Hepatic steatosis (fatty liver)-associated chronic liver disease is the main cause of liver-related morbidity and mortality worldwide, with a steadily increasing incidence and prevalence. With the advent of highly effective direct-acting antiviral regimes for chronic hepatitis C infection, fatty liver is the most prevalent etiology of early and advanced chronic liver disease.1
The two main causes of fatty liver disease are alcohol-associated liver disease (ALD) and nonalcoholic fatty liver disease (NAFLD). Moreover, many patients have both conditions, so they are considered to have dual etiology fatty liver disease.2 Although there is some agreement on the amount of alcohol required to define the existence of ALD, whether fatty liver disease associated with the metabolic syndrome should exclude any alcohol intake is a matter of controversy. This debate is based on the fact that alcohol intake is present in up to 28% of patients diagnosed with NAFLD and alcohol consumption appears to play a role in disease progression.3 Since its first description in 19804 until the mid-2019, the term “NAFLD” had not been formally revisited. The need for a revised nomenclature to redefine patients with NAFLD was initially proposed by Eslam et al in 2019.5 One year later, the new term “metabolic-associated fatty liver disease” (MAFLD) was coined in a consensus-driven proposal.6
The term NAFLD was initially defined by excessive hepatic fat accumulation associated with obesity and/or insulin resistance.4 Additionally, the exclusion of both secondary causes and a daily alcohol consumption >30 g for men and >20 g for women was required to establish the diagnosis.7 However, there have been several calls for a “positive criteria” rather than “exclusion criteria”-based approach to accurately define the entity as a whole and link it to its pathogenesis. Thus, the term MAFLD was proposed to replace NAFLD, based on evidence of hepatic steatosis proven by imaging techniques, blood biomarkers, or liver histology, in addition to one of the following three criteria: (1) overweight/obesity, (2) type 2 diabetes mellitus, or (3) two cardiometabolic risk factors.8 Although several statements have supported the use of the name MAFLD,8, 9, 10, 11, 12 there is no consensus regarding the revised nomenclature of NAFLD.13, 14, 15 To address these issues, a recent joint AASLD-EASL conference along with multiple interested parties, including other liver associations and patient organizations, was recently held to reach an agreement on new nomenclature (https://www.aasld.org/news/reaching-consensus-nafld-nomenclature).
One of the unmet needs in this new effort to revise the current nomenclature is defining the amount of alcohol that would be included as part of the new definition, that would likely replace the NAFLD acronym. Although there is near universal agreement on that heavy daily alcohol intake (>60 g/day in men and >40 g/day in women) should be considered positive criteria for defining ALD etiology, it is unclear what level of alcohol consumption should be included in a novel disease terminology that will likely replace the diagnosis of NAFLD (i.e., MAFLD or an alternative name). To wit: what is the threshold of daily/weekly alcohol intake that should be used to differentiate between NAFLD/MAFLD alone, dual etiology of fatty liver disease, and ALD alone? Since the management and prognosis of these three entities differ, it is important to clearly define these consumption ranges. This commentary discusses the current use of the newly proposed MAFLD acronym and the different alcohol consumption ranges defined under that acronym in original manuscripts during the last 3 years. Because its use is heavily influenced by different opinion leaders and continental liver societies, we also performed an analysis per continent and the countries where this term has been adopted.
Use of the New Term MAFLD: Geographical Differences
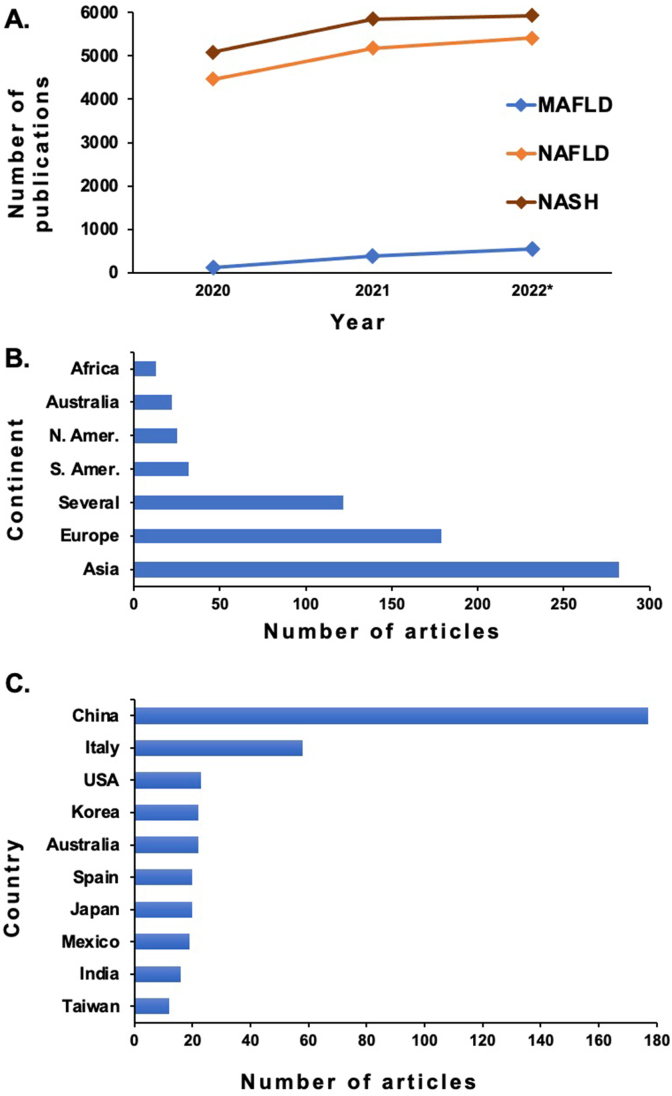
We first assessed the use of the acronym “MAFLD” during the last 3 years (the initial publication was in 2020) using PubMed. All manuscripts published in English from May 2020 until July 2022 were included. Articles in PubMed are freely accessible and the PubMed identifiers of the selected publications are provided (see supplementary material). The literature retrieved in this systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses reporting guidelines.16 Specifically, the terms “MAFLD” vs “NAFLD” vs “NASH” (nonalcoholic steatohepatitis) were used to retrieve all articles that contained those terms. Data regarding the country and the continent (if more than one continent, the publication was considered as global) were collected. Figure 1A shows the number of papers published using different nomenclatures. It clearly shows that the usage of the terms NAFLD and NASH has slightly increased during these years and that the new terminology did not decrease their use. The use of the term MAFLD increased from 2020 (119 manuscripts) to an estimate of 536 manuscripts in 2022. Although the relative contribution of the term MAFLD to publications is steadily increasing, it still only represented ∼4.5% of manuscripts related to fatty liver disease associated with the metabolic syndrome.
Figure 1.
Usage of MAFLD acronym in scientific publications (PubMed). (A) Usage comparison of the terms NASH, NAFLD, and MAFLD in all specialties scientific publications (PubMed). Studies during pregnancy and those that included pediatric populations were excluded. (∗) Estimated rate of publications for the second half of 2022 based on the first half trend. (B) Usage of MAFLD acronym by continent. (C) Usage of MAFLD acronym by the top-ten countries. Countries with less than 12 publications per year are not shown.
We further analyzed the details of MAFLD publications. Out of the 676 publications selected (from 2020 to mid-July 2022), a total of 654 papers were scrutinized. Papers were sorted by geographical region considering the continents and/or countries of the authors' institutions. When publications were considered by continent, the largest number were from Asia (275 out of 654 publications, 42%), followed by Europe (169 papers, 26%), the Americas (South and North, 5% and 4%, respectively), Australia (3%) and Africa (2%) (Figure 1B). The remaining 18% was considered global since more than one continent was involved. Regarding the allocation by country, 504 (77%) of the publications could be assigned to a specific country. China was the dominant contributor country in Asia (174 publications within the three-year period), followed by Italy (58 papers) in Europe. The use of this new term was similar in the remaining countries as indicated in the figure, with a total number of manuscripts that ranges from 11 to 23 publications per year (Figure 1C).
Alcohol Consumption Thresholds in Papers Using the Term MAFLD
Despite the rising prevalence of harmful alcohol use, there is no global consensus on the definition of “at-risk drinking.” The UK Chief Medical Officers consider as an easy and comprehensible threshold for hazardous alcohol intake the strict amount of 14 units per week, equal for men and women. Per this definition, the equivalent amount of alcohol in units would be 1 unit = 8 g of pure alcohol (eg, a small glass of wine is 1.5 units, or a pint of beer is 2 units).17 Other reports on alcohol use different measures (volume, grams per day, and so on). However, the National Institute on Alcohol Abuse and Alcoholism (NIAAA) defines alcohol use disorder as a consumption of >28 drinks per week in men and >21 drinks per week in women, or binge drinking (defined as >5 drinks in males and >4 drinks in females, consumed over a 2-hour period). Moreover, the NIAAA states that a “standard drink” in the United States contains 14 g of alcohol (12 oz of beer, 5 oz of wine, or 1 oz of liquor).18 Given the global prevalence and rising incidence of fatty liver disease, reaching a consensus on the standard units to measure alcohol intake has become an urgent priority as highlighted in several manuscripts.17,19
In our analysis, the amount of alcohol intake included in studies/manuscripts using the MAFLD nomenclature was categorized as “not well-defined” when no mention of the amount of alcohol consumption was specified, either in the methods or the result section. When specified, alcohol intake included under the MAFLD umbrella was stratified into two levels: “nonrestricted threshold” (g of alcohol: >20 g/day for women or >30 g/day for men; or number of drinks: >2 drinks/day or >3 drinks/day, respectively) or “restricted cutoff” that included moderate drinking (<20 g/day for woman or <30 g/day for men; or <2 drinks/day or <3 drinks/day, respectively). Binge drinking or other patterns of alcohol consumption were rarely considered throughout the different studies. Of note, the implemented cutoffs in this study were selected based values that are considered by both EASL and AASLD clinical guidelines.20,21
Use of Different Definitions of Alcohol Intake in Manuscripts Using “MAFLD”
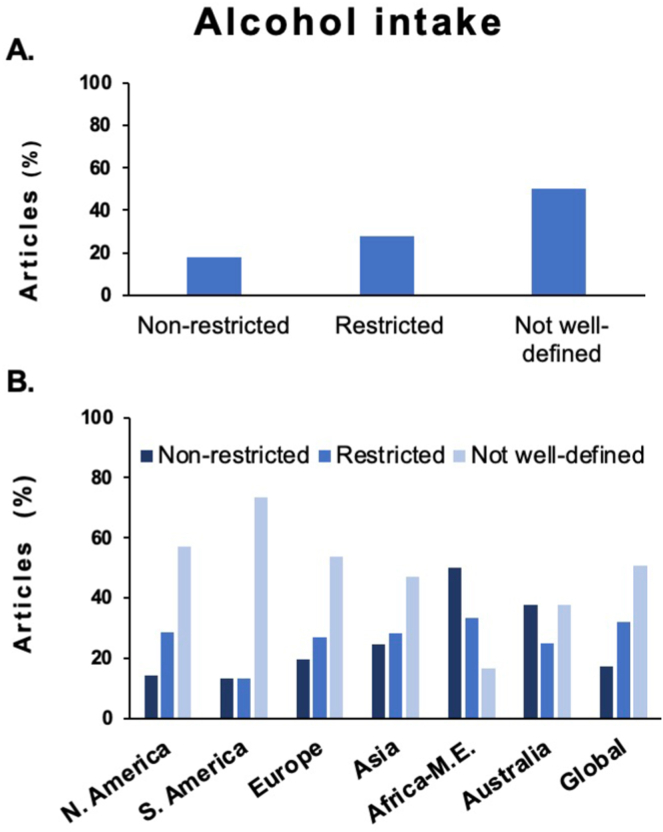
A total of 654 studies were scrutinized in terms of alcohol threshold to define MAFLD vs dual etiology MAFLD/ALD. Studies were allocated into three groups: those allowing any alcohol consumption (“nonrestricted threshold”; 18%), those including restrictive alcohol intake (“restricted cutoff”; 28%), and studies without alcohol intake threshold definition (“not well-defined”; 50%) (Figure 2A). The results indicate that most papers did not include a clear definition and that there was high variability in the threshold used to include patients with significant alcohol intake. Next, the geographical location (stratifying by continent) was collected to identify potential differences in MAFLD definition (Figure 2B). When comparing by continent, significant differences were observed in Africa-Middle East, where the percentage of publication that included a “nonrestricted threshold” was higher than in other continents. These data suggest that the threshold of alcohol intake in the MAFLD definition remains unclear regardless the geographical location.
Figure 2.
Alcohol intake categorization in studies using MAFLD nomenclature. (A) Global thresholds of alcohol intake. (B) Thresholds of alcohol by continent.
Urgent Need for Consensus on Alcohol Consumption Thresholds in the New Nomenclature of Fatty Liver Disease
The number of publications using the new MAFLD nomenclature is rapidly increasing since 2020, yet they only represent a minority of papers referring to fatty liver diseases due to metabolic syndrome. Importantly, the amount of alcohol that is allowed in the new nomenclature is either poorly defined or very heterogeneous. Thus, it is imperative that the international effort to update the nomenclature of fatty liver disease, including the recent joint AASLD-EASL conference, should also focus on the amount of alcohol intake that defines ALD, NAFLD/MAFLD, and dual etiology of fatty liver disease. A multinational Delphi consensus might be an extremely useful approach to address this unmet need. Given the high global prevalence of concurrent metabolic syndrome and alcohol consumption, the subcategory of dual etiology fatty liver disease with clearly defined alcohol consumption thresholds should be promptly included in the revised nomenclature. Large natural history studies of alcohol intake suggest that the threshold of safe alcohol intake may be lower than previously believed, particularly for liver-related complications.22 Besides the synergistic effect between alcohol consumption and metabolic syndrome,21,23 there is individual susceptibility to progress into advanced fibrosis influenced by the combination of behavioral, environmental, genetic, and epigenetic factors.24
In addition, a special consideration on how alcohol intake should be evaluated in the design of clinical trials must be taken. In this regard, patients being enrolled in NASH clinical trials should be carefully assessed for alcohol intake using both self-reports methods (i.e., Alcohol Use Disorders Identification Test) and alcohol biomarkers (ie, either urine-based [ethyl glucuronide/ethyl sulfate] or blood-based [phosphatidyl ethanol]). A recent study already has demonstrated the potential usefulness of alcohol biomarkers in unmasking the unnoticed alcohol consumption in NAFLD patients.3 Ideally, alcohol assessment should be performed at enrollment and during follow-up. In addition, the role of any alcohol intake in treatment or placebo responses should be incorporated in NAFLD clinical trials.
In summary, we think that if MAFLD refers to patients traditionally considered with NAFLD, those with a very high alcohol intake should not be included in this definition. Otherwise, the role of alcohol and the need to treat the underlying alcohol use disorder would be overlooked. Rather, patients with both metabolic syndrome and high intake of alcohol should be considered to have a “dual etiology” disease, concept that is being increasingly used in the medical literature.24 Thus, a “restricted” alcohol consumption should be globally adopted to differentiate MAFLD from dual etiology fatty liver disease.3
Footnotes
Authors’ Contributions: Maria Hernandez-Tejero: Conceptualization, data curation, formal analysis, writing – original draft, writing – review & editing. Samhita Ravi: Data curation. Jaideep Behari: Writing – review & editing. Gavin E. Arteel: Writing – review & editing. Ramon Bataller: Conceptualization, writing – original draft, writing – review & editing.
Conflicts of Interest: The author discloses the following: Jaideep Behari has received research grant funding from Gilead, Pfizer, and Endra Life Sciences. His institution has clinical research contracts with Intercept, Pfizer, Galectin, Exact Sciences, and Inventiva, Enanta, Shire, Gilead, Allergan, Celgene, Galmed Rhythm, and Genentech. The remaining authors disclose no conflicts.
Funding: This article was partially supported by NIH grants 1R01CA255809, 4UH3TR003289, and 5U01AA026978 (to JB); the Chilean government through the Fondo Nacional de Desarrollo Cientifico y Tecnologico (FONDECYT 1200227 to JPA); NIH/NIAAA grants RO1AA018873, 1U01AA026978-01, 1U01AA026972-01, 1U01AA026264-01 and NIDDK grant 1R01DK117881-01 (to RB); and NIH grants R01AA021978 and P30DKDK120531 (GEA).
Ethical Statement: The corresponding author, on behalf of all authors, jointly and severally, certifies that their institution has approved the protocol for any investigation involving humans or animals and that all experimentation was conducted in conformity with ethical and humane principles of research.
Reporting Guidelines: PRISMA.
Material associated with this article can be found in the online version at https://doi.org/10.1016/j.gastha.2023.08.016.
Supplementary Materials
References
- 1.Estes C., Razavi H., Loomba R., et al. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123–133. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyle M., Masson S., Anstee Q.M. The bidirectional impacts of alcohol consumption and the metabolic syndrome: cofactors for progressive fatty liver disease. J Hepatol. 2018;68(2):251–267. doi: 10.1016/j.jhep.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Staufer K., Huber-Schönauer U., Strebinger G., et al. Ethyl glucuronide in hair detects a high rate of harmful alcohol consumption in presumed non-alcoholic fatty liver disease. J Hepatol. 2022;77(4):918–930. doi: 10.1016/j.jhep.2022.04.040. [DOI] [PubMed] [Google Scholar]
- 4.Ludwig J., Viggiano T.R., McGill D.B., et al. Nonalcoholic steatohepatitis: mayo clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55(7):434–438. [PubMed] [Google Scholar]
- 5.Eslam M., Sanyal A.J., George J. Toward more accurate nomenclature for fatty liver diseases. Gastroenterology. 2019;157(3):590–593. doi: 10.1053/j.gastro.2019.05.064. [DOI] [PubMed] [Google Scholar]
- 6.Eslam M., Sanyal A.J., George J. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 7.EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia. 2016;59(6):1121–1140. doi: 10.1007/s00125-016-3902-y. [DOI] [PubMed] [Google Scholar]
- 8.Eslam M., Newsome P.N., Sarin S.K., et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 9.Méndez-Sánchez N., Bugianesi E., Gish R.G., et al. Global multi-stakeholder endorsement of the MAFLD definition. Lancet Gastroenterol Hepatol. 2022;7(5):388–390. doi: 10.1016/S2468-1253(22)00062-0. [DOI] [PubMed] [Google Scholar]
- 10.Mendez-Sanchez N., Arrese M., Gadano A., et al. The Latin American Association for the Study of the Liver (ALEH) position statement on the redefinition of fatty liver disease. Lancet Gastroenterol Hepatol. 2021;6(1):65–72. doi: 10.1016/S2468-1253(20)30340-X. [DOI] [PubMed] [Google Scholar]
- 11.Shiha G., Alswat K., Al Khatry M., et al. Nomenclature and definition of metabolic-associated fatty liver disease: a consensus from the Middle East and North Africa. Lancet Gastroenterol Hepatol. 2021;6(1):57–64. doi: 10.1016/S2468-1253(20)30213-2. [DOI] [PubMed] [Google Scholar]
- 12.Nan Y., An J., Bao J., et al. The Chinese Society of Hepatology position statement on the redefinition of fatty liver disease. J Hepatol. 2021;75(2):454–461. doi: 10.1016/j.jhep.2021.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Fouad Y., Dufour J.F., Zheng M.H., et al. The NAFLD-MAFLD debate: is there a consensus-on-Consensus methodology? Liver Int. 2022;42(4):742–748. doi: 10.1111/liv.15197. [DOI] [PubMed] [Google Scholar]
- 14.Devi J., Raees A., Butt A.S. Redefining non-alcoholic fatty liver disease to metabolic associated fatty liver disease: is this plausible? World J Hepatol. 2022;14(1):158–167. doi: 10.4254/wjh.v14.i1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanchez N.M., Pal S.C. New terms for fatty liver disease other than MAFLD: time for a reality check. J Hepatol. 2022;77(6):1716–1717. doi: 10.1016/j.jhep.2022.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cabezas J., Bataller R. Alcoholic liver disease: new UK alcohol guidelines and dry January: enough to give up boozing? Nat Rev Gastroenterol Hepatol. 2016;13(4):191–192. doi: 10.1038/nrgastro.2016.39. [DOI] [PubMed] [Google Scholar]
- 18.Crabb D.W., Bataller R., Chalasani N.P., et al. Standard definitions and common data elements for clinical trials in patients with alcoholic hepatitis: recommendation from the NIAAA alcoholic hepatitis consortia. Gastroenterology. 2016;150(4):785–790. doi: 10.1053/j.gastro.2016.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bataller R., Arab J.P., Shah V.H. Alcohol-associated hepatitis. N Engl J Med. 2022;387(26):2436–2448. doi: 10.1056/NEJMra2207599. [DOI] [PubMed] [Google Scholar]
- 20.EASL clinical practice guidelines: management of alcohol-related liver disease. J Hepatol. 2018;69(1):154–181. doi: 10.1016/j.jhep.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 21.Rinella M.E., Neuschwander-Tetri B.A., Siddiqui M.S., et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77(5):1797–1835. doi: 10.1097/HEP.0000000000000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Åberg F., Puukka P., Salomaa V., et al. Combined effects of alcohol and metabolic disorders in patients with chronic liver disease. Clin Gastroenterol Hepatol. 2020;18(4):995–997.e2. doi: 10.1016/j.cgh.2019.06.036. [DOI] [PubMed] [Google Scholar]
- 23.Whitfield J.B., Schwantes-An T.H., Darlay R., et al. A genetic risk score and diabetes predict development of alcohol-related cirrhosis in drinkers. J Hepatol. 2022;76(2):275–282. doi: 10.1016/j.jhep.2021.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott E., Anstee Q.M. Genetics of alcoholic liver disease and non-alcoholic steatohepatitis. Clin Med. 2018;18(Suppl 2):s54–s59. doi: 10.7861/clinmedicine.18-2s-s54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.