association of exposures of interest with TNBC was quantified with weighted odds ratios (ORs). Weighting was done using a restricted maximum likelihood random effects model, as described by Hardy and Thompson (18). Heterogeneity was assessed by computing Higgins’s I2 statistic using Higgins and Thompson’s (19) method. For Higgins’s I2, we considered the values of I2 between 0% and 40% as low heterogeneity, 41% to 65% as moderate, and above 65% as high levels of statistical heterogeneity, provided the P value was less than .05. Sensitivity analysis was performed by carrying out iterations of the meta-analysis leaving one study out at a time, using the leave one out meta-analysis method. Continuity correction by adding a value of .5 to all the cells was performed at the estimation stage in studies that had any cells with a zero. Publication bias was assessed visually by contour enhanced funnel plots of log odds-ratio estimates and standard errors of individual studies. Quantitatively, small study effects were studied using Egger’s test. Statistical significance was assessed using 95% confidence intervals (CIs) for odds ratios, and P values for I2. Results were considered significant at P values less than .05. Only 2-sided P values have been used and reported in this article. All analyses were performed using STATA 18 (College Station, TX).
Results
Our initial search yielded 1238 studies; after removal of 136 duplicates, 1102 studies were screened on the basis of title and abstract. A total of 89 studies from this list matched the inclusion criteria and underwent full-text screening. At this stage, from the reference lists of these studies and from a Google Scholar search, we identified 4 additional studies that matched the inclusion criteria and had the required estimates for meta-analysis. Thirty-three studies met inclusion criteria and were included in the meta-analysis (Table 1). The PRISMA flow diagram depicting inclusions and exclusions is present in Supplementary Figure 31 (available online).
Table 1.
Characteristics of included studiesa
| ID | Authors | Year | Design | Location | Menopausal status | Sample size | # TNBC cases | Race specification | Exposures studied |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Akinyemiju et al. (20) | 2021 | Case-control | Nigeria | Any | 338 | 52 | Black | BMI, MHT use |
| 2 | Ambrosone et al. (21) | 2015 | Case-control | USA | Any | 36 304 | 1356 | Black | Age at menarche, age at first live birth |
| 3 | Ambrosone et al. (22) | 2014 | Case-control | USA | Any | 1146 | 131 | Black | Breastfeeding, parity |
| 4 | Atkinson et al. (23) | 2016 | Case-control | USA | Any | 620 | 64 | Not specified | Breastfeeding, family history, parity |
| 5 | Azubuike et al. (24) | 2022 | Case-control | Nigeria | Any | 759 | 71 | Black | OC use, parity |
| 6 | Beaber et al. (25) | 2014 | Case-control | USA | Any | 1867 | 171 | Not specified | Duration of OC use, OC use |
| 7 | Bethea et al. (26) | 2016 | Case-control | USA | Any | 21 940 | 696 | Black | Family history |
| 8 | Bethea et al. (27) | 2015 | Case-control | USA | Any | 12 935 | 494 | Black | Duration of OC use, OC use |
| 9 | Bigman et al. (28) | 2022 | Case-control | Nigeria | Any | 1400 | 123 | Black | Alcohol, BMI, breastfeeding, parity, smoking |
| 10 | Ellingjord-Dale et al. (29) | 2017 | Case-control | Norway | Any | 26 236 | 386 | Not specified | Age at first live birth, breastfeeding, duration of breastfeeding, MHT use, OC use, parity |
| 11 | Figueroa et al. (30) | 2020 | Case-control | Ghana | Any | 2198 | 102 | Black | Parity |
| 12 | Gaudet et al. (31) | 2011 | Case-control | USA | Post-menopausal | 3678 | 246 | Not specified | BMI, family history, OC use |
| 13 | Gomes et al. (32) | 2022 | Case-control | Brazil | Any | 634 | 43 | Hispanic | Alcohol, BMI, family history, OC use, parity, smoking |
| 14 | Islam et al. (33) | 2012 | Case-control | Japan | Any | 1415 | 68 | East Asian | Age at first live birth, alcohol, BMI, breastfeeding, family history, parity |
| 15 | John et al. (34) | 2018 | Case-control | USA | Any | 5669 | 558 | Black/East Asian/Hispanic | Age at menarche, breastfeeding, duration of breastfeeding, parity |
| 16 | Kleinstern et al. (35) | 2021 | Case-control | USA | Any | 13 640 | 369 | Not specified | BMI, breast density, family history, MHT use |
| 17 | Klintman et al. (36) | 2022 | Cohort | Sweden | Post-menopausal | 31 510 | 60 | Not specified | BMI |
| 18 | Lee et al. (37) | 2020 | Case-control | Hong Kong | Any | 1150 | 137 | East Asian | BMI, duration of breastfeeding, parity |
| 19 | Li et al. (38) | 2013 | Case-control | USA | Any | 1125 | 184 | Not specified | Age at menarche, age at first live birth, breastfeeding, duration of breastfeeding, parity |
| 20 | Ma et al. (39) | 2009 | Case-control | USA | Post-menopausal | 482 | 106 | Not specified | Breast density |
| 21 | Ma et al. (40) | 2017 | Case-control | USA | Any | 3002 | 554 | Not specified | Age at menarche, age at first live birth, duration of breastfeeding, parity |
| 22 | Ma et al. (41) | 2018 | Case-control | USA | Post-menopausal | 3416 | 1030 | Not specified | BMI |
| 23 | McCarthy et al. (3) | 2021 | Cohort | USA | Any | 198 278 | 300 | Not specified | Age at menarche, age at first live birth, BMI, breast density, family history, parity |
| 24 | Palmer et al. (42) | 2014 | Case-control | USA | Not mentioned | 14 747 | 567 | Black | Breastfeeding, parity |
| 25 | Park et al. (43) | 2016 | Case-control | USA | Any | 18 070 | 694 | Black | Smoking |
| 26 | Phipps, Buist, et al. (44) | 2011 | Cohort | USA | Not mentioned | 1 055 171 | 705 | Not specified | Family history |
| 27 | Phipps et al. (45) | 2011 | Cohort | USA | 155 723 | 307 | Not specified | Age at menarche, age at first live birth, BMI, duration of breastfeeding, duration of OC use, family history, MHT use, OC use, parity, smoking | |
| 28 | Phipps et al. (46) | 2008 | Case-control | USA | Post-menopausal | 1554 | 78 | Not specified | Age at menarche, age at first live birth, breastfeeding, family history, MHT use, parity |
| 29 | Razzaghi et al. (47) | 2013 | Case-control | USA | Any | 1019 | 48 | Not specified | Breast feeding, family history, OC use |
| 30 | Shin et al. (48) | 2018 | Case-control | Korea | Any | 1883 | 31 | East Asian | Breast density |
| 31 | Wang et al. (49) | 2022 | Cohort | USA | Post-menopausal | 121 744 | 529 | Not specified | Age at menarche, breastfeeding, BMI, family history, parity, smoking |
| 32 | Wang et al. (50) | 2020 | Case-control | China | Any | 7974 | 448 | East Asian | Age at menarche, age at first live birth, breastfeeding, duration of breastfeeding, parity |
| 33 | Williams et al. (51) | 2016 | Case-control | USA | Any | 1495 | 229 | Black | Alcohol |
BMI = body mass index; MHT = menopausal hormone therapy; OC = oral contraceptive; TNBC = triple-negative breast cancer.
Overview of included studies
Of the 33 included studies (Table 1), 22 were from the United States, 4 from Africa (3 Nigeria, 1 Ghana), 4 from Asia (Japan, Hong Kong, China, and South Korea), 2 from Scandinavia (Norway and Sweden), and 1 from South America (Brazil). With respect to racial/ethnic distribution, 11 studies included exclusively Black participants, 4 Asian participants, 1 Hispanic participants, and the remaining 17 studies had participants from multiple racial/ethnic backgrounds. There were 4 cohort studies and 29 case-control studies included.
Risk of bias
The summary of risk of bias assessment scores for all studies has been summarized in Supplementary Figure 4 (available online). Risk of bias assessment was performed for 3 elements in each study: 1) participant selection, 2) comparability of cases/controls, and 3) ascertainment of exposure/outcome. Most studies scored quite well in the comparability of cases and controls section, with a median score of 100%. The section on participant selection had a median score of 75% (interquartile range = 25%, 100%) indicating most studies had moderately good scores and were mostly free of selection bias. The section on exposure/outcome ascertainment had least scores compared to the other 2 sections with a median score of 34% (interquartile range = 34%, 67%).
Age at first live birth
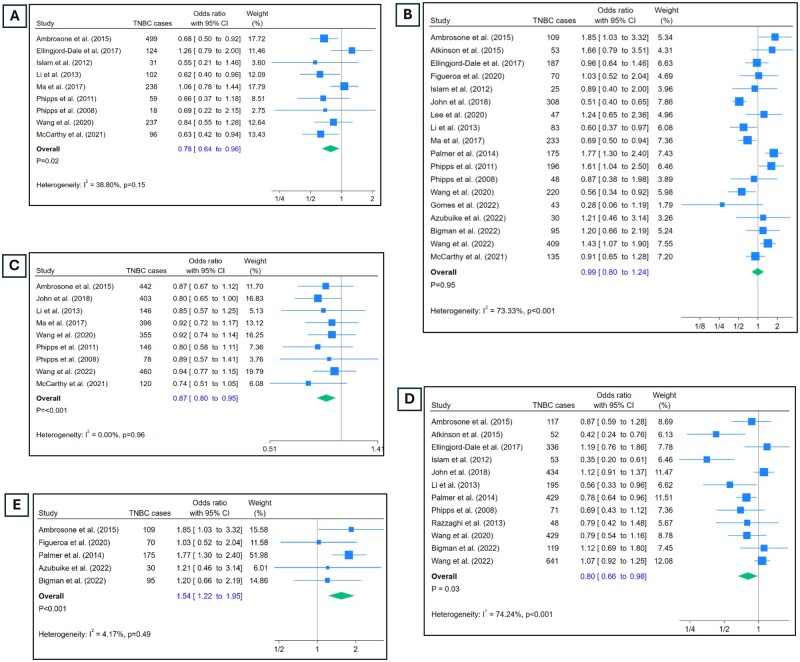
Nine studies report data on the age at first live birth (Figure 1, A). Having a first birth at 30 years of age or older was associated with lower odds of TNBC compared with age 19 years or younger at first birth (OR = 0.78, 95% CI = 0.64 to 0.96). Heterogeneity in the estimates was low (I2 = 38%), and sensitivity analysis using leave-one-out meta-analysis method revealed there were no outliers in the included studies (Supplementary Figure 10, available online).
Figure 1.
Odds of triple-negative breast cancer (TNBC) by reproductive risk factors. A) Odds of TNBC in those who had first live birth at 30 years of age or older vs those who had it at 19 years or younger. B) Odds of TNBC in those with 3 or more live births vs 0 live births. C) Odds of TNBC in those who had menarche at 14 years or older vs those who had it at 11 years or younger. D) Odds of TNBC in those who ever breastfed vs those who never did. E) Odds of TNBC in Black participants with 3 or more live births vs 0 live births.
Parity
Eighteen studies contributed estimates for parity (Figure 1, B). Participants with 3 or more live births had similar odds of developing TNBC compared with those had no live births (OR = 0.99, 95% CI = 0.8 to 1.24). There was high level of heterogeneity in the estimates (I2 = 73.3%, P < .00). From the sensitivity analysis, it appeared that considerable heterogeneity was contributed by 2 studies: John et al. (34) and Palmer et al. (42) (Supplementary Figure 5, A, available online). Omitting these 2 studies and running the meta-analysis again (Supplementary Figure 5, B, available online) reduced the heterogeneity to 57% while not changing the estimates much (OR = 1.01, 95% CI = 0.8 to 0.23). Restricting the analysis to studies with only Black participants (24,28,30,42) revealed highly significant association of 3 or more live births and occurrence of TNBC with OR = 1.54 and 95% CI: 1.22 to 1.95 (Figure 1, E). This also brought down the heterogeneity significantly to 4.17% (P = .49).
Age at menarche
Nine studies reported odds of developing TNBC in those who experienced menarche at 14 years of age or older vs those who experienced it at 11 years of age or younger (Figure 1, C). Being older at menarche was associated with significantly lower odds of developing TNBC compared with those who were younger (OR = 0.87, 95% CI = 0.80 to 0.95). There was no heterogeneity in the estimates (I2 = 0%), and none of the studies were outliers (Supplementary Figure 9, available online).
Breastfeeding
Exposure to breastfeeding in a binary form (ever/never) was reported by 12 studies (Figure 1, D). The odds of developing TNBC were significantly lower in those who reported as having ever breastfed vs those who reported never breastfeeding (OR = 0.80, 95% CI = 0.66 to 0.98). There was high level of heterogeneity in the estimates as indicated by an I2 of 74.2% (P < .01). As per the sensitivity analysis (Supplementary Figure 6, A, available online), omitting any study would not change the estimates or heterogeneity much (Supplementary Figure 6, B, available online).
Duration of breastfeeding
Duration of breastfeeding was measured by 7 studies. The odds of developing TNBC were similar in participants who breastfed more than 12 months and those who never breastfed (Supplementary Figure 32, available online) with a pooled odds ratio of 1.02 (95% CI = 0.59 to 1.76). The level of heterogeneity in the estimates was high with I2 = 92.8%. Estimates from Phipps et al. (45) seemed to contribute sizeable heterogeneity; running the meta-analysis after omitting this study (Supplementary Figure 7, A and B, available online) reduced the heterogeneity to 53% and did not change the pooled estimate much (OR = 0.83, 95% CI = 0.66 to 1.05).
OC use
Six studies contributed estimates for oral contraceptive use (Supplementary Figure 33, available online). Those who ever used OCs had similar odds of TNBC compared with those who never used them (OR = 1.16, 95% CI = 0.92 to 1.46). The estimates had moderate heterogeneity (I2 = 62.7%, P = .04). The heterogeneity was contributed mostly by Gaudet et al. (31) as assessed in sensitivity analysis (Supplementary Figure 11, A, available online), where removal of this study significantly improved the precision (Supplementary Figure 11, B, available online) as well as reduced the heterogeneity to 0% while not changing the overall effect estimate (OR = 1.18, 95% CI = 1.04 to 1.35, P = .012).
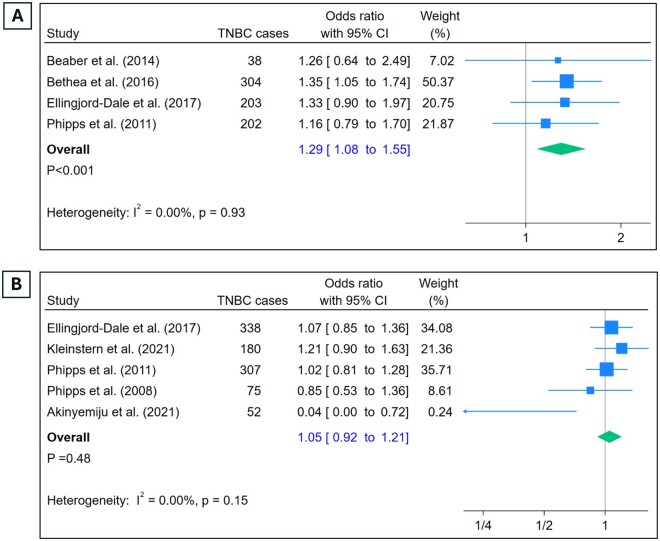
Duration of OC use
Duration of OC use was reported by 4 studies (Figure 2, A). Comparison of odds of developing TNBC in those with 10 years or more of use of OCs vs those who never used OCs revealed a clear pattern of significantly increased odds in the users (OR = 1.29, 95% CI = 1.08 to 1.55). Sensitivity analysis did not indicate presence of outliers (Supplementary Figure 12, available online).
Figure 2.
Odds of TNBC and use of hormones. A) Odds of TNBC in those with 10 or more years of OC use vs. no use. B) Odds of TNBC in those who ever used MHT vs those who never did. OC = oral contraceptive; MHT = menopausal hormone therapy; TNBC = triple-negative breast cancer.
Menopausal hormone therapy use
Five studies reported the use of MHT (Figure 2, B). The association between MHT use and TNBC was not statistically significant (OR = 1.05, 95% CI = 0.92 to 1.21). There was no heterogeneity in the estimates (I2 = 0%), and the sensitivity analysis did not indicate presence of outliers (Supplementary Figure 13, available online).
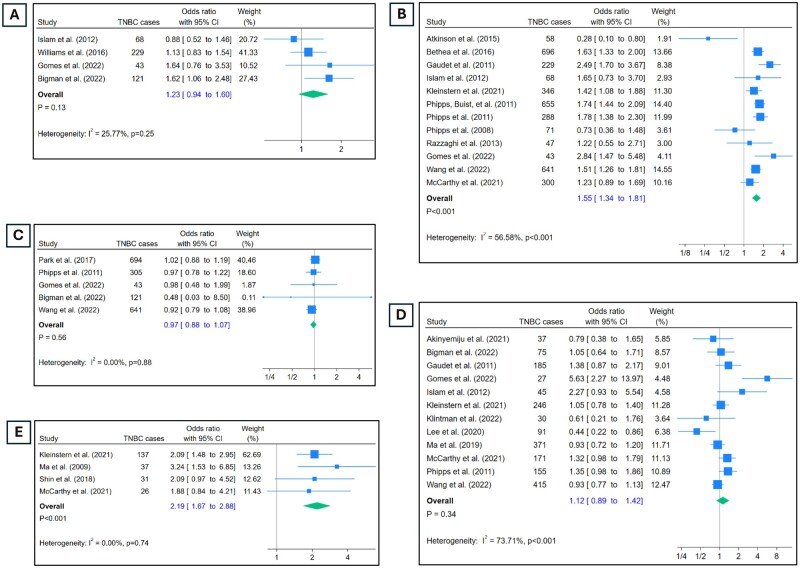
Alcohol use
Use of alcohol was analyzed in a binary form as “ever used” vs “never used” (Figure 3, A). This comparison did not reveal any difference in the odds of developing TNBC (OR = 1.23, 95% CI = 0.94 to 1.6). Heterogeneity in the estimates was low (I2 = 25%), and there were no significant outliers (Supplementary Figure 14, available online).
Figure 3.
Odds of TNBC by lifestyle factors, family history, BMI, and breast density. A) Odds of TNBC in participants who ever consumed alcohol vs those who never did. B) Odds of TNBC in those with history of breast cancer in 1° relative vs those without. C) Odds of TNBC in ever smokers vs never smokers. D) Odds of TNBC in those with BMI ≥30 kg/m2 vs BMI <25 kg/m2, stratified by menopausal status. E) Odds of TNBC in those with ≥75% mammographic density vs ≤25%. BMI = body mass index.
Family history
Twelve studies contributed estimates on family history of breast cancer (Figure 3, B). Participants with family history of breast cancer in an immediate family member had significantly higher odds of developing TNBC compared with those who did not (OR = 1.55, 95% CI = 1.34 to 1.81). There was moderate heterogeneity in the estimates (I2 = 56.5%, P < .01). Although 2 studies reported a beneficial effect of having family history on developing TNBC [Atkinson et al. (23) and Phipps et al. (46)], sensitivity analysis did not indicate these to significantly influence the overall estimate (Supplementary Figure 8, available online).
Smoking
Among 5 studies, participants who reported ever smoking had similar odds of developing TNBC (Figure 3, C) compared with those who reported being never smokers (OR = 0.97, 95% CI = 0.88 to 1.07). Estimates did not have heterogeneity (I2 = 0%), and sensitivity analysis did not reveal presence of significant outliers (Supplementary Figure 15, available online).
BMI
Overall, estimates on BMI and TNBC were contributed by 12 studies (Figure 3, D). The pooled estimates revealed that a BMI of 30 or higher at diagnosis was not associated with TNBC (OR = 1.12, 95% CI = 0.89 to 1.42). The level of heterogeneity was high (I2 = 73.7%, P < .01), and one particular study, Gomes et al. (32), contributed a significant portion of it. Upon omitting estimates from this study (Supplementary Figure 17, A and B, available online), the heterogeneity reduced significantly to 30%, and the pooled odds ratio did not change significantly (OR = 1.03, 95% CI = 0.88 to 1.21). Restricting the analysis to post-menopausal women only (3,31,36,41,45,49) did not change the estimate much (OR = 1.07, 95% CI = 0.87 to 1.30). Higher BMI was found not be associated with TNBC within postmenopausal women or group of mixed menopausal status women.
Mammographic breast density
Among 4 studies, higher breast density was significantly associated with higher odds of TNBC (Figure 3, E; OR = 2.19, 95% CI = 1.67 to 2.88). There was no heterogeneity (I2 = 0%) or presence of outliers (Supplementary Figure 16, available online).
Publication bias
Visual asymmetry in contour-enhanced funnel plots was not seen in any of the exposures, except in OC use (Supplementary Figures 18-30, available online). However, Egger’s test for presence of small-study effects was not significant in any of the exposures, except breastfeeding (Supplementary Figure 19, A, available online) and MHT use (Supplementary Figure 25, A, available online) with P = .016 and P = .025, respectively. Assessing the extent of missing studies in both these using the trim and fill method indicated 2 studies being imputed for breastfeeding with observed 12 studies (Supplementary Figure 19, B, available online). For MHT as well, the trim and fill algorithm indicated imputation of 2 missing studies with observed 5 studies (Supplementary Figure 25, B, available online).
Discussion
This systematic review and meta-analysis examined the association of established breast cancer risk factors with TNBC. Family history, longer duration of oral contraceptive use, and higher breast density were significantly associated with increased risk for TNBC, whereas later age at menarche and breastfeeding were protective against TNBC (Table 2). In contrast to published associations with overall breast cancer, later age at first birth was protective against TNBC. Parity, MHT, alcohol, smoking, and BMI were not significantly associated with risk for TNBC. These findings further clarify differences in etiology between TNBC and breast cancer overall and highlight the need to identify additional actionable risk factors for TNBC to improve prevention and early detection of these poor prognosis breast tumors.
Table 2.
Summary of findingsa
| Exposure | Pooled OR (95% CI) for TNBC | Heterogeneity I2 (P value) | # Studies | #TNBC cases |
|---|---|---|---|---|
| Reproductive Risk Factors | ||||
| Parity (≥3 live births/0 live births) | 0.99 (0.80, 1.24) | 73% (<.001) | 18 | 2466 |
| Breastfeeding (Yes/No) | 0.80 (0.66, 0.98) | 74% (<.001) | 8 | 2924 |
| Duration of breastfeeding (≥12 m/0 m) | 1.02 (0.59, 1.76) | 92% (<.001) | 7 | 1294 |
| Age at menarche (≥14 yr/<12 yr) | 0.87 (0.80, 0.95) | 0% (.96) | 9 | 2546 |
| Age at first live birth (≥30 yr/<20 yr) | 0.78 (0.64, 0.96) | 38% (.15) | 9 | 1404 |
| Hormone use | ||||
| OC use (Yes/No) | 1.16 (0.92, 1.46) | 62% (.04) | 6 | 1310 |
| Duration of OC use (≥10 yr/0 yr) | 1.29 (1.08, 1.55) | 0% (.93) | 4 | 747 |
| MHT use (Yes/No) | 1.05 (0.92, 1.21) | 0% (.15) | 5 | 952 |
| Other Factors | ||||
| Family history (Yes/No) | 1.55 (1.34, 1.81) | 56% (<.001) | 12 | 3442 |
| Alcohol (Yes/No) | 1.23 (0.94, 1.60) | 25% (.25) | 4 | 461 |
| Smoking (Yes/No) | 0.97 (0.88, 1.07) | 0% (.88) | 5 | 1804 |
| BMI (≥30/<25) | 1.12 (0.89, 1.42) | 73% (<.001) | 12 | 1848 |
| Breast density (≥75%/≤25%) | 2.19 (1.67, 2.88) | 0% (0.74) | 4 | 231 |
CI = confidence intervals; TNBC = triple negative breast cancer; BMI = body mass index; OC = oral contraceptive; MHT = menopausal hormone therapy.
Both family history and breast density were strongly associated with increased risk of TNBC, with similar associations to those reported for breast cancer overall (9-11). This suggests that breast cancer prevention and screening recommendations targeted to patients with family history and dense breasts are relevant for TNBC prevention and early detection. Similarly, later age at menarche and ever breastfeeding were associated with reduced risk of TNBC, similar to breast cancer overall (12). We found no significant association between parity and TNBC overall, but having high parity was associated with increased TNBC risk among Black women. This finding is consistent with prior findings from the Black Women’s Health Study that that TNBC risk was particularly high among women with high parity who had not breastfed (42). However, we were unable to evaluate the interaction between parity and breastfeeding, which has been previously reported (34,42), as these data were unavailable in most studies (42).
We found use of OCs for at least 10 years was associated with nearly 30% increased risk of TNBC. Studies have consistently shown a small but significant increased risk of breast cancer overall due to OC use, which is highest when patients are using OCs and declines with increased time since last use of OCs (52,53). The level of risk estimated in this study for long-term OC use is higher than what was reported for the risk of long-term OC use with breast cancer overall in a meta-analysis of cohort studies (54), but consistent with a large study of OC use and breast cancer risk overall among 1.8 million Danish women (55).
Contrary to studies in breast cancer overall, having a first child at age 30 or older decreased risk for TNBC by more than 20% compared with early age at first birth (<20). Maternal ages at first birth have been increasing, with most recent data showing a median age at first birth in the United States of 30 years overall, and only slightly lower, age 28, for Black women (13). These reproductive patterns would suggest decreasing risk for TNBC over time on the population level.
We observed no statistically significant association between BMI and risk of TNBC, either overall or when limiting to studies of postmenopausal women. This is in direct contrast to the well-established positive association between higher BMI and risk of postmenopausal breast cancer overall and ER/PR+ breast cancers (14). A prior meta-analysis similarly found no association between postmenopausal BMI and hormone receptor negative tumors (14). There were too few studies with enough information to evaluate the association of BMI and TNBC among premenopausal women.
Black women in the United States have significantly higher rates of TNBC than White women (56). Black women on average have earlier age at menarche (57), are an earlier age at first birth (13), and are less likely to breastfeed (58) than White women. All of these risk factor distributions are consistent with observed higher population rates of TNBC for Black women compared with White women. Although age at menarche is not easily modifiable, recent trends toward older age at first birth as well as interventions to increase breastfeeding rates among Black women may help reduce risk of TNBC.
Strengths and limitations
This is the first comprehensive meta-analysis to report the association of 13 known risk factors of breast cancer with TNBC and, to our knowledge, the largest meta-analysis of risk factors for TNBC published to date. One of the main strengths of this analysis is the large sample size in all the included analyses and, consequently, the precision in the estimates.
Of the 13 exposures meta-analyzed in this article, most had low to moderate levels of heterogeneity. Even among the exposures with higher levels of heterogeneity, the heterogeneity went down to 30% for BMI and 53% for duration of breastfeeding upon excluding 1 study. For breastfeeding, however, the heterogeneity was more spread out across the studies. This is possibly because of the broad definition of breastfeeding categories as “ever” and “never.” Owing to this binary categorization, most participants who fell under the “ever” category would still likely have varying levels of breastfeeding—this is bound to introduce between-study differences in the estimates.
One limitation of the existing literature was the dearth of studies that stratified effect estimates by menopause status or age at menopause, making evaluations of differences in risk factor associations by menopause status, particularly among premenopausal women, difficult. Given that only 2 (25,31) of the included studies reported the formulation of OCs used, it was not possible to examine the effects of different formulations of OCs. Similarly, we were unable to evaluate the effects of duration and formulation of MHT use. In addition, there was a lack of sufficient data to evaluate risk factor associations by race/ethnicity. Of the included studies, only 2 (3,40) reported race stratified estimates for age at first live birth, breastfeeding, and parity, which was not sufficient to meta-analyze. The presence of BRCA1 mutation is associated with increased risk of TNBC. With the exception of McCarthy et al. (3), who excluded BRCA1 carriers, none of the included studies disclosed the BRCA1 status of the participants. Consequently, it is not possible to completely rule out the potential effect of BRCA1 mutations in the estimates. All included studies except 2 (35 and 41) excluded participants with secondary breast cancer. Because these two studies contributed estimates for parity and OC use, it cannot be ruled out that associations seen are partly because of inclusion of secondary breast cancer cases. Finally, given the pooled estimates were computed using unadjusted count data extracted from individual studies, it was not possible to compute adjusted pooled estimates. Because of this, the strength of associations may be overstated.
In conclusion, our findings confirm family history, breast density, and longer use of oral contraceptive hormones are associated with increased odds of TNBC. Age at first birth was inversely associated with TNBC, which is contrary to the association seen with breast cancer overall. We did not find a significant association of parity, MHT, alcohol, smoking, and BMI with overall TNBC risk; however, a significant association of high parity with TNBC among Black women was seen. Given the new insight from this study that TNBC has a unique risk-factor profile compared with breast cancer overall, this should inform screening and risk reduction strategies. Further research should evaluate whether additional lifestyle and environmental risk factors such as diet, physical activity, environmental exposures, and medication use contribute to TNBC risk in order to design policy interventions for prevention.
Supplementary Material
Acknowledgments
This work was presented at the American Association of Cancer Research (AACR) conference on Advances in Breast Cancer Research, held in San Diego in October 2023. The funders were not involved in any stage of the study conception, design, data collection, analysis, interpretation, manuscript writing, or the decision to submit the manuscript for publication.
Contributor Information
Nitya Kumar, Department of Medicine, Royal College of Surgeons in Ireland - Bahrain, Busaiteen, Bahrain.
Sarah Ehsan, Department of Biostatistics, Epidemiology & Informatics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Shahana Banerjee, Department of Biostatistics, Epidemiology & Informatics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Claudia Fernandez Perez, Department of Biostatistics, Epidemiology & Informatics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Isabelle Lhuilier, Department of Biostatistics, Epidemiology & Informatics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Jillian Neuner, Department of Biostatistics, Epidemiology & Informatics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Tara Friebel-Klingner, Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, USA.
Oluwadamilola M Fayanju, Department of Biostatistics, Epidemiology & Informatics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Bindhu Nair, Department of Medicine, Royal College of Surgeons in Ireland - Bahrain, Busaiteen, Bahrain.
Sara Anjum Niinuma, Department of Medicine, Royal College of Surgeons in Ireland - Bahrain, Busaiteen, Bahrain.
Shivangi Nampoothiri, Science Department, Christ University, Bangalore, KA, India.
Anne Marie McCarthy, Department of Biostatistics, Epidemiology & Informatics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Data availability
No new/primary data were generated for this work. The data extracted for conducting the analysis presented in this work will be available upon reasonable request.
Author contributions
Nitya Kumar, PhD, MS, MFCSc, BSc, FHEA (Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Software; Supervision; Validation; Visualization; Writing—original draft; Writing—review & editing), Sarah Ehsan, MPH, BA (Data curation; Investigation; Project administration; Resources; Validation; Writing—original draft; Writing—review & editing), Shahana Banerjee, BA (Data curation; Investigation; Writing—review & editing), Claudia Fernandez Perez, MHS, BS (Data curation; Investigation; Validation; Writing—review & editing), Isabelle Lhuilier, BA (Data curation; Investigation; Visualization; Writing—review & editing), Jilian Neuner, BA (Data curation; Investigation; Validation; Writing—review & editing), Tara Friebel-Klingner, PhD, MPH (Data curation; Investigation; Validation; Writing—review & editing), Oluwadamilola M. Fayanju, MD, MA, MPHS (Conceptualization; Validation; Writing—review & editing), Bindhu Nair, PhD, MSc, BSc (Data curation; Investigation; Methodology; Software; Writing—review & editing), Sara Anjum Niinuma, MB, BCH, BAO (Data curation; Investigation; Validation; Writing—review & editing), Shivangi Nampoothiri, BSc- BCZ (Data curation; Investigation; Writing—review & editing), Anne Marie McCarthy, PhD, ScM (Conceptualization; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Writing—original draft; Writing—review & editing).
Funding
This work was supported by grants from RCSI-MUB Bahrain (PI: NKumar, project number: 151/13-Apr-2021) and the American Cancer Society (PI McCarthy MSRG-17-144-01-CCE). Dr. Fayanju is supported by the National Institutes of Health (NIH) under Award Numbers 7K08CA241390-03 (PI: Fayanju) and P50CA244690 (PIs: Bekelman), the Breast Cancer Research Foundation, and philanthropic funds from the Haas family. She also reports research support unrelated to this work from Gilead Sciences, Inc.
Conflicts of interest
The authors report no conflicting interests associated with this work.
References
- 1. Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rakha EA, Elsheikh SE, Aleskandarany MA, et al. Triple-negative breast cancer: distinguishing between basal and nonbasal subtypes. Clin Cancer Res. 2009;15(7):2302-2310. [DOI] [PubMed] [Google Scholar]
- 3. McCarthy AM, Friebel-Klingner T, Ehsan S, et al. Relationship of established risk factors with breast cancer subtypes. Cancer Med. 2021;10(18):6456-6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dai X, Li T, Bai Z, et al. Breast cancer intrinsic subtype classification, clinical use and future trends. Am J Cancer Res. 2015;5(10):2929-2943. [PMC free article] [PubMed] [Google Scholar]
- 5. Haque R, Ahmed SA, Inzhakova G, et al. Impact of breast cancer subtypes and treatment on survival: an analysis spanning two decades. Cancer Epidemiol Biomarkers Prev. 2012;21(10):1848-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parise CA, Caggiano V.. Breast cancer survival defined by the ER/PR/HER2 subtypes and a surrogate classification according to tumor grade and immunohistochemical biomarkers. J Cancer Epidemiol. 2014;2014:469251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stark A, Kleer CG, Martin I, et al. African ancestry and higher prevalence of triple-negative breast cancer: findings from an international study. Cancer. 2010;116(21):4926-4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barnard ME, Boeke CE, Tamimi RM.. Established breast cancer risk factors and risk of intrinsic tumor subtypes. Biochim Biophys Acta. 2015;1856(1):73-85. [DOI] [PubMed] [Google Scholar]
- 9. Pettersson A, Graff RE, Ursin G, et al. Mammographic density phenotypes and risk of breast cancer: a meta-analysis. J Natl Cancer Inst. 2014;106(5):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bond-Smith D, Stone J.. Methodological challenges and updated findings from a meta-analysis of the association between mammographic density and breast cancer. Cancer Epidemiol Biomarkers Prev. 2019;28(1):22-31. [DOI] [PubMed] [Google Scholar]
- 11. Pharoah PD, Day NE, Duffy S, Easton DF, Ponder BA.. Family history and the risk of breast cancer: a systematic review and meta-analysis. Int J Cancer. 1997;71(5):800-809. [DOI] [PubMed] [Google Scholar]
- 12. Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012;13(11):1141-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ely DM, Hamilton BE.. Trends in fertility and mother’s age at first birth among rural and metropolitan counties: United States, 2007-2017. NCHS Data Brief. 2018;323:1-8. [PubMed] [Google Scholar]
- 14. Chan DSM, Abar L, Cariolou M, et al. World Cancer Research Fund International: Continuous Update Project—systematic literature review and meta-analysis of observational cohort studies on physical activity, sedentary behavior, adiposity, and weight change and breast cancer risk. Cancer Causes Control. 2019;30(11):1183-1200. [DOI] [PubMed] [Google Scholar]
- 15. Kumar N, Ehsan S, McCarthy AM. Are the established breast cancer risk factors associated with the triple negative intrinsic subtype of breast cancer in adult females? A systematic review and meta-analysis. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021254594. Accessed June 12, 2021.
- 16. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A.. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wells GSB, Shea BO’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. https://www.ohri.ca/programs/clinical_epidemiology/ oxford.asp. Accessed November 2022.
- 18. Hardy RJ, Thompson SG.. A likelihood approach to meta-analysis with random effects. Statist Med 1996;15(6):619-629. [DOI] [PubMed] [Google Scholar]
- 19. Higgins JP, Thompson SG.. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539-1558. [DOI] [PubMed] [Google Scholar]
- 20. Akinyemiju T, Jones K, Gupta A, et al. ; H3 Africa Kidney Research Network. Association of body composition with odds of breast cancer by molecular subtype: analysis of the Mechanisms for Established and Novel Risk Factors for Breast Cancer in Nigerian Women (MEND) study. BMC Cancer. 2021;21(1):1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ambrosone CB, Zirpoli G, Hong CC, et al. Important role of menarche in development of estrogen receptor-negative breast cancer in African American women. J Natl Cancer Inst. 2015;107(9):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ambrosone CB, Zirpoli G, Ruszczyk M, et al. Parity and breastfeeding among African-American women: Differential effects on breast cancer risk by estrogen receptor status in the Women’s Circle of Health Study. Cancer Causes Control. 2014;25(2):259-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Atkinson RL, El-Zein R, Valero V, et al. Epidemiological risk factors associated with inflammatory breast cancer subtypes. Cancer Causes Control. 2016;27(3):359-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Azubuike SO, Hayes L, Sharp L, McNally R.. Reproductive factors and the risk of breast cancer among Nigerian women by age and oestrogen receptor status. Cancer Causes Control. 2022;33(12):1401-1412. [DOI] [PubMed] [Google Scholar]
- 25. Beaber EF, Malone KE, Tang MT, et al. Oral contraceptives and breast cancer risk overall and by molecular subtype among young women. Cancer Epidemiol Biomarkers Prev. 2014;23(5):755-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bethea TN, Rosenberg L, Castro-Webb N, et al. Family history of cancer in relation to breast cancer subtypes in African American women. Cancer Epidemiol Biomarkers Prev. 2016;25(2):366-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bethea TN, Rosenberg L, Hong CC, et al. A case-control analysis of oral contraceptive use and breast cancer subtypes in the African American Breast Cancer Epidemiology and Risk Consortium. Breast Cancer Res. 2015;17(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bigman G, Adebamowo SN, Yawe KT, et al. Leisure-time physical activity is associated with reduced risks of breast cancer and triple negative breast cancer in Nigerian women. Cancer Epidemiol. 2022;79:102195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ellingjord-Dale M, Vos L, Tretli S, Hofvind S, Dos-Santos-Silva I, Ursin G.. Parity, hormones and breast cancer subtypes—results from a large nested case-control study in a national screening program. Breast Cancer Res. 2017;19(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Figueroa JD, Davis Lynn BC, Edusei L, et al. ; Ghana Breast Health Study Team Reproductive factors and risk of breast cancer by tumor subtypes among Ghanaian women: a population-based case-control study. Int J Cancer. 2020;147(6):1535-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gaudet MM, Press MF, Haile RW, et al. Risk factors by molecular subtypes of breast cancer across a population-based study of women 56 years or younger. Breast Cancer Res Treat. 2011;130(2):587-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gomes KAL, de Araujo Jeronimo AF, Guimaraes CMC, de Oliveira Ramos R, Dos Santos Andrade LS, Weller M.. Risk factors for breast cancer and their association with molecular subtypes in a population of northeast Brazil. Cancer Epidemiol. 2022;78:102166. [DOI] [PubMed] [Google Scholar]
- 33. Islam T, Matsuo K, Ito H, et al. Reproductive and hormonal risk factors for luminal, HER2-overexpressing, and triple-negative breast cancer in Japanese women. Ann Oncol. 2012;23(9):2435-2441. [DOI] [PubMed] [Google Scholar]
- 34. John EM, Hines LM, Phipps AI, et al. Reproductive history, breast-feeding and risk of triple negative breast cancer: the Breast Cancer Etiology in Minorities (BEM) study. Int J Cancer. 2018;142(11):2273-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kleinstern G, Scott CG, Tamimi RM, et al. Association of mammographic density measures and breast cancer “intrinsic” molecular subtypes. Breast Cancer Res Treat. 2021;187(1):215-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Klintman M, Rosendahl AH, Randeris B, et al. Postmenopausal overweight and breast cancer risk; results from the KARMA cohort. Breast Cancer Res Treat. 2022;196(1):185-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee PMY, Kwok CH, Chan WC, et al. Heterogeneous associations between obesity and reproductive-related factors and specific breast cancer subtypes among Hong Kong Chinese women. Horm Cancer. 2020;11(3-4):191-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li CI, Beaber EF, Tang MT, Porter PL, Daling JR, Malone KE.. Reproductive factors and risk of estrogen receptor positive, triple-negative, and HER2-neu overexpressing breast cancer among women 20-44 years of age. Breast Cancer Res Treat. 2013;137(2):579-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ma H, Luo J, Press MF, Wang Y, Bernstein L, Ursin G.. Is there a difference in the association between percent mammographic density and subtypes of breast cancer? Luminal A and triple-negative breast cancer. Cancer Epidemiol Biomarkers Prev. 2009;18(2):479-485. [DOI] [PubMed] [Google Scholar]
- 40. Ma H, Ursin G, Xu X, et al. Reproductive factors and the risk of triple-negative breast cancer in white women and African-American women: a pooled analysis. Breast Cancer Res. 2017;19(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ma H, Ursin G, Xu X, et al. Body mass index at age 18 years and recent body mass index in relation to risk of breast cancer overall and ER/PR/HER2-defined subtypes in White women and African-American women: a pooled analysis. Breast Cancer Res. 2018;20(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Palmer JR, Viscidi E, Troester MA, et al. Parity, lactation, and breast cancer subtypes in African American women: results from the AMBER Consortium. J Natl Cancer Inst. 2014;106(10):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Park SY, Palmer JR, Rosenberg L, et al. A case-control analysis of smoking and breast cancer in African American women: findings from the AMBER consortium. Carcinogenesis. 2016;37(6):607-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Phipps AI, Buist DS, Malone KE, et al. Family history of breast cancer in first-degree relatives and triple-negative breast cancer risk. Breast Cancer Res Treat. 2011;126(3):671-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Phipps AI, Chlebowski RT, Prentice R, et al. Reproductive history and oral contraceptive use in relation to risk of triple-negative breast cancer. J Natl Cancer Inst. 2011;103(6):470-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Phipps AI, Malone KE, Porter PL, Daling JR, Li CI.. Reproductive and hormonal risk factors for postmenopausal luminal, HER-2-overexpressing, and triple-negative breast cancer. Cancer. 2008;113(7):1521-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Razzaghi H, Troester MA, Gierach GL, Olshan AF, Yankaskas BC, Millikan RC.. Association between mammographic density and basal-like and luminal A breast cancer subtypes. Breast Cancer Res. 2013;15(5):R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shin J, Lee JE, Ko HY, et al. Association between mammographic density and tumor marker-defined breast cancer subtypes: a case-control study. Eur J Cancer Prev. 2018;27(3):239-247. [DOI] [PubMed] [Google Scholar]
- 49. Wang F, Kroenke CH, Pan K, et al. Racial differences in anthropometric measures as risk factors for triple-negative breast cancer. Cancer Causes Control. 2022;33(12):1413-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang JM, Wang J, Zhao HG, Liu TT, Wang FY.. Reproductive risk factors associated with breast cancer molecular subtypes among young women in northern China. Biomed Res Int. 2020;2020:5931529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Williams LA, Olshan AF, Tse CK, Bell ME, Troester MA.. Alcohol intake and invasive breast cancer risk by molecular subtype and race in the Carolina Breast Cancer Study. Cancer Causes Control. 2016;27(2):259-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347(9017):1713-1727. [DOI] [PubMed] [Google Scholar]
- 53. Fitzpatrick D, Pirie K, Reeves G, Green J, Beral V.. Combined and progestagen-only hormonal contraceptives and breast cancer risk: a UK nested case-control study and meta-analysis. PLoS Med. 2023;20(3):e1004188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhu H, Lei X, Feng J, Wang Y.. Oral contraceptive use and risk of breast cancer: a meta-analysis of prospective cohort studies. Eur J Contracept Reprod Health Care. 2012;17(6):402-414. [DOI] [PubMed] [Google Scholar]
- 55. Morch LS, Skovlund CW, Hannaford PC, Iversen L, Fielding S, Lidegaard O.. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377(23):2228-2239. [DOI] [PubMed] [Google Scholar]
- 56. Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(5):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Martinez GM. Trends and patterns in menarche in the United States: 1995 through 2013-2017. Natl Health Stat Rep. 2020;(146):1-12. [PubMed] [Google Scholar]
- 58. Chiang KV, Li R, Anstey EH, Perrine CG.. Racial and ethnic disparities in breastfeeding initiation – United States, 2019. MMWR Morb Mortal Wkly Rep. 2021;70(21):769-774. doi: 10.15585/mmwr.mm7021a1external. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new/primary data were generated for this work. The data extracted for conducting the analysis presented in this work will be available upon reasonable request.