Abstract
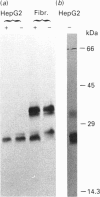
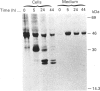
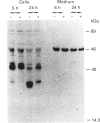
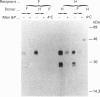
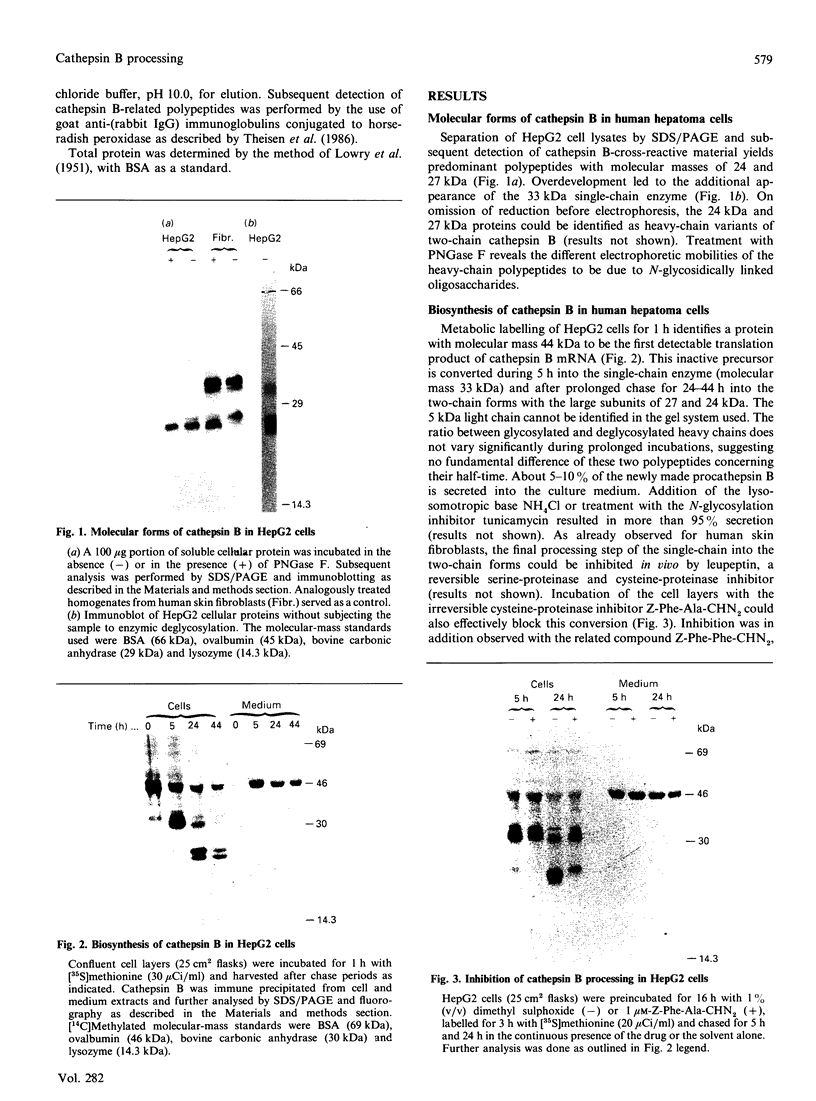
The lysosomal cysteine proteinase cathepsin B is synthesized in cultured human hepatoma HepG2 cells as an inactive 44 kDa precursor and subsequently processed to the mature single-chain enzyme with a molecular mass of 33 kDa. Intralysosomal conversion into the two-chain form results in subunits of 27 kDa, 24 kDa (heavy chain) and 5 kDa (light chain). Enzymic deglycosylation reveals that the 27 kDa polypeptide is the glycosylated variant of the carbohydrate-free 24 kDa heavy-chain form. The intracellular transport to the lysosomes is dependent upon mannose 6-phosphate-containing N-linked oligosaccharides. Receptor-mediated endocytosis of human skin-fibroblast-derived procathepsin B by HepG2 cells resulted in processed molecular forms that are not distinguishable from endogenous cathepsin B, thus favouring rather a cell-type-specific processing than structural differences due to the source of the proenzyme. The conversion step of single-chain catehpsin B into the two-chain enzyme is inhibited in vivo by the irreversible cysteine-proteinase inhibitors Z-Phe-Ala-CHN2 and, albeit weaker, Z-Phe-Phe-CHN2. Both substances have no effect on the activation of procathepsin B to the mature enzyme. The carbohydrate moiety of cathepsin B exerts no significant influence on the stability and the enzymatic activity of the enzyme.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achkar C., Gong Q. M., Frankfater A., Bajkowski A. S. Differences in targeting and secretion of cathepsins B and L by BALB/3T3 fibroblasts and Moloney murine sarcoma virus-transformed BALB/3T3 fibroblasts. J Biol Chem. 1990 Aug 15;265(23):13650–13654. [PubMed] [Google Scholar]
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Buttle D. J., Bonner B. C., Burnett D., Barrett A. J. A catalytically active high-Mr form of human cathepsin B from sputum. Biochem J. 1988 Sep 15;254(3):693–699. doi: 10.1042/bj2540693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. J., San Segundo B., McCormick M. B., Steiner D. F. Nucleotide and predicted amino acid sequences of cloned human and mouse preprocathepsin B cDNAs. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7721–7725. doi: 10.1073/pnas.83.20.7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner G. E., Udey J. A. Expression and refolding of recombinant human fibroblast procathepsin D. DNA Cell Biol. 1990 Jan-Feb;9(1):1–9. doi: 10.1089/dna.1990.9.1. [DOI] [PubMed] [Google Scholar]
- Dong J. M., Sahagian G. G. Basis for low affinity binding of a lysosomal cysteine protease to the cation-independent mannose 6-phosphate receptor. J Biol Chem. 1990 Mar 15;265(8):4210–4217. [PubMed] [Google Scholar]
- Felleisen R., Klinkert M. Q. In vitro translation and processing of cathepsin B of Schistosoma mansoni. EMBO J. 1990 Feb;9(2):371–377. doi: 10.1002/j.1460-2075.1990.tb08120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong D., Calhoun D. H., Hsieh W. T., Lee B., Wells R. D. Isolation of a cDNA clone for the human lysosomal proteinase cathepsin B. Proc Natl Acad Sci U S A. 1986 May;83(9):2909–2913. doi: 10.1073/pnas.83.9.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieselmann V., Pohlmann R., Hasilik A., Von Figura K. Biosynthesis and transport of cathepsin D in cultured human fibroblasts. J Cell Biol. 1983 Jul;97(1):1–5. doi: 10.1083/jcb.97.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinde B. The thiol proteinase inhibitors, Z-Phe-PheCHN2 and Z-Phe-AlaCHN2, inhibit lysosomal protein degradation in isolated rat hepatocytes. Biochim Biophys Acta. 1983 May 4;757(1):15–20. doi: 10.1016/0304-4165(83)90147-2. [DOI] [PubMed] [Google Scholar]
- Hanewinkel H., Glössl J., Kresse H. Biosynthesis of cathepsin B in cultured normal and I-cell fibroblasts. J Biol Chem. 1987 Sep 5;262(25):12351–12355. [PubMed] [Google Scholar]
- Hara K., Kominami E., Katunuma N. Effect of proteinase inhibitors on intracellular processing of cathepsin B, H and L in rat macrophages. FEBS Lett. 1988 Apr 11;231(1):229–231. doi: 10.1016/0014-5793(88)80737-3. [DOI] [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Synthesis as precursors of higher molecular weight. J Biol Chem. 1980 May 25;255(10):4937–4945. [PubMed] [Google Scholar]
- Ii K., Hizawa K., Kominami E., Bando Y., Katunuma N. Different immunolocalizations of cathepsins B, H, and L in the liver. J Histochem Cytochem. 1985 Nov;33(11):1173–1175. doi: 10.1177/33.11.4056381. [DOI] [PubMed] [Google Scholar]
- Knight C. G. Human cathepsin B. Application of the substrate N-benzyloxycarbonyl-L-arginyl-L-arginine 2-naphthylamide to a study of the inhibition by leupeptin. Biochem J. 1980 Sep 1;189(3):447–453. doi: 10.1042/bj1890447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazzarino D., Gabel C. A. Protein determinants impair recognition of procathepsin L phosphorylated oligosaccharides by the cation-independent mannose 6-phosphate receptor. J Biol Chem. 1990 Jul 15;265(20):11864–11871. [PubMed] [Google Scholar]
- Mason R. W., Gal S., Gottesman M. M. The identification of the major excreted protein (MEP) from a transformed mouse fibroblast cell line as a catalytically active precursor form of cathepsin L. Biochem J. 1987 Dec 1;248(2):449–454. doi: 10.1042/bj2480449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moin K., Rozhin J., McKernan T. B., Sanders V. J., Fong D., Honn K. V., Sloane B. F. Enhanced levels of cathepsin B mRNA in murine tumors. FEBS Lett. 1989 Feb 13;244(1):61–64. doi: 10.1016/0014-5793(89)81162-7. [DOI] [PubMed] [Google Scholar]
- Mort J. S., Leduc M. S., Recklies A. D. Characterization of a latent cysteine proteinase from ascitic fluid as a high molecular weight form of cathepsin B. Biochim Biophys Acta. 1983 Feb 22;755(3):369–375. doi: 10.1016/0304-4165(83)90240-4. [DOI] [PubMed] [Google Scholar]
- Mort J. S., Leduc M., Recklies A. D. A latent thiol proteinase from ascitic fluid of patients with neoplasia. Biochim Biophys Acta. 1981 Dec 15;662(2):173–180. doi: 10.1016/0005-2744(81)90027-9. [DOI] [PubMed] [Google Scholar]
- Mort J. S., Poole A. R., Decker R. S. Immunofluorescent localization of cathepsins B and D in human fibroblasts. J Histochem Cytochem. 1981 May;29(5):649–657. doi: 10.1177/29.5.6788835. [DOI] [PubMed] [Google Scholar]
- Mort J. S., Recklies A. D. Interrelationship of active and latent secreted human cathepsin B precursors. Biochem J. 1986 Jan 1;233(1):57–63. doi: 10.1042/bj2330057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort J. S., Recklies A. D., Poole A. R. Extracellular presence of the lysosomal proteinase cathepsin B in rheumatoid synovium and its activity at neutral pH. Arthritis Rheum. 1984 May;27(5):509–515. doi: 10.1002/art.1780270505. [DOI] [PubMed] [Google Scholar]
- Mort J. S., Tam A., Steiner D. F., Chan S. J. Expression of rat and mouse cathepsin B precursors in Escherichia coli. Biol Chem Hoppe Seyler. 1988 May;369 (Suppl):163–167. [PubMed] [Google Scholar]
- Nishimura Y., Amano J., Sato H., Tsuji H., Kato K. Biosynthesis of lysosomal cathepsins B and H in cultured rat hepatocytes. Arch Biochem Biophys. 1988 Apr;262(1):159–170. doi: 10.1016/0003-9861(88)90178-6. [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Kato K. Intracellular transport and processing of lysosomal cathepsin B. Biochem Biophys Res Commun. 1987 Oct 14;148(1):254–259. doi: 10.1016/0006-291x(87)91103-x. [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Kawabata T., Kato K. Identification of latent procathepsins B and L in microsomal lumen: characterization of enzymatic activation and proteolytic processing in vitro. Arch Biochem Biophys. 1988 Feb 15;261(1):64–71. doi: 10.1016/0003-9861(88)90104-x. [DOI] [PubMed] [Google Scholar]
- Prence E. M., Dong J. M., Sahagian G. G. Modulation of the transport of a lysosomal enzyme by PDGF. J Cell Biol. 1990 Feb;110(2):319–326. doi: 10.1083/jcb.110.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian F., Bajkowski A. S., Steiner D. F., Chan S. J., Frankfater A. Expression of five cathepsins in murine melanomas of varying metastatic potential and normal tissues. Cancer Res. 1989 Sep 1;49(17):4870–4875. [PubMed] [Google Scholar]
- Rademacher T. W., Parekh R. B., Dwek R. A. Glycobiology. Annu Rev Biochem. 1988;57:785–838. doi: 10.1146/annurev.bi.57.070188.004033. [DOI] [PubMed] [Google Scholar]
- Recklies A. D., Poole A. R., Mort J. S. A cysteine proteinase secreted from human breast tumours is immunologically related to cathepsin B. Biochem J. 1982 Dec 1;207(3):633–636. doi: 10.1042/bj2070633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly J. J., Jr, Mason R. W., Chen P., Joseph L. J., Sukhatme V. P., Yee R., Chapman H. A., Jr Synthesis and processing of cathepsin L, an elastase, by human alveolar macrophages. Biochem J. 1989 Jan 15;257(2):493–498. doi: 10.1042/bj2570493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich D. H., Brown M. A., Barrett A. J. Purification of cathepsin B by a new form of affinity chromatography. Biochem J. 1986 May 1;235(3):731–734. doi: 10.1042/bj2350731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozhin J., Wade R. L., Honn K. V., Sloane B. F. Membrane-associated cathepsin L: a role in metastasis of melanomas. Biochem Biophys Res Commun. 1989 Oct 16;164(1):556–561. doi: 10.1016/0006-291x(89)91755-5. [DOI] [PubMed] [Google Scholar]
- Salminen A., Gottesman M. M. Inhibitor studies indicate that active cathepsin L is probably essential to its own processing in cultured fibroblasts. Biochem J. 1990 Nov 15;272(1):39–44. doi: 10.1042/bj2720039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw E., Dean R. T. The inhibition of macrophage protein turnover by a selective inhibitor of thiol proteinases. Biochem J. 1980 Feb 15;186(2):385–390. doi: 10.1042/bj1860385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw E., Wikstrom P., Ruscica J. An exploration of the primary specificity site of cathepsin B. Arch Biochem Biophys. 1983 Apr 15;222(2):424–429. doi: 10.1016/0003-9861(83)90540-4. [DOI] [PubMed] [Google Scholar]
- Sloane B. F., Rozhin J., Hatfield J. S., Crissman J. D., Honn K. V. Plasma membrane-associated cysteine proteinases in human and animal tumors. Exp Cell Biol. 1987;55(4):209–224. doi: 10.1159/000163420. [DOI] [PubMed] [Google Scholar]
- Smith S. M., Gottesman M. M. Activity and deletion analysis of recombinant human cathepsin L expressed in Escherichia coli. J Biol Chem. 1989 Dec 5;264(34):20487–20495. [PubMed] [Google Scholar]
- Smith S. M., Kane S. E., Gal S., Mason R. W., Gottesman M. M. Glycosylation of procathepsin L does not account for species molecular-mass differences and is not required for proteolytic activity. Biochem J. 1989 Sep 15;262(3):931–938. doi: 10.1042/bj2620931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Schmidt P. G., Tang J. Novel carbohydrate structures of cathepsin B from porcine spleen. J Biol Chem. 1984 May 25;259(10):6059–6062. [PubMed] [Google Scholar]
- Takio K., Towatari T., Katunuma N., Teller D. C., Titani K. Homology of amino acid sequences of rat liver cathepsins B and H with that of papain. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3666–3670. doi: 10.1073/pnas.80.12.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T., Mizuochi T., Towatari T., Katunuma N., Kobata A. Structural studies on the carbohydrate moieties of rat liver cathepsins B and H. J Biochem. 1985 Mar;97(3):973–976. doi: 10.1093/oxfordjournals.jbchem.a135141. [DOI] [PubMed] [Google Scholar]
- Theisen N., Lohoff E. S., von Figura K., Hasilik A. Sequential detection of antigens in Western blots with differently colored products. Anal Biochem. 1986 Feb 1;152(2):211–214. doi: 10.1016/0003-2697(86)90399-4. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Figura K., Hasilik A. Lysosomal enzymes and their receptors. Annu Rev Biochem. 1986;55:167–193. doi: 10.1146/annurev.bi.55.070186.001123. [DOI] [PubMed] [Google Scholar]
- von Figura K., Steckel F., Hasilik A. Juvenile and adult metachromatic leukodystrophy: partial restoration of arylsulfatase A (cerebroside sulfatase) activity by inhibitors of thiol proteinases. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6066–6070. doi: 10.1073/pnas.80.19.6066. [DOI] [PMC free article] [PubMed] [Google Scholar]