Abstract
Coronary microvascular dysfunction (CMD) can cause myocardial ischemia in patients presenting with angina without obstructive coronary artery disease (ANOCA). Evaluating for CMD by using the thermodilution technique offers a widely accessible means of assessing microvascular resistance. Through this technique, 2 validated indices, namely coronary flow reserve and the index of microcirculatory resistance, can be computed, facilitating investigation of the coronary microcirculation. The index of microcirculatory resistance specifically estimates minimum achievable microvascular resistance within the coronary microcirculation. We aim to review the bolus thermodilution method, outlining the fundamental steps for conducting measurements and introducing an algorithmic approach (CATH CMD) to systematically evaluate the coronary microcirculation. Embracing a standardized approach, exemplified by the CATH CMD algorithm, will facilitate adoption of this technique and streamline the diagnosis of CMD.
Keywords: CATH CMD, coronary flow reserve, coronary microvascular dysfunction, coronary microvascular function, index of microvascular dysfunction
Introduction
Coronary microvascular dysfunction (CMD) can cause ischemia in patients presenting with angina without obstructive coronary artery disease (ANOCA).1 Observational studies have revealed that up to half of the patients with ANOCA exhibit CMD, with a higher prevalence observed among women.2 The presence of CMD has been linked to a worse prognosis compared to individuals with normal microcirculatory function.3 Although the precise pathophysiological mechanisms underlying CMD remain under investigation, promising advancements have been achieved in the invasive assessment of this condition. The CORMICA (CORonary MICrovascular Angina) study showed that an invasive diagnostic evaluation using thermodilution in patients without obstructive CAD linked with medical therapy improved angina in patients with no obstructive CAD.4
The thermodilution technique offers a widely accessible means of assessing microvascular resistance. Similar to measuring cardiac output with the Swan-Ganz catheter, this method involves the use of an intracoronary wire equipped with temperature sensors to determine coronary flow by thermodilution of an indicator fluid.5 The intracoronary wire is also equipped with a distal coronary pressure sensor. Through this technique, 2 validated indices, namely coronary flow reserve (CFR) and the index of microcirculatory resistance (IMR), can be computed, both playing vital roles in investigating the coronary microcirculation. CFR quantifies the increase in blood flow from resting to hyperemic conditions across the entire coronary circulation, encompassing both the epicardial compartment and the microcirculation. In contrast, IMR estimates hyperemic minimal resistance of the coronary microcirculation.6,7
In this manuscript, we detail the bolus thermodilution method comprehensively and outline the fundamental steps and setup for conducting measurements, introducing an algorithmic approach to systematically evaluate the coronary microcirculation.
Bolus thermodilution: Theoretical framework and clinical validation
The bolus thermodilution technique provides an estimate of flow by leveraging the indicator-dilution theory. In this approach, a bolus of an indicator liquid is injected into the proximal coronary circulation and the temperature is registered on the shaft of the coronary wire. As the indicator (saline) mixes with the blood and travels down the coronary circulation, its temperature is also measured using a distal temperature sensor. By analyzing the resulting dilution curves, which depict the change in temperature over time, the transit time (Tmn) of the indicator can be determined. The start of Tmn (t = 0) is defined as the time halfway through the injection, indicated by the temperature change at the ostium of the catheter. The end time is based on the temperature curve registered by the distal sensor. Subsequently, by dividing the volume by the transit time, the flow in mL/s can be reliably estimated. This method has proven to be effective in providing valuable insights into blood flow dynamics and is used in various clinical and research settings.5,8
De Bruyne et al8 and Pijls et al6 conducted pivotal studies to validate the bolus thermodilution technique for assessing coronary blood flow. These initial investigations revealed a close correlation between Tmn (transit time) and absolute flow measurements obtained using an external Doppler probe. Additionally, the calculation of CFR as the ratio of resting to hyperemic Tmn demonstrated a robust correlation with CFR derived from flow velocities measured by a Doppler wire.6 These findings showed the potential utility of the bolus thermodilution method for assessing coronary blood flow changes.3 It is important to highlight that CFR stands out as one of the most extensively validated metrics related to the coronary circulation, and its clinical significance spans across various scenarios, including patients with nonobstructive coronary artery disease. Numerous studies have consistently demonstrated the strong association between CFR and major cardiovascular events.3
Analogous to Ohm's law for electric circuits, the microvascular bed's resistance (R) can be represented as the ratio of the pressure gradient across a specific vascular territory (ΔP; in mm Hg) to the blood flow traversing this territory (Q; in mL/min). In this context, ΔP signifies the difference between the distal pressure in the coronary (Pd) and the coronary venous pressure (Pv). As Pv is typically considered negligible compared to Pd, the microvascular resistance equation can be succinctly expressed as follows:
As earlier demonstrated by De Bruyne et al,8 flow can be effectively estimated using bolus thermodilution as 1/Tmn. It is essential to highlight that flow is expressed in milliliters per minute, representing the ratio of volume to time; however, during thermodilution assessment, the vascular volume remains unmeasured. Fearon et al7 made a contribution by introducing the IMR as a novel application of bolus thermodilution. The formula for the IMR can be expressed as follows:
The same formula can be applied to resting conditions allowing us to assess the baseline resistance index.9 IMR, is, therefore, a metric surrogate of minimal microvascular resistance.
Since its introduction, the IMR has garnered validation across various clinical scenarios. In patients presenting with acute myocardial infarction, IMR has shown significant associations with indicators of microvascular function, including microvascular obstruction as assessed by magnetic resonance imaging, infarct size, and mortality.3,10,11
In stable patients undergoing percutaneous coronary intervention, IMR also has proven clinical relevance. Studies have demonstrated an association between IMR and peri-procedural myocardial infarction, subsequent myocardial infarction as well as cardiac death.12, 13, 14 IMR has demonstrated its usefulness as a prognostic marker in patients with cardiomyopathies and posttransplant settings.15,16 Nevertheless, it is relevant to recognize that an isolated increase of IMR with normal CFR does not portend an increased risk of events and that, in contrast to CFR, there is limited prognostic value of IMR in ANOCA patients.3,17,18
Invasive procedure
A dedicated wire that measures pressure and temperature is required to evaluate the coronary microcirculation invasively using the bolus thermodilution technique (PressureWire X, Abbott Vascular). The shaft of the wire, on which the temperature-dependent electrical resistance is monitored, acts as a proximal thermistor, which allows for the detection of the start of the injections. A microsensor is mounted 3 cm from the tip at the transition of the radiopaque part of the wire and acts as a distal sensor enabling simultaneous recording of high-fidelity coronary pressure measurement as well as temperature measurement, with an accuracy of 0.02 °C. The proximal temperature sensor allows recording the time the saline bolus takes from the exit of the guide catheter until the distal coronary segment where the distal sensor is located. The bolus thermodilution injections are processed online using dedicated software (CoroFlow, Coroventis Research AB).
The mean transit times (Tmn) are derived from 3 brisk 3 mL saline injections, which are averaged, and the mean of the 3 values is used to calculate CFR and/or IMR. When injecting saline at room temperature through the guide catheter, a brisk injection leads to time-shifted, U-shaped temperature response curves in the proximal and distal temperature sensors. On occasion, due to a variety of factors discussed in the troubleshooting section, the 3 recordings may not always closely pair. In this case, outlier recordings may be easily corrected by repeating the injections in the same conditions. Because the quality of the thermodilution curves depends on the hand injection technique, we recommend using a 3 mL Luer Lock syringe attached to the manifold or autoinjector 3-way stop-cock. For CFR, the 3 Tmn are obtained in resting and hyperemic conditions. In the case of IMR, only Tmn obtained during hyperemic conditions is used for the calculation. For reliable CFR and IMR measurements, it is essential to have a steady hyperemic state.
CATH CMD algorithm
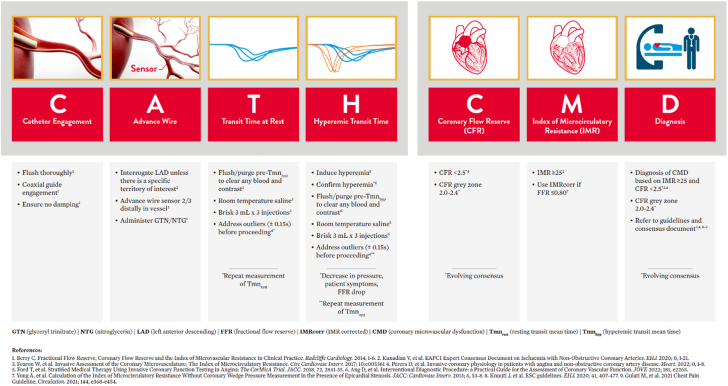
To systematize bolus thermodilution measurements, we recommend following the CATH CMD algorithm (Central Illustration). This mnemonic aims at structuring the procedure while providing tips and tricks to acquire high-quality curves and correct results. The preparation of the pressure-temperature wire follows the same procedure as for fractional flow reserve (FFR) measurements with equalization of the pressure at the tip of the guide catheter before advancement into the distal segment of the coronary artery.
Central Illustration.
CATH CMD algorithm.
Catheter engagement
Coaxial catheter engagement is a key factor in obtaining optimal temperature curves. Lack of coaxiality results in a spill into the aorta, resulting in insufficient saline reaching the thermistor located in the distal segment of the vessel and erroneous elevations in measurements of Tmn. Extra backup guide catheters can improve catheter engagement for thermodilution measurements, and constant assessment of the morphology of the aortic pressure curve to ensure the presence of a dicrotic notch and avoidance of pressure curve ventricularization is critical.
Advance wire
In view of its larger subtended left ventricular mass and the fact that the majority of existing coronary physiology data in ANOCA were obtained from within this vessel, we advise interrogating the left anterior descending (LAD) artery unless anatomical scenarios hinder the measurement (eg, severe vessel tortuosity, small vessel, etc.). Of note, CMD may, however, develop in other territories (left circumflex, right coronary artery), and interrogation of the LAD artery is not necessarily representative of other circulations.19 Systemic anticoagulation is recommended before instrumentation of the coronary artery with a guide wire with control of activated clotting time. Before the wire is advanced, epicardial vasodilation with intracoronary nitrate administration is mandatory. The wire should be advanced, and the sensor should be placed in the transition between the mid and distal segments of the vessel, two-thirds along the vessel length (at least 6 cm down the vessel). Too proximal or distal a position affects the transit times and may influence the measurements. Subsequently, the proximal and distal thermistors are “equalized” by clicking “Zero Temperature” in the CoroFlow console.
Transit time at rest
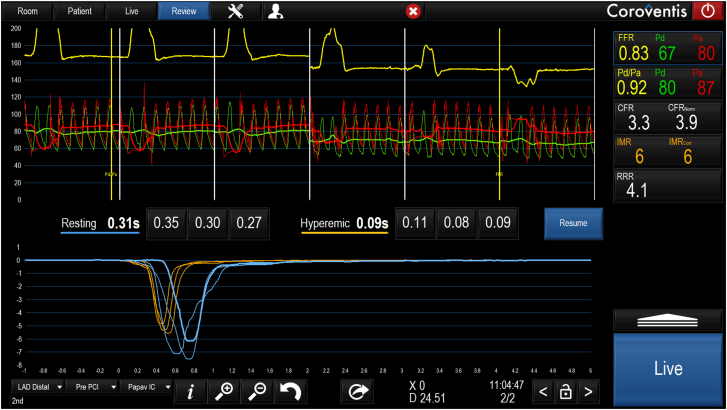
Before starting bolus thermodilution measurements, it is recommended to flush with saline to clear blood and contrast from the system and the coronary artery. After clicking on “Start,” the first bolus thermodilution injection can be performed as indicated by the system with the message “Inject now.” While performing the injection, it is useful to assess the shapes of the temperature curves and compare them visually. Outliers should be repeated (see below for definition). The quantification of the transit times will appear automatically on the screen after each injection, and at the end of the 3 injections, the average of the 3 injections will also appear automatically on the screen (Figure 1).9,20
Figure 1.
Output after bolus thermodilution assessment in resting and hyperemic conditions. Several pressure and thermodilution-based indexes will be displayed on the right panel of the Coroflow screen. The values are linked with the positioning of 2 yellow cursors (“Pd/Pa” and fractional flow reserve [“FFR”]) along the pressure recording, which is divided into 6 panels by white lines; the 3 on the left-hand side show the pressure during the 3 injections in resting conditions, and the 3 on the right obtained during hyperemic conditions. The Pd/Pa and FFR are shown from top to bottom. The coronary flow reserve (CFR) and index of microcirculatory resistance (IMR) follow these; both CFR and IMR values show their corrected values as CFRnorm and IMRcorr. A CFR <2.5 is considered abnormal; values between 2.0 and 2.4 are considered to fall in the gray zone. An IMR >25 is considered indicative of high microvascular resistance. In cases of hemodynamically significant disease (FFR ≤0.80), it is advisable to use the IMR value corrected by FFR. This method is derived from the formula: Pa × Tmn × ([1.35 × Pd/Pa] – 0.32) and enables assessment of coronary microcirculatory in the presence of epicardial stenosis and collateral flow.20 The resistive reserve ratio (RRR) indicates the number of times that the index of resistance drops, and it is calculated as the ratio of basal resistance index and IMR. RRR has been shown to correlate with clinical outcomes in patients presenting with acute coronary syndromes.9 Finally, the value is also shown normalized by FFR as CFRnorm.
Hyperemic transit times
After the 3 resting injections have been completed, the system will instruct to induce hyperemia, generally performed pharmacologically with adenosine via continuous infusion, although other approaches, including papaverine, nicorandil, nicardipine, regadenoson, or exercise, are also possible (Table 1). Hyperemia can be confirmed by a mild decrease in mean aortic pressure and a decrease in Pd/Pa or, in cases where intravenous adenosine is used, may also be identified by the patient’s symptoms, such as shortness of breath, hot flashes, or flushing. Once hyperemia is achieved, flush with saline again to clear any blood and contrast from the system. During hyperemia, 3 new 3 mL injections are performed to assess hyperemic flow. As with the resting injections, the quantification of transit times will appear automatically on the screen after each injection, and at the end of the 3 injections, the average of the 3 injections will be displayed next to the individual values. The injection of saline in the resting condition may itself elicit some hyperemia. Thus, it is important to wait 20 to 30 seconds between each resting injection to allow this effect to level off.
Table 1.
Commonly used hyperemic agents.
| Drug | Dose | Hyperemia duration | Half-life | Side effects | Advantages | Disadvantages |
|---|---|---|---|---|---|---|
| Adenosine IV | 140 μg/kg/min | 1-2 min | <10 s |
|
|
|
| Adenosine IC | 36-120 μg LCA 18-60 μg RCA |
3-8 s | <10 s |
|
|
|
| Papaverine IC | 10 mg LCA 15 mg RCA |
30-60 s | 2 min |
|
|
|
| Nicorandil IC | 2 mg LCA 2 mg RCA |
30 ± 15 s | 6 min |
|
|
|
| Regadenoson IV | 400 μg bolus | Variable (10 s to 10 min) Mean 163 s |
2-4 min |
|
|
|
AV, atrioventricular; COPD, chronic obstructive pulmonary disease; IC, introcoronary; IMR, index of microvascular resistance; IV, intravenous; LCA, left coronary artery; RCA, right coronary artery.
CFR
An abnormal CFR (<2.5) in patients with nonobstructive CAD (FFR > 0.80) suggests the presence of impaired vasodilatory reserve and CMD.1 Bolus thermodilution may slightly overestimate the CFR value compared to Doppler measures of CFR.21,22 Therefore, CFR values between 2.0 and 2.5 should be considered to fall in the gray zone, and clinical judgment should be used in the interpretation of the findings.
Microcirculatory resistance
IMR represents the minimal microvascular resistance, calculated as the distal coronary pressure multiplied by the hyperemic transit time. The current consensus recommends a cutoff of 25 IMR units to define CMD with high microvascular resistance in stable patients without coronary artery disease.7 In the presence of epicardial stenosis with FFR <0.80, the corrected IMR (IMRc), taking into account collateral flow, is displayed next to the IMR value and should be used.
Diagnosis
The etiology of CMD is multifactorial and remains the subject of ongoing research. Endothelial dysfunction, inflammation, and alterations in the autonomic nervous system are all relevant pathophysiological mechanisms. Reduced nitric oxide bioavailability and increased oxidative stress contribute to endothelial dysfunction, leading to impaired vasodilation and microvascular constriction.23 Additionally, inflammatory processes within the coronary microvasculature may further compromise blood flow regulation in specific clinical scenarios. The dysregulation of the autonomic nervous system, characterized by sympathetic overactivity and parasympathetic withdrawal, may also play a role in CMD pathogenesis. Furthermore, heart failure, systemic factors, including raised left ventricular end-diastolic pressure (LVEDP), microvascular rarefaction or compression, platelet plugging, and distal embolization of thrombus in the setting of myocardial infarction, are important structural noncoronary contributors to elevated microvascular resistance.24 Understanding these mechanisms is vital for the development of targeted therapeutic interventions.
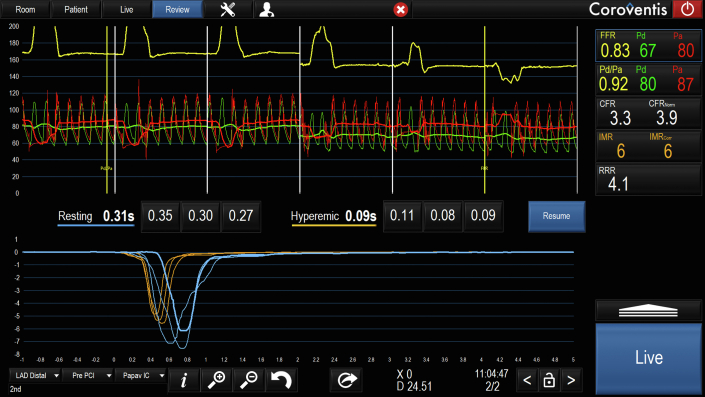
In clinical practice, low CFR can arise from 2 distinct mechanisms: firstly, from increased resting coronary artery blood flow, indicating reduced microvascular resistance at rest; and secondly, from reduced hyperemic flow due to elevated hyperemic microvascular resistance.25 These 2 scenarios give rise to different outcomes when using CFR and IMR as a diagnostic tool. In cases where low CFR results from increased resting blood flow (short resting Tmn), IMR will typically appear normal. Conversely, when low CFR results from prolonged hyperemic transit time, leading to elevated IMR values (IMR > 25), it indicates abnormal microvascular function. Case examples of different scenarios with bolus thermodilution are shown in Figure 2.
Figure 2.
Case examples of patients with normal and abnormal microvascular function. (A) shows a case of normal microvascular function with normal coronary flow reserve (CFR), index of microcirculatory resistance (IMR), and fractional flow reserve (FFR). (B) shows a case of borderline FFR (0.80), low CFR, and normal IMR; this is an example of functional coronary microvascular dysfunction. (C) shows a case of low CFR driven by high microvascular resistance (IMR of 37); this is an example of structural coronary microvascular dysfunction. (D) shows a case of normal CFR but high IMR.
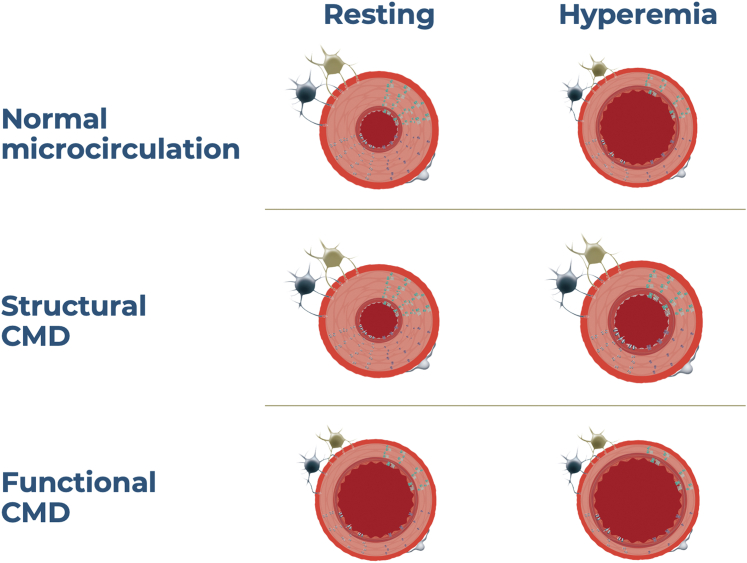
Evolving consensus suggests that these 2 distinct presentations represent separate entities of CMD, classified as functional and structural CMD, respectively (Figure 3). Patients with low CFR due to short Tmn at rest can be identified as having functional CMD, whereas those with low CFR resulting from prolonged hyperemic Tmn and high IMR are classified as having structural CMD. The clinical implications and long-term prognosis of these separate CMD subtypes are the subject of intense investigation. A specific scenario that deserves to be mentioned is normal CFR (>2.5) with high IMR. In these cases, it becomes crucial to recognize that both resting and hyperemic transit times are considerably prolonged. This clinical presentation suggests that the microvascular bed possesses sufficient vasodilator capacity to increase coronary flow by more than 2.5 times during hyperemia. However, despite this capability, the hyperemic flow remains lower than expected due to persistently elevated microvascular resistance. This clinical scenario appears not to pose additional risk to patients.17
Figure 3.
Coronary microvascular dysfunction (CMD) endotypes.
The therapeutic approach to patients with CMD is a field of active investigation, and pharmacological therapies are principally directed at reducing oxygen demand with beta-blockers and calcium channel antagonists. However, the EDIT-CMD trial (Efficacy of Diltiazem to Improve Coronary Microvascular Dysfunction) showed no benefit of diltiazem, when compared with a placebo, in patients with CMD. However, diltiazem reduced the prevalence of epicardial spasms.26 More recently, the ChaMP-CMD (Characterizing Mechanisms in Patients with Coronary Microvascular Disease to Stratify Therapy) study demonstrated the clinical benefit of amlodipine and ranolazine in patients with CMD with a documented improvement in exercise capacity.27 The potential treatment strategies for CMD are beyond the scope of this manuscript.28
Tips, tricks, and troubleshooting
Patient preparation plays a pivotal role in ensuring an accurate assessment of the coronary circulation; however, considerable operator variation exists. We recommend withholding vaso-active drugs, such as calcium channel blockers and calcium antagonists, for 24 to 48 hours before the procedure. Additionally, as with any hyperemic physiological assessment, patients should be advised to abstain from consuming caffeine and its derivatives on the day prior to the assessment, particularly in cases where adenosine is utilized as the hyperemic agent.
Coronary microvascular testing is optimally done as part of more comprehensive invasive testing, which includes acetylcholine infusion for endothelial function assessment and provocation test for coronary vasospasm. In situations where a concomitant test for the evaluation of endothelial dysfunction or vasospastic angina with acetylcholine is planned, consideration should be given to the potential impact of upfront use of vasodilator cocktails, which are commonly employed to minimize radial spasms. The order of the examination remains a matter of debate, some centers advocate for acetylcholine testing first, without prior nitrates and vessel instrumentation, followed by nonendothelial-dependent evaluation. There is ample experience using radial access with 6 French guides for comprehensive invasive physiologic testing. In some situations, such as the presence of radial spasm, 5 French guides may be considered; however, this may increase the errors detected by the software during bolus thermodilution injections.
To obtain accurate physiological measurements, stability of hemodynamic conditions is critical, as the coronary circulation autoregulates in response to changes in blood pressure. These mechanisms can impact the evaluation of coronary flow and microvascular resistance. When assessing resting blood flow, it becomes essential for the patient's conditions to closely resemble a true resting state. However, achieving this can be challenging, as many patients may experience heightened distress during invasive procedures. Additionally, specific factors such as pain at the arterial access site can further deviate from a genuine resting state. To mitigate these challenges, it is advisable to conduct measurements in a quiet environment. Conscious sedation can be considered. However, when this is not possible, it is important to account for these factors when interpreting the results, particularly in supposedly “resting” conditions. Elevated LVEDP increases mechanical compression of the subendocardial layer and is associated with CMD, impairments in cardiac function, and heart failure with preserved ejection fraction. LVEDP is also prognostically important.29 For these reasons, measuring LVEDP complements the assessment of coronary microvascular function in the catheter laboratory and should be integrated into the interpretation of the resistance measurements. There are several potential situations during the procedure where the CoroFlow system can detect errors related to the technique. The most frequent errors and corrective actions are shown in Table 2. One of the most frequently encountered situations is variability between Tmn. Variability is physiological, as coronary blood flow changes rapidly between systole and diastole. Hence, the need for an average of measurements, and also for a resting state environment. The CoroFlow software will identify this by displaying a yellow box around the transit times. Any transit time value deviating by more than 25% from the average value will be flagged automatically by the system with a yellow box around the Tmn. The system employs an automatic adjustment of the threshold for detecting high variability in Tmn based on the average Tmn value. For average Tmn values greater than or equal to 0.25, a cutoff of 30% variability was utilized, whereas in cases where Tmn was less than 0.25, a threshold for maximal variability of 50% was adopted. We recommend replacing these values by clicking on the outlying value and performing an extra injection. It should be noted that injection of saline in the resting state itself can introduce hyperemia.30 Thus, subsequent resting Tmn may be artificially too short. A time interval of 20 to 30 seconds between resting injections is therefore advised. After the third saline injection, the recording is saved, and the results are provided for clinical interpretation.
Table 2.
Potential errors during bolus thermodilution measurements.
| Type of error | Reason | Corrective actions |
|---|---|---|
| Slow inject | Injection too slow with >0.60 s between the start of the decline in temperature and the nadir at the proximal thermistor |
|
| Timeout | Temperature did not return to baseline in time (> 8 s) at the distal thermistor |
|
| Low amp | The temperature response did not reach the minimum limit during injection (<–1 °C) at the distal thermistor |
|
Finally, it is very important to identify and record the vessel assessed, the phase of the procedure, and the hyperemic agent used in all recordings. This is facilitated in the CoroFlow platform with specific fields in the bottom left corner of the screen.
Current limitations and future perspective
Knowledge on the diagnosis, clinical implication, and management of CMD is rapidly evolving, but significant uncertainties persist. In this document, we advocate for the systematic assessment of the LAD artery; however, the potential added clinical value of multivessel interrogation remains a question for ongoing research. Studies have shown that CFR derived from bolus thermodilution may differ from that obtained through Doppler or continuous thermodilution measurements.22 Furthermore, bolus thermodilution-derived parameters can vary by up to 20% with repeated measurements.31 Therefore, it is essential to use the appropriate technique, emphasizing the rationale for standardizing this procedure, as outlined in the present document.
Although IMR has been proposed as a metric specific to the coronary microcirculation, it has been shown to depend on the subtended myocardial mass, which is strongly related to vessel volume.32 Given the assumptions embedded in the IMR calculation, more research is needed to understand the influence of mass and vessel volume on IMR and better the clinical implications of this finding. Several prospective studies are ongoing to increase our understanding of how to diagnose and manage CMD.
Conclusion
Bolus thermodilution represents a reliable and efficient approach to assessing the coronary microcirculation. Thus, embracing a standardized approach, exemplified by the CATH CMD algorithm, will facilitate the widespread adoption of this technique and streamline high-quality diagnosis of CMD in patients with nonobstructive coronary artery disease. The increasing awareness of CMD and standardization of diagnostic tools have the potential to improve the outcomes in patients with nonobstructive coronary artery disease.
Acknowledgments
Declaration of competing interest
Carlos Collet reports receiving research grants from Biosensors, Coroventis Research AB, Medis Medical Imaging, Pie Medical Imaging, CathWorks, Boston Scientific, Siemens, HeartFlow, and Abbott Vascular; and consultancy fees from HeartFlow, Abbott Vascular, and Philips Volcano, and has patents pending on diagnostic methods for coronary artery disease. Andy Yong has received minor honoraria from Abbott Vascular and research grants from Abbott Vascular and Philips. Daniel Munhoz reports a research grant provided by the CardioPaTh PhD program and speaker fees from Abbott Vascular. Takashi Akasaka is a medical advisor of Terumo Corp, and report speaker fees from Abbott Medical Japan and consulting fees from Nipro Corp Colin Berry receives research funding from the British Heart Foundation (RE/18/6134217, BHF/FS/17/26/32744, PG/19/28/34310) and is employed by the University of Glasgow which holds consultancy and research agreements for his work with Abbott Vascular, AstraZeneca, Boehringer Ingelheim, Coroventis Research AB, GlaxoSmithKline, HeartFlow, Menarini, Novartis, Servier, Siemens Healthcare, and Valo Health. Damien Collison has received consulting fees from Abbott. John E.A. Blair has received speaker fees from Abbott and Boston Scientific. Javier Escaned is supported by the Intensification of Research Activity project INT22/00088 from the Spanish Instituto de Salud Carlos III and received speaker and advisory board member fees from Abbott and Philips. William F. Fearon receives institutional research support from Abbott, Boston Scientific, and Medtronic, has consulting relationships with CathWorks and Siemens, and stock options from HeartFlow. Thomas Engstrøm reports speaker and advisory board fees from Abbott, Boston Scientific, and Novo Nordisk. Tommaso Gori reports receiving speaker fees and institutional support from Abbott. Adrian F. Low reports receiving speaker and advisory board fees from Abbott. Bon-Kwon Koo received an institutional research grant from St. Jude Medical (Abbott Vascular) and Philips Volcano. Martin K.C. Ng reports speaker/advisory board fees for Abbott and reports receiving research funding from Abbott. Takuya Mizukami reports receiving research grants from Boston Scientific and speaker fees from Abbott Vascular, CathWorks, and Boston Scientific. Nathaniel R. Smilowitz reports advisory board fees from Abbott. Nadia R. Sutton reports advisory and speaking fees from Abbott, honoraria for speaking for Zoll, and advisory board fees from Philips. Jennifer Tremmel reports honoraria and advisory boards for Abbott Vascular; honoraria, advisory board, and research support from Boston Scientific; honoraria from Shockwave Medical, consultant for Avinger. Johan Svanerud is the CEO and founder of Coroventis Research AB. Takayuki Warisawa reports receiving speaker fees from Abbott Medical Japan, Philips Japan, and Boston Scientific Japan. Nick E.J. West is an employee and owns stock in Abbott Vascular, holding a former position of chief medical officer and deputy vice president. Ziad A. Ali reports institutional grant support from Abbott, Abiomed, Acist, Amgen, Boston Scientific, CathWorks, Canon Medical, Conavi, HeartFlow, Inari, Medtronic, the U.S. National Institutes of Health, Nipro, OpSens Medical, Medis, Philips, Shockwave Medical, Siemens, SpectraWAVE, Teleflex. Consulting fees from Abiomed, AstraZeneca, Boston Scientific, CathWorks, OpSens Medical, Philips, Shockwave Medical, and equity in Elucid, Lifelink, SpectraWAVE, Shockwave Medical, VitalConnect. The other authors have no conflicts of interest to declare.
Funding sources
This work was not supported by funding agencies in the public, commercial, or not-for-profit sectors.
Ethics statement and patient consent
This manuscript does not report on patients or patient data.
References
- 1.Kunadian V., Chieffo A., Camici P.G., et al. An EAPCI expert consensus document on ischaemia with non-obstructive coronary arteries in collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation endorsed by Coronary Vasomotor Disorders International Study Group. EuroIntervention. 2021;16(13):1049–1069. doi: 10.4244/EIJY20M07_01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mileva N., Nagumo S., Mizukami T., et al. Prevalence of coronary microvascular disease and coronary vasospasm in patients with nonobstructive coronary artery disease: systematic review and meta-analysis. J Am Heart Assoc. 2022;11(7) doi: 10.1161/JAHA.121.023207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelshiker M.A., Seligman H., Howard J.P., et al. Coronary flow reserve and cardiovascular outcomes: a systematic review and meta-analysis. Eur Heart J. 2022;43(16):1582–1593. doi: 10.1093/eurheartj/ehab775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford T.J., Stanley B., Good R., et al. Stratified medical therapy using invasive coronary function testing in angina: the CorMicA trial. J Am Coll Cardiol. 2018;72(23 Pt A):2841–2855. doi: 10.1016/j.jacc.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Candreva A., van Gallinoro E., van ’t Veer M., et al. Basics of coronary thermodilution. JACC Cardiovasc Interv. 2021;14(6):595–605. doi: 10.1016/j.jcin.2020.12.037. [DOI] [PubMed] [Google Scholar]
- 6.Pijls N.H., De Bruyne B., Smith L., et al. Coronary thermodilution to assess flow reserve: validation in humans. Circulation. 2002;105(21):2482–2486. doi: 10.1161/01.cir.0000017199.09457.3d. [DOI] [PubMed] [Google Scholar]
- 7.Fearon W.F., Balsam L.B., Farouque H.M., et al. Novel index for invasively assessing the coronary microcirculation. Circulation. 2003;107(25):3129–3132. doi: 10.1161/01.CIR.0000080700.98607.D1. [DOI] [PubMed] [Google Scholar]
- 8.De Bruyne B., Pijls N.H., Smith L., Wievegg M., Heyndrickx G.R. Coronary thermodilution to assess flow reserve: experimental validation. Circulation. 2001;104(17):2003–2006. doi: 10.1161/hc4201.099223. [DOI] [PubMed] [Google Scholar]
- 9.Maznyczka A.M., Oldroyd K.G., Greenwood J.P., et al. Comparative significance of invasive measures of microvascular injury in acute myocardial infarction. Circ Cardiovasc Interv. 2020;13(5) doi: 10.1161/CIRCINTERVENTIONS.119.008505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Maria G.L., Alkhalil M., Wolfrum M., et al. Index of microcirculatory resistance as a tool to characterize microvascular obstruction and to predict infarct size regression in patients with STEMI undergoing primary PCI. JACC Cardiovasc Imaging. 2019;12(5):837–848. doi: 10.1016/j.jcmg.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 11.Ahn S.G., Hung O.Y., Lee J.W., et al. Combination of the thermodilution-derived index of microcirculatory resistance and coronary flow reserve is highly predictive of microvascular obstruction on cardiac magnetic resonance imaging after ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2016;9(8):793–801. doi: 10.1016/j.jcin.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 12.Layland J.J., Whitbourn R.J., Burns A.T., et al. The index of microvascular resistance identifies patients with periprocedural myocardial infarction in elective percutaneous coronary intervention. Heart. 2012;98(20):1492–1497. doi: 10.1136/heartjnl-2012-302252. [DOI] [PubMed] [Google Scholar]
- 13.Fearon W.F., Low A.F., Yong A.S., et al. Prognostic value of the Index of Microcirculatory Resistance measured after primary percutaneous coronary intervention. Circulation. 2013;127(24):2436–2441. doi: 10.1161/CIRCULATIONAHA.112.000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishi T., Murai T., Ciccarelli G., et al. Prognostic value of coronary microvascular function measured immediately after percutaneous coronary intervention in stable coronary artery disease: an international multicenter study. Circ Cardiovasc Interv. 2019;12(9) doi: 10.1161/CIRCINTERVENTIONS.119.007889. [DOI] [PubMed] [Google Scholar]
- 15.Aguiar Rosa S., Mota Carmo M., Rocha Lopes L., et al. Index of microcirculatory resistance in the assessment of coronary microvascular dysfunction in hypertrophic cardiomyopathy. Rev Port Cardiol. 2022;41(9):761–767. doi: 10.1016/j.repc.2021.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Ahn J.M., Zimmermann F.M., Gullestad L., et al. Microcirculatory resistance predicts allograft rejection and cardiac events after heart transplantation. J Am Coll Cardiol. 2021;78(24):2425–2435. doi: 10.1016/j.jacc.2021.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Hong D., Shin D., Lee S.H., et al. Prognostic impact of coronary microvascular dysfunction according to different patterns by invasive physiologic indexes in symptomatic patients with intermediate coronary stenosis. Circ Cardiovasc Interv. 2023;16(3) doi: 10.1161/CIRCINTERVENTIONS.122.012621. [DOI] [PubMed] [Google Scholar]
- 18.Boerhout C.K.M., de Waard G.A., Lee J.M., et al. Prognostic value of structural and functional coronary microvascular dysfunction in patients with non-obstructive coronary artery disease; from the multicentre international ILIAS registry. EuroIntervention. 2022;18(9):719–728. doi: 10.4244/EIJ-D-22-00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi Y., Lee J.M., Fearon W.F., et al. Three-vessel assessment of coronary microvascular dysfunction in patients with clinical suspicion of ischemia: prospective observational study with the index of microcirculatory resistance. Circ Cardiovasc Interv. 2017;10(11) doi: 10.1161/CIRCINTERVENTIONS.117.005445. [DOI] [PubMed] [Google Scholar]
- 20.Yong A.S., Layland J., Fearon W.F., et al. Calculation of the index of microcirculatory resistance without coronary wedge pressure measurement in the presence of epicardial stenosis. JACC Cardiovasc Interv. 2013;6(1):53–58. doi: 10.1016/j.jcin.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 21.Demir O.M., Boerhout C.K.M., de Waard G.A., et al. Comparison of Doppler flow velocity and thermodilution derived indexes of coronary physiology. JACC Cardiovasc Interv. 2022;15(10):1060–1070. doi: 10.1016/j.jcin.2022.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fearon W.F., Farouque H.M., Balsam L.B., et al. Comparison of coronary thermodilution and Doppler velocity for assessing coronary flow reserve. Circulation. 2003;108(18):2198–2200. doi: 10.1161/01.CIR.0000099521.31396.9D. [DOI] [PubMed] [Google Scholar]
- 23.Boden W.E., Marzilli M., Crea F., et al. Evolving management paradigm for stable ischemic heart disease patients: JACC review topic of the week. J Am Coll Cardiol. 2023;81(5):505–514. doi: 10.1016/j.jacc.2022.08.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samuels B.A., Shah S.M., Widmer R.J., et al. Comprehensive management of ANOCA, part 1—definition, patient population, and diagnosis: JACC state-of-the-art review. J Am Coll Cardiol. 2023;82(12):1245–1263. doi: 10.1016/j.jacc.2023.06.043. [DOI] [PubMed] [Google Scholar]
- 25.Rahman H., Demir O.M., Khan F., et al. Physiological stratification of patients with angina due to coronary microvascular dysfunction. J Am Coll Cardiol. 2020;75(20):2538–2549. doi: 10.1016/j.jacc.2020.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jansen T.P.J., Konst R.E., de Vos A., et al. Efficacy of diltiazem to improve coronary vasomotor dysfunction in ANOCA: the EDIT-CMD randomized clinical trial. JACC Cardiovasc Imaging. 2022;15(8):1473–1484. doi: 10.1016/j.jcmg.2022.03.012. [DOI] [PubMed] [Google Scholar]
- 27.Sinha A., Rahman H., Douiri A., et al. ChaMP-CMD: a phenotype-blinded, randomized controlled, cross-over trial. Circulation. 2024;149(1):36–47. doi: 10.1161/CIRCULATIONAHA.123.066680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smilowitz N.R., Prasad M., Widmer R.J., et al. Comprehensive management of ANOCA, part 2—program development, treatment, and research initiatives: JACC state-of-the-art review. J Am Coll Cardiol. 2023;82(12):1264–1279. doi: 10.1016/j.jacc.2023.06.044. [DOI] [PubMed] [Google Scholar]
- 29.Maznyczka A.M., McCartney P.J., Oldroyd K.G., et al. Risk stratification guided by the index of microcirculatory resistance and left ventricular end-diastolic pressure in acute myocardial infarction. Circ Cardiovasc Interv. 2021;14(2) doi: 10.1161/CIRCINTERVENTIONS.120.009529. [DOI] [PubMed] [Google Scholar]
- 30.Fujimori Y., Baba T., Yamazaki K., et al. Saline-induced Pd/Pa ratio predicts functional significance of coronary stenosis assessed using fractional flow reserve. EuroIntervention. 2018;14(8):898–906. doi: 10.4244/EIJ-D-17-01010. [DOI] [PubMed] [Google Scholar]
- 31.Gallinoro E., Bertolone D.T., Fernandez-Peregrina E., et al. Reproducibility of bolus versus continuous thermodilution for assessment of coronary microvascular function in patients with ANOCA. EuroIntervention. 2023;19(2):e155–e166. doi: 10.4244/EIJ-D-22-00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Echavarría-Pinto M., van de Hoef T.P., Nijjer S., et al. Influence of the amount of myocardium subtended to a coronary stenosis on the index of microcirculatory resistance. Implications for the invasive assessment of microcirculatory function in ischaemic heart disease. EuroIntervention. 2017;13(8):944–952. doi: 10.4244/EIJ-D-16-00525. [DOI] [PubMed] [Google Scholar]