Abstract
Background
Catheter-directed thrombolysis (CDT) and large-bore mechanical thrombectomy (MT) are the leading percutaneous-based therapies for the management of intermediate-risk pulmonary embolism (PE). While previous studies have demonstrated their procedural safety and efficacy, the cost implications of these interventions remain unclear. This study aims to conduct a cost-benefit analysis to evaluate the economic advantages associated with CDT and MT from the perspective of the treating hospital.
Methods
A total of 372 consecutive patients with intermediate-risk acute PE who underwent either MT or CDT at 3 academic centers between 2013 and 2021 were included in this analysis. The costs of care incurred during the index hospitalization for the 2 treatment groups were collected and compared using an adjusted cost model.
Results
This study compared the hospital costs of 226 patients who underwent CDT and 146 patients who underwent MT. In the unadjusted overall cohort, the use of CDT was associated with a numerical but nonsignificant increase in costs amounting to $5120 relative to MT (P = .062). This cost difference was primarily driven by the longer length of stay in the intensive care unit and hospital for CDT patients, particularly earlier in the studied timeframe. However, when accounting for confounders including variations between the treating institutions and the timing of treatment during the study period, the adjusted cost differential between CDT and MT narrowed to $1351 (P = .71).
Conclusions
This multicenter cost analysis does not reveal a clear cost advantage of 1 treatment over the other for intermediate-risk PE. The observed cost differences were influenced by variations in practice patterns across the study period and among the 3 participating institutions. Future efforts should also focus on strategies to reduce the length of stay, improve efficiency, and minimize the overall cost of care for intermediate-risk PE patients.
Keywords: catheter-directed thrombolysis, cost analysis, mechanical thrombectomy, pulmonary embolism
Central Illustration
Highlights
-
•
Catheter-directed thrombolysis (CDT) and large-bore mechanical thrombectomy (MT) are the leading percutaneous-based therapies for intermediate-risk pulmonary embolism (PE).
-
•
Prior studies have demonstrated both treatments’ procedural safety and efficacy, but the financial implications of the interventions remain unclear.
-
•
This study aims to conduct a cost-benefit analysis to evaluate the economic advantages associated with CDT and MT from the perspective of the treating hospital.
-
•
This multicenter cost analysis does not reveal a distinct cost advantage between CDT and MT for intermediate-risk PE.
-
•
The observed cost differences were influenced by variations in practice patterns overtime and between institutions.
Introduction
The management of intermediate-risk pulmonary embolism (PE) has seen significant advancements with the emergence of 2 percutaneous catheter-based therapies: catheter-directed thrombolysis (CDT) and mechanical thrombectomy (MT). These interventions have gained momentum over the past decade due to their demonstrated safety and efficacy in improving both right ventricle function and hemodynamics.1, 2, 3
CDT involves the slow infusion of a thrombolytic agent directly into the pulmonary arteries through a small-caliber perfusion catheter, utilizing lower dosages than those required for systemic administration. The hemodynamic benefits of CDT are seen over the course of hours,4, 5, 6, 7 during which patients are monitored in the intensive care unit (ICU). On the other hand, MT employs larger bore catheters to directly extract clots from the pulmonary arteries, resulting in a more immediate improvement in hemodynamics.8, 9, 10 A larger randomized clinical trial powered for clinical outcomes comparing these 2 treatments is underway, but results are not yet available.11
To provide guidance for managing intermediate-risk PE patients, a large multicenter database of patients who underwent CDT and MT was established. Analysis of nonrandomized data from this database revealed that both treatment options are effective, with no statistically significant differences in-hospital mortality, catheter-related complications, or postprocedure hemodynamics.2 Using this database, we performed a cost analysis to compare the financial implications associated with CDT and MT in patients with intermediate-risk PE to determine if 1 strategy is associated with higher cost savings from the perspective of the treating hospital.
Methods
Study population
A multicenter patient database from 3 large academic institutions with extensive experience in treating PE was created to evaluate CDT and MT therapies. The initial database contained a total of 454 PE patients who underwent catheter-based treatment from 2013 to 2021, of which 372 patients were included in this analysis. These were patients with intermediate-risk acute PE who met the inclusion criteria and underwent MT or CDT in the cardiac catheterization laboratory. Intermediate-risk PE is defined per the European Society of Cardiology and American Heart Association criteria as hemodynamically stable patients with acute PE associated with objective right ventricular dysfunction by imaging studies and/or cardiac biomarker elevations.
This analysis included patients who were at least 18 years of age, diagnosed with symptomatic proximal PE by computed tomography (CT), and classified in the intermediate-risk category. Only patients who were admitted with a primary diagnosis of PE were included, while those with delayed diagnoses made beyond 24 hours after admission were excluded. Each institution has an active, multidisciplinary pulmonary embolism response team (PERT) responsible for determining the need for invasive procedures in these patients. The choice between CDT or MT was at the discretion of the interventionist and PERT and not determined by randomization. Patients who experienced complications during CDT or MT treatment, such as intracranial bleeding, vascular complications, significant bleeds, and catheter site complications, or those who required a second rescue procedure were included in the analysis. This study primarily focused on patient data collected during the hospital stay and did not incorporate longer-term follow-up data after discharge.
Patients who presented with massive PE and experienced cardiac arrest, received systemic thrombolytics, required extracorporeal membrane oxygenation, or were administered vasopressors were not included in the analysis. Patients who underwent surgical thrombectomy or required intubation during the hospital stay were excluded. Four patients with prolonged hospital courses for conditions unrelated to PE were also excluded from the study. After removing patients according to these exclusion criteria, with several patients meeting multiple criteria, the final cohort size was 372 patients.
Treatment and procedure
All patients received unfractionated heparin at the time of diagnosis and remained on heparin infusion throughout the procedure. The initial admission location to either a medical-surgical telemetry bed or ICU bed was at the discretion of the admitting team and bed availability. The decision to treat a patient and the choice of treatment were made in accordance with the recommendation of the local PERT.
MT was performed using the FlowTriever (FT) Aspiration System (Inari Medical). CDT was performed by using either a Cragg–McNamara (CM) perfusion catheter (Medtronic) or EKOS ultrasound-facilitated thrombolysis system (Boston Scientific). No patients were treated with any of the other available devices for MT or CDT. All procedures were performed in the cardiac catheterization laboratory under conscious sedation and fluoroscopy. Access was obtained either through the femoral or internal jugular veins.
For MT, either 20F or 24F FT catheters were used for the procedure. Baseline hemodynamics were measured using a balloon-tipped right-heart catheter. Pulmonary artery angiograms were performed with a standard pigtail or Arrow Berman pulmonary angiogram catheters (Teleflex) as determined by the institution. The use of additional FT disks and smaller catheters from the Inari treatment line was at the discretion of the operator. The additional FT disks and catheters are included in the 1-time cost per FT procedure bundle; therefore, utilizing additional equipment did not increase the price of the FT. At the conclusion of the case, hemostasis was achieved using either a simple mattress suture or a Perclose suture (Abbott Vascular). Following the procedure, patients were placed in either an ICU or medical-surgical telemetry hospital bed at the discretion of the treating team and bed availability.
For CDT, baseline hemodynamics were measured using a balloon-tipped catheter. Pulmonary angiography was performed at the discretion of the interventionalist. CDT was then performed by utilizing either a CM infusion catheter or EKOS endovascular catheter for ultrasound-facilitated thrombolysis. The choice of CDT catheter was determined by the treating physician and institutional protocol. The thrombolytic agent alteplase (tPA) was infused at 1 mg per hour, either into 1 lung or divided between both lungs. The use of a bolus and the duration of tPA infusion were also at the discretion of the treating team. All patients who received CDT were placed in the ICU after the procedure for at least the duration of the infusion. After completion of the tPA infusion, the catheters were removed and manual pressure was applied for hemostasis. A chest x-ray radiograph was obtained the day after the CDT procedure. Fibrinogen levels, coagulation studies, and blood gas to monitor cardiac output were tested every 8 hours and at the conclusion of the duration of the infusion.
Costs
The direct costs of care for the index hospitalization after admission were collected from the finance and accounting departments based on hospital costs which were then adjusted to 2022 US dollars based on the universal consumer price index. Indirect costs associated with a hospital stay were not accounted for or included in the analysis. Patient care costs were calculated based on imaging, pharmaceutical costs, laboratory costs, procedural costs, and hospital stay costs. These were the costs incurred by the hospitals and not the charges to patients. All costs were categorized by whether they were fixed, variable, or center-specific. Fixed costs were defined as costs incurred by every patient undergoing the treatment. Variable costs were defined as costs dependent on the patient outcome and additional resources utilized during the treatment. Center-specific costs accounted for differences in treatment protocols between the centers.
Imaging costs were based on protocols for all PE patients in both MT and CDT treatment cohorts to undergo chest CT scans, lower extremity venous dopplers, and echocardiogram. Patients receiving CDT had a follow-up chest x-ray and some patients had a follow-up echocardiogram at the discretion of the treating team. Pharmaceutical costs included unfractionated heparin drip used prior to and after the procedure. In addition, intraprocedural medications such as conscious sedation, lidocaine, and additional heparin were also included. Discharge anticoagulation medications were not included in the cost of the index hospitalization. Laboratory costs included routine labs performed on all patients during admission as well as daily complete blood count, basic metabolic panel, and coagulation tests. In patients undergoing CDT, costs for a follow-up venous blood gas sample were also included. Procedural costs included the cost of time in the catheterization laboratory, the cost of devices, and other miscellaneous procedure-related costs.
Cost variables for MT treatment were defined as follows: (1) fixed cost variables which were standard components for MT treatment for every patient including the imaging costs, cardiac catheterization lab costs, standard sterile cardiac catheterization packet, FT product itself, Gore DrySeal sheath (Gore Medical), 7F sheath, balloon-tipped right-heart catheter, Amplatz super stiff wire (Boston Scientific), Soren dilator kit (LivaNova), drugs required for the catheterization lab, contrast dye, micropuncture access kit, cardiac catheterization sterile packet, and ultrasound probe cover; (2) patient variable cost items which included the procedural costs incorporating days in the ICU and hospital bed days, pharmaceutical costs including intraprocedural medications, laboratory costs, blood transfusion units, and any additional echocardiograms performed after the procedure; and (3) center-specific costs which accounted for slight differences in procedure protocols such as percentage of patients receiving either the mattress suture or Perclose for hemostasis and the type of catheter used for pulmonary artery angiogram.
Cost variables of CDT treatment were defined similarly: (1) fixed cost variables which were standard components for CDT treatment based on the institution’s protocols, including imaging costs, catheterization laboratory costs, Swan Ganz catheter, cardiac catheterization sterile packet, vascular access sheaths, micropuncture access kit, ultrasound probe cover, drugs required for the catheterization lab, x-ray after the procedure, 240 cm exchange 0.035-inch intravascular wire, catheter for pulmonary artery angiogram, and contrast dye; (2) patient variable cost items which included procedural costs incorporating days in the ICU and hospital bed days, pharmaceutical costs including intraprocedural medications, laboratory costs, blood transfusion units, and additional lab testing such as fibrinogen levels; and (3) center-specific costs which included the variation in tPA costs and type of catheter used for CDT. Two institutions used 10 mg tPA vials while 1 institution used 50 mg vials to create the infusion solutions. For the CDT treatment, either the CM or EKOS catheter was utilized during the operation.
The costs of a second procedure were also captured for those who required a second intervention. Hospital stay costs were based on the number of days in the hospital including the number of days in the ICU. Hospital complications were captured based on the cost of blood transfusions and increased length of stay since no patients required a separate surgery related to procedural complications. We excluded the cost of supplemental oxygen in both groups since documentation and cost data were inconsistent. All costs are listed in Supplemental Table S1.
Statistical analysis
All analyses were conducted using Stata version 17.0 (StataCorp) and SAS version 9.4 for Windows (SAS Institute). Statistical significance was recognized as P values less than the alpha-value of 0.05.
For analysis of individual continuous covariates, normality was assessed using Shapiro Wilk’s test and visual inspection of the respective histograms and quantile–quantile plots; differences across treatments were tested using t-tests for approximately normal variables (age and body mass index) while Kruskal–Wallis tests were used for significantly nonnormal variables (days from admission to intervention, days from diagnosis to intervention, hospital LOS, ICU LOS, and tPA dosage). Univariate regressions were built to test for changes over time, and linearity was assessed and found nonsignificant in all cases. Fisher exact tests were used to test for significant differences between treatments and categorical patient characteristics due to sample size restrictions for certain variables.
Our main outcome cost was handled separately based on its distribution. We used nonparametric permutation tests to test for differences between groups. Our adjusted cost model utilized a generalized linear model with a gamma distribution and log link, and the covariates were chosen a priori based on previous research and clinical expertise. It controlled for treatment, center, time (defined as the number of months since January 2013), active cancer, age, body mass index, congestive heart failure, chronic lung disease, race, recent surgery, and sex. Continuous variables were centered and subsequently tested for nonlinearity and found nonsignificant. We tested and found a possible, and thus nonignorable, 3-way dependence between treatment, center, and time. Although technically nonsignificant (P = .054), including the 3-way interaction allowed us to dissect differences in treatment costs across centers at disparate time points, which proved essential given the changes in treatment use over time. Marginal differences calculated from the model were averaged across all covariates. Since the distribution of treatment use varied drastically over the course of the study, treatments were compared based on relative time points (ie, quartiles) rather than concurrent time points.
Results
Patient population
A total of 372 patients with intermediate-risk PE, of which 226 underwent CDT and 146 underwent MT, were included from a multicenter database. The baseline characteristics of the patients who underwent CDT or MT are included in Table 1. The 2 groups had fairly similar baseline demographics but notable differences were a slightly older median age in the MT group than in the CDT group (59.5 years vs 57.0 years; P = .007) and fewer patients who identified as white in the MT group than in the CDT group (49% vs 56%; P = .045). Of the 372 patients, 78% were classified as intermediate-high risk defined as having both positive troponin and right ventricular dilatation. The remaining 22% of patients were classified as intermediate-low risk defined as having either a positive troponin or right ventricular dilatation but not both.
Table 1.
Patient demographics and characteristics
| CDT (n = 226) | MT (n = 146) | P value | |
|---|---|---|---|
| Female sex | 105 (46%) | 83 (57%) | .056 |
| Age, y | 57 (21) | 59.5 (21) | .007 |
| Body mass index, kg/m2 | 34.1 (11.9) | 32.5 (11.5) | .216 |
| Days from admission to intervention | 1 (0) | 1 (0) | .551 |
| Active cancer | 25 (11%) | 20 (14%) | .515 |
| Bilateral pulmonary embolism | 205 (91%) | 134 (92%) | 1.000 |
| History of CAD | 16 (7%) | 11 (8%) | 1.000 |
| History of CHF | 10 (4%) | 11 (8%) | .251 |
| History of CLD | 46 (20%) | 21 (14%) | .168 |
| History of anemia | 39 (17%) | 34 (23%) | .181 |
| History of CKD | 34 (15%) | 17 (12%) | .441 |
| History of diabetes | 43 (19%) | 30 (21%) | .789 |
| History of hypertension | 107 (47%) | 81 (55%) | .138 |
| History of stroke | 4 (2%) | 7 (5%) | .119 |
| Race | .045 | ||
| African American | 86 (38%) | 56 (38%) | |
| White | 127 (56%) | 71 (49%) | |
| Other | 13 (6%) | 19 (13%) |
Values are median (IQR) or n (%).
CAD, coronary artery disease; CDT, catheter-directed thrombolysis; CHF, chronic heart failure; CKD, chronic kidney disease; CLD, chronic lung disease; MT, mechanical thrombectomy.
The median number of days from diagnosis to treatment was 1 day for both treatment groups (P = .43). This means that 88% of patients were treated either on the day of the presentation or on their first hospital day. The overall median hospital LOS was 5 days for both treatment groups (P = .45). In the MT group, only 69% of patients required a stay in the ICU whereas all patients in the CDT had a duration of stay in the ICU (P < .001). The median LOS over the entire study period in the ICU was 3.1 days for the CDT group and 1.6 days for the MT group (P < .001). The hospital LOS decreased by 0.2 days per year and ICU LOS decreased by 0.3 days per year in both groups over the study period (P < .001).
Table 2 summarizes the complications seen in both treatment groups. The in-hospital complications and mortality were similar for both groups. No patients required surgery due to a procedural or treatment complication. The additional costs of these complications including blood transfusions, longer hospital stays, and imaging were captured. A total of 17 patients (5%) needed a second procedure during the initial hospital stay. Specifically, 11 patients receiving MT and 6 patients receiving CDT required a second procedure. The costs of the second procedure and the longer hospital stay were captured and associated with the first treatment strategy.
Table 2.
Complications associated with treatment.
| CDT | MT | P value | |
|---|---|---|---|
| Any blood transfusion | 11 (5%) | 11 (8%) | .368 |
| Catheter site access complication | 13 (6%) | 5 (3%) | .459 |
| In-hospital mortality | 2 (2%) | 1 (1%) | 1.000 |
| Intracranial bleed | 1 (0%) | 0 (0%) | 1.000 |
| Significant bleed | 7 (3%) | 3 (2%) | .746 |
| Vascular complication (not access site-related) | 2 (1%) | 1 (1%) | 1.000 |
CDT, catheter-directed thrombolysis; MT, mechanical thrombectomy.
In patients treated with CDT, 83% received 2 perfusion catheters for bilateral infusion. Fifty-seven percent of patients who underwent CDT were treated with EKOS and the remaining patients with a CM standard infusion catheter. The median tPA dosage was 18 mg in the EKOS group and 24 mg in the CM group. However, the choice of CDT catheter and the tPA dosage varied by institution. Institution 1 used CM 20% of the time with an average tPA dosage of 18 mg, Institution 2 used CM 100% of the time with an average tPA dosage of 24 mg, and Institution 3 used CM 6% of the time with an average tPA dosage of 12 mg (P < .001).
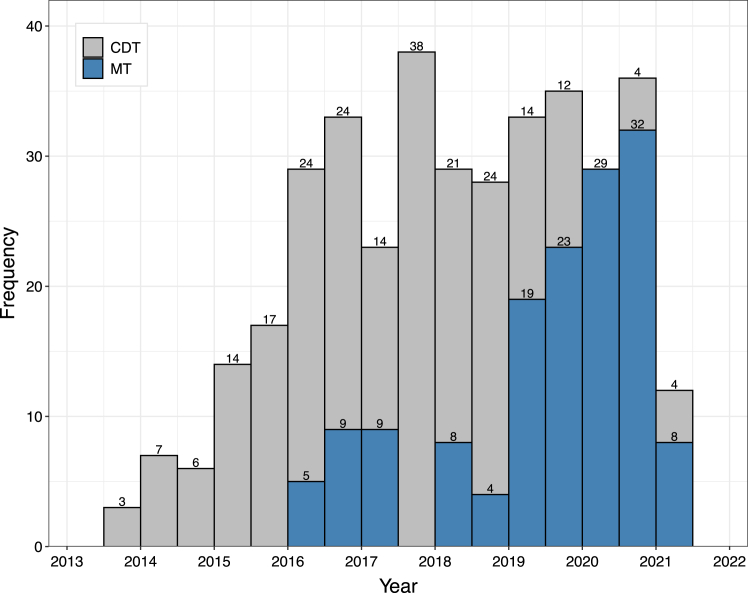
The number of patients treated by MT or CDT also changed over time. Earlier on, more patients were treated with CDT. However, over time, the use of MT increased and the use of CDT decreased as seen in Figure 1.
Figure 1.
Overall, we can see a general increase in the number of total procedures from 2013 through 2016 while the number of total procedures remained relatively constant from 2016 through 2020. CDT was the most common procedure from 2013 through 2019 while MT was the most common procedure from 2019 through 2021. CDT, catheter-directed thrombolysis; MT, mechanical thrombectomy.
Overall costs
The observed hospital costs for each treatment group at each of the 3 hospitals are summarized in Table 3. In the unadjusted overall cohort, the use of CDT compared to MT was associated with a higher but nonsignificant increase in cost of $5120 (P = .062). This cost difference was primarily driven by the longer LOS in the ICU and hospital for CDT patients early in the study period.
Table 3.
Unadjusted cost and model adjusted costs per treatment and institution.
| Unadjusted mean cost per treatment by institution | ||||
|---|---|---|---|---|
| CDT | MT | Difference | P value | |
| Institution 1 | $73,197 | $70,252 | $2946 | .456 |
| Institution 2 | $74,794 | $64,241 | $10,553 | .009 |
| Institution 3 | $78,040 | $80,124 | ($2085) | .790 |
| Overall | $74,727 | $69,607 | $5120 | .062 |
| Adjusted mean cost per treatment by institution | ||||
|---|---|---|---|---|
| CDT | MT | Difference | P value | |
| Institution 1 | $72,899 | $70,751 | $2147 | .625 |
| Institution 2 | $71,282 | $59,437 | $11,844 | .058 |
| Institution 3 | $81,149 | $89,353 | ($8204) | .400 |
| Overall | $73,108 | $71,756 | $1351 | .705 |
CDT, catheter-directed thrombolysis; MT, mechanical thrombectomy.
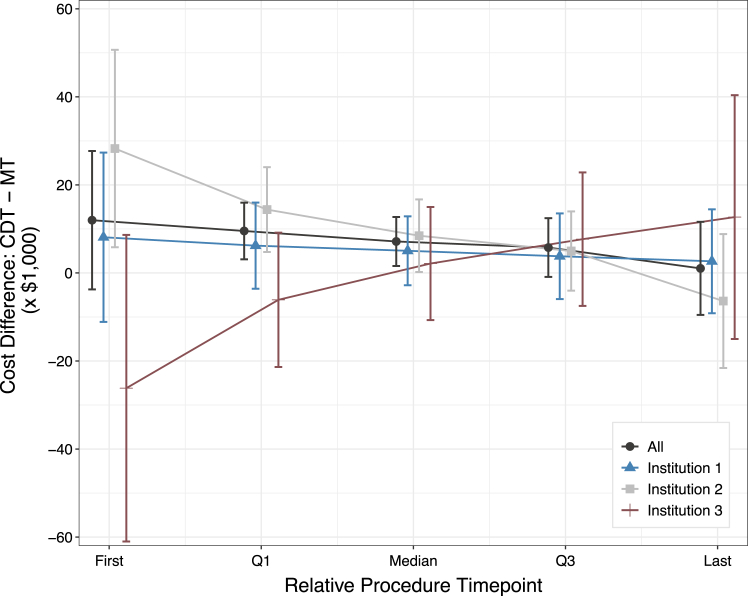
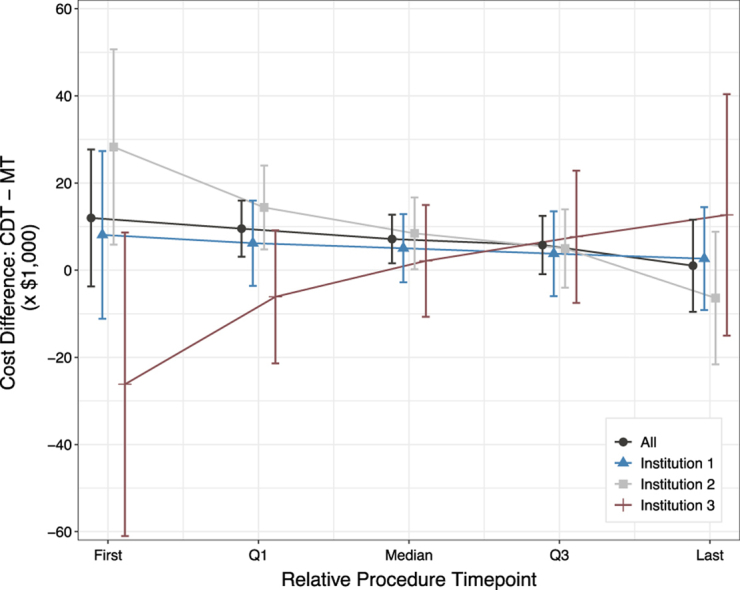
However, when comparing costs between MT and CDT, the total cost of each procedure was influenced by confounders including the time point at which the procedure took place and by the treatment center. Since the distribution of treatment use varied drastically over the course of the study, treatments were compared based on relative time points (ie, quartiles) rather than concurrent time points. Weighted across time and controlling for all covariates in the model, the adjusted cost difference between treatments was $1351 (P = .71), as seen in Table 3. Central Illustration and Supplemental Table S2 illustrate the difference in cost between MT and CDT over time by treating institutions. By the second half of the study period, the cost difference between CDT and MT narrowed and was not significant.
Central Illustration.
Because the use of procedures varied over time (see Figure 1) and varied per institution over time, we compared the cost of each treatment at respective quartiles. For example, consider the dark gray marker at the first time point. The first MT procedure occurred 31 months after the first CDT procedure; therefore, the overall expected cost difference between CDT and MT at their first time points (October 2013 and June 2016, respectively) was $11,983 (95% CI, −$3736 to $27,703). For a full breakdown of costs and relative time points, see Supplemental Table S2. CDT, catheter-directed thrombolysis; MT, mechanical thrombectomy.
CDT catheter choice
Due to the significant cost difference between the CDT catheters, further analysis based on catheter choice was conducted. Comparing the patients who were treated with CM versus EKOS, the mean tPA dosage (26.6 mg vs 19.4 mg), ICU LOS (3.4 days vs 2.9 days), and hospital LOS (6.5 days vs 6.5 days) were not dissimilar. Despite this large price difference in the EKOS and CM catheters, we observed a nonsignificant cost benefit of only $413 (P = .90) for the CM catheter when abstracted across all 3 centers. The costs associated with CDT are not due to catheter type, but rather the treating hospital, duration of hospital stay, and ICU stay. Therefore, comparing MT to the entire CDT group was performed without differentiation of catheter type.
Discussion
A total of 372 patients diagnosed with intermediate-risk PE at 3 large academic centers between 2013 and 2021 and who underwent treatment with MT or CDT were compared in this study. A recent publication from this patient cohort demonstrated that both treatment strategies were similarly effective and safe.2 Consequently, the objective of this study was to evaluate potential cost differences between CDT and MT to determine if 1 treatment was associated with savings from the perspective of the treating hospital.
Based on our initial observed unadjusted data, there appeared to be a trend toward $5120 cost savings with the use of MT. However, further analysis revealed several confounders including a nonnegligible 3-way interaction between the initial treatment strategy (CDT vs MT), the treating center, and the time point at which the patient was treated in the study. After adjusting for these factors, the cost difference between CDT and MT narrowed and was not significant. Therefore, based on the adjusted cost analysis, both treatments were found to be associated with similar costs, with neither showing a clear advantage in terms of savings.
We believe there are several factors contributing to the convergence of costs between the 2 treatments and the absence of a significant cost difference. This cohort of patients spanned 8 years, during which the field of PE interventions witnessed rapid advancements in technology, treatment protocols, and strategies for both CDT and MT.12 Initially, most PE patients were treated with CDT as the MT technology was still in its early stages and often reserved for patients ineligible for thrombolysis. However, as the MT device became larger and generated more suction force and as operators became more comfortable with the device, MT became a preferred first-line treatment option. While there are more years of experience with CDT, there is still no consensus best practice on the optimal thrombolytic infusion duration or if a particular delivery catheter is superior.7,13 The variation in CDT protocols between centers and over time makes direct comparison of CDT difficult.
As local PERT became more comfortable with treating intermediate-risk PE patients and the treatment options evolved, total days in the ICU and hospital decreased. The hospital LOS decreased by 0.2 days per year and the ICU LOS decreased by 0.3 days per year over the study period. While all patients treated with CDT were in the ICU during the infusion period, their overall ICU LOS decreased significantly over time. This is similar to other trends in cardiovascular care such as ST-segment myocardial infarctions which have seen a reduction in LOS and a decrease in patients needing ICU level of care over time.14,15 The reduction in ICU and hospital days, particularly for the CDT group, was the primary factor leading to the convergence of costs for the 2 strategies in the latter half of the study period.
While it may be tempting to discuss best-case treatment scenarios in which MT patients do not require ICU stays and CDT patients undergo short durations of tPA infusion, our real-world data of patients showed that both groups had similar total hospital LOS that decreased over time. Moreover, there was significant variability in costs for the procedures between each of the institutions for CDT and MT. Treatment protocols for PE vary greatly and are primarily based on local PERT recommendations. For example, the total dosage of tPA decreased over time at Institutions 1 and 2, whereas Institution 3 consistently treated patients with 12 mg of tPA throughout the study period. This analysis further reveals the lack of best-practice algorithms and emphasizes the need to establish standardized protocols to optimize benefits and minimize costs.
Given the proven safety profile of these interventions, our analysis underscores the important potential for future cost and bed availability savings opportunities with these therapies. Traditionally, intermediate-risk PE patients were conservatively managed with anticoagulation and bedrest, while catheter-based interventions were reserved for those who decompensated or failed conservative therapy.16 With recent improvements in technology and increasing comfort in utilizing catheter-based interventions, there is a growing trend toward early catheter-based therapy for treating intermediate-risk PE patients, which may lead to reduced hospital LOS and potential cost savings.
Limitations
The primary limitation of this study is largely attributed to its retrospective nature and lack of randomization. Treatment decisions were based on the discretion of the local PERT without standardized protocols, leading to significant variations in treatment protocols across institutions and changes over time. For example, there were differences in the choice of catheters and infusion durations for CDT at each center. Although these variations were adjusted for in the cost model, there is a possibility of bias and other confounders that have not been accounted for. These variations suggest the need for further standardization and optimization of treatment approaches.
The MT group was limited to treatment with only the Inari Medical FT system and did not utilize other commercially available systems. The CDT group included only 2 devices: the EKOS catheter and the CM perfusion catheter. Other perfusion catheters and systems are available but were not part of the protocol during the study time period at these 3 institutions. There was also no medical therapy alone arm in this study. The cost of the EKOS does not include any initial 1-time cost for purchasing the infusion and ultrasound console. This price was not included because different hospitals likely have different purchasing agreements and contract pricing for the consoles and EKOS catheters with the manufacturer.
The costs were obtained from hospital charges and adjusted to 2022 US dollars with possible underreporting. While the total cost may be underestimated due to the inability to find specific costs associated with certain items, this would equally apply to both groups. Therefore, the cost difference between the groups remains constant. The economic analysis was also performed from the perspective of the US healthcare system and costs in other countries may alter the findings. Hospital billing data was not utilized due to variations in markups based on time, patient characteristics, and differences in charges at US hospitals. Indirect costs, such as labor-related costs, salaries, and the cost of supplemental oxygen, were not accurately captured and therefore not included in either group. The analysis also did not include reimbursement rates for each procedure given this can vary greatly between payors and different regions.
Given similar in-hospital outcomes in both groups, cost-effectiveness modeling was not performed. However, there are ongoing randomized clinical trials comparing medical therapy between CDT and MT, which will hopefully help answer if 1 treatment is superior to the other in the acute setting with short-term follow-up. This analysis does not include follow-up after discharge from the hospital, and data on whether 1 treatment is better long-term is also not available. Since data postdischarge was not captured, quality-adjusted life years and cost models were not calculated. An additional limitation is that the database does not distinguish patients who presented directly to the treating hospital from patients who initially presented to an outside hospital before transferring to the treating hospital. Regardless of the initial admission hospital, patients diagnosed with PE beyond 24 hours of presentation to the treating hospital were excluded from the analysis. Cost of transfer or medical care outside the treating hospital was not included in the analysis.
Conclusions
Our objective was to assess if treatment of intermediate-risk PE patients with CDT or MT was associated with a cost benefit. The findings of this study demonstrate that both strategies yield comparable results without a clear advantage in terms of cost savings. The median length of stay in the hospital was 5 days in both groups. In a time where managing health care costs is of utmost importance, this data presents an opportunity to improve the cost of care for intermediate-risk PE patients.
Acknowledgments
Declaration of competing interest
Sameer Khandhar is a consultant for Inari Medical. Catalin Toma is a consultant for Medtronic and Neptune Medical. Wissam Jaber is a consultant for Inari Medical, Medtronic, and RapidAI and receives educational grants from Medtronic and Abbott Vascular. Jay Giri has served on advisory boards and received research funding to the institution from Boston Scientific, Abbott Vascular, Recor Medical, Inari Medical, Edwards Lifesciences, and Abiomed, and has equity in Endovascular Engineering. Taisei Kobayashi reports that the institution receives funding for research from Inari Medical and Endovascular Engineering. Ashwin Nathan receives speaking fees from Edwards Lifesciences and Abiomed and institutional research funds from Abiomed, Biosense Webster, and Edwards Lifesciences.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial or non-for-profit sectors.
Ethics statement and patient consent
The study was conducted in accordance with the Declaration of Helsinki. All patient information was deidentified and no specific consent was needed for statistical analyses of aggregated deidentified data.
Footnotes
To access the supplementary material accompanying this article, visit the online version of the Journal of the Society for Cardiovascular Angiography & Interventions at 10.1016/j.jscai.2023.101187.
Supplementary material
Months refer to the number of months since study start date. We present the cost differences between treatments at the respective institutional and treatment time points along with their respective confidence interval lower and upper bounds. LB = Lower bound of range. UB = Upper bound of range.
References
- 1.Graif A., Patel K.D., Wimmer N.J., et al. Large-bore aspiration thrombectomy versus catheter directed thrombolysis for acute pulmonary embolism: a propensity score-matched comparison. J Vasc Interv Radiol. 2020;31(12):2052–2059. doi: 10.1016/j.jvir.2020.08.028. [DOI] [PubMed] [Google Scholar]
- 2.Inci E.K., Khandhar S.J., Toma C., et al. Mechanical thrombectomy versus catheter directed thrombolysis in patients with pulmonary embolism: a multicenter experience. Catheter Cardiovasc Interv. 2023;101(1):140–146. doi: 10.1002/ccd.30505. [DOI] [PubMed] [Google Scholar]
- 3.Feroze R., Arora S., Tashtish N., et al. Comparison of large-bore thrombectomy with catheter-directed thrombolysis for the treatment of pulmonary embolism. J Soc Cardiovasc Angiogr Interv. 2023;2(1) doi: 10.1016/j.jscai.2022.100453. [DOI] [Google Scholar]
- 4.Khandhar S.J., Mehta M., Cilia L., et al. Invasive hemodynamic assessment of patients with submassive pulmonary embolism. Catheter Cardiovasc Interv. 2020;95(1):13–18. doi: 10.1002/ccd.28491. [DOI] [PubMed] [Google Scholar]
- 5.Piazza G., Hohlfelder B., Jaff M.R., et al. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: the SEATTLE II study. JACC Cardiovasc Intv. 2015;8(10):1382–1392. doi: 10.1016/j.jcin.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 6.Chiarello M.A., Sista A.K. Catheter-directed thrombolysis for submassive pulmonary embolism. Semin Intervent Radiol. 2018;35(2):122–128. doi: 10.1055/s-0038-1642041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tapson V.F., Sterling K., Jones N., et al. A randomized trial of the optimum duration of acoustic pulse thrombolysis procedure in acute intermediate-risk pulmonary embolism: the OPTALYSE PE trial. JACC Cardiovasc Intv. 2018;11(14):1401–1410. doi: 10.1016/j.jcin.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Tu T., Toma C., Tapson V.F., et al. A prospective, single-arm, multicenter trial of catheter-directed mechanical thrombectomy for intermediate-risk acute pulmonary embolism: the FLARE study. JACC Cardiovasc Intv. 2019;12(9):859–869. doi: 10.1016/j.jcin.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 9.Toma C., Bunte M.C., Cho K.H., et al. Percutaneous mechanical thrombectomy in a real-world pulmonary embolism population: interim results of the FLASH registry. Catheter Cardiovasc Interv. 2022;99(4):1345–1355. doi: 10.1002/ccd.30091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toma C., Jaber W.A., Weinberg M.D., et al. Acute outcomes for the full US cohort of the FLASH mechanical thrombectomy registry in pulmonary embolism. EuroIntervention. 2023;18(14):1201–1212. doi: 10.4244/EIJ-D-22-00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The PEERLESS Study (PEERLESS) ClinicalTrials.gov Identifier: NCT05111613. https://clinicaltrials.gov/ct2/show/NCT05111613
- 12.Kabrhel C., Rosovsky R., Channick R., et al. A multidisciplinary pulmonary embolism response team: initial 30-month experience with a novel approach to delivery of care to patients with submassive and massive pulmonary embolism. Chest. 2016;150(20):384–393. doi: 10.1016/j.chest.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Avgerinos E.D., Jaber W., Lacomis J., et al. Randomized trial comparing standard versus ultrasound-assisted thrombolysis for submassive pulmonary embolism: the SUNSET sPE Trial. JACC Cardiovasc Intv. 2021;14(12):1364–1373. doi: 10.1016/j.jcin.2021.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shavadia J.S., Chen A.Y., Fanaroff A.C., de Lemos J.A., Kontos M.C., Wang T.Y. Intensive care utilization in stable patients with ST-segment elevation myocardial infarction treated with rapid reperfusion. JACC Cardiovasc Intv. 2019;12(8):709–717. doi: 10.1016/j.jcin.2019.01.230. [DOI] [PubMed] [Google Scholar]
- 15.Seto A.H., Shroff A., Abu-Fadel M., et al. Length of stay following percutaneous coronary intervention: an expert consensus document update from the society for cardiovascular angiography and interventions. Catheter Cardiovasc Interv. 2018;92(4):717–731. doi: 10.1002/ccd.27637. [DOI] [PubMed] [Google Scholar]
- 16.Stevens S.M., Woller S.C., Kreuziger L.B., et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160(6):e545–e608. doi: 10.1016/j.chest.2021.07.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Months refer to the number of months since study start date. We present the cost differences between treatments at the respective institutional and treatment time points along with their respective confidence interval lower and upper bounds. LB = Lower bound of range. UB = Upper bound of range.