Abstract
Introduction
Stroke is the leading cause of death and disability among adults and elderly individuals worldwide. Although several primary studies have been conducted to determine the prevalence of poststroke cognitive impairment among stroke survivors in sub-Saharan Africa, these studies have presented inconsistent findings. Therefore, this study aimed to determine the pooled prevalence of poststroke cognitive impairment and identify its associated factors among stroke survivors in sub-Saharan Africa.
Methods
The studies were retrieved from the Google Scholar, Scopus, PubMed, and Web of Science databases. A manual search of the reference lists of the included studies was performed. A random-effects DerSimonian-Laird model was used to compute the pooled prevalence of poststroke cognitive impairment among stroke survivors in sub-Saharan Africa.
Results
A total of 10 primary studies with a sample size of 1,709 stroke survivors were included in the final meta-analysis. The pooled prevalence of PSCI was obtained from the 9 included studies with a sample size of 1,566. In contrast, the data regarding the associated factors were obtained from all the 10 included studies with a sample size of 1,709. The pooled prevalence of poststroke cognitive impairment among stroke survivors was 59.61% (95% CI: 46.87, 72.35); I2 = 96.47%; P < 0.001). Increased age (≥ 45 years) [AOR = 1.23, 95% CI: 1.09, 1.40], lower educational level [AOR = 4.35, 95% CI: 2.87, 6.61], poor functional recovery [AOR = 1.75, 95% CI: 1.42, 2.15], and left hemisphere stroke [AOR = 4.88, 95% CI: 2.98, 7.99] were significantly associated with poststroke cognitive impairment.
Conclusions
The pooled prevalence of poststroke cognitive impairment was considerably high among stroke survivors in sub-Saharan Africa. Increased age, lower educational level, poor functional recovery, and left hemisphere stroke were the pooled independent predictors of poststroke cognitive impairment in sub-Saharan Africa. Stakeholders should focus on empowering education and lifestyle modifications, keeping their minds engaged, staying connected with social activities and introducing rehabilitative services for stroke survivors with these identified factors to reduce the risk of developing poststroke cognitive impairment.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-024-19684-3.
Keywords: Poststroke cognitive impairment, Stroke survivors, Sub-Saharan Africa, Meta-analysis
Introduction
Stroke is a leading cause of death and disability among adults and elderly individuals worldwide, particularly in low- and middle-income countries (LMICs) in sub-Saharan Africa (SSA) [1–4]. It affects more than 80 million people globally [5], and results in approximately 6,500,000 stroke-related deaths annually [6]. Most of the stroke-related deaths occur in LMICs [7].
Stroke causes cell damage and cell death in the brain, which may result in acute neuronal loss, disruption of brain functional networks, inflammation, social isolation, and poststroke cognitive impairment (PSCI) [8]. PSCI is a cognitive decline usually evidenced by a Montreal Cognitive Assessment (MoCA) score < 26/30 [9, 10]. Globally, the prevalence of PSCI varies and has been reported to range between 32 to 92% [11–13] with an estimated 64% of stroke survivors developing PSCI [14, 15].
PSCI involves multidomain disruptions including disruptions in attention, concentration, executive function, language, memory, and visuospatial function, with executive dysfunction being the earliest and predominantly affected domain [14, 16–18]. The PSCI significantly affects the victim’s quality of life, family, and community at large [19]. It is associated with reduced quality of life, disability, high dependency, increased likelihood of depressive symptoms, increased healthcare costs, lost wages, and social isolation [19–24].
The cognitive profile of stroke survivors depends on the demographic characteristics of the patient, such as differences in age, pre-stroke cognitive status and comorbidities, and stroke-related clinical characteristics such as the extent and site of brain injury [14, 25–28]. Identifying these factors early and managing them where possible can help improve the cognitive profile of stroke survivors [26].
Although several primary studies have been conducted to determine the prevalence of PSCI among stroke survivors in SSA, these studies have presented inconsistent findings. Therefore, this study aimed to determine the pooled prevalence of PSCI and identify its associated factors among stroke survivors in SSA.
Methods
Reporting and registration protocol
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) format [29] was used to report the findings of the study (Supplemental Table 1). The review protocol was registered with the Prospero database: (PROSPERO, 2023: CRD42023494791).
Table 1.
Subgroup analyses of poststroke cognitive impairment among stroke survivors in SSA, 2023
| Variables | Outcome | Subgroup | No. of studies | Model | Prevalence (95%CI) |
I2 | P = value |
|---|---|---|---|---|---|---|---|
| Study design | PSCI | CS | 6(n = 1,062) | Random | 62.27 (54.79, 69.75) | 79.84% | < 0.001 |
| Cohort | 3(n = 647) | Random | 55.09 (20.60, 89.58) | 96.39% | < 0.001 | ||
| Sample size | PSCI | < 171 | 8(n = 884) | Random | 64.57 (55.58, 73.57) | 86.13% | < 0.001 |
| ≥ 171 | 2(n = 825) | Random | 44.29 (16.85, 71.73) | 98.60% | < 0.001 |
CS Cross-sectional, CI Confidence interval, n Sample size
Databases and search strategy
The studies were subsequently retrieved from the Google Scholar, Scopus, PubMed, and Web of Science databases using search terms and phrases. Moreover, a manual search of the reference lists of the included studies was performed (Supplemental file 1). All studies were conducted in SSA and published in English between 2011 and 2023.
Eligibility criteria
All observational (cross-sectional, cohort, and case–control) studies that were conducted among stroke survivors in SSA, reported the prevalence of PSCI and/or at least one associated factor of PSCI and were written in English included in the study. However, citations without abstracts, full texts, anonymous reports, editorials, systematic reviews, meta-analyses, or qualitative studies were excluded from the study.
Study selection
All the retrieved studies were exported to the EndNote version 7 reference manager to remove the duplicate studies. Primarily, two independent authors (TMA and SA) screened the titles and abstracts, followed by the full-text reviews to determine the eligibility of each study. Disagreements between the reviewers were resolved through discussion.
Quality appraisal of the included studies
Two independent authors (TMA and SA) appraised the quality of the included primary studies. The quality of each study was appraised using the Joanna Briggs Institute (JBI) quality appraisal criteria [30]. Six studies [28, 31–35], three [36–38], and two [14, 39] were appraised using the JBI checklist for cross-sectional, cohort, and case–control respectively. Studies were classified as “high quality” if 50% or higher on the quality assessment indicators scored “Yes” and as “low quality” if lower than 50% on the quality assessment indicators scored “Yes”. Thus, among the 6 cross-sectional studies, 4 studies scored “Yes” on 7 of the 8 questions (high quality), 1 study scored “Yes” on 6 of the 8 questions (high quality), and the remaining study scored “Yes” on 5 of the 8 questions (high quality). Similarly, among the 3 cohort studies, two studies scored “Yes” on 8 of the 10 questions (high quality), and the third study scored “Yes” on 7 of the 10 questions (high quality). Moreover, of the 2 case–control studies, one study scored “Yes” on 8 of the 10 questions (high quality). However, the second case–control study [39] scored “Yes” on 4 of the 10 questions (low quality). The cross-sectional studies scored “Yes” on 5 to 7 of the 8 points, whereas the cohort and case–control studies scored “Yes” on 4 to 8 of the 10 points (Supplemental Table 2). Therefore, the second case–control study [39] was of lower quality, and was excluded from the study.
Risk of bias assessment of the included studies
The risk of bias was assessed using the adopted standardized assessment tool [40]. The tool consists of ten items that assess four areas of bias. To assess the risk of bias in the included studies, studies were classified as “low risk of bias” if 8 and above of the 10 questions scored “Yes”, as “moderate risk of bias” if 6 to 7 of the 10 questions scored “Yes” and as “high risk of bias” if ≤ 5 of the 10 questions scored “Yes”. Consequently, of the total of the 10 included studies, 8 studies scored “Yes” on 8 of the 10 questions (low risk of bias), and the 2 studies scored “Yes” on 7 of the 10 questions (moderate risk of bias) (Supplemental Table 3).
Data extraction
Two independent authors (TMA and AK) extracted the data using a structured Microsoft Excel sheet. When differences were detected in the extracted data, the step was repeated. When discrepancies between the extracted data were continued, the third reviewer (SDK) was consulted. The name of the first authors, year of publication, study area, study design, sample size, response rate, and effect size of the included primary studies were extracted.
Outcome measures
The study focused on two outcome measures. The primary outcome measure of interest was the prevalence of PSCI among stroke survivors in SSA. Additionally, the second outcome measure was identifying factors associated with PSCI among stroke survivors.
Operational definition of variables
“The total score of the MoCA ranges from 0 to 30. A score < 26/30 indicates cognitive impairment, while a MoCA score ≥ 26/30 indicates normal cognitive function” [10]. Similarly, the total score of the Mini-Mental State Examination (MMSE) ranges from 0 to 30, and a score < 25/30 indicates cognitive impairment, whereas a MMSE score ≥ 25/30 indicates normal cognitive function” [33]. These tools were translated into local African languages in each included study so as not to favor study participants with higher educational levels.
Data analysis
The extracted data were exported to STATA version 17 for statistical analysis. The pooled prevalence of PSCI was computed using a random-effects DerSimonian-Laird model [41]. The pooled prevalence of PSCI was obtained from the 9 included primary studies with a sample size of 1,566 [28, 31–38]. In contrast, the data regarding the associated factors were obtained from all the 10 included primary studies [14, 28, 31–38] with a sample size of 1,709. A funnel plot was used to determine publication bias, and Egger’s test with a P = value of < 0.05 was used to determine significant publication bias [42]. The heterogeneity across the included primary studies was assessed using I2 statistics [43]. The values of I2 statistic range from 0 to 100%, and I2 statistic values of 0, 25, 50, and 75% denote no, low, moderate, and high heterogeneity respectively [43].
A P = value of the chi-square test on the Cochrane’s Q statistic < 0.05 was used to indicate a significant heterogeneity [44, 45]. Sensitivity analysis was employed to determine the effect of a single study on the overall estimate. A forest plot was used to display the effect of independent factors on the outcome and a measure of association with a 95% CI was generated. The adjusted odds ratio (AOR) was the reported measure of association in the included primary studies.
Results
Search results
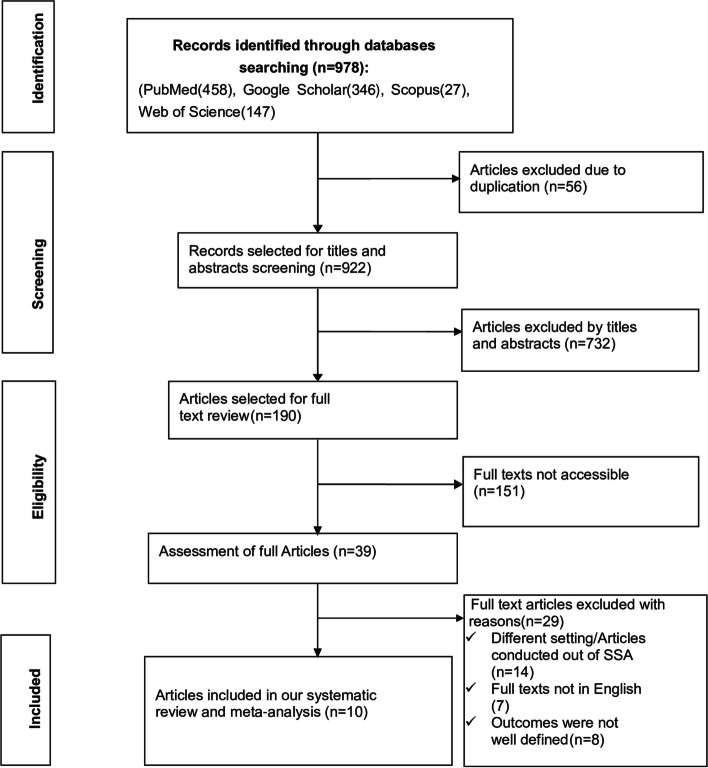
The search strategy retrieved a total of 978 studies from PubMed (458), Google Scholar (346), Scopus (27), and Web of Science (147). After removing the irrelevant studies based on their titles and abstracts (732) and duplicated studies (56), a total of 190 studies were selected for full-text review.
Afterward, full-text reviews were conducted, resulting in the removal of 151 studies due to a lack of complete texts. Then, 39 studies were assessed for full article review and 29 studies were excluded. Finally, 10 studies were found to be relevant for determining the pooled prevalence of PSCI and identifying its associated factors. We have constructed the PRISMA flow chart [46] to show the selection process from the initially identified records to the ultimately included studies (Fig. 1).
Fig. 1.
PRISMA flow chart showing the studies selection process, 2024
Characteristics of the included studies
Six studies [28, 31–35], three studies [36–38], and one study [14] were conducted using cross-sectional, cohort, and case–control study designs respectively. Concerning geographical regions, four studies [31, 34, 35, 37] were conducted in Ethiopia, two [32, 33] in Uganda, one [28] in Ghana, one [36] in Tanzania, one [14] in Nigeria, and one [38] was conducted in the Democratic Republic of the Congo (DRC). The total sample size of the included studies was 1,709, where the smallest and largest sample sizes were 67 [34, 35] and 422 [31] respectively, among studies conducted in Ethiopia. The response rate of the included studies ranged from 61.96 to 100% (Supplemental Table 4).
Meta-analysis
Pooled prevalence of poststroke cognitive impairment
Finally, the 10 eligible primary studies [14, 28, 31–38] were included in the final meta-analysis, and the pooled prevalence of PSCI was 59.61% (95% CI: 46.87, 72.35); I2 = 96.47%; P < 0.001) (Fig. 2).
Fig. 2.
Forest plot showing the pooled prevalence of PSCI with 95% CIs in SSA, 2024
Publication bias
The asymmetric distribution of the funnel plot indicated the presence of publication bias in the study (Fig. 3).
Fig. 3.
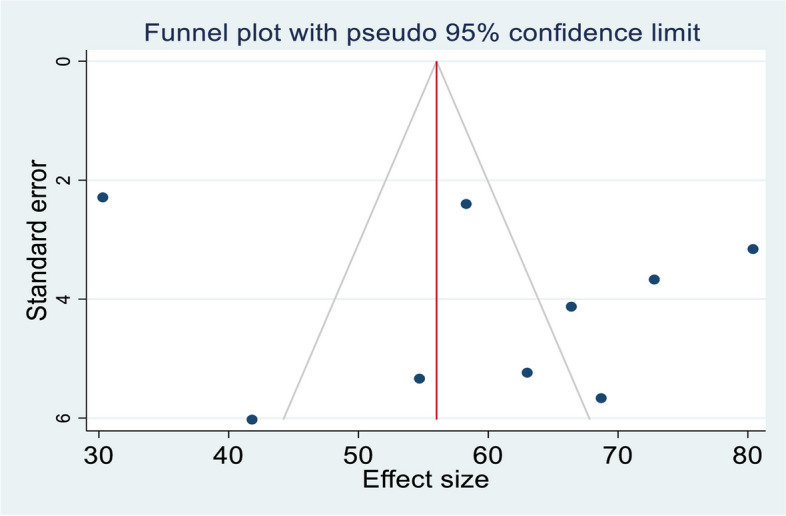
Funnel plot showing the publication bias of PSCI among stroke survivors in SSA, 2024
Investigation of heterogeneity
The percentage of I2 statistics in the forest plot indicated a considerable heterogeneity among the included primary studies (I2 = 96.47%; P < 0.001) (Fig. 2). Therefore, sensitivity and subgroup analyses were performed to investigate potential sources of heterogeneity.
Sensitivity analysis
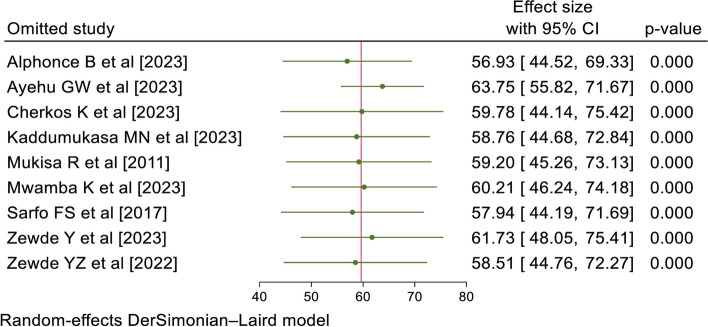
A sensitivity analysis was also conducted to determine the influence of a single study on the overall meta-analysis. The forest plot showed that the estimate of a single study was closer to the combined estimate, indicating the absence of a single study effect on the overall pooled estimate (Fig. 4).
Fig. 4.
Sensitivity analysis of PSCI among stroke survivors in SSA, 2024
Subgroup analysis
Subgroup analysis was performed based on the study design and sample size. The subgroup analysis performed using the study design revealed that the higher pooled prevalence of PSCI was detected among studies conducted with a cross-sectional study design [62.27, 95% CI: 54.79, 69.75; I2 = 79.84%, P < 0.001], and the lower pooled prevalence was among studies conducted with a cohort study design [55.09, 95% CI: 20.60, 89.58; I2 = 96.39%; P < 0.001]. Similarly, the subgroup analysis performed using the sample size indicated that the higher pooled prevalence of PSCI was among studies with a sample size < 171 [64.57, 95% CI: 55.58, 73.57; I2 = 86.13%; P < 0.001] followed by studies with a sample size ≥ 171 [44.29, 95% CI: 16.85, 71.73; I2 = 98.60%; P < 0.001] (Table 1). Thus, the heterogeneity of the study could be attributed to variances in study design and sample size across the primary studies.
Factors associated with poststroke cognitive impairment
In the study, eight studies [14, 28, 31–34, 36, 37] indicated that increased age (≥ 45 years) was significantly associated with PSCI. The pooled AOR of PSCI for stroke survivors aged ≥ 45 years was 1.23 (95% CI: 1.09, 1.40; I2 = 90.70%; P < 0.001) (Fig. 5).
Fig. 5.
Forest plot of the AORs with 95% CIs of studies on the association of increased age and PSCI among stroke survivors in SSA, 2024
Five studies [14, 28, 31, 32, 34] reported a significant association between lower educational levels and PSCI. The pooled AOR of PSCI for stroke survivors with a lower educational level was 4.35 (95% CI: 2.87, 6.61; I2 = 0.00%; P < 0.89) (Fig. 6).
Fig. 6.
Forest plot of the AORs with 95% CIs of studies on the association of lower educational level and PSCI among stroke survivors in SSA, 2024
Five studies [28, 32, 34, 35, 37] showed that poor functional recovery was significantly associated with PSCI. The pooled AOR of PSCI for stroke survivors with poor functional recovery was 1.75 (95% CI: 1.42, 2.15; I2 = 45.33%; P < 0.12) (Fig. 7).
Fig. 7.
Forest plot of the AORs with 95% CIs of studies on the association of poor functional recovery and PSCI among stroke survivors in SSA, 2024
Three studies [31, 35, 36] also revealed a significant association between left hemisphere stroke and PSCI. The pooled AOR of PSCI for stroke survivors with left hemisphere stroke was 4.88 (95% CI: 2.98, 7.99; I2 = 0.00%; P < 0.99) (Fig. 8).
Fig. 8.
Forest plot of the AORs with 95% CIs of studies on the association of left hemisphere stroke and PSCI among stroke survivors in SSA, 2024
Discussion
The study findings indicated that the pooled prevalence of PSCI was 59.61% (95% CI: 46.87, 72.35); I2 = 96.47%; P < 0.001). This finding is consistent with/higher than that of studies conducted in Japan (48%) [47], Austria (56.3%) [48], Norway (57%) [49], Finland (61.7%) [50], Indonesia (68.2%) [51], Holland (70%) [52], France (16.3%) [53], USA (19.3%) [54], Benin (20%) [55], Egypt (25.3%) [56], Chile (39%) [57], and China (39.4%) [58]. However, this finding is lower than that of a study conducted in China (80%) [59]. These discrepancies could be due to variations in the cognitive assessment tool used, inconsistency of treatment approaches, the cutoff point for diagnosis, or sample sizes across the studies [37, 60].
In addition, the findings of the study indicated that stroke survivors aged ≥ 45 years were 1.23 fold more likely to develop PSCI than those aged < 45 years. The finding of this study was consistent with the finding of a study conducted in Egypt [56]. This could be due to the nature of stroke, which brings cognitive decline in line with increased age [61]. Hence, encouraging lifestyle modifications could be a way to reduce the occurrence of cognitive impairment with increased age after stroke.
The findings of this study also revealed that stroke survivors with lower educational levels were 4.35 times more likely to develop PSCI than stroke survivors with higher educational levels. This study finding was supported by a study conducted in China [59]. Education increases the cognitive reserve and protects against cognitive impairment [62]. This could be the reason why a lower educational level favors the onset of PSCI. Therefore, improving access to education could be a way of decreasing the occurrence of cognitive impairment and dementia after stroke.
Similarly, the findings of this study indicated that stroke survivors with poor functional recovery were 1.75 times more likely to experience PSCI than their counterparts. This study finding was congruent with the study conducted in China [63]. A possible reason could be poor functional recovery is an indicator of stroke severity, clinical deficit, and vascular burden [64]. Consequently, initiating rehabilitative services early could enhance functional recovery, which may be a mean to decrease the occurrence of cognitive impairment after a stroke.
Moreover, the findings of this study revealed that stroke survivors with left hemisphere stroke were 4.88 times more likely to experience PSCI than stroke survivors with right hemisphere stroke. This finding was similar to those of a study conducted in Egypt [65]. Language is a key domain in the MoCA assessment [66], and it is a left hemispheric cognitive domain for more than 90% of the population worldwide and is potentially affected by left hemisphere stroke [65, 67, 68]. Thus, delivering rehabilitative services early could maintain the cognitive domain of left hemisphere, which could be a way to decrease the occurrence of cognitive impairment after a stroke.
Strengths and limitations of the study
To the best of our knowledge, this was the first systematic review and meta-analysis conducted to pool the results of various primary studies that have been conducted in SSA, providing strong evidence of PSCI among stroke survivors. Although the included studies were of good quality, most of the included primary studies were cross-sectional. Similarly, there was heterogeneity in PCSI diagnostic methods, absence of data indicating stroke severity, and a high proportion of loss to follow-up across the included studies. Moreover, this study included only studies that have been conducted using the English language.
Conclusions
The pooled prevalence of PSCI was considerably high among stroke survivors in SSA. Increased age, lower educational level, poor functional recovery, and left hemisphere stroke were the pooled independent predictors of PSCI in SSA. Stakeholders should focus on empowering education and lifestyle modifications, keeping their minds engaged, staying connected with social activities and introducing rehabilitative services for stroke survivors with these identified factors to reduce the risk of developing poststroke cognitive impairment.
Supplementary Information
Additional file 1: Supplemental file 1. Search strategy.
Additional file 2: Supplemental Table 1. PRISMA checklist.
Additional file 3: Supplemental Table 3. Quality assessment of the included studies..
Additional file 4: Supplemental Table 4. Risk of bias assessment of the included studies.
Additional file 5: Supplemental Table 4. General characteristics of the included studies.
Acknowledgements
We would like to extend our deepest gratitude to Mr. Henok Andualem for his unreserved support throughout the study.
Abbreviations
- AOR
Adjusted odds ratio
- CI
Confidence interval
- CSID
Community Screening Instrument for Dementia
- DRC
Democratic Republic of the Congo
- GCS
Glasgow Coma Scale
- JBI
Joanna Briggs Institute
- MMSE
Mini-Mental State Examination
- MoCA
Montreal Cognitive Assessment
- PICO
Population, Intervention, Context and Outcome
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSCI
Poststroke Cognitive Impairment
- USA
United States of America
Authors’ contributions
TMA has generated the idea for this review. DK, BMB, SDK, WNA, and AK were involved in the data collection and statistical analysis. TMA wrote the first draft of this manuscript. YMT, GL, AG, BB, and SA revised the manuscript. All the authors were responsible for the accuracy of the analysis, and the contents of the study. Finally, all the authors read and approved the final version of the manuscript for publication.
Funding
Not applicable.
Availability of data and materials
All the necessary data and materials were included in the manuscript.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All the authors declare that they have no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Adeloye D. An estimate of the incidence and prevalence of stroke in Africa: a systematic review and meta-analysis. PLoS ONE. 2014J 26;9(6). 10.1371/journal.pone.0100724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L, Truelsen T, O’Donnell M. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. The lancet. 2014J 18;383(9913):245–55. 10.1016/S0140-6736(13)61953-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Owolabi M O, Arulogun O, Melikam S, Adeoye A M, Akarolo-Anthony S, Akinyemi R, Arnett D, Tiwari H, Gebregziabher M, Jenkins C, Lackland D. The burden of stroke in Africa: a glance at the present and a glimpse into the future. Cardiovasc J Afr. 2015;26(2):S27-38. 10.5830/CVJA-2015-038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu Z, Chen L, Guo D, Zhong C, Wang A, Bu X, Xu T, Zhang J, Ju Z, Guo L, Zhang J. Serum rheumatoid factor levels at acute phase of ischemic stroke are associated with poststroke cognitive impairment. J Stroke Cerebrovasc Dis. 2019A 1;28(4):1133–40. 10.1016/j.jstrokecerebrovasdis.2018.12.049 [DOI] [PubMed] [Google Scholar]
- 5.Saini V, Guada L, Yavagal DR. Global Epidemiology of Stroke and Access to Acute Ischemic Stroke Interventions. Neurology. 2021;97(20):S6–16. [DOI] [PubMed] [Google Scholar]
- 6.Katan M, Luft A. Global burden of stroke. InSeminars in neurology 2018 Apr (Vol. 38, No. 02, pp. 208–211). Thieme Medical Publishers. [DOI] [PubMed]
- 7.Abba MA, Usman MY. Prevalence and pattern of post-stroke cognitive impairment in Kano, Nigeria. Archives of Physiotherapy and Global Researches. 2020F 5;24(1):7–11. [Google Scholar]
- 8.Srinivas S, Rk B V, Ayinapudi V N, Govindarajan A, Sundaram S S, Priyathersini N. Neurological Consequences of Cardiac Arrhythmias: Relationship Between Stroke, Cognitive Decline, and Heart Rhythm Disorders. Cureus. 2024;16(3):e57159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JY, Lee DW, Cho SJ, Na DL, Jeon HJ, Kim SK, Lee YR, Youn JH, Kwon M, Lee JH, Cho MJ. Brief screening for mild cognitive impairment in elderly outpatient clinic: validation of the Korean version of the Montreal Cognitive Assessment. J Geriatr Psychiatry Neurol. 2008J;21(2):104–10. 10.1177/0891988708316855 [DOI] [PubMed] [Google Scholar]
- 10.Pendlebury ST, Mariz J, Bull L, Mehta Z, Rothwell PM. MoCA, ACE-R, and MMSE versus the National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards neuropsychological battery after tia and stroke. Stroke. 2012F;43(2):464–9. 10.1161/STROKEAHA.111.633586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong L, Gu Y, Yu Q, Wang H, Zhu X, Dong Q, Xu R, Zhao Y, Liu X. Prognostic factors for cognitive recovery beyond early poststroke cognitive impairment (PSCI): a prospective cohort study of spontaneous intracerebral hemorrhage. Front Neurol. 2020A;28(11):278. 10.3389/fneur.2020.00278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nys GM, Van Zandvoort MJ, De Kort PL, Jansen BP, De Haan EH, Kappelle LJ. Cognitive disorders in acute stroke: prevalence and clinical determinants. Cerebrovasc Dis. 2007A 2;23(5–6):408–16. 10.1159/000101464 [DOI] [PubMed] [Google Scholar]
- 13.Sharma R, Mallick D, Llinas RH, Marsh EB. Early post-stroke cognition: in-hospital predictors and the association with functional outcome. Front Neurol. 2020D;23(11). 10.3389/fneur.2020.613607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akinyemi RO, Allan L, Owolabi MO, Akinyemi JO, Ogbole G, Ajani A, Firbank M, Ogunniyi A, Kalaria RN. Profile and determinants of vascular cognitive impairment in African stroke survivors: the CogFAST Nigeria Study. J Neurol Sci. 2014N 15;346(1–2):241–9. 10.1016/j.jns.2014.08.042 [DOI] [PubMed] [Google Scholar]
- 15.Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, Powers WJ, DeCarli C, Merino JG, Kalaria RN, Vinters HV. National Institute of Neurological Disorders and Stroke-Canadian stroke network vascular cognitive impairment harmonization standards. Stroke. 2006S 1;37(9):2220–41. 10.1161/01.STR.0000237236.88823.47 [DOI] [PubMed] [Google Scholar]
- 16.Ballard C, Rowan E, Stephens S, Kalaria R, Kenny RA. Prospective follow-up study between 3 and 15 months after stroke: improvements and decline in cognitive function among dementia-free stroke survivors> 75 years of age. Stroke. 2003O 1;34(10):2440–4. 10.1161/01.STR.0000089923.29724.CE [DOI] [PubMed] [Google Scholar]
- 17.Gorelick P B, Scuteri A, Black S E, DeCarli C, Greenberg S M, Iadecola C, Launer L J, Laurent S, Lopez O L, Nyenhuis D, Petersen RC. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. stroke. 2011;42(9):2672–713. 10.1161/STR.0b013e3182299496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henon H, Pasquier F, Leys D. Poststroke dementia. Cerebrovasc Dis. 2006A 26;22(1):61–70. 10.1159/000092923 [DOI] [PubMed] [Google Scholar]
- 19.J Jeffares I, Rohde D, Doyle F, Horgan F, Hickey A. The impact of stroke, cognitive function and post-stroke cognitive impairment (PSCI) on healthcare utilisation in Ireland: a cross-sectional nationally representative study. BMC Health Services Research. 2022;22(1):1–3. 10.1186/s12913-022-07837-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fride Y, Adamit T, Maeir A, Ben Assayag E, Bornstein NM, Korczyn AD, Katz N. What are the correlates of cognition and participation to return to work after first ever mild stroke? Top Stroke Rehabil. 2015O 1;22(5):317–25. 10.1179/1074935714Z.0000000013 [DOI] [PubMed] [Google Scholar]
- 21.Lees RA, Hendry BAK, Broomfield N, Stott D, Larner AJ, Quinn TJ. Cognitive assessment in stroke: feasibility and test properties using differing approaches to scoring of incomplete items. Int J Geriatr Psychiatry. 2017O;32(10):1072–8. 10.1002/gps.4568 [DOI] [PubMed] [Google Scholar]
- 22.Levine DA, Wadley VG, Langa KM, Unverzagt FW, Kabeto MU, Giordani B, Howard G, Howard VJ, Cushman M, Judd SE, Galecki AT. Risk factors for poststroke cognitive decline: the REGARDS study (reasons for geographic and racial differences in stroke). Stroke. 2018A;49(4):987–94. 10.1161/STROKEAHA.117.018529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollock A, St George B, Fenton M, Firkins L. Top ten research priorities relating to life after stroke. The Lancet Neurology. 2012M 1;11(3):209. 10.1016/S1474-4422(12)70029-7 [DOI] [PubMed] [Google Scholar]
- 24.Zulkifly MF, Ghazali SE, Din NC, Subramaniam P. The influence of demographic, clinical, psychological and functional determinants on post-stroke cognitive impairment at day care stroke center, Malaysia. The Malaysian Journal of Medical Sciences: MJMS. 2016M;23(2):53. [PMC free article] [PubMed] [Google Scholar]
- 25.Burton EJ, Kenny RA, O’Brien J, Stephens S, Bradbury M, Rowan E, Kalaria R, Firbank M, Wesnes K, Ballard C. White matter hyperintensities are associated with impairment of memory, attention, and global cognitive performance in older stroke patients. Stroke. 2004J 1;35(6):1270–5. 10.1161/01.STR.0000126041.99024.86 [DOI] [PubMed] [Google Scholar]
- 26.Danovska M, Peychinska D. Danovska M, Peychinska D. Post-stroke cognitive impairment–phenomenology and prognostic factors. Journal of IMAB–Annual Proceeding Scientific Papers. 2012;18(3):290–7. 10.5272/jimab.2012183.290 [DOI] [Google Scholar]
- 27.Gottesman RF, Hillis AE. Predictors and assessment of cognitive dysfunction resulting from ischaemic stroke. The Lancet Neurology. 2010S 1;9(9):895–905. 10.1016/S1474-4422(10)70164-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarfo FS, Akassi J, Adamu S, Obese V, Ovbiagele B. Burden and predictors of poststroke cognitive impairment in a sample of Ghanaian stroke survivors. J Stroke Cerebrovasc Dis. 2017N 1;26(11):2553–62. 10.1016/j.jstrokecerebrovasdis.2017.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021A;1(88). 10.1016/j.ijsu.2021.105906 [DOI] [PubMed] [Google Scholar]
- 30.Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. JBI Evid Implementation. 2015;13(3):141–6. [DOI] [PubMed] [Google Scholar]
- 31.Cherkos K, Jember G, Mihret T, Fentanew M. Prevalence and Associated Factors of Cognitive Impairment Among Stroke Survivors at Comprehensive Specialized Hospitals in Northwest Ethiopia: Multi-Centered Cross-Sectional Study. Vascular Health and Risk Management. 2023D;31:265–77. 10.2147/VHRM.S405357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaddumukasa MN, Kaddumukasa M, Katabira E, Sewankambo N, Namujju LD, Goldstein LB. Prevalence and predictors of post-stroke cognitive impairment among stroke survivors in Uganda. BMC Neurol. 2023D;23(1):1–8. 10.1186/s12883-023-03212-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukisa R, Ddumba E, Musisi S, Kiwuwa SM. Prevalence and types of cognitive impairment among patients with stroke attending a referral hospital in Uganda. Afr J Neurol Sci. 2011;30(2).
- 34.Zewde Y, Alem A, Seeger SK. Magnitude and predictors of post-stroke cognitive impairment among Ethiopian stroke survivors: A facility-based cross-sectional study. Res Sq. 2023.
- 35.Zewde YZ. Post-stroke dementia and its determinant factors among Ethiopian stroke survivors: A cross-sectional study. Alzheimer’s & Dementia. 2022D;18. 10.1002/alz.060612 [DOI] [Google Scholar]
- 36.Alphonce B, Meda J, Nyundo A. Incidence and predictors of post-stroke cognitive impairment among patients admitted with first stroke at tertiary hospitals in Dodoma, Tanzania: A prospective cohort study. PLoS One. 2024;19(4):e0287952. 10.1371/journal.pone.0287952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ayehu GW, Admasu FT, Yitbarek GY, Agegnehu Teshome A, Amare AT, Atlaw D, Sharma S. Early post-stroke cognitive impairment and in-hospital predicting factors among stroke survivors in Ethiopia. Front Neurol. 2023M;22(14):1163812. 10.3389/fneur.2023.1163812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mwamba K, Kazenza B, Mbenza BL, Kalula TK, Ayanne MT, Bumoko G. Evolving Profile and Determinants of Post-Stroke Cognitive Impairment in the 3rd Month among Kinshasa’s Survivors (Democratic Republic of the Congo). World Journal of Neuroscience. 2023J 28;13(3):160–72. 10.4236/wjns.2023.133011 [DOI] [Google Scholar]
- 39.Fatoye FO, Komolafe MA, Eegunranti BA, Adewuya AO, Mosaku SK, Fatoye GK. Cognitive impairment and quality of life among stroke survivors in Nigeria. Psychol Rep. 2007J;100(3):876–82. 10.2466/pr0.100.3.876-882 [DOI] [PubMed] [Google Scholar]
- 40.Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, Baker P, Smith E, Buchbinder R. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012S 1;65(9):934–9. 10.1016/j.jclinepi.2011.11.014 [DOI] [PubMed] [Google Scholar]
- 41.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007F 1;28(2):105–14. 10.1016/j.cct.2006.04.004 [DOI] [PubMed] [Google Scholar]
- 42.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006F 8;295(6):676–80. 10.1001/jama.295.6.676 [DOI] [PubMed] [Google Scholar]
- 43.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003S 4;327(7414):557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Research synthesis methods. 2010A;1(2):97–111. 10.1002/jrsm.12 [DOI] [PubMed] [Google Scholar]
- 45.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002J 15;21(11):1539–58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 46.Stovold E, Beecher D, Foxlee R, Noel-Storr A. Study flow diagrams in Cochrane systematic review updates: an adapted PRISMA flow diagram. Syst Rev. 2014;29(3):54. 10.1186/2046-4053-3-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naoki MO, Otaka Y, Honaga K, Matsuura D, Kondo K, Meigen L I, Tsuji T. Factors associated with cognitive improvement in subacute stroke survivors. J Rehabil Med. 2021;53(8):jrm00220. 10.2340/16501977-2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ladurner G, Iliff LD, Lechner H. Clinical factors associated with dementia in ischaemic stroke. J Neurol Neurosurg Psychiatry. 1982F;45(2):97. 10.1136/jnnp.45.2.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ihle-Hansen H, Thommessen B, Bruun Wyller T, Engedal K, Øksengård AR, Stenset V, Løken K, Aaberg M, Fure B. Incidence and subtypes of MCI and dementia 1 year after first-ever stroke in patients without pre-existing cognitive impairment. Dement Geriatr Cogn Disord. 2012F 3;32(6):401–7. 10.1159/000335361 [DOI] [PubMed] [Google Scholar]
- 50.Pohjasvaara T, Erkinjuntti T, Vataja R, Kaste M. Dementia three months after stroke: baseline frequency and effect of different definitions of dementia in the Helsinki Stroke Aging Memory Study (SAM) cohort. Stroke. 1997A;28(4):785–92. 10.1161/01.STR.28.4.785 [DOI] [PubMed] [Google Scholar]
- 51.Pinzon RT, Sanyasi RD, Totting S. The prevalence and determinant factors of post-stroke cognitive impairment. Asian Pacific J Heal Sci. 2018;5(1):78–83. 10.21276/apjhs.2018.5.1.17 [DOI] [Google Scholar]
- 52.Rasquin S, Verhey F, Van Oostenbrugge RJ, Lousberg R, Lodder J. Demographic and CT scan features related to cognitive impairment in the first year after stroke. J Neurol Neurosurg Psychiatry. 2004N;75(11):1562. 10.1136/jnnp.2003.024190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jacquin A, Binquet C, Rouaud O, Graule-Petot A, Daubail B, Osseby GV, Bonithon-Kopp C, Giroud M, Béjot Y. Post-stroke cognitive impairment: high prevalence and determining factors in a cohort of mild stroke. Journal of Alzheimer’s Disease. 2014J 1;40(4):1029–38. 10.3233/JAD-131580 [DOI] [PubMed] [Google Scholar]
- 54.Ivan CS, Seshadri S, Beiser A, Au R, Kase CS, Kelly-Hayes M, Wolf PA. Dementia after stroke: the Framingham Study. Stroke. 2004J 1;35(6):1264–8. 10.1161/01.STR.0000127810.92616.78 [DOI] [PubMed] [Google Scholar]
- 55.Gnonlonfoun DD, Ossou-Nguiet PM, Diallo LL, Adjien C, Avlessi I, Goudjinou G, Houannou O, Houinato D, Avode GD. Post-Stroke Cognitive Disorders and Associated Factors in French Speaking West Africa. Benin Case Neuroscience and Medicine. 2014M 7;5(01):32–41. 10.4236/nm.2014.51006 [DOI] [Google Scholar]
- 56.Esmael A, Elsherief M, Eltoukhy K. Prevalence of cognitive impairment in acute ischaemic stroke and use of Alberta Stroke Programme Early CT Score (ASPECTS) for early prediction of post-stroke cognitive impairment. Neurol Neurochir Pol. 2021;55(2):179–85. 10.5603/PJNNS.a2021.0006 [DOI] [PubMed] [Google Scholar]
- 57.Delgado C, Donoso A, Orellana P, Vásquez C, Díaz V, Behrens MI. Frequency and determinants of poststroke cognitive impairment at three and twelve months in Chile. Dement Geriatr Cogn Disord. 2010M 19;29(5):397–405. 10.1159/000305097 [DOI] [PubMed] [Google Scholar]
- 58.Huang Y, Yang S, Jia J. Factors related to long-term post-stroke cognitive impairment in young adult ischemic stroke. Medical science monitor: international medical journal of experimental and clinical research. 2015;21:654. 10.12659/MSM.892554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qu Y, Zhuo L, Li N, Hu Y, Chen W, Zhou Y, Wang J, Tao Q, Hu J, Nie X, Zhan S. Prevalence of post-stroke cognitive impairment in china: a community-based, cross-sectional study. PLoS ONE. 2015A 13;10(4). 10.1371/journal.pone.0122864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun JH, Tan L, Yu JT. Post-stroke cognitive impairment: epidemiology, mechanisms and management. Annals of translational medicine. 2014 Aug;2(8). [DOI] [PMC free article] [PubMed]
- 61.De Ronchi D, Palmer K, Pioggiosi P, Atti AR, Berardi D, Ferrari B, Dalmonte E, Fratiglioni L. The combined effect of age, education, and stroke on dementia and cognitive impairment no dementia in the elderly. Dement Geriatr Cogn Disord. 2007S 1;24(4):266–73. 10.1159/000107102 [DOI] [PubMed] [Google Scholar]
- 62.Meng X, D’arcy C. Education and dementia in the context of the cognitive reserve hypothesis: a systematic review with meta-analyses and qualitative analyses. PLoS ONE. 2012J 4;7(6). 10.1371/journal.pone.0038268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li J, Wang J, Wu B, Xu H, Wu X, Zhou L, Deng B. Association between early cognitive impairment and midterm functional outcomes among Chinese acute ischemic stroke patients: a longitudinal study. Front Neurol. 2020F;26(11):20. 10.3389/fneur.2020.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leys D, Hénon H, Mackowiak-Cordoliani MA, Pasquier F. Poststroke dementia. The Lancet Neurology. 2005N 1;4(11):752–9. 10.1016/S1474-4422(05)70221-0 [DOI] [PubMed] [Google Scholar]
- 65.Lo Coco D, Lopez G, Corrao S. Cognitive impairment and stroke in elderly patients. Vascular health and risk management. 2016M;24:105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hobson J. The montreal cognitive assessment (MoCA). Occup Med. 2015D 1;65(9):764–5. 10.1093/occmed/kqv078 [DOI] [PubMed] [Google Scholar]
- 67.Fridriksson J, den Ouden DB, Hillis AE, Hickok G, Rorden C, Basilakos A, Yourganov G, Bonilha L. Anatomy of aphasia revisited. Brain. 2018M 1;141(3):848–62. 10.1093/brain/awx363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Packheiser J, Schmitz J, Arning L, Beste C, Güntürkün O, Ocklenburg S. A large-scale estimate on the relationship between language and motor lateralization. Sci Rep. 2020A 3;10(1):13027. 10.1038/s41598-020-70057-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplemental file 1. Search strategy.
Additional file 2: Supplemental Table 1. PRISMA checklist.
Additional file 3: Supplemental Table 3. Quality assessment of the included studies..
Additional file 4: Supplemental Table 4. Risk of bias assessment of the included studies.
Additional file 5: Supplemental Table 4. General characteristics of the included studies.
Data Availability Statement
All the necessary data and materials were included in the manuscript.