Abstract
Background
Chronic total occlusion (CTO) remains the most complex anatomical subset of lesions in percutaneous coronary intervention (PCI), often requiring advanced techniques and technologies, including the use of microcatheters.
Methods
The BIOMICS study is a premarket first-in-human prospective, multicenter, open-label, single-arm trial investigating the safety and efficacy of a novel coronary microcatheter (BioMC, Biosensors International) in 100 patients with symptoms of ischemia undergoing elective CTO-PCI. The primary efficacy end point of the study was device success defined according to the CTO-ARC (Chronic Total Occlusion Academic Research Consortium) criteria namely the ability of the microcatheter to successfully facilitate placement of a guide wire beyond the occluded coronary segment. The primary safety end point was the incidence of in-hospital cardiac death or myocardial infarction at hospital discharge.
Results
Hundred patients were recruited between March 2022 and January 2023. The primary efficacy end point was achieved in 75% of patients (95% CI, 65.3%-83.1%; P < .0001 for superiority compared to the prespecified performance goal of 54%). The primary safety end point of in-hospital cardiac death or myocardial infarction was observed in 2% of the patients. There were no study device-related coronary perforations or device failures.
Conclusions
The use of a novel coronary microcatheter during CTO-PCI was associated with a high device success and an excellent safety profile.
Keywords: chronic total occlusion, microcatheter, percutaneous coronary intervention
Introduction
Chronic total occlusion (CTO) remains the most complex anatomical subset of lesions in percutaneous coronary intervention (PCI). The prevalence of CTO in patients undergoing angiography for the investigation of symptomatic coronary heart disease is as high as 30%, and up to 13% of cases exhibit more than 1 CTO.1 Coronary microcatheters are often required to facilitate initial wire crossing in subjects undergoing PCI of complex lesions including severely tortuous and/or calcified stenoses and CTO.2 The hybrid algorithm for CTO-PCI3 includes both antegrade and retrograde (via collaterals) approaches and dissection/reentry techniques, with the primary approach dictated by CTO anatomy. These techniques, along with wire exchange during CTO-PCI, are facilitated using dedicated microcatheters. The development of CTO-PCI–specific devices, including microcatheters, has contributed to an increase in the success rate of CTO-PCI. A weighted meta-analysis4 of 18,061 subjects from 65 studies showed that angiographic success increased from 68.2% in 2000-2002 to 79.4% in 2008-2011, with a significant decrease in major complications from 1.6% in 2000-2002 to 0.5% in 2009-2011. A 2018 consensus statement from a group of 113 CTO-PCI experts from 56 countries stated that “a microcatheter should be routinely used for supporting the coronary guidewire and allowing rapid guidewire switching during both antegrade and retrograde wire manipulation.”5
Multiple studies have demonstrated the efficacy and safety of different microcatheters in CTO-PCI. In the PROGRESS-CTO registry,6 between 2010 and 2016, the most frequently used microcatheters were the Caravel, Corsair and Corsair Pro (Asahi), FineCross (Terumo), and the Turnpike (Vascular Solutions). The Corsair (Asahi) has been shown to be associated with a procedural success rate of 85.3%7 in antegrade CTO-PCI and 86%8 in retrograde CTO-PCI. Procedural success in 150 patients undergoing CTO-PCI utilizing specialized guide wires, microcatheters, and guide extensions (Teleflex) was 75.3%, with an in-hospital major adverse cardiovascular event rate of 19.3%.9 Similarly, the procedural success rate of the Tornus (Asahi) microcatheter in a series of 14 complex CTO was 78.6%10 with no reported complications.
The BioMC (Biosensors International) device is a new single-lumen coronary microcatheter designed for use in patients undergoing CTO-PCI and other complex PCI procedures. In many health care systems, the need to use multiple expensive microcatheters may restrict access to complex PCI in general and CTO-PCI in particular. One of the design goals for this new device was to create a microcatheter with an improved trade-off between performance and cost as this could potentially increase accessibility to and improve the cost-effectiveness of complex (CTO) PCI. The novel features of this new device include a graduated outer shaft for improved force transmission and a uniquely configured single flat stainless steel thread wound around the wire lumen to provide torquability (Figure 1).
Figure 1.
Schematic diagram of the BioMC coronary microcatheter (Biosensors International).
The BIOMICS study is a premarket evaluation of the safety and effectiveness of this new device in patients undergoing CTO-PCI.
Methods
Study design
The BIOMICS study is a premarket, first-in-human, prospective, multicenter, open-label, single-arm trial investigating the safety and effectiveness of the BioMC coronary microcatheter in 100 patients undergoing attempted CTO-PCI at 9 centers in the United Kingdom (11 operators). The study was conducted to comply with the requirements of the new European Union Medical Device Regulations No. 2017/745 and registered on ClinicalTrials.gov (NCT04966273).
Study population
Patients with symptomatic ischemic heart disease undergoing clinically indicated nonemergent PCI were screened. The inclusion and exclusion criteria are shown in Supplemental Table S1.
Study device design
The BioMC microcatheter (Figure 1) is an over-the-wire, single-lumen microcatheter available in lengths of 135 cm or 150 cm. The catheter is constructed of a graduated braided Pebax jacket for good force transmission and support with a fluoropolymer lining to facilitate smooth guide wire exchange. The distal end of the microcatheter has a radiopaque tip and the microcatheter wall incorporates tungsten braiding and a single flat stainless steel thread to provide torquability and visibility under fluoroscopy. The distal tip and shaft have a lubricious hydrophilic coating for smooth navigation. It also features a tapered tip that has a gradual reduction in diameter to a minimum of 0.55 mm which improves the crossability.
The study device is indicated for use in the coronary vasculature to provide support to facilitate the placement and exchange of guide wires, to deliver contrast media, to provide additional guide wire support, and to increase guide wire penetration force to facilitate crossing of the occluded segment in CTO-PCI.
Study protocol
Prior to the index procedure, each patient’s medical history and medication were documented. Physical examination, assessments of cardiac troponin levels, creatinine levels, and an electrocardiogram (ECG) were performed. The initial microcatheter used had to be a study device and a maximum of 1 additional study device could be employed. Thereafter, if required, nonstudy microcatheters could be used to complete the procedure. Staged completion of the CTO-PCI was allowed but device, technical, and procedural success were assessed on the first procedure only. All nonstudy microcatheter usage was documented.
Following the procedure, a predischarge ECG was performed. Additional ECG was performed to document any episodes of potential cardiac ischemia. At least 1 measurement of cardiac troponin level and creatinine level was taken predischarge. In patients with signs or symptoms of myocardial infarction (MI), serial cardiac troponin measurements were taken according to local protocols. The peak value was documented in the case of multiple measurements. Clinical status was also assessed and recorded at discharge. Patients were followed up to a maximum of 7 days from their index hospitalization or until discharge, whichever came first.
Primary end points
The primary efficacy end point was the successful placement of a guide wire across the occluded coronary segment. The primary safety end point was the incidence of in-hospital cardiac death or MI. MI was defined by the Fourth Universal Definition of Myocardial Infarction,11 as an elevation of troponin values >5× the 99th percentile upper reference limit in patients with normal values, and a rise in troponin of >20% in patients with elevated preprocedure troponin values. Both primary end points were compared to performance goals (PG) derived from prior published studies of CTO-PCI, 3 large scale studies12, 13, 14 for the efficacy end point and several additional studies9,15 for the safety end point.
Secondary end points
The prespecified secondary end points were as follows:
Technical success—defined as achievement of Thrombolysis in Myocardial Infarction (TIMI) ≥2 antegrade flow with <30% residual stenosis of the target CTO lesion at the end of the procedure.
Procedural success—defined as technical success without in-hospital major adverse cardiovascular events, which included death, MI, or clinical-driven target vessel revascularization (TVR).
Crossing success—defined as the study devices crossing the lesion.
Device-related perforation at the site of the target coronary lesion and/or its proximal reference segment including donor artery or collateral.
Device failure—defined as any evidence of fracture of any part of the study microcatheter, evidence of wire puncture of any part of the device, or evidence of a fragment being retained in the body of the patient after a procedure.
Adjudicated adverse events
Safety was monitored throughout the study and adverse events and device deficiencies were reported according to the EN ISO 14155:2020 Clinical Investigation of Medical Devices for Human Patients–Good Clinical Practice guidelines while recognizing and following other specific laws, regulations, directives, standards, and/or guidelines as appropriate. A safety management plan was created which described the definitions followed, the specific reporting requirements, and a list of foreseeable adverse events and anticipated adverse device effects, together with their likely incidence.
An independent data safety monitoring board (DSMB) performed comprehensive safety reviews of the data. A total of 3 meetings were held at the last of which, the final dataset including all monitored and adjudicated data was provided to the DSMB members. The DSMB ruled that there were no safety concerns at any time point. There was also an independent clinical events committee (CEC) made up of 2 interventional cardiologists who were not participants in the study. The CEC was responsible for adjudicating all adverse events following preestablished rules outlined in the CEC charter which summarized the type of data required from the site per event (blinded source documentation) and the definitions followed in order to classify a clinical event. All records of adverse events included a description of the event, date of onset, date of resolution (when known), severity, action taken, relationship to study device or procedure, and seriousness criteria. The CEC also had access to the angiographic images of the index and any subsequent repeat procedures as required. Every repeat PCI angiographic film was sent to a core lab for assessment prior to transfer to the CEC for adjudication.
Statistical analyses
The primary statistical hypothesis is that the device success rate of the investigational device is greater than the PG of 54%12, 14:
H0: AB ≤ 0.54
H1: AB > 0.54
where AB is the observed device success. A 1-group Clopper–Pearson exact test was used to test whether the observed device’s success was greater than 54%. If the P value from the 2-sided Clopper–Pearson test was <.05, the investigational device was concluded to meet the PG with respect to the device success rate. This corresponds to the 2-sided 95% lower confidence bound on the observed device success rate being more than the PG. Continuous summaries include the number of values (n), number of patients with missing data (missing), mean, standard deviation, median, ranges (minimum and maximum), and first and third quartile, 95% (unless otherwise specified) CI, which will be calculated if appropriate. Categorical summaries include the frequency and percentage of patients who are in each particular category. In general, the denominator for the percentage calculation is based upon the number of nonmissing values available, unless otherwise specified. The corresponding 95% CI (unless otherwise specified) are calculated, if appropriate.
For time-to-event analysis, Kaplan-Meier survival curves as well as CI based on the log-log transformation are reported.
Results
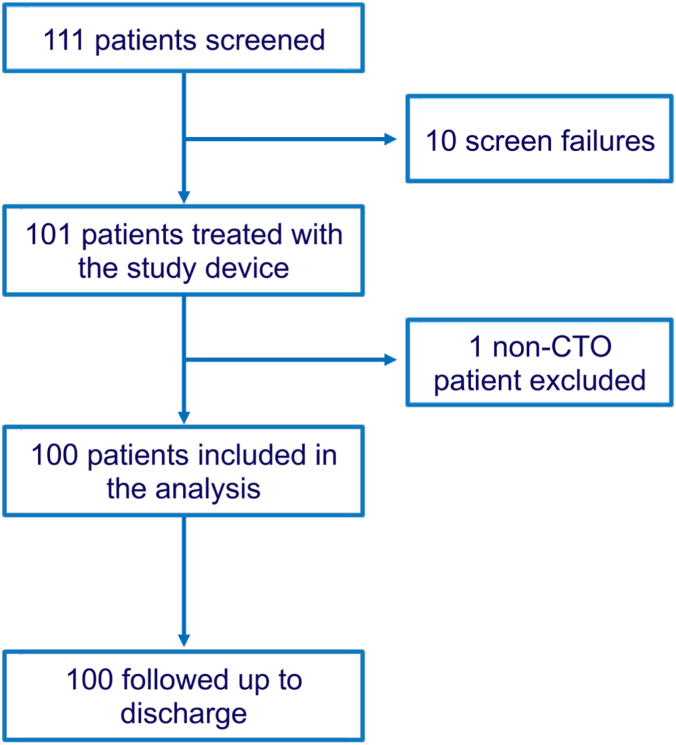
Between 31 March 2022 and 3 January 2023, 111 patients were screened for inclusion (Figure 2). There were 10 screen failures and 1 patient was enrolled but subsequently identified to have an exclusion criterion. This left 100 patients in the intention-to-treat population, of which 3 were removed due to major protocol deviations leaving 97 in the per protocol population.
Figure 2.
Flow diagram of the study. A total of 111 patients were screened, 100 of whom met the requirement to be included in the intention-to-treat population (excluding the 10 screen failure patients and the patients who did not meet all inclusion/exclusion criteria). Out of the remaining 100 patients in the intention-to-treat population, 3 were removed due to major protocol deviations resulting in 97 in the per protocol population. CTO, chronic total occlusion.
Baseline characteristics
Table 1 shows the demographics of the study population. The mean age was 67.7 ± 10.5 years, and most of the patients (90%) were male. Previous cardiac history and cardiac risk factors were prevalent, with 44% of patients having had a previous MI and a majority of patients having hypertension (60%) and/or dyslipidemia (71%). The vast majority of patients presented with stable angina, predominantly with Canadian Cardiovascular Society class II (49%) and III (35%) symptoms.
Table 1.
Patient demographics.
| N = 100 | |
|---|---|
| Age, y | 62.72 ± 10.50 |
| Male sex | 90 (90%) |
| Weight, kg | 89.64 ± 19.09 |
| Body mass index, kg/m2 | 30.45 ± 5.52 |
| Systolic BP, mm Hg | 128.92 ± 20.96 |
| Diastolic BP, mm Hg | 71.50 ± 11.14 |
| Serum creatinine, μmol/L | 84.73 ± 20.52 |
| Diabetes mellitus | 24 (24%) |
| Smoking | |
| Current | 13 (13%) |
| Former | 42 (42%) |
| Renal insufficiency | 4 (4%) |
| Dyslipidemia | 71 (71%) |
| Hypertension | 60 (60%) |
| Previous cerebrovascular event | 10 (10%) |
| Previous cancer | 10 (10%) |
| Previous MI | 44 (44%) |
| Previous PCI | 47 (47%) |
| Previous CABG | 18 (18%) |
| Congestive heart failure | 4 (4%) |
| NYHA Class | |
| I | 2 (50%) |
| II | 1 (25%) |
| III | 1 (25%) |
| IV | 0 |
| Peripheral vascular disease | 4 (4%) |
| Atrial fibrillation | 8 (8%) |
| Presentation | |
| Silent ischemia | 4 (4%) |
| Stable angina | 96 (96%) |
| CCS class | |
| I | 11 (11%) |
| II | 49 (49%) |
| III | 35 (35%) |
| IV | 3 (3%) |
| Unknown | 2 (2%) |
Values are mean ± SD or n (%).
BP, blood pressure; CABG, coronary artery bypass grafting; CCS, Canadian Cardiovascular Society; MI, myocardial infarction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
Angiographic and lesion characteristics
The Japanese Chronic Total Occlusion (J-CTO)16 and RECHARGE12 scores are shown in Table 2.12,16 Thirty-four (34%) patients had a J-CTO score of 0 to 1 and 66 (66%) a J-CTO score of 2 to 5. The mean J-CTO score was 2.3 ± 1.4. The RECHARGE scores showed a similar pattern. The majority of lesions treated were in the right coronary artery (63%) followed by the left anterior descending artery (24%) and circumflex artery (11%) (Table 2). Calcification was absent in 22% of lesions, 30% had mild calcification, 25% had moderate calcification, and 23% had severe calcification. A bifurcation lesion was observed in 31% of cases. The average lesion length was 31.5 ± 22.6 mm and the mean reference vessel diameter was 3.3 ± 0.5 mm. Overall procedure duration was 126.1 ± 59.7 minutes (Table 2).
Table 2.
Angiographic and procedural characteristics.
| N = 100 | |
|---|---|
| J-CTO Score16 | |
| 0 | 11 (11%) |
| 1 | 23 (23%) |
| 2 | 22 (22%) |
| 3 | 23 (23%) |
| 4 | 16 (16%) |
| 5 | 5 (5%) |
| RECHARGE Score12 | |
| 0 | 9 (9%) |
| 1 | 11 (11%) |
| 2 | 26 (26%) |
| 3 | 18 (18%) |
| 4 | 21 (21%) |
| 5 | 11 (11%) |
| 6 | 4 (4%) |
| 7 | 0 (0%) |
| Lesion location | |
| RCA | 63 (63%) |
| LM | 2 (2%) |
| LAD | 24 (24%) |
| LCx | 11 (11%) |
| Ramus intermedius | 0 (0%) |
| SVG | 0 (0%) |
| Arterial graft | 0 (0%) |
| Occlusion lengtha, mm | 31.52 ± 22.55 |
| CTO diametera, mm | 33.31 ± 0.49 |
| Calcification | |
| Absent | 22 (22%) |
| Mild | 30 (30%) |
| Moderate | 25 (25%) |
| Severe | 23 (23%) |
| Bifurcation | 31 (31%) |
| Procedure duration, min | 126.14 ± 59.73 |
| Number of study device(s) used | 1.19 ± 0.39 |
| 1 | 81 (81%) |
| 2 | 19 (19%) |
| Number of nonstudy device(s) used | 0.92 ± 0.96 |
| 0 | 41 (41%) |
| 1 | 33 (33%) |
| 2 | 20 (20%) |
| 3 | 3 (4%) |
| 4 | 4 (2%) |
| Procedure with study device used only | 41 (41%) |
| Postprocedure antegrade TIMI flow | |
| 0 | 11 (11%) |
| 1 | 1 (1%) |
| 2 | 1 (1%) |
| 3 | 87 (87%) |
| Postprocedure % stenosisa | 13.7 ± 31.48 |
| Contrast used, mL | 237.40 ± 85.40 |
Values are mean ± SD or n (%).
CTO, chronic total occlusion; J-CTO, Japanese Chronic Total Occlusion; LAD, left anterior descending artery; LCx, circumflex artery; LM, left main stem; RCA, right coronary artery; RECHARGE, REgistry of Crossboss and Hybrid procedures in FrAnce, the NetheRlands, BelGium and UnitEd Kingdom; SVG, saphenous vein graft; TIMI, Thrombolysis in Myocardial Infarction.
Visual estimate.
Study and nonstudy microcatheter usage
There were 119 study microcatheters used in the 100 procedures (Table 3). The 135 cm length was used in 90 (75.6%) patients and the 150 cm length in 29 patients (24.4%). One study microcatheter was used in 81 patients (81%) with 2 study devices being used in the remaining 19 (19%). Overall, 41 patients (41%) had their procedures completed using the study microcatheter only. One additional nonstudy microcatheter was used in 33 (33%) patients, 2 in 20 patients (20%), 3 in 4 patients (4%), and 4 in 2 patients (2%), giving a total of 93 nonstudy microcatheters overall.
Table 3.
Device characteristics and procedure strategies.
| N = 119a | |
|---|---|
| Device length | |
| 135 cm | 90 (75.6%) |
| 150 cm | 29 (24.4%) |
| Initial strategy | |
| Antegrade wiring | 79 (66.4%) |
| Antegrade dissection reentry | 9 (7.6%) |
| Retrograde wiring | 6 (5%) |
| Retrograde dissection reentry | 25 (21%) |
| Final strategy | |
| Antegrade wiring | 58 (49.6%) |
| Antegrade dissection reentry | 18 (15.4%) |
| Retrograde wiring | 16 (13.7%) |
| Retrograde dissection reentry | 25 (21.4%) |
| Use of device | |
| Antegrade | 82 (68.9%) |
| Retrograde | 15 (12.6%) |
| Both | 22 (18.5%) |
| Contrast injection via study device | 7 (5.9%) |
| Adverse event caused by contrast injection | 0 |
Values are n (%).
Analyses performed on 119 study devices used in 100 procedures.
Procedure strategies and outcomes
The initial strategy was antegrade wiring in 66.4% followed by retrograde dissection reentry (21%). Antegrade wiring was the final strategy in half (49.6%) of the cases. The study devices were predominantly used antegradely (68.9%), with retrograde use in 12.6% and both in 18.5%. Postprocedure TIMI flow of 3 was achieved in 87% of the patients and the site-reported residual diameter stenosis postprocedure was 13.7 ± 31%.
Study end points
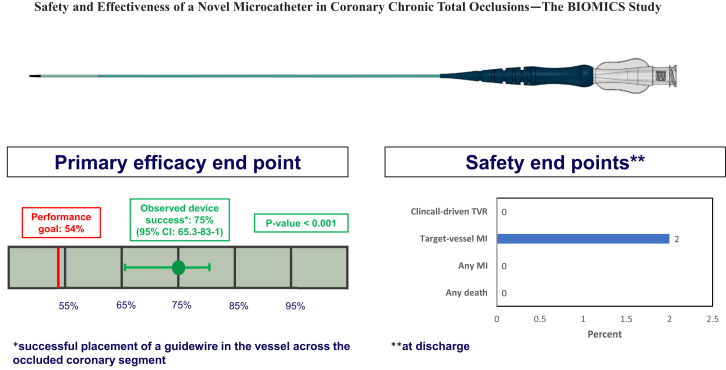
Considering the study device only, the primary efficacy end point defined as the percent of patients with successful placement of a guide wire in the distal lumen across the occluded coronary segment was achieved in 75/100 (75%) patients (95% CI, 65.3%-83.1%; P < .0001 vs prespecified PG) (Central Illustration). The primary safety end point defined as the composite of in-hospital cardiac death or MI was observed in 2 (2%) of patients (95% CI, 0.5%-7.8%) consisting of 2 periprocedural MI and no deaths (Table 4). Including the use of nonstudy microcatheters, the primary efficacy end point was achieved in 97/100 patients. In the 41 (41%) patients in whom only study microcatheters were employed, procedural success was 100%, with all procedures utilizing an antegrade approach only.
Central Illustration.
Primary and secondary efficacy end points for the BIOMICS study. MI, myocardial infarction; TVR, target vessel revascularization.
Table 4.
Primary efficacy end point and safety end points.
| N = 100 | P value | |
|---|---|---|
| Efficacy end point | ||
| Successful placement of guidewire across CTO | 97 (97%) | <.0001a |
| Safety end points | ||
| Any death | 0 | |
| Any MI | 2 (2%) | |
| Target vessel MI | 2 (2%) | |
| Clinically-driven TVR | 0 | |
| Composite of in-hospital cardiac death or MI | 2 (2%) | |
| Composite of death or MI or clinically-driven TVR (MACE) | 2 (2%) |
Values are n (%).
CTO, chronic total occlusion; MACE, major adverse cardiovascular events; MI, myocardial infarction; TVR, target vessel revascularization.
Compared to the hypothesized performance goal of 54%.
The prespecified secondary end points of technical and procedural success were reported in 85 (85%) and 83 (83%) of patients respectively. Of the 119 study microcatheters employed, 67 (56.3%) were able to cross the occluded segment. There were 3 coronary perforations but none of these were related to the study devices. In a post hoc analysis using a PG derived from the Asahi Intecc Chronic Total Occlusion study which utilized mainly Corsair (Asahi Intecc) coronary microcatheters, procedural success rates were similar (BioMC 83% [95% CI, 74.2%-89.8%] vs Corsair 73% [66.2%-79.8%]; P = .024).17
Discussion
The use of microcatheters in CTO-PCI is considered fundamental in achieving high levels of procedural success, while maintaining procedural efficiency. In addition to facilitating wire manipulation and exchange, they augment wire penetration force and improve lesion crossability. As techniques have expanded to include retrograde lesion crossing, so have the demands for microcatheters, which now need to be flexible and lubricious in addition to supportive. The present study aimed to clarify how the study device would meet these challenges.
In this cohort of 100 patients undergoing CTO-PCI, baseline and procedure characteristics were in line with those expected in a contemporary CTO population. In these procedures, a strategy of starting with at least 1 (maximum 2, average 1.2 per case) BioMC microcatheters was associated with procedural success rates of 41% without the need for any other microcatheters. It also facilitated successful wire crossing into the distal vessel in 75% of patients and this rate increased to 97% with the use of a mean of 1.6 additional nonstudy microcatheters (93 in 59 patients). Overall technical success was 85%. There were no in-hospital deaths with 2 (2%) cases of periprocedural MI (Fourth Universal Definition) as adjudicated by an independent CEC, neither of which was due to acute stent thrombosis. This resulted in an overall procedural success of 83%. Despite a recent move toward using the SCAI definition of periprocedural MI18 we chose to employ the more sensitive Fourth Universal Definition as this was a premarket study in which patient safety is of paramount importance.
This prospective multicenter study represents a comprehensive examination of procedural methods, resource utilization, and outcomes in a contemporary CTO population treated by highly experienced operators. Despite extensive lesion length and significant complexity, a high level of procedural success was achieved through application of antegrade and retrograde techniques, and a good safety profile of the novel microcatheter was demonstrated. To the best of our knowledge, this is the first study of a coronary microcatheter conducted to comply with the new European Union Medical Device Regulations No 2017/745.
Study limitations
As a nonrandomized study, it is possible that measured or unmeasured confounders may have affected the comparison of outcomes with the PG derived from prior CTO trials. Although CTO-PCI procedures often involve the use of multiple microcatheters, it is important to acknowledge that at least 1 nonstudy microcatheter was used in 59% of procedures and this will have contributed to the overall technical and procedural success rates. By design, a maximum of 2 study microcatheters were allowed and so we cannot estimate what these success rates would have been if there had been no limit on the number of study devices allowed or if only study devices had been allowed.
Acknowledgments
The authors thank Taina Carreaux (Biosensors), Deepa Venkiteswara (Biosensors), and Diane Pieloni (Biosensors) for study management and study oversight.
Declaration of competing interest
Keith Oldroyd, Diana Schuette, and Samuel Copt are full-time employees of Biosensors Europe SA. Margaret McEntegart provides consultancy for Biosensors, Boston Scientific, Shockwave Medical, and Teleflex, and has received honoraria from Biosensors, Boston Scientific, and Medtronic. All other authors report no conflicts of interest relevant to this work.
Funding sources
This study was fully sponsored by Biosensors Europe SA.
Ethics statement and patient consent
The study was performed according to the Declaration of Helsinki and ISO14155:2020. It was approved by the ethics committees of participating centers and all patients provided written informed consent before enrolling into the study.
Footnotes
To access the supplementary material accompanying this article, visit the online version of the Journal of the Society for Cardiovascular Angiography & Interventions at 10.1016/j.jscai.2024.102017.
Supplementary material
References
- 1.Young M.N., Secemsky E.A., Kaltenbach L.A., et al. Examining the operator learning curve for percutaneous coronary intervention of chronic total occlusions. Circ Cardiovasc Interv. 2019;12(8) doi: 10.1161/CIRCINTERVENTIONS.119.007877. [DOI] [PubMed] [Google Scholar]
- 2.Goel P.K., Sahu A.K., Kasturi S., et al. Guiding principles for the clinical use and selection of microcatheters in complex coronary interventions. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.724608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brilakis E.S., Grantham J.A., Rinfret S., et al. A percutaneous treatment algorithm for crossing coronary chronic total occlusions. JACC Cardiovasc Interv. 2012;5(4):367–379. doi: 10.1016/j.jcin.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Patel V.G., Brayton K.M., Tamayo A., et al. Angiographic success and procedural complications in patients undergoing percutaneous coronary chronic total occlusion interventions: a weighted meta-analysis of 18,061 patients from 65 studies. JACC Cardiovasc Interv. 2013;6(2):128–136. doi: 10.1016/j.jcin.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Brilakis E.S., Mashayekhi K., Tsuchikane E., et al. Guiding principles for chronic total occlusion percutaneous coronary intervention. Circulation. 2019;140(5):420–433. doi: 10.1161/CIRCULATIONAHA.119.039797. [DOI] [PubMed] [Google Scholar]
- 6.Nikolakopoulos I., Choi J.W., Alaswad K., et al. Equipment utilization in chronic total occlusion percutaneous coronary interventions: insights from the PROGRESS-CTO registry. Catheter Cardiovasc Interv. 2021;97(4):658–667. doi: 10.1002/ccd.29106. [DOI] [PubMed] [Google Scholar]
- 7.Obata J.E., Nakamura T., Kitta Y., et al. Usefulness of a collateral channel dilator for antegrade treatment of chronic total occlusion of a coronary artery. J Interv Cardiol. 2012;25(6):533–539. doi: 10.1111/j.1540-8183.2012.00758.x. [DOI] [PubMed] [Google Scholar]
- 8.Joseph G., Thomson V.S., Radhakrishnan S. Corsair microcatheter for retrograde coronary chronic total occlusion recanalization: early experience outside the realm of dedicated recanalization specialists. Indian Heart J. 2012;64(4):388–393. doi: 10.1016/j.ihj.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kandzari D.E., Alaswad K., Jaffer F.A., et al. Safety and efficacy of dedicated guidewire, microcatheter, and guide catheter extension technologies for chronic total coronary occlusion revascularization: primary results of the Teleflex Chronic Total Occlusion Study. Catheter Cardiovasc Interv. 2022;99(2):263–270. doi: 10.1002/ccd.29962. [DOI] [PubMed] [Google Scholar]
- 10.Mohandes M., Rojas S., Guarinos J., et al. Efficacy and safety of Tornus catheter in percutaneous coronary intervention of hard or balloon-uncrossable chronic total occlusion. ARYA Atheroscler. 2016;12(4):206–211. [PMC free article] [PubMed] [Google Scholar]
- 11.Thygesen K., Alpert J.S., Jaffe A.S., et al. Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018;72(18):2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 12.Maeremans J., Walsh S., Knaapen P., et al. The hybrid algorithm for treating chronic total occlusions in Europe: the RECHARGE registry. J Am Coll Cardiol. 2016;68(18):1958–1970. doi: 10.1016/j.jacc.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 13.Tajti P., Karmpaliotis D., Alaswad K., et al. The hybrid approach to chronic total occlusion percutaneous coronary intervention: update from the PROGRESS CTO registry. JACC Cardiovasc Interv. 2018;11(14):1325–1335. doi: 10.1016/j.jcin.2018.02.036. [DOI] [PubMed] [Google Scholar]
- 14.Wilson W.M., Walsh S.J., Yan A.T., et al. Hybrid approach improves success of chronic total occlusion angioplasty. Heart. 2016;102(18):1486–1493. doi: 10.1136/heartjnl-2015-308891. [DOI] [PubMed] [Google Scholar]
- 15.Walsh S.J., Dudek D., Bryniarski L., et al. Safety and efficacy of the NovaCross microcatheter in facilitating crossing of chronic total occlusion coronary lesions: a multicenter, single-arm clinical trial. Coron Artery Dis. 2020;31(7):573–577. doi: 10.1097/MCA.0000000000000947. [DOI] [PubMed] [Google Scholar]
- 16.Morino Y., Abe M., Morimoto T., et al. Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 minutes: the J-CTO (Multicenter CTO Registry in Japan) score as a difficulty grading and time assessment tool. JACC Cardiovasc Interv. 2011;4(2):213–221. doi: 10.1016/j.jcin.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 17.Kandzari D.E., Grantham J.A., Karmpaliotis D., et al. Safety and efficacy of dedicated guidewire and microcatheter technology for chronic total coronary occlusion revascularization: principal results of the Asahi Intecc Chronic Total Occlusion Study. Coron Artery Dis. 2018;29(8):618–623. doi: 10.1097/MCA.0000000000000668. [DOI] [PubMed] [Google Scholar]
- 18.Moussa I.D., Klein L.W., Shah B., et al. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: an expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI) J Am Coll Cardiol. 2013;62(17):1563–1570. doi: 10.1016/j.jacc.2013.08.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.