Abstract
Background:
Evidence-based insertion and maintenance strategies for neonatal vascular access devices (VAD) exist to reduce the causes of VAD failure and complications in neonates. Peripheral intravenous catheter failure and complications including, infiltration, extravasation, phlebitis, dislodgement with/without removal, and infection are majorly influenced by catheter securement methods.
Methods:
A retrospective, observational study using routinely collected data on intravenous device use in a large neonatal intensive care unit in Qatar. A 6-month historical cohort was compared with a 6-month cohort after the introduction of an octyl-butyl-cyanoacrylate glue (CG). In the historical cohort, the catheter was secured using a semi-permeable transparent membrane dressing while in the CG cohort, CG was applied at the insertion site on initial insertion and after any dressing change. This was the only variable intervention between both groups.
Results:
A total of 8330 peripheral catheters were inserted. All catheters were inserted and monitored by members of the NeoVAT team. 4457 (53.5%) were secured with just a semi-permeable transparent dressing and 3873 (46.5%) secured a semi-permeable transparent dressing with the addition of CG. The odds ratio for premature failure after securement with CG was 0.59 (0.54–0.65) when compared to the catheters secured with a semi-permeable transparent dressing, which was statistically significant (p < 0.001). The correlation between the occurrence of a complication and the use of CG for device securement was significant (p < 0.001).
Conclusions:
The risk of developing device-related phlebitis and premature device removal, increased significantly if CG was not used for adjunct catheter securement. In parallel with the currently published literature, this study’s findings support the use of CG for vascular device securement. When device securement and stabilization concerns are most pertinent CG is a safe and effective adjunct to reducing therapy failures in the neonatal patient population.
Keywords: Neonate, cyanoacrylate, vascular access, infection prevention, peripheral intravenous devices, complications
Discussion/Recommendations
What do we know?
Currently, PIVCs are predominantly used to provide infusion therapy in neonatal intensive care.
Despite a high complication risk there is little progress to be noticed in improvements and/or innovations (benchmark studies by Petit 2002–2003).
CG does contribute to the overall reduction of therapy failure and especially phlebitis.
What needs further investigation?
New sensor technologies for early recognition of infiltration/extravasation in a combination of the touch-look-compare observation and the use of the PIVIE tool might reduce the severity of the complications in patients.
What can we do today?
Implement a decision chart for vascular access devices in which the 5Rights for vascular access are represented that is, the right vein, for the right device, with the right therapy, and the right duration should be selected for the right patient.
Introduction
Vascular access devices (VADs), particularly peripheral intravenous catheters (PIVCs), play a vital role within the neonatal intensive care unit (NICU). The support and management of critically ill neonatal conditions rely upon reliable vascular access for the administration of fluids, nutrition, medications, and blood products.1,2 However, VAD use is not without risk, and complications may occur frequently, leading to failure of the device, interruption of required therapies, and lead to potential patient harm or injury. Both peripheral and central venous vascular access-related complications in this population have been previously published, with initial benchmark studies published by Pettit.3–5 More recent studies have shown similarities among reported complication rates.6–10 It is widely known and yet accepted, that neonatal short PIVCs often develop complications before therapy has been completed. Reported incidences of peripheral IV complications in these patients range from 16% to 78%.7–15
Evidence-based insertion and maintenance strategies including securement and stabilization of vascular devices have been developed to reduce the preventable causes of VAD failure and complications.12,14,15 This study aims to describe the prevalence of premature PIVC failure and complications associated with catheter securement (e.g. infiltration/extravasation, leaking, phlebitis, device occlusion, dislodgement and accidental removal, discoloration, and infection) before and after the introduction of octyl-butyl-cyanoacrylate glue as an adjunct catheter securement tool in the NICU. Furthermore, this study aims to evaluate the efficacy and identify modifiable risk factors to help inform innovation, practice, and policy development.
An octyl-butyl-cyanoacrylate catheter securement adhesive glue (SecurePortIV®, Adhezion Biomedical, Wyomissing, PA, United States) has been approved by the U.S. Food and Drug Administration for use with vascular access devices. Once the cyanoacrylate glue (CG) is applied, it may prevent catheter movement, migration, and dislodgement. Additionally, it may help to seal the insertion site, by reducing bleeding and improving dressing longevity, consequently reducing dressing changes and risk of contamination. Literature has described the antimicrobial characteristics of CG against micro-organisms usually associated with bloodstream infections related to the use of intravascular catheters. Cyanoacrylate glue for securement may mitigate the spread of microorganisms by immobilizing the device at the insertion site along with any skin flora. It can be used safely both with premature infants and with chlorhexidine-sensitive patients.16–18 The fact that the CG can be applied on a small area, in addition to that occupied at the insertion site, allows the adaption of the catheter to any position. International studies have shown significant results and related benefits of using a catheter securement glue for intravenous catheters.8,15,19–23
The 2021 Infusion Therapy Standards of Practice acknowledge cyanoacrylate glue as a standard of care for VAD securement. 24 Evidence on peripheral IV catheter securement glue and its applicability in the neonatal population is slowly increasing.
Methods
Design and setting
This retrospective observational study uses routinely collected anonymized data on intravenous device use. Across the whole study, 24 or 26 Gage SuperCath™ Safety (ICU Medical, San Clemente, CA, USA) catheters were used. A 6-month historical cohort was compared with a 6-month cohort after the introduction of octyl-butyl-cyanoacrylate glue (SecurePortIV®, Adhezion Biomedical, Wyomissing, PA, United States). In the historical cohort, the PIVC was secured using a Clik-FIX® Neonatal fixation device (BBraun, Melsungen, Germany) and a semi-permeable transparent membrane dressing (3M™ Tegaderm™). In the CG cohort, the application of CG was performed directly after the insertion of the PIVC and after every dressing change (See Figure 1). This was the only intervention variable in this group. In both cohorts, the skin was disinfected with either 3M™ SoluPrep™ 0.65 ml single-use wipes (2% chlorhexidine gluconate in 70% isopropyl alcohol) for neonates weighing 1500 g or more or was ⩾14 days of age, while 3M™ SoluPrep™ 1.5 ml single-use wipes (2% chlorhexidine gluconate aqueous) was used for all neonates weighing <1500 g and <14 days old after birth. In both groups, a liquid skin barrier wipe was applied before the application of CG, the dressing, and the stabilization device to provide an additional protective layer between the epidermis and the securement devices. If required (e.g. if contaminated or loose) the dressing was changed by a neonatal vascular access team (NeoVAT) team member and a bedside nurse, using single-use acetone-free adhesive remover pads (Medline™) if required, and then CG was reapplied.
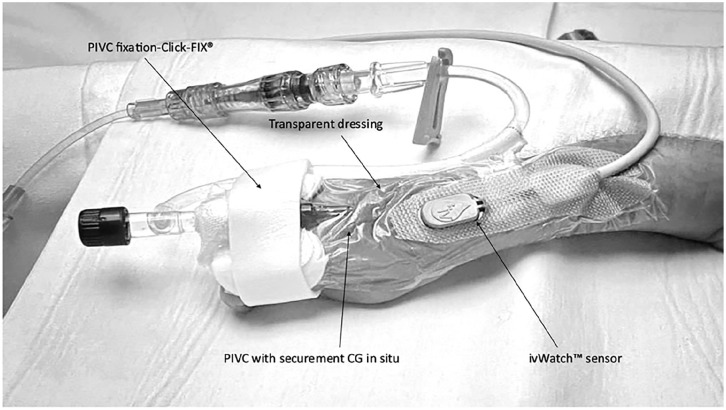
Figure 1.
Image showing the typical securement in our unit using CG, dressing and fixation of a PIVC, an ivWatch™ SmartTouch Sensor is in situ 34 (permission for use in publication obtained).
The outcome of interest was the occurrence of any complication with PIVC use, leading to the unplanned removal of the device before the completion of the intended intravenous therapy. This study was carried out in a large NICU (112 cots) of the Women’s Wellness and Research Centre (WWRC) of Hamad Medical Corporation (HMC), Doha, Qatar. This NICU is the largest in the country and has an annual average of approximately 3500 in- and out-born admissions. Approximately 9000 PIVCs catheters are placed per annum.
The study protocol (MRC-01-22-553) was approved by the local Institutional Review Board (IRB). As the data source was retrospectively collected and fully anonymized, the facility’s ethics committee deemed participant consent was not feasible nor required, classifying the study as a quality review.
Patient and public involvement statement
Study participants, nor parents were not involved in the design, conduct or reporting of this study.
Participants and sample size
All infants who were admitted to the NICU and who required intravenous therapy were included in this study. Participants were excluded from the sample if the data collection was incomplete or if it related to the use of other vascular access devices than peripheral IV catheters (PIVC).
Procedure
In response to the challenges faced in reducing the incidence and potential harms related to PIVC use, 2016 evidence-based care bundles and further preventative measures were developed and implemented in 2017 by NICU staff. During every patient assessment phase, the team followed a locally developed mnemonic the “5Rights for Vascular Access,” based upon Steere et al. 25 This mnemonic consists of ensuring the right device, for the right vein, with the right therapy, for the right duration and selected for the right patient. Currently, peripheral intravenous cannulation is performed according to hospital policy, based on the latest international evidence and the availability of products in the country. Furthermore, this process directed new guidance on undertaking vascular assessment before cannulation with the saphenous and elbow veins frequently avoided, and utilizing near infra-red technologies for appropriate site assessment, along with appropriate catheter selection, use of a securement device, use of in-line filters, in-line pressure monitoring, infusate risk assessment and vigilant hourly observation of the insertion site, and surrounding tissue using the Infusion Nursing Society recommendation of “Touch, Look, and Compare.” 24
The standard PIVC devices utilized in the NICU and used in the study are 24 or 26 Ga. SuperCath™ Safety (ICU Medical, San Clemente, USA) polyurethane IV catheters. In the study setting, peripheral IV cannulation and securement (at the time using a transparent, semi-permeable dressing, CG, and flexible universal IV supports (Clik-FIX®)) were routinely performed by a dedicated neonatal vascular access team (NeoVAT).
In the NeoVAT, several nurses (six per 12-h shift) received specialized simulation training to develop expertise in vascular access and transformed into a specialized team, the neonatal vascular access team. 26 This nurse-led team is highly dedicated and shoulders several clinical and managerial responsibilities for continuous data collection, review, and monthly analysis. 8
In this unit’s practices, short PIVCs are used for therapy anticipated to be required for 2 days or less. Extended dwell PIVCs (8 cm Vygon Premicath® without stylet) is inserted when the duration of therapy is expected to be 5 days or less. In situations where intravenous therapy is expected for >5 days, central venous access devices are preferred. The selection of suitable veins was done using the VeinViewer™ (Christie Medical Holdings, Lake Mary, Florida, USA). Vein length, valves and potential for the vein to fill and empty itself were previously assessed using a standardized approach to the appraisal of the potential site.
Measurements and data collection
Our primary outcome measure consisted of preterm failure of the device and interruption of the required peripheral IV therapy and the impact of the use of glue on this. Patients’ demographics and baseline data (gender, gestational age at birth in weeks and days, birth weight in grams) were collected. Data regarding the procedure of peripheral intravenous cannulation (date and time of cannulation, the number of attempts needed for successful cannulation, cannulation side, extremity of cannulation and the site on the extremity, size of the inserted device) were registered during or directly after intravenous cannulation. The same applies to data about the removal of the device (date and time of removal, catheter dwell time, reason for removal of the device).
Statistical analyses
Descriptive statistics were used to summarize the outcomes with a mean and its standard deviation or median and its minimum and maximum for continuous variables regarding its normal distribution, and absolute numbers with percentages for discrete variables. The assumption of normal distribution was proved with Kolmogorov-Smirnoff testing. χ2 and unpaired t-testing was used to identify differences between study outcomes as appropriate. Spearman ρ testing was used to identify correlations. Log Rank (Mantel-Cox) testing was used to denote differences in time-related factors with the outcomes of interest. Any relation between independent variables with the outcome of interest was identified using univariate logistic regression analysis. Variables with significance in the univariate analysis (p < 0.05) were used for multivariate logistic regression analyses. Using a backward elimination process based on the highest Wald score and lowest p value, the smallest set of factors with a significant relationship in which the occurrence of complications was identified. The odds ratio with its 95% confidence interval (95% CI) was identified in these analyses. Throughout this study, a p < 0.05 was denoted to be statistically significant. SPSS version 27.0 was used for statistical analyses, and a p < 0.05 was denoted as statistically significant.
Results
A total of 8330 PIVCs were inserted. All PIVCs were inserted and monitored by members of the NeoVAT team. Of the inserted catheters, 4457 (53.5%) were secured with a conventional securement method and in 3873 (46.5%) the PIVC was additionally secured with CG. Analysis of patient demographics and baseline characteristics demonstrated no significant differences between the two groups (Table 1).
Table 1.
Patient demographics of both cohort groups.
| Non-CG group n = 4457 (%) | CG group n = 3873 (%) | p-Value | |
|---|---|---|---|
| Gender | |||
| Male | 2581 (58) | 1880 (49) | <0.001 |
| Female | 1870 (42) | 1990 (51) | |
| Age at birth | |||
| Weeks | 34.8 ± 4.5 | 34.8 ± 4.5 | 0.717 |
| Age at birth (categorized) (weeks) | |||
| <28 | 502 (11) | 499 (13) | <0.001 |
| 28–31.9 | 546 (12) | 395 (10) | |
| 32–36.9 | 1576 (35) | 1268 (33) | |
| >37 | 1833 (41) | 1711 (44) | |
| Current age | |||
| Weeks | 36.0 ± 4.0 | 35.9 ± 3.8 | 0.149 |
| Current age (categorized) (weeks) | |||
| <28 | 128 (3) | 120 (3) | 0.060 |
| 28–31.9 | 453 (10) | 437 (11) | |
| 32–36.9 | 1814 (41) | 1472 (38) | |
| >37 | 2062 (46) | 1844 (48) | |
| Age from birth | |||
| Days | 9.3 ± 22.2 | 8.8 ± 17.8 | 0.222 |
| Weight at birth | |||
| Grams | 2337 ± 971 | 2375 ± 998 | 0.081 |
| Weight at birth (categorized) (g) | |||
| <1000 | 576 (13) | 471 (12) | <0.001 |
| 1000–1499 | 280 (6) | 316 (8) | |
| 1500–2499 | 1633 (37) | 1254 (32) | |
| >2500 | 1968 (44) | 1832 (47) | |
| Current weight | |||
| Grams | 2432 ± 921 | 2450 ± 918 | 0.372 |
| Current weight (categorized) (g) | |||
| <1000 | 222 (5) | 222 (6) | 0.023 |
| 1000–1499 | 441 (10) | 382 (10) | |
| 1500–2499 | 1741 (39) | 1396 (36) | |
| >2500 | 2035 (46) | 1837 (47) | |
Data are represented as mean ± standard deviation, or as absolute numbers (percentages rounded to the nearest full number where appropriate). Data is tested with χ2 testing or with unpaired t-testing, as appropriate.
First-attempt insertion success was 75% across the total cohort, with a facility maximum of two attempts per clinician, which did not exceed the number allowed for by hospital policy. Other data regarding the procedure of intravenous cannulation are shown in Table 2.
Table 2.
Intravenous access data for cohort groups.
| Non-CG group n = 4457 (%) | CG group n = 3873 (%) | p-Value | |
|---|---|---|---|
| Catheter size (Ga.) | |||
| 24. | 50 (1) | 4 (1) | <0.001 |
| 26 | 4407 (99) | 3869 (99) | |
| Indication | |||
| Blood products | 160 (4) | 170 (4) | <0.001 |
| Continuous infusion | 3980 (89) | 3203 (83) | |
| Intermittent infusion | 250 (6) | 429 (11) | |
| Procedure* | 67 (1) | 71 (2) | |
| Side of cannulation | |||
| Left | 2417 (54) | 2204 (57) | 0.014 |
| Right | 2040 (46) | 1669 (43) | |
| Site of cannulation | |||
| Elbow | 3 (<1) | 0 (0) | <0.001 |
| Foot | 630 (14) | 331 (8) | |
| Hand | 3656 (82) | 3430 (89) | |
| Lower arm | 157 (3) | 105 (3) | |
| Lower leg | 5 (<1) | 4 (<1) | |
| Upper arm | 4 (<1) | 1 (<1) | |
| Upper leg | 2 (<1) | 2 (<1) | |
| Attempts | Skin punctures | 1.3 ± 0.5 | 1.4 ± 0.7 |
| Reason of removal | |||
| Elective | 1655 (37) | 1919 (49) | <0.001 |
| Accidental | 130 (3) | 105 (3) | |
| Leaking | 445 (10) | 386 (10) | |
| Occlusion | 219 (5) | 160 (4) | |
| Phlebitis | 594 (13) | 123 (3) | |
| Infiltration/extravasation | 1365 (31) | 1121 (29) | |
| Lost to follow-up | 49 (1) | 59 (2) | |
| Dwell time | |||
| Hours | 31.0 ± 24.3 | 37.1 ± 31.1 | <0.001 |
Ga.: gage; PIVIE: peripheral intravenous infiltration/extravasation.
Data are represented as mean ± standard deviation, or as absolute numbers (percentages rounded to the nearest full number where appropriate). Data is tested with χ2 testing or with unpaired t-testing, as appropriate.
VAD insertion related to a procedure if required in diagnostic imaging like MRI or CT-scan.
The odds ratio for premature removal of the PIVC after securement with CG was 0.59 (0.54–0.65) when compared to those in which the PIVC was secured with a conventional securement method (χ2 = 135.51, df = 1, p < 0.001). The indwelling time varied from 1 h to 14 days, with a mean dwell time of 31 h for the non-CG group and 37 h for the CG group per inserted PIVC (χ2 = 210.62, df = 1, p < 0.001). A correlation between the occurrence of a complication and the use of CG for device fixation was detected (ρ = 0.126, p < 0.001).
Logistic regression analyses were performed including 11 variables to detect their relationship with the occurrence of a complication leading to premature removal of the PIVC (Table 3). From the univariate analyses, five variables with a significant relationship with the outcome of interest were included in the multivariate logistic regression analyses. As a result of this analysis, three factors had a significant relationship with the outcome of interest: the use of CG for device securement (odds ratio 0.59 [0.54–0.65], p < 0.001), size of the inserted PIVC (odds ratio 2.19 [1.43–4.33], p = 0.001), and the site of cannulation on the body (odds ratio 0.81 [0.73–0.91], p < 0.001).
Table 3.
Univariate logistic regression analyses.
| Factor | Exp (B) | 95% CI | Wald | p-Value |
|---|---|---|---|---|
| Gender | 1.07 | 0.98–1.16 | 2.061 | 0.151 |
| Age at birth | 1.02 | 0.98–1.07 | 1.183 | 0.277 |
| Current age | 0.96 | 0.91–1.02 | 1.715 | 0.190 |
| Age from birth | 0.99 | 0.98–0.99 | 9.127 | 0.003 |
| Weight at birth | 1.03 | 0.98–1.07 | 1.281 | 0.258 |
| Current weight | 0.98 | 0.93–1.03 | 0.786 | 0.375 |
| Size of the PIVC | 1.99 | 0.15–3.45 | 6.062 | 0.014 |
| First attempt success | 0.92 | 0.86–0.99 | 5.608 | 0.018 |
| Site of cannulation | 0.78 | 0.70–0.88 | 18.841 | <0.001 |
| Side of cannulation | 0.99 | 0.91–1.08 | 0.061 | 0.805 |
| CG securement | 0.59 | 0.54–0.65 | 137.691 | <0.001 |
Data is represented with odd ratios and its 95% CI after performing univariate logistic regression analyses, in which any relation with the occurrence of a complication leading to premature removal of the PIVC was identified.
Discussion
Premature VAD failure in the clinical setting is influenced by numerous unmodifiable patient characteristics and potentially modifiable factors and impacts upon patient experience, their well-being and outcomes, and hospital economics.8,27 Appropriate securement of VADs is an essential component of effective infusion therapy and quality care. The strategies employed to ensure the securement of VADs have a crucial role in preventing the development of device-related failures, infusion-related complications and associated injuries within all patient populations. Intravenous device failures may have a far greater impact on neonates than on pediatric, or even adult patients. 8 In part, this is due to age/gestation-related differences in physiology, the smaller nature of peripheral arterial and venous blood vessels, and the ratios of muscle–soft tissue mass.2,8–12
Our findings support the contention that applying CG to secure PIVCs is an effective intervention for the neonatal population and could be added to existing insertion care bundles. Several publications have recently evaluated outcomes along with the use of CG for both peripherally and centrally inserted central catheters (PICC/CICC) with various securement methods,16–18,27–30 outlining the noteworthiness and clinical impact CG has in preventing device-related failures and loss of venous access, corroborating it is safe and effective with regular use. Cyanoacrylates are well established as being very biocompatible and their ability to undergo polymerization with moisture allows for better bonding to the skin. 31 This makes it an invaluable tool to enhance dressing adherence and device securement in any diaphoretic patient. Highlighting its superior bonding strength substantiates that CG can provide superior adhesive properties to prevent unwarranted device failure and dressing loss, including the prevention of infectious complications, which has also been confirmed within the adult population.32,33
The choice to reapply the CG at every dressing change was pragmatic to preserve the VAD, but this practice requires further empirical research. At this moment this choice is not fully supported by the available data. 23 However, our results are supportive of this practice. Implementing prevention strategies is an important consideration for clinicians, with early detection impacting outcomes by reducing patient harm or injury from device-related complications. Recent research on continuous infusion site monitoring using new sensor technology offers the potential to detect infiltration/extravasation events earlier than relying on intermittent observation alone, which is currently still the standard of care. 34
Strength and limitations
All eligible neonates were included ensuring a large sample size that was representative of the neonatal PIVC population. This increased the statistical power of the study’s findings, helping to minimize selection bias and increase the generalizability of the findings to similar settings.
Despite these strengths, there are limitations to this research. This study was from a single center and used retrospective data. Whilst the NeoVAT members are highly experienced inserter practice variability, particularly over vein selection may have affected dwell time results. To optimize venous catheterization, the adoption of a standardized mnemonic like RaSuVA (Rapid Superficial Vein Assessment)35–37 might provide opportunities for a more methodic pre-assessment of vein suitability to reduce this possible variability.
Conclusion
The results from this study show that PIVC failure rates are high amongst neonatal populations with relatively short catheter dwell times and higher associated incidences of complications. This poses significant challenges for healthcare practitioners in delivering safe and effective vascular access care. Despite the interventions and preliminary data suggesting significant decreases in the incidence of complications, the authors consider a higher reduction should have possibly been achieved.
The risk for the development of device-related phlebitis, which leads to premature device removal, increased significantly if CG was not used for adjunct catheter securement. Using CG in the sterile dressing for fixation and stabilization was not found to cause any negative effects. In parallel with currently published literature, this study’s findings support the use of CG with vascular device securement. When device securement and stabilization are most pertinent, the use of CG is a safe and effective method to reduce therapy failures.
In the world of neonatology, newly introduced vascular access practices and clinical advancement with minimally invasive methods, play an important role in improving patient and device-related outcomes. The use of a medically approved CG for vascular device securement is an important step for clinicians who place and care for VADs in this patient population. To the authors’ knowledge, this is the first published research for the use of cyanoacrylate glue with PIVCs in neonatal patients, and future research is necessary to ensure this securement choice provides impactful clinical benefits and outcomes when used for vascular device securement and stabilization.
Acknowledgments
The authors would like to acknowledge all members of the neoVAT, the nursing, and medical staff of the NICU at WWRC for their support and Binsy Padinharayil for her assistance with the database and statistics during the study period.
Footnotes
Author contributions: MvR, MAAB, and KH contributed to the conceptualization and design of the study. ALVF collected the data. MvR, MAAB, and KH drafted the initial manuscript; FvL provided statistical analysis; MvR, MAAB, KH, FvL, and TRS provided all editorial content, reviews, and all revisions to the manuscript; all authors critically reviewed and approved the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Matheus FPT van Rens  https://orcid.org/0000-0002-9595-0265
https://orcid.org/0000-0002-9595-0265
Timothy R Spencer  https://orcid.org/0000-0002-3128-2034
https://orcid.org/0000-0002-3128-2034
Fredericus HJ van Loon  https://orcid.org/0000-0003-3854-6976
https://orcid.org/0000-0003-3854-6976
Mohammad AA Bayoumi  https://orcid.org/0000-0002-2627-4806
https://orcid.org/0000-0002-2627-4806
References
- 1. Rocha G, Soares P, Pissarra S, et al. Vascular access in neonates. Minerva Pediatr 2017; 69(1): 72–82. [DOI] [PubMed] [Google Scholar]
- 2. Hugill K. Vascular access in neonatal care settings: selecting the appropriate device. Br J Nurs 2016; 25(3): 171–176. [DOI] [PubMed] [Google Scholar]
- 3. Pettit J. Assessment of infants with peripherally inserted central catheters: part 1. detecting the most frequently occurring complications. Adv Neonatal Care 2002; 2(6): 304–315. [DOI] [PubMed] [Google Scholar]
- 4. Pettit J. Assessment of infants with peripherally inserted central catheters: part 2. Detecting less frequently occurring complications. Adv Neonatal Care 2003; 3(1): 14–26. [DOI] [PubMed] [Google Scholar]
- 5. Pettit J. Assessment of the infant with a peripheral intravenous device. Adv Neonatal Care 2003; 3(5): 230–240. [PubMed] [Google Scholar]
- 6. Helm RE, Klausner JD, Klemperer JD, et al. Accepted but unacceptable: peripheral IV catheter failure. J Infus Nurs 2015; 38(3): 189–203. [DOI] [PubMed] [Google Scholar]
- 7. Legemaat M, Carr PJ, van Rens RM, et al. Peripheral intravenous cannulation: complication rates in the neonatal population: a multicenter observational study. J Vasc Access 2016; 17(4): 360–365. [DOI] [PubMed] [Google Scholar]
- 8. van Rens MFPT, Hugill K, Mahmah MA, et al. Evaluation of unmodifiable and potentially modifiable factors affecting peripheral intravenous device-related complications in neonates: a retrospective observational study. BMJ Open 2021; 11(9): e047788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Atay S, Sen S, Cukurlu D. Incidence of infiltration/extravasation in newborns using peripheral venous catheter and affecting factors. Rev Esc Enferm USP 2018; 52: e03360. [DOI] [PubMed] [Google Scholar]
- 10. Unbeck M, Förberg U, Ygge BM, et al. Peripheral venous catheter related complications are common among paediatric and neonatal patients. Acta Paediatr 2015; 104(6): 566–574. [DOI] [PubMed] [Google Scholar]
- 11. McIntyre C, August D, Cobbald L, et al. Neonatal vascular access practice and complications: an observational study of 1,375 catheter days. J Perinat Neonatal Nurs. Epub ahead of print 11 February 2022. DOI: 10.1097/jpn.0000000000000589 [DOI] [PubMed] [Google Scholar]
- 12. Odom B, Lowe L, Yates C. Peripheral infiltration and extravasation injury methodology: a retrospective study. J Infus Nurs 2018; 41(4): 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chenoweth KB, Guo JW, Chan B. The extended dwell peripheral intravenous catheter is an alternative method of NICU intravenous access. Adv Neonatal Care 2018; 18(4): 295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Corley A, Ullman AJ, Marsh N, et al. A pilot randomized controlled trial of securement bundles to reduce peripheral intravenous catheter failure. Heart Lung 2023; 57: 45–53. [DOI] [PubMed] [Google Scholar]
- 15. Zhang S, Lingle BS, Phelps S. A revolutionary, proven solution to vascular access concerns: a review of the advantageous properties and benefits of catheter securement cyanoacrylate adhesives. J Infus Nurs 2022; 45(3): 154–164. [DOI] [PubMed] [Google Scholar]
- 16. D’Andrea V, Barone G, Pezza L, et al. Securement of central venous catheters by subcutaneously anchored suturless devices in neonates. J Matern Fetal Neonatal Med 2022; 35: 6747–6750. [DOI] [PubMed] [Google Scholar]
- 17. D’Andrea V, Pezza L, Barone G, et al. Use of cyanoacrylate glue for the sutureless securement of epicutaneo-caval catheters in neonates. J Vasc Access 2022; 23: 801–804. [DOI] [PubMed] [Google Scholar]
- 18. van Rens M, Nimeri AMA, Spencer TR, et al. Cyanoacrylate securement in neonatal PICC use: a 4-year observational study. Adv Neonatal Care 2022; 22(3): 270–279. [DOI] [PubMed] [Google Scholar]
- 19. Widmer AF. Sterilization of skin and catheters before drawing blood cultures. J Clin Microbiol 2003; 41(10): 4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ullman AJ, Kleidon T, Gibson V, et al. Innovative dressing and securement of tunneled central venous access devices in pediatrics: a pilot randomized controlled trial. BMC Cancer 2017; 17(1): 595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pabon DF, Yost MJ, Melendez GC, et al. Novel bacterial immobilization compound effectively decreases bacterial counts in healthy volunteers. Am Surg 2010; 76(1): 15–19. [PubMed] [Google Scholar]
- 22. Kleidon TM, Ullman AJ, Gibson V, et al. A pilot randomized controlled trial of novel dressing and securement techniques in 101 pediatric patients. J Vasc Interv Radiol 2017; 28(11): 1548–1556.e1. [DOI] [PubMed] [Google Scholar]
- 23. Pittiruti M, Annetta MG, Marche B, et al. Ten years of clinical experience with cyanoacrylate glue for venous access in a 1300-bed university hospital. Br J Nurs 2022; 31(8): S4–S13. [DOI] [PubMed] [Google Scholar]
- 24. Gorski LA, Hadaway L, Hagle ME, et al. Infusion therapy standards of practice, 8th Edition. J Infus Nurs 2021; 44(1S Suppl 1): S1–S224. [DOI] [PubMed] [Google Scholar]
- 25. Steere L, Ficara C, Davis M, et al. Reaching one peripheral intravenous catheter (PIVC) per patient visit with Lean Multimodal Strategy: the piv5rights™ Bundle. J Assoc Vasc Access 2019; 24(3): 31–43. [Google Scholar]
- 26. Bayoumi MAA, Elmalik EE, Ali H, et al. Neonatal simulation program: a 5 years educational journey from Qatar. Front Pediatr 2022; 10: 843147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Rens MF, Hugill K, Mahmah MA, et al. Effect of peripheral intravenous catheter type and material on therapy failure in a neonatal population. J Vasc Access 2022. Feb 23: 11297298221080071. DOI: 10.1177/11297298221080071 [DOI] [PubMed] [Google Scholar]
- 28. Pinelli F, Pittiruti M, Van Boxtel T, et al. GAVeCeLT-WoCoVA consensus on subcutaneously anchored securement devices for the securement of venous catheters: current evidence and recommendations for future research. J Vasc Access 2021; 22(5): 716–725. [DOI] [PubMed] [Google Scholar]
- 29. Ostroff M, Zauk A, Chowdhury S, et al. A retrospective analysis of the clinical effectiveness of subcutaneously tunneled femoral vein cannulations at the bedside: A low risk central venous access approach in the neonatal intensive care unit. J Vasc Access 2021; 22(6): 926–934. [DOI] [PubMed] [Google Scholar]
- 30. Bierlaire S, Danhaive O, Carkeek K, et al. How to minimize central line–associated bloodstream infections in a neonatal intensive care unit: a quality improvement intervention based on a retrospective analysis and the adoption of an evidence-based bundle. Eur J Pediatr 2021; 180(2): 449–460. [DOI] [PubMed] [Google Scholar]
- 31. Korde Jm, Kandasubramanian B. Biocompatible alkyl cyanoacrylates and their derivatives as bio-adhesives. Biomater Sci 2018; 6(7): 1691–1711. [DOI] [PubMed] [Google Scholar]
- 32. Bahl A, Gibson SM, Jankowski D, et al. Short peripheral intravenous catheter securement with cyanoacrylate glue compared to conventional dressing: a randomized controlled trial. J Vasc Access 2023; 24: 52–63. [DOI] [PubMed] [Google Scholar]
- 33. Gilardi E, Piano A, Chellini P, et al. Reduction of bacterial colonization at the exit site of peripherally inserted central catheters: a comparison between chlorhexidine-releasing sponge dressings and cyano-acrylate. J Vasc Access 2021; 22(4): 597–601. [DOI] [PubMed] [Google Scholar]
- 34. van Rens M, Hugill K, Velez Francia A. A new approach for early recognition of peripheral intravenous (Piv) infiltration: a pilot appraisal of a sensor technology in a neonatal population. Vascular Access 2019; 5(2): 38–41. [Google Scholar]
- 35. D’Andrea V, Prontera G, Pezza L, et al. Rapid Superficial Vein Assessment (RaSuVA): a pre-procedural systematic evaluation of superficial veins to optimize venous catheterization in neonates. J Vasc Access 2022; 20: 11297298221098481. [DOI] [PubMed] [Google Scholar]
- 36. Bayoumi MAA, van Rens MFPT, Chandra P, et al. Does the antimicrobial-impregnated peripherally inserted central catheter decrease the CLABSI rate in neonates? Results from a retrospective cohort study. Front Pediatr 2022; 10: 1012800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van Rens MFPT, Bayoumi MAA, van de Hoogen A, et al. The ABBA project (assess better before access): a retrospective cohort study of neonatal intravascular device outcomes. Front Pediatr 2022; 10: 980725. [DOI] [PMC free article] [PubMed] [Google Scholar]