Abstract
Sindbis virus (SV) is an alphavirus that causes encephalitis in mice and can lead to the apoptotic death of infected cells. To determine the step in virus replication during which apoptosis is triggered, we used UV-inactivated SV, chemicals that block virus fusion or protein synthesis, and cells that do and do not express heparan sulfate, the initial binding molecule for SV infection of many cells. In initial experiments, UV-inactivated neuroadapted SV (NSV) induced apoptosis in Chinese hamster ovary (CHO) cells lacking heparan sulfate in the presence of cycloheximide. When fusion of prebound UV-inactivated NSV was rapidly induced at the plasma membrane by exposure to acidic pH, apoptosis was induced in CHO cells with or without heparan sulfate in the presence or absence of cycloheximide in a virus dose-dependent manner. In N18 neuroblastoma cells, the relative virulence of the virus strain was an important determinant of apoptosis induced by UV-inactivated SV. Treatment of N18 cells with monensin to prevent endosomal acidification an hour before, but not 2 h after, exposure to live NSV blocked the induction of cell death, as did treatment with NH4Cl or bafilomycin A1. These studies indicate that SV can induce apoptosis at the time of fusion with the cell membrane and that virus replication is not required.
The interactions between viruses and host cells have several possible outcomes: transformation, persistent productive infection, latency, or cell death. Sindbis virus (SV), a mosquito-borne alphavirus, is an enveloped, plus-strand RNA virus that causes infection of neurons and age-dependent encephalomyelitis in mice (20, 24). SV causes persistent infections in most mosquito cell lines, but it induces apoptosis in most vertebrate cell lines, a property that may be relevant to its maintenance in natural transmission cycles (15). Viral and host cell factors that regulate the process of apoptosis determine the outcome of infection for the cell and ultimately for the host. Studies of SV infection of the central nervous system in vivo and of primary dorsal root ganglion neurons in vitro have shown that neurons become increasingly resistant to SV-induced apoptosis as they mature, which correlates with the age dependence of fatal infection (27, 28). The mechanism of this increased resistance is not known, but a number of studies have shown that apoptosis induced by SV can be blocked by caspase inhibitors and by members of the Bcl-2 family of antiapoptotic proteins (8, 26, 27, 36), so a variety of cellular inhibitors of apoptosis can prevent or slow SV-induced apoptosis. Neurovirulent strains of SV can overcome some cellular inhibitors of apoptosis and cause cell death (49), suggesting that a balance of cellular factors regulating cell death and viral factors regulating virulence determines the outcome of infection. However, how this apoptotic pathway is triggered during SV infection is not understood.
For some viruses, specific viral proteins (e.g., adenovirus E1A [41], parvovirus NS [7, 25], reovirus sigma 1 [48], retrovirus Env and Vpr [4, 43], or chicken anemia virus VP3 [38]) have been associated with the induction of apoptosis. Some of these proteins act at the cell membrane, presumably through interaction with a cellular receptor for the virus, while others act intracellularly (4, 33, 48). SV, like other alphaviruses, encodes four nonstructural proteins (nsPs) and three major structural proteins: an internal capsid protein and two surface glycoproteins, E1 and E2 (44). E1 and E2 exist on the surface of the virion as a heterodimer that is trimerized to form spikes which attach the virus to cells to initiate infection. Receptor-mediated endocytosis brings the virion into the endosome, where exposure to acidic pH induces a conformational change in the E1-E2 heterodimer, leading to fusion of the viral envelope with the endosomal membrane. Delivery of the capped and polyadenylated genomic RNA to the cytoplasm is followed by translation of the nsPs and formation of membrane-bound replication complexes. At approximately the time that structural protein synthesis is initiated, the synthesis of nonstructural viral and cellular proteins ceases. Virions are assembled at, and bud from, the plasma membrane (44). Although a role for nsPs in the establishment of persistent SV infection has been suggested (11), infection of cells with SV replicons has shown that the structural proteins dramatically accelerate cell death (14). The most recent data gathered from transient-transfection assays suggest that the induction of apoptosis may involve the transmembrane regions of the surface glycoproteins (23).
To begin to determine the step in the SV life cycle that triggers the signal for cell death, we used UV-inactivated SV, chemicals that block virus fusion or protein synthesis, and cell lines that do and do not express heparan sulfate (the initial virus binding molecule) for studies of SV-induced apoptosis. We found that UV-inactivated SV is able to induce apoptosis. This was most easily demonstrated when the cells lacked heparan sulfate and when virus-cell membrane fusion was rapidly and synchronously induced by exposure of cells with bound virions to acidic pH. Apoptosis was blocked by treating cells exposed to infectious virus with inhibitors of endosomal acidification. It is proposed that the process of apoptosis is initiated by an interaction between the viral glycoprotein(s) and a cell surface receptor and that it is specifically triggered during acid-induced virus fusion with the cell membrane.
MATERIALS AND METHODS
Cell lines.
Chinese hamster ovary (CHO-22) cells (18) and mutant CHO-18.4m cells lacking cell surface sulfated proteoglycans (21) were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Grand Island, N.Y.) supplemented with 10% fetal bovine serum (FBS) (Gibco) and 1.5× nonessential amino acids (Gibco). Mouse neuroblastoma (N18) cells (2) and baby hamster kidney (BHK-21) cells (American Type Culture Collection, Manassas, Va.) were grown in DMEM supplemented with 10% FBS.
Virus strains, purification, and inactivation.
AR339 was originally isolated from mosquitoes (45) and obtained from the American Type Culture Collection. A neuroadapted strain of SV (NSV) was derived by serial passages of AR339 in mouse brain (16). The heat-resistant small-plaque strain of SV (HRSP) was derived from serial passages of the HR strain (derived from AR339) in chicken embryo fibroblasts (6). Virus stocks were grown and assayed in BHK-21 cells and contained 108 to 109 PFU/ml.
Radiolabeling and purification of virions.
BHK-21 cells were infected at a multiplicity of infection (MOI) of 5, and 1 h later, cells were fed with methionine- and cysteine-free MEM to which was added 10 μCi of [35S]methionine-cysteine (Tran35S-label; ICN) per ml. When a >90% cytopathic effect was evident, supernatant fluid was harvested and clarified. For purification, virus was precipitated in 10% (wt/vol) polyethylene glycol 8000 in 0.5 M NaCl, resuspended in NET buffer (10 mM Tris, 3 mM EDTA, 150 mM NaCl [pH 7.4]), and banded in a 15 to 40% potassium tartrate gradient as previously described (42). Banded virus was collected, dialyzed against 0.05 M Tris-HCl (pH 7.4), and stored in aliquots at −70°C.
Virus binding assay.
Cells were grown in 12-well plates and washed twice in cold binding medium (RPMI 1640 without NaHCO3, 0.2% bovine serum albumin, 20 mM HEPES [pH 7.4]). Radiolabeled virus (4,000 cpm per well in 0.4 ml of cold binding medium) was added, and the plates were rocked gently at 4°C. At various times after addition of virus, supernatant fluids were collected from triplicate wells and cells were washed twice. The wash fluids were added to the supernatant fluids and counted to determine the amount of unbound virus. Cells were lysed in 1% sodium dodecyl sulfate (SDS) and counted to determine the amount of bound virus (46).
UV inactivation of SV.
SV was inactivated by exposing the virus to a germicidal lamp (wavelength, 254 nm) at a distance of 5 cm for 30 min at 4°C. Inactivation was confirmed by the inoculation of BHK-21 cells before use and, in individual experiments, by monitoring the exposed cells for synthesis of nsPs and the supernatant fluids from these cells for the presence of infectious virus at 24 h.
Assessment of DNA degradation.
A total of 2 × 106 cells infected with untreated SV or UV-treated SV were harvested after a 48- to 72-h exposure to the virus. Genomic DNA was isolated with DNAzol (Gibco BRL), incubated with RNase (1 μg/ml) at 50°C for 1 h, and then loaded onto a 1.2% agarose gel containing ethidium bromide. After electrophoresis, the gel was photographed and examined for the presence of DNA laddering.
Drug treatments and assessment of viral protein synthesis.
Confluent cells were pretreated with NH4Cl (20 mM), monensin (10 μM), bafilomycin A1 (10 μM), or cycloheximide (CHX) (10 μg/ml; Sigma Chemical Co., St. Louis, Mo.) beginning 1 h before or 2 h after exposure to SV. At various times after infection, 2 ml of labeling medium containing [35S]methionine-cysteine (50 μCi/ml) was added to the cells for 1 h. After removal of the labeling medium, cells were washed twice with phosphate-buffered saline (PBS) and lysed with radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-Cl [pH 7.5], 150 mM NaCl, 10 mM EDTA, 0.1% SDS, 1% Triton X-100, 1% deoxycholate). Equal amounts of cell lysates were analyzed on an SDS–15% polyacrylamide gel.
To monitor the synthesis of nsPs as evidence of infection, N18 cells were infected with NSV diluted in DMEM at an MOI of 50 or were exposed to UV-inactivated NSV at an MOI equivalent of 500 at 4°C for 2 h. Cells exposed to live virus were washed, fed with DMEM–2% FBS, and shifted to 37°C. Cells exposed to UV-inactivated virus were treated with DMEM (pH 5.0) at 37°C for 1 h, and then they were washed and fed with DMEM–2% FBS. At 24 h, cells were washed with PBS, fixed with acetone-methanol (1:1), and stained overnight at 4°C with rabbit anti-nsP1 (diluted 1:500), followed by fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin G (1:2,000) at 37°C for 2 h.
Western blot analysis.
Cells were washed twice with PBS and lysed in RIPA buffer. The protein concentrations were determined by the Bradford protein assay (Bio-Rad, Hercules, Calif.). Proteins (50 to 100 μg) were electrophoresed in an SDS–15% polyacrylamide gel, blotted onto a Hybond-C extra membrane (Amersham, Arlington Heights, Ill.), and reacted with antibody. Rabbit polyclonal antiserum to SV (19) was used to detect the E1, E2, and capsid proteins.
RESULTS
Induction of apoptosis in CHO-18.4m cells by UV-inactivated NSV.
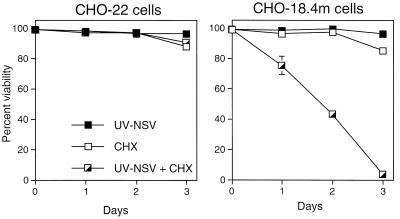
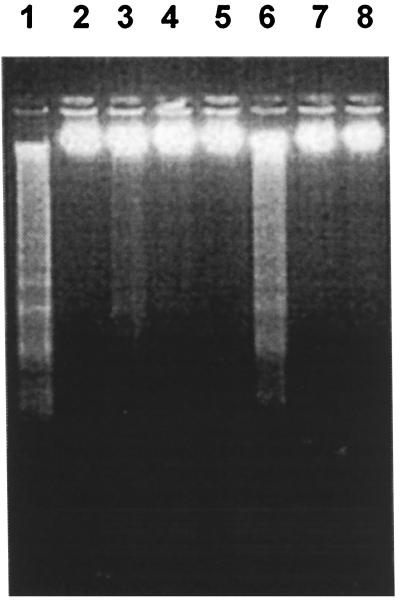
To begin to determine the step in SV replication that induces apoptosis, we investigated whether virus replication was required. CHO-18.4m cells, which lack heparan sulfate (21) and therefore allow virus to interact directly with a postulated receptor, and parental CHO-22 cells were used. Cells were treated with UV-inactivated NSV at a BHK cell MOI equivalent of 500 in the presence and absence of CHX to inhibit the synthesis of viral and cellular proteins (Fig. 1). UV-inactivated NSV induced the death of CHO-18.4m cells, but not that of CHO-22 cells, in the presence of CHX. CHX alone did not induce significant cell death in these cell lines within 3 days. The death of CHO-18.4m cells induced by UV-inactivated NSV was due to apoptosis, as indicated by the presence of DNA laddering (Fig. 2). These data suggest that the induction of cell death did not require virus replication or virus protein synthesis, was optimized by interaction of the virus with a cell surface molecule other than heparan sulfate, and, surprisingly, appeared to require the inhibition of cellular protein synthesis by CHX.
FIG. 1.
Effect of UV-inactivated NSV (UV-NSV) on CHO-22 and CHO-18.4m cells in the presence or absence of CHX. Cells were or were not pretreated with CHX (10 μg/ml) for 1 h, after which UV-inactivated NSV (MOI equivalent = 500) was added with additional CHX. Cell viabilities were assessed at 1, 2, and 3 days by trypan blue exclusion. The bar indicates standard deviation.
FIG. 2.
Integrity of DNA of CHO-18.4m cells after exposure to NSV. Lane 1, cells infected with viable NSV (MOI equivalent = 5, harvested at 48 h); lane 2, uninfected cells; lanes 3, 4, 6, and 7, cells exposed to UV-inactivated NSV (MOI equivalent = 500) in the presence of CHX (10 μg/ml) (lane 3, 48 h; lane 6, 72 h) or without CHX (lane 4, 48 h; lane 7, 72 h); lanes 5 and 8, cells exposed to CHX alone (lanes 5, 48 h; lane 8, 72 h). DNA was analyzed on a 1.2% agarose gel stained with ethidium bromide.
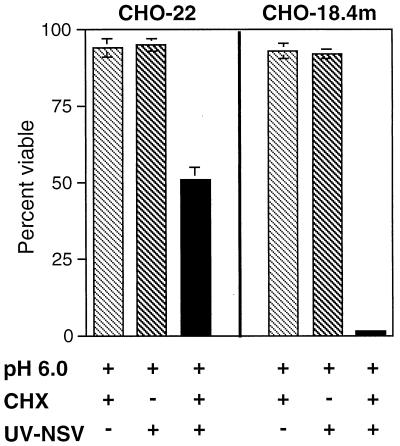
To determine whether apoptosis could be enhanced by inducing coordinated fusion of UV-inactivated virus at the plasma membrane, CHO-18.4m and CHO-22 cells, with UV-inactivated NSV bound to the surface at 4°C, were exposed to pH 6.0 and shifted to 37°C, and their viabilities were assessed at 48 h (Fig. 3). With this experimental paradigm, both CHO-18.4m and CHO-22 cells pretreated with CHX to inhibit viral and cellular protein synthesis died, although CHO-18.4m cells were killed more efficiently. Neither CHX nor acidic medium, alone or together, induced cell death.
FIG. 3.
Effect of exposure to pH 6.0 on viabilities of CHO-22 and CHO-18.4m cells after treatment with UV-inactivated NSV (UV-NSV) in the presence or absence of CHX. Cells were pretreated with CHX (10 μg/ml) for 1 h, UV-inactivated NSV (MOI equivalent = 500) was added, and then the plates were kept at 4°C to allow virus binding for 2 h. Culture medium was then replaced with fresh medium adjusted to pH 6.0, and cells were shifted to 37°C. Viabilities were assessed 48 h later by trypan blue exclusion. Bars indicate standard deviations.
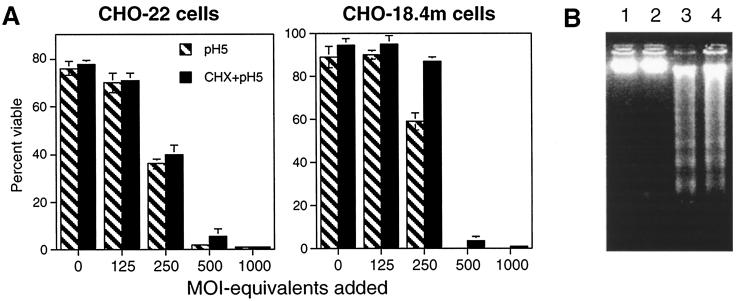
To determine the effects of altering the amount of bound UV-inactivated NSV and of inducing fusion at the plasma membrane more rapidly by exposure to a lower pH, gradient-purified UV-inactivated NSV at MOI equivalents ranging from 0 to 1,000 was bound to CHO-22 and CHO-18.4m cells at 4°C for 2 h. The pH was then shifted to 5.0 for an hour, and the temperature was shifted to 37°C (Fig. 4A). Cell death induced by the acid-induced fusion of UV-inactivated NSV with the plasma membrane was dose dependent, was equally efficient in the two types of CHO cells, and was not influenced by pretreatment with CHX. An MOI equivalent of at least 500 was required for the death of all cells, but some cell death also occurred at an MOI equivalent of 250. Cell death was characterized as apoptosis by the presence of DNA laddering (Fig. 4B).
FIG. 4.
UV-inactivated NSV induces apoptosis in the absence of CHX in CHO-22 and CHO-18.4m cells exposed to pH 5.0. (A) UV-inactivated, gradient-purified NSV at various MOI equivalents was allowed to bind to cells for 2 h at 4°C. Culture medium was then replaced with medium of pH 5.0 and incubated at 37°C for 1 h. The acidic medium was then replaced with medium of neutral pH. Cell viabilities were assessed 48 h later by trypan blue exclusion. (B) DNA extracted from cells exposed only to pH 5.0 (lane 1, CHO-18.4m cells; lane 2, CHO-22 cells) or to UV-inactivated NSV (MOI equivalent = 500) and pH 5.0 (lane 3, CHO-18.4m cells; lane 4, CHO-22 cells) and analyzed on a 1.2% agarose gel stained with ethidium bromide.
UV-inactivated NSV also induces apoptosis of N18 cells.
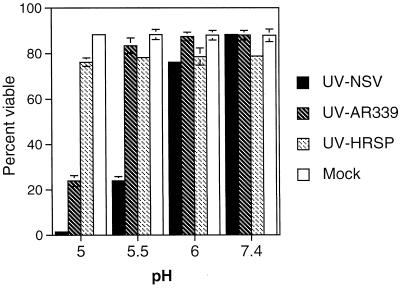
Strains of SV vary in their neurovirulence for mice, and these differences are often reflected in the efficiency with which these strains bind to and replicate in N18 cells (10, 46, 47). To determine whether UV-inactivated SV could induce apoptosis in N18 cells and whether this varied with virus virulence, UV-inactivated NSV, AR339, and HRSP (high, medium, and low virulence, respectively) were prebound to N18 cells at 4°C and then exposed to various pHs at 37°C (Fig. 5). Both UV-inactivated NSV and UV-inactivated AR339 induced cell death at pH 5.0; however, at pH 5.5, only UV-inactivated NSV induced cell death. UV-inactivated HRSP did not induce cell death at any pH. The lack of virus replication was confirmed in cells treated with UV-inactivated NSV by monitoring the supernatant fluids for infectious virus and monitoring cells for synthesis of nsPs (Fig. 6).
FIG. 5.
Viabilities of N18 cells bound with different strains of UV-inactivated SV and exposed to acidic pH. N18 cells were left untreated (Mock) or were treated with UV-inactivated NSV (UV-NSV), AR339 (UV-AR339), or HRSP (UV-HRSP) for 2 h at 4°C at an MOI equivalent of 500. Virus inocula were then replaced with media of pH 5.0, 5.5, 6.0, and 7.4 and incubated at 37°C for 1 h. Subsequent incubation was in medium of neutral pH. Cell viabilities were assessed at 48 h by trypan blue exclusion. Bars indicate standard deviations.
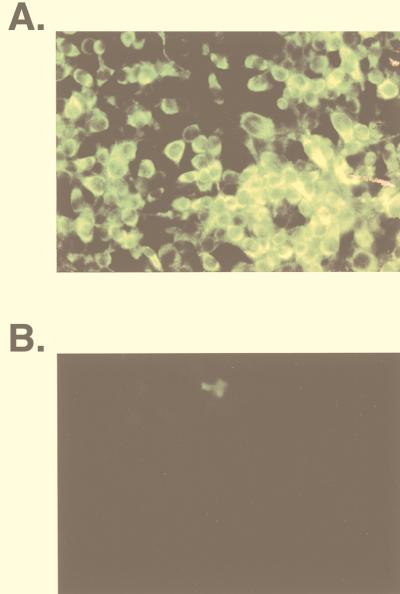
FIG. 6.
Lack of virus replication in N18 cells exposed to UV-inactivated NSV and pH 5. N18 cells exposed to live NSV (MOI = 50) (A) or UV-inactivated NSV (MOI equivalent = 500) and pH 5 (B) were stained 24 h later to detect the synthesis of nsP1. N18 cells infected with live virus, but not those infected with UV-inactivated virus, synthesized nsP1.
Inhibition of endosomal acidification blocks NSV-induced apoptosis.
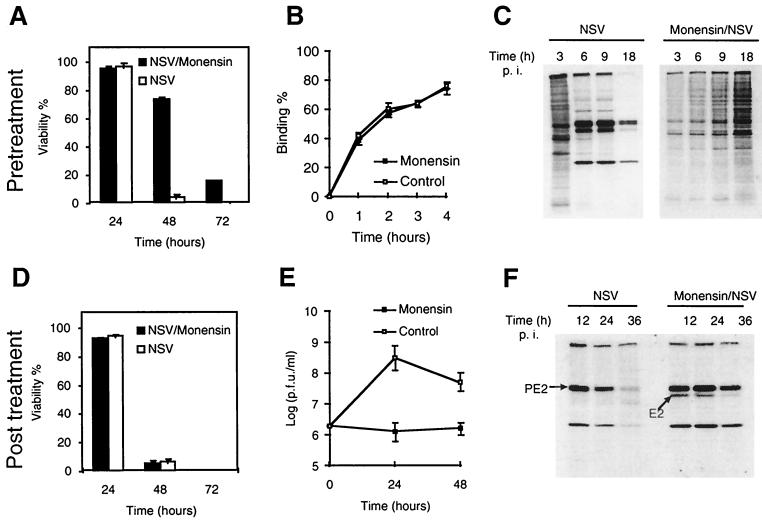
Monensin is an ionophore that blocks endosomal acidification and the transport of glycoproteins through the Golgi complex. Pretreatment of N18 cells with monensin protected them against NSV-induced cell death (Fig. 7A). Monensin did not inhibit NSV binding (Fig. 7B), but it did inhibit virus entry, as evidenced by the absence of viral protein synthesis (Fig. 7C). Treatment of N18 cells with monensin 2 h after infection did not protect them against cell death (Fig. 7D), but it did inhibit virus production (Fig. 7E) due to the inhibition of PE2-E1 transport through the Golgi complex, as evidenced by the failure of PE2 to be processed into E2 (Fig. 7F).
FIG. 7.
Treatment with monensin before, but not after, infection protects N18 cells from NSV-induced cell death. (A) Effect of monensin pretreatment on NSV-induced cell death. N18 cells were pretreated with monensin for 1 h and then infected with NSV (MOI = 10). (B) Effect of monensin on virus binding. N18 cells were exposed to 35S-labeled NSV at 4°C in the presence or absence of monensin (10 μM). (C) Effect of monensin pretreatment on viral protein synthesis. Lysates from 35S-labeled cells were assessed by SDS-polyacrylamide gel electrophoresis and autoradiography. (D) Effect of monensin posttreatment on NSV-induced cell death. N18 cells were infected with NSV (MOI = 10), and monensin (10 μM) was added 2 h later. Cell viabilities at different time points were assessed by trypan blue exclusion. (E) Plaque assays of supernatant fluids described for panel D. (F) Lysate from NSV-infected cells with or without monensin posttreatment analyzed by Western blot analysis with rabbit anti-SV antiserum and showing inhibition of PE2 processing into E2. p.i., postinfection.
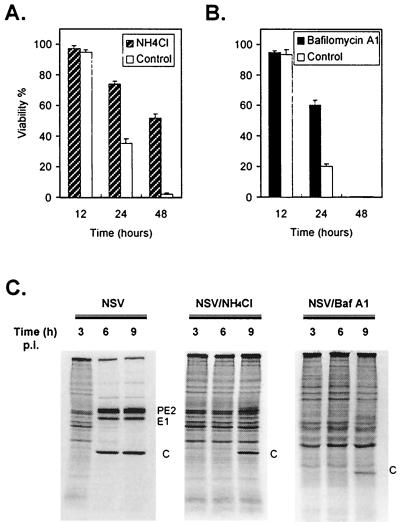
The base NH4Cl and the vacuolar H+-ATPase inhibitor bafilomycin A1 (3) also prevent endosomal acidification. Therefore, these drugs also inhibit the acid-induced conformational change of E1-E2 and the subsequent fusion of the virus envelope and cell membrane. Pretreatment of N18 cells with either NH4Cl or bafilomycin A1 protected them against infection and NSV-induced death (Fig. 8).
FIG. 8.
Pretreatment with NH4Cl or bafilomycin A1 protects N18 cells against NSV-induced cell death. N18 cells were pretreated with NH4Cl (20 mM) (A) or bafilomycin A1 (10 μM) (B) for 1 h and then infected with NSV (MOI = 10). Cell viabilities were assessed at the indicated times by trypan blue exclusion. (C) Viral protein synthesis in NH4Cl- or bafilomycin A1 (Baf A1)-treated N18 cells. At the indicated times, cells were labeled with methionine- and cysteine-free medium to which was added [35S]methionine-cysteine (10 μCi/ml) for 1 h. Cell lysates were run on an SDS–15% polyacrylamide gel and analyzed by autoradiography. p.i., postinfection.
DISCUSSION
Virus-induced apoptotic cell death is an important determinant of the outcome of infection. However, the viral and cellular proteins that participate in the induction and execution of the apoptotic program are just beginning to be identified, and for many virus-cell interactions, multiple factors may contribute to determining the outcome. For many types of viruses, replication in the host cell is necessary for induction of apoptosis, while for others, replication is not necessary. In these studies of the alphavirus SV, we showed that characteristics of the virus strain and type of cell studied were important determinants of how easily apoptosis was induced in the absence of virus replication. The induction of programmed cell death was most efficient when a virulent strain of virus was able to interact optimally with a cellular receptor other than heparan sulfate. The data support the hypothesis that apoptosis can be initiated during acid-induced fusion of the viral glycoproteins with the cell membrane.
Induction of cell death in the absence of viral replication has been reported for primary cells or cell lines exposed to type 3 reovirus (48), avian leukosis virus (4), bovine herpesvirus 1 (17), and vaccinia virus (40). The mechanism(s) by which any of these viruses induces apoptosis has not been definitively determined. For reovirus and vaccinia virus, it is known to be related to the sigma-1 attachment protein (48) and to a postattachment entry step (40), respectively. The Env protein of avian leukosis virus interacts with a member of the tumor necrosis factor (TNF) receptor family (4). For SV to enter a cell, a continuous and uninterrupted interaction with cell membrane molecules, facilitated by a series of conformational changes in the viral envelope proteins and cell membrane molecules, is essential. SV binds cells through its surface glycoproteins. The E2 and E1 proteins of SV are primarily responsible for binding and fusion, respectively. Conformational changes of viral envelope proteins occur with binding to the cell surface and may be required for subsequent internalization (12). With CHO-18.4m cells, cell death due to apoptosis was easily induced by UV-inactivated NSV, indicating that virus replication was not essential. We hypothesize that CHO-18.4m cells were more susceptible because they lack the heparan sulfate used for initial attachment, allowing more direct access to a cell surface molecule specifically involved in virus entry and induction of apoptosis.
Interactions between incoming virus and cell membrane molecules also occur in the endosome. After virus is internalized into the endosome, the pH in the endosome decreases through the action of vacuolar H+-ATPase. The acidic environment of the endosome induces further conformational changes (50). Cholesterol in the endosomal membrane is involved in low-pH-dependent binding of alphaviruses to the lipid membrane, and sphingolipids are essential for low-pH-induced fusion (5, 37). Therefore, interactions between SV and the cell membrane are changed as the entry process progresses from binding to fusion. To determine whether SV could trigger cell death under the acidic conditions of the endosome, we treated cells bound with UV-inactivated virus with media of low pH. The results imply that the acid-induced interaction between virus and cell membrane which triggers fusion and delivery of the nucleocapsid into the cytoplasm is capable of inducing cell death, and they are consistent with the observations that the transmembrane portion of the E1 glycoprotein is likely to be essential for fusion (9) and that the transmembrane portion of either the E1 or E2 glycoprotein can induce apoptosis (23).
Monensin blocks both virus entry and the transport of the PE2-E1 proteins of Semliki Forest virus (34, 39), an alphavirus related to SV, through the Golgi complex to the trans-Golgi network. Therefore, it is a useful chemical to determine whether SV triggers cell death at the time between fusion and viral protein transport or at the time of virus assembly and budding. Pretreatment with monensin to block virus fusion is protective; however, treatment with monensin later to block viral protein transport is not. Therefore, the death signal is triggered sometime between initiation of fusion and PE2-E1 protein transport. Protection by pretreatment with NH4Cl or bafilomycin A1, which also block decreases in endosomal pH, further supports the conclusion that fusion or activities accompanying or following fusion are important for the induction of apoptosis.
The role of CHX in SV-induced apoptosis is unclear and potentially confusing. CHX was originally included to determine whether viral protein synthesis was required for initiating apoptosis, but surprisingly, CHX itself appeared to be required, as has been shown in a number of experimental paradigms for inducing apoptosis (35). CHX is often required for apoptosis induced by TNF (32). In TNF-sensitive cells, neither TNF nor CHX alone induces cell death, but when they are combined, apoptosis occurs. CHX treatment may decrease the amount of some short-lived protein that otherwise protects the cell from apoptosis. In other paradigms of apoptosis, CHX protects against apoptosis by stimulating cytoprotective pathways (35). SV infection inhibits host protein synthesis within a few hours after infection, so CHX would mimic this aspect of infection. In cells with bound UV-inactivated NSV, CHX-treated cells had consistently higher, rather than lower, viabilities than those of untreated cells when they were exposed to pH 5.0, suggesting that the cells were protected. It is likely that these apparently contradictory observations are linked to the strength of the apoptotic signal being delivered in different experimental situations. If virus fusion occurs over a relatively extended period of time (through the endosomal pathway) or if fusion is inefficient (higher pH), then decreasing the availability of short-lived antiapoptotic cellular factors will enhance the apoptotic process. However, if the apoptotic signal is delivered in a coordinated fashion, then such antiapoptotic cellular factors will be insufficient to prevent initiation of the apoptotic process. This balance between cellular and viral factors also likely explains why relatively large amounts of inactivated virus, compared to those of live virus, are required to induce apoptosis. UV irradiation has a deleterious effect on viral proteins, as well as viral RNA, resulting in less efficient binding and probably less efficient fusion than that which occurs with virus that has not been UV inactivated.
Although these experiments demonstrate that SV can induce apoptosis at the time of entry without a requirement for virus replication, it is likely that other steps in the virus replication cycle can also lead to cell death and can possibly induce apoptosis. Studies with SV replicons have shown that expressing only the nsPs can lead to the shutoff of cellular protein synthesis and eventually to cell death (1, 13). Furthermore, mutations in the C-terminal portion of nsP2 that decrease RNA synthesis also decrease cytopathology and increase the likelihood of establishing persistent infection by an as-yet-unknown mechanism (11). How fusion and entry induce apoptosis is also unknown and may be related to previously described signalling roles for Ras, NF-κB, or superoxide in SV-induced apoptosis (22, 29–31).
ACKNOWLEDGMENTS
We thank Charles Rice for the gift of rabbit antibody to nsP1.
This work was supported by research grant NS18596 from the National Institutes of Health (D.E.G.) and a predoctoral scholarship from the National Defense Medical Center, Taiwan (J.-T.J.).
REFERENCES
- 1.Agapov E V, Frolov I, Lindenbach B D, Pragai B M, Schlesinger S, Rice C M. Noncytopathic Sindbis virus RNA vectors for heterologous gene expression. Proc Natl Acad Sci USA. 1998;95:12989–12994. doi: 10.1073/pnas.95.22.12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amano T, Richelson E, Nirenberg M. Neurotransmitter synthesis by neuroblastoma clones. Proc Natl Acad Sci USA. 1972;69:258–263. doi: 10.1073/pnas.69.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowman E M, Siebers A, Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci USA. 1988;85:7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brojatsch J, Naughton J, Rolls M M, Zingler K, Young J A T. CAR1, a TNFR-related protein, is a cellular receptor for cytopathic avian leukosis-sarcoma viruses and mediates apoptosis. Cell. 1996;87:845–855. doi: 10.1016/s0092-8674(00)81992-3. [DOI] [PubMed] [Google Scholar]
- 5.Bron R, Wahlberg J M, Garoff H, Wilschut J. Membrane fusion of Semliki Forest virus in a model system: correlation between fusion kinetics and structural changes in the envelope glycoprotein. EMBO J. 1993;12:693–701. doi: 10.1002/j.1460-2075.1993.tb05703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burge B, Pfefferkorn E. Isolation and characterization of conditional lethal mutants of Sindbis virus. Virology. 1966;30:204–213. doi: 10.1016/0042-6822(66)90096-1. [DOI] [PubMed] [Google Scholar]
- 7.Caillet-Fauquet P, Perros M, Brandenburger A, Spegelaere P, Rommelaere J. Programmed killing of human cells by means of an inducible clone of parvoviral genes encoding non-structural proteins. EMBO J. 1990;9:2989–2995. doi: 10.1002/j.1460-2075.1990.tb07491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng E H, Levine B, Boise L H, Thompson C B, Hardwick J M. Bax-independent inhibition of apoptosis by Bcl-xL. Nature. 1996;379:554–556. doi: 10.1038/379554a0. [DOI] [PubMed] [Google Scholar]
- 9.Cleverley D Z, Lenard J. The transmembrane domain in viral fusion: essential role for a conserved glycine residue in vesicular stomatitis virus G protein. Proc Natl Acad Sci USA. 1998;95:3425–3430. doi: 10.1073/pnas.95.7.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dropulic L K, Hardwick J M, Griffin D E. A single amino acid change in the E2 glycoprotein of Sindbis virus confers neurovirulence by altering an early step of virus replication. J Virol. 1997;71:6100–6105. doi: 10.1128/jvi.71.8.6100-6105.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dryga S A, Dryga O A, Schlesinger S. Identification of mutations in a Sindbis virus variant able to establish persistent infection in BHK cells: the importance of a mutation in the nsP2 gene. Virology. 1997;228:74–83. doi: 10.1006/viro.1996.8364. [DOI] [PubMed] [Google Scholar]
- 12.Flynn D C, Meyer W J, Mackenzie J M, Jr, Johnston R E. A conformational change in Sindbis virus glycoproteins E1 and E2 is detected at the plasma membrane as a consequence of early virus-cell interaction. J Virol. 1990;64:3643–3653. doi: 10.1128/jvi.64.8.3643-3653.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frolov I, Agapov E, Hoffman T A, Jr, Prágai B M, Lippa M, Schlesinger S, Rice C M. Selection of RNA replicons capable of persistent noncytopathic replication in mammalian cells. J Virol. 1999;73:3854–3865. doi: 10.1128/jvi.73.5.3854-3865.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frolov I, Schlesinger S. Comparison of the effects of Sindbis virus and Sindbis virus replicons on host cell protein synthesis and cytopathogenicity in BHK cells. J Virol. 1994;68:1721–1727. doi: 10.1128/jvi.68.3.1721-1727.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffin D E, Hardwick J M. Regulators of apoptosis on the road to persistent alphavirus infection. Annu Rev Microbiol. 1997;51:565–592. doi: 10.1146/annurev.micro.51.1.565. [DOI] [PubMed] [Google Scholar]
- 16.Griffin D E, Johnson R T. Role of the immune response in recovery from Sindbis virus encephalitis in mice. J Immunol. 1977;118:1070–1075. [PubMed] [Google Scholar]
- 17.Hanon E, Meyer G, Vanderplasschen A, Dessy-Doizé C, Thiry E, Pastoret P-P. Attachment but not penetration of bovine herpesvirus 1 is necessary to induce apoptosis in target cells. J Virol. 1998;72:7638–7641. doi: 10.1128/jvi.72.9.7638-7641.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hubbard S C, Walls L, Ruley H E, Muchmore E A. Generation of Chinese hamster ovary cell glycosylation mutants by retroviral insertional mutagenesis. J Biol Chem. 1994;269:3717–3724. [PubMed] [Google Scholar]
- 19.Jackson A C, Moench T R, Griffin D E. The pathogenesis of spinal cord involvement in the encephalomyelitis of mice caused by neuroadapted Sindbis virus infection. Lab Investig. 1987;56:418–423. [PubMed] [Google Scholar]
- 20.Jackson A C, Moench T R, Trapp B D, Griffin D E. Basis of neurovirulence in Sindbis virus encephalomyelitis of mice. Lab Investig. 1988;58:503–509. [PubMed] [Google Scholar]
- 21.Jan J-T, Byrnes A P, Griffin D E. Characterization of a Chinese hamster ovary cell line developed by retroviral insertional mutagenesis that is resistant to Sindbis virus infection. J Virol. 1999;73:4919–4924. doi: 10.1128/jvi.73.6.4919-4924.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joe A K, Ferrari G, Jiang H H, Liang X H, Levine B. Dominant inhibitory Ras delays Sindbis virus-induced apoptosis in neuronal cells. J Virol. 1996;70:7744–7751. doi: 10.1128/jvi.70.11.7744-7751.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joe A K, Foo H H, Kleeman L, Levine B. The transmembrane domains of Sindbis virus envelope glycoproteins induce cell death. J Virol. 1998;72:3935–3943. doi: 10.1128/jvi.72.5.3935-3943.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson R T, McFarland H F, Levy S E. Age-dependent resistance to viral encephalitis: studies of infections due to Sindbis virus in mice. J Infect Dis. 1972;125:257–262. doi: 10.1093/infdis/125.3.257. [DOI] [PubMed] [Google Scholar]
- 25.Legrand C, Rommelaere J, Caillet-Fauquet P. MVM(p) NS-2 protein expression is required with NS-1 for maximal cytotoxicity in human transformed cells. Virology. 1993;195:149–155. doi: 10.1006/viro.1993.1355. [DOI] [PubMed] [Google Scholar]
- 26.Levine B, Goldman J E, Jiang H H, Griffin D E, Hardwick J M. Bcl-2 protects mice against fatal alphavirus encephalitis. Proc Natl Acad Sci USA. 1996;93:4810–4815. doi: 10.1073/pnas.93.10.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levine B, Huang Q, Isaacs J T, Reed J C, Griffin D E, Hardwick J M. Conversion of lytic to persistent alphavirus infection by the bcl-2 cellular oncogene. Nature. 1993;361:739–742. doi: 10.1038/361739a0. [DOI] [PubMed] [Google Scholar]
- 28.Lewis J, Wesselingh S L, Griffin D E, Hardwick J M. Alphavirus-induced apoptosis in mouse brains correlates with neurovirulence. J Virol. 1996;70:1828–1835. doi: 10.1128/jvi.70.3.1828-1835.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin K-I, DiDonato J A, Hoffman A, Hardwick J M, Ratan R R. Suppression of steady-state, but not stimulus-induced NF-κB activity inhibits alphavirus-induced apoptosis. J Cell Biol. 1998;141:1479–1487. doi: 10.1083/jcb.141.7.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin K-I, Pasinelli P, Brown R H, Hardwick J M, Ratan R R. Decreased intracellular superoxide levels activate Sindbis virus-induced apoptosis. J Biol Chem. 1999;274:13650–13655. doi: 10.1074/jbc.274.19.13650. [DOI] [PubMed] [Google Scholar]
- 31.Lin K-I, Lee S H, Narayanan R, Baraban J M, Hardwick J M, Ratan R R. Thiol agents and Bcl-2 identify an alphavirus-induced apoptotic pathway that requires activation of the transcription factor NF-kappa B. J Cell Biol. 1995;131:1–14. doi: 10.1083/jcb.131.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Z G, Hsu H, Goeddel D V, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-κB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 33.Lowe S W, Ruley H E. Stabilization of the p53 tumor suppressor is induced by adenovirus 5 E1A and accompanies apoptosis. Genes Dev. 1993;7:535–545. doi: 10.1101/gad.7.4.535. [DOI] [PubMed] [Google Scholar]
- 34.Marsh M, Wellsteed J, Kern H, Harms E, Helenius A. Monensin inhibits Semliki Forest virus penetration into culture cells. Proc Natl Acad Sci USA. 1982;79:5297–5301. doi: 10.1073/pnas.79.17.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mattson M P, Furukawa K. Anti-apoptotic actions of cycloheximide: blockade of programmed cell death or induction of programmed cell life? Apoptosis. 1997;2:257–264. doi: 10.1023/a:1026433019210. [DOI] [PubMed] [Google Scholar]
- 36.Nava V E, Rosen A, Veliuona M A, Clem R J, Levine B, Hardwick J M. Sindbis virus induces apoptosis through a caspase-dependent, CrmA-sensitive pathway. J Virol. 1998;72:452–459. doi: 10.1128/jvi.72.1.452-459.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nieva J-L, Bron R, Corver J, Wilschut J. Membrane fusion of Semliki Forest virus requires sphingolipids in the target membrane. EMBO J. 1994;13:2797–2804. doi: 10.1002/j.1460-2075.1994.tb06573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noteborn M H M, Todd D, Verschueren C A J, de Gauw H W F M, Curran W L, Veldkamp S, Douglas A J, McNulty M S, van der Eb A J, Koch G. A single chicken anemia virus protein induces apoptosis. J Virol. 1994;68:346–351. doi: 10.1128/jvi.68.1.346-351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pesonen M, Kaariainen L. Incomplete complex oligosaccharides in Semliki Forest virus envelope proteins arrested within the cell in the presence of monensin. J Mol Biol. 1982;158:213–230. doi: 10.1016/0022-2836(82)90430-2. [DOI] [PubMed] [Google Scholar]
- 40.Ramsey-Ewing A, Moss B. Apoptosis induced by a postbinding step of vaccinia virus entry into Chinese hamster ovary cells. Virology. 1998;242:138–149. doi: 10.1006/viro.1997.8985. [DOI] [PubMed] [Google Scholar]
- 41.Rao L, Debbas M, Sabbatini P, Hockenberry D, Korsmeyer S, White E. The adenovirus E1A proteins induce apoptosis, which is inhibited by the E1B 19-kDa and Bcl-2 proteins. Proc Natl Acad Sci USA. 1992;89:7742–7746. doi: 10.1073/pnas.89.16.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmaljohn A L. Protective monoclonal antibodies define maturational and pH-dependent antigenic changes in Sindbis virus E1 glycoprotein. Virology. 1983;130:144–154. doi: 10.1016/0042-6822(83)90124-1. [DOI] [PubMed] [Google Scholar]
- 43.Stewart S A, Poon B, Jowett J B M, Chen I S Y. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J Virol. 1997;71:5579–5592. doi: 10.1128/jvi.71.7.5579-5592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strauss J H, Strauss E G. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor R M, Hurlbut H S, Work T H, Kingsbury J R, Frothingham T E. Sindbis virus: a newly recognized arthropod-transmitted virus. Am J Trop Med Hyg. 1955;4:844–846. doi: 10.4269/ajtmh.1955.4.844. [DOI] [PubMed] [Google Scholar]
- 46.Tucker P C, Griffin D E. Mechanism of altered Sindbis virus neurovirulence associated with a single-amino-acid change in the E2 glycoprotein. J Virol. 1991;65:1551–1557. doi: 10.1128/jvi.65.3.1551-1557.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tucker P C, Lee S H, Bui N, Martinie D, Griffin D E. Amino acid changes in the Sindbis virus E2 glycoprotein that increase neurovirulence improve entry into neuroblastoma cells. J Virol. 1997;71:6106–6112. doi: 10.1128/jvi.71.8.6106-6112.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tyler K L, Squier M K T, Rodgers S E, Schneider B E, Oberhaus S M, Grdina T A, Cohen J J, Dermody T S. Differences in the capacity of reovirus strains to induce apoptosis are determined by the viral attachment protein ς1. J Virol. 1995;69:6972–6979. doi: 10.1128/jvi.69.11.6972-6979.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ubol S, Tucker P C, Griffin D E, Hardwick J M. Neurovirulent strains of alphavirus induce apoptosis in bcl-2-expressing cells; role of a single amino acid change in the E2 glycoprotein. Proc Natl Acad Sci USA. 1994;91:5202–5206. doi: 10.1073/pnas.91.11.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wahlberg J M, Garoff H. Membrane fusion process of Semliki Forest virus. I. Low pH-induced rearrangement in spike protein quaternary structure precedes virus penetration into cells. J Cell Biol. 1992;116:339–348. doi: 10.1083/jcb.116.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]