Abstract
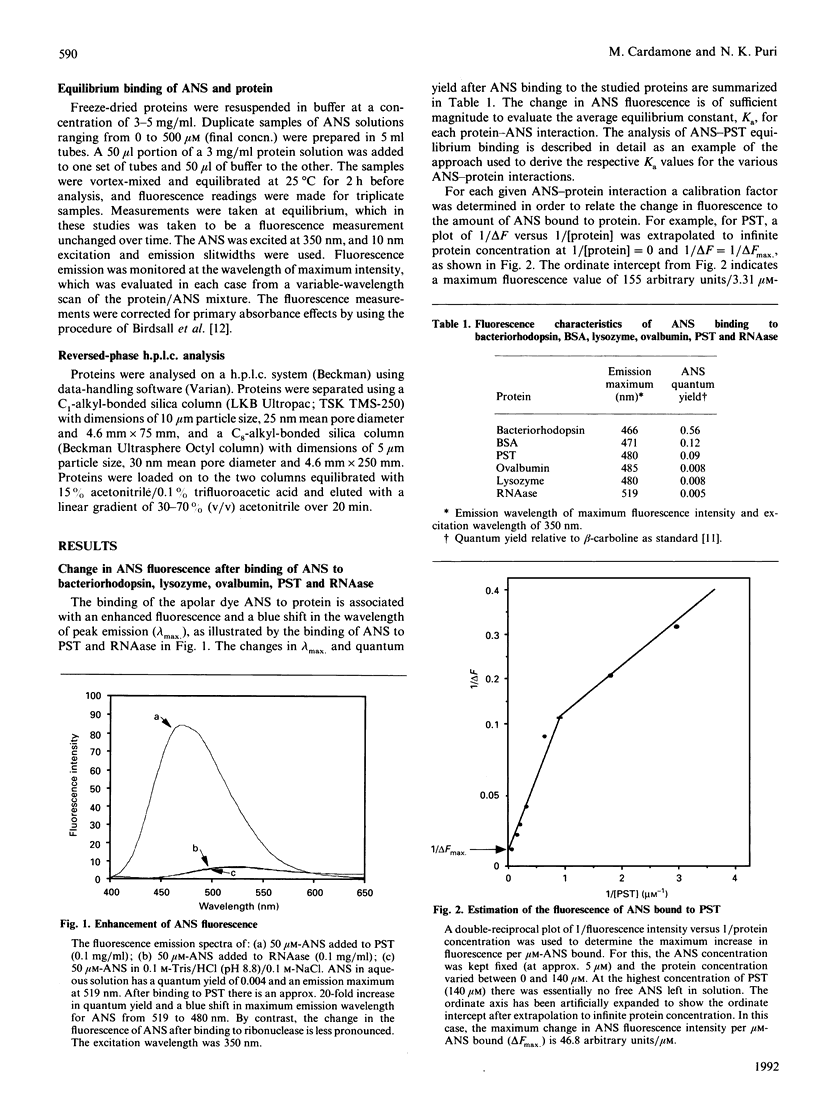
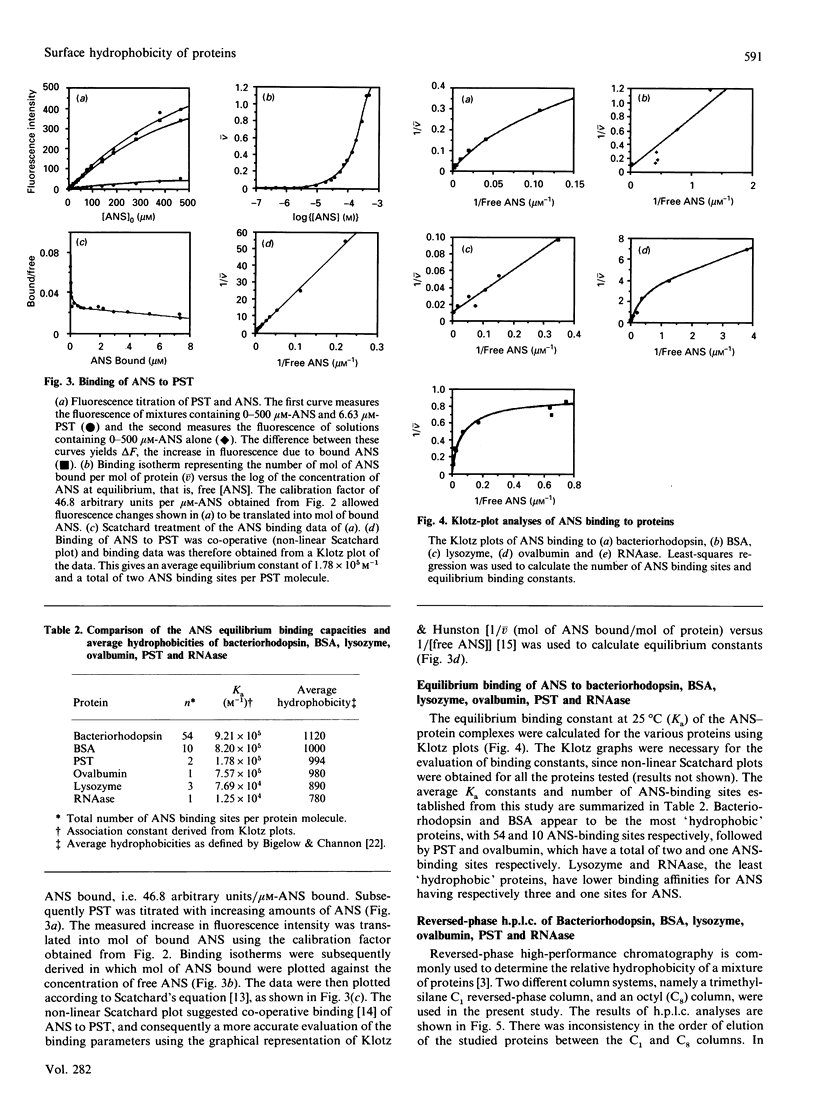
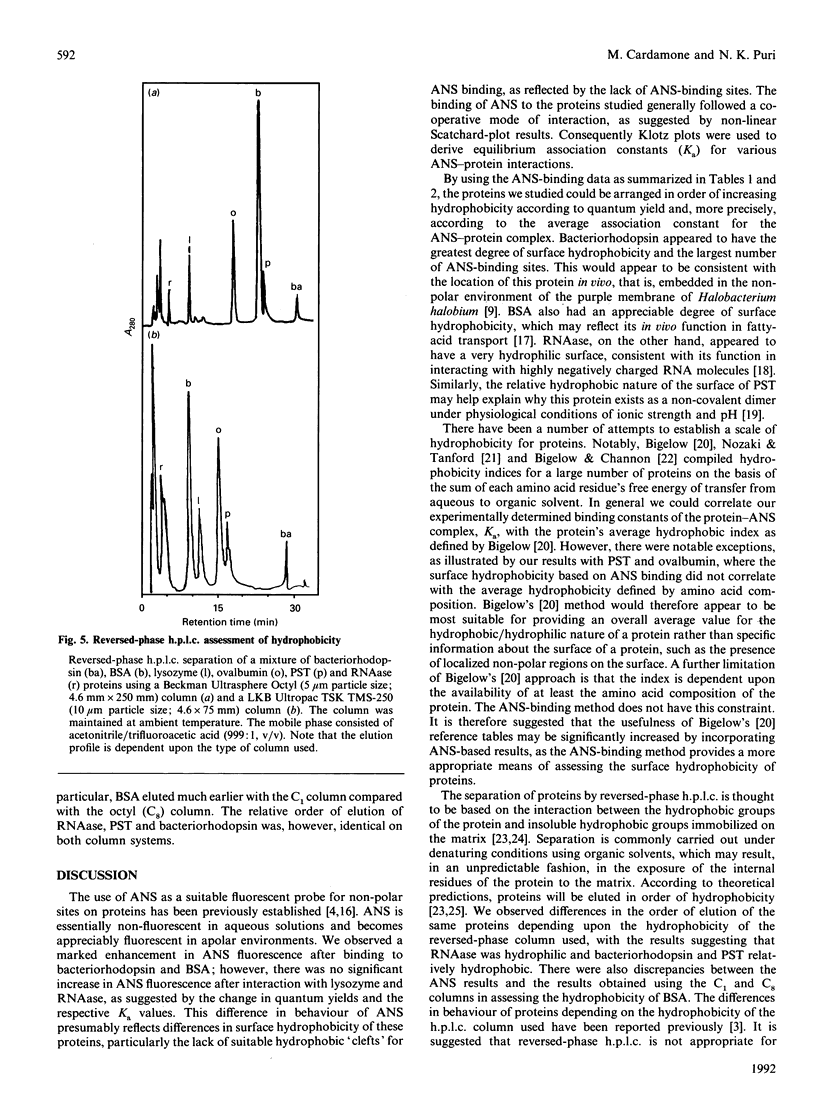
The equilibrium binding of the apolar fluorescent dye 1-anilinonaphthalene-8-sulphonate (ANS) to bacteriorhodopsin, BSA, chicken egg lysozyme, ovalbumin, porcine somatotrophin (PST) and bovine pancreatic ribonuclease (RNAase) was quantitatively evaluated using Scatchard- and Klotz-plot analyses. On the basis of the average association constant for ANS binding sites (Ka), the proteins could be ranked in order of surface hydrophobicity as: Bacteriorhodopsin greater than BSA greater than ovalbumin greater than PST greater than lysozyme greater than RNAase. The number of protein-ANS binding sites was determined as 54, 10, 3, 1, 2 and 1 respectively. The ANS-based assessment of the surface hydrophobicity of these proteins was generally in agreement with the average hydrophobicity based on amino acid sequence [Bigelow (1967) J. Theor. Biol. 16, 187-211], except for results with PST and ovalbumin. The proteins were also analysed by reversed-phase h.p.l.c. using C1 and C8 columns. There was no significant correlation between ANS and reversed-phase-h.p.l.c. assessment of hydrophobicity, with the results obtained by h.p.l.c. being dependent upon the column used. ANS-based measurement of surface hydrophobicity appears to be the most appropriate means for assessing proteins such as to reflect their overall three-dimensional structure in solution.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bigelow C. C. On the average hydrophobicity of proteins and the relation between it and protein structure. J Theor Biol. 1967 Aug;16(2):187–211. doi: 10.1016/0022-5193(67)90004-5. [DOI] [PubMed] [Google Scholar]
- Birdsall B., King R. W., Wheeler M. R., Lewis C. A., Jr, Goode S. R., Dunlap R. B., Roberts G. C. Correction for light absorption in fluorescence studies of protein-ligand interactions. Anal Biochem. 1983 Jul 15;132(2):353–361. doi: 10.1016/0003-2697(83)90020-9. [DOI] [PubMed] [Google Scholar]
- Davies R. C., Neuberger A., Wilson B. M. The dependence of lysozyme activity on pH and ionic strength. Biochim Biophys Acta. 1969 Apr 22;178(2):294–305. doi: 10.1016/0005-2744(69)90397-0. [DOI] [PubMed] [Google Scholar]
- Fausnaugh J. L., Kennedy L. A., Regnier F. E. Comparison of hydrophobic-interaction and reversed-phase chromatography of proteins. J Chromatogr. 1984 Dec 28;317:141–155. doi: 10.1016/s0021-9673(01)91654-1. [DOI] [PubMed] [Google Scholar]
- Joniau M., Bloemmen J., Lontie R. The reaction of 4-(p-sulfophenylazo)-2-mercuriphenol with the thiol groups of proteins. Biochim Biophys Acta. 1970 Sep 29;214(3):468–477. doi: 10.1016/0005-2795(70)90306-5. [DOI] [PubMed] [Google Scholar]
- KAUZMANN W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- Kennedy R. M. Hydrophobic chromatography. Methods Enzymol. 1990;182:339–343. doi: 10.1016/0076-6879(90)82029-2. [DOI] [PubMed] [Google Scholar]
- Klotz I. M., Hunston D. L. Properties of graphical representations of multiple classes of binding sites. Biochemistry. 1971 Aug 3;10(16):3065–3069. doi: 10.1021/bi00792a013. [DOI] [PubMed] [Google Scholar]
- Murphy K. P., Privalov P. L., Gill S. J. Common features of protein unfolding and dissolution of hydrophobic compounds. Science. 1990 Feb 2;247(4942):559–561. doi: 10.1126/science.2300815. [DOI] [PubMed] [Google Scholar]
- Nozaki Y., Tanford C. The solubility of amino acids and two glycine peptides in aqueous ethanol and dioxane solutions. Establishment of a hydrophobicity scale. J Biol Chem. 1971 Apr 10;246(7):2211–2217. [PubMed] [Google Scholar]
- Schein C. H. Solubility as a function of protein structure and solvent components. Biotechnology (N Y) 1990 Apr;8(4):308–317. doi: 10.1038/nbt0490-308. [DOI] [PubMed] [Google Scholar]
- Stryer L. The interaction of a naphthalene dye with apomyoglobin and apohemoglobin. A fluorescent probe of non-polar binding sites. J Mol Biol. 1965 Sep;13(2):482–495. doi: 10.1016/s0022-2836(65)80111-5. [DOI] [PubMed] [Google Scholar]
- TABORSKY G. Inactivation of ribonuclease by phosphorylation. J Biol Chem. 1959 Nov;234:2915–2920. [PubMed] [Google Scholar]
- Thakur A. K., Jaffe M. L., Rodbard D. Graphical analysis of ligand-binding systems: evaluation by Monte Carlo studies. Anal Biochem. 1980 Sep 15;107(2):279–295. doi: 10.1016/0003-2697(80)90385-1. [DOI] [PubMed] [Google Scholar]
- WEBER G., LAURENCE D. J. Fluorescent indicators of adsorption in aqueous solution and on the solid phase. Biochem J. 1954 Jan 16;56(325TH):xxxi–xxxi. [PubMed] [Google Scholar]


