Abstract
Captan dislodgeable foliar residues (DFRs) were determined by following the applications of this fungicide in an apple orchard. The study comprised an investigation of the variability of captan DFR values and 14 days of DFR monitoring to assess kinetic modeling. A method combining solid-phase microextraction (SPME) gas chromatography and high-resolution mass spectrometry (GC-QTOF-MS) was developed for the quantification of captan residues from DFR aqueous extracts. The results evidenced that (1) sampling parameters such as the position of the tree in a row and the height of foliar significantly influenced captan DFR levels (247–1450 ng·cm–2), highlighting the need to implement a comprehensive sampling strategy; (2) the DFR captan dissipation kinetic model best matched with a biphasic one, with half-lives of DFRcaptan of 3.4 and 12.8 days, respectively, for the initial rapid phase 1 decline (day 0–5) and the slower phase 2 decline phase (day 6–14). Furthermore, through DFR measurements, the potential dermal exposure (PDE) of workers was assessed using transfer coefficients (TCs) from the literature. Compared to the acceptable operator exposure levels (AOELs), the results showed that the re-entry interval for captan may not sufficiently protect workers whose arms, hands, and legs are not covered.
Keywords: dislodgeable foliar residues, kinetic, re-entry, worker exposure, apple growing, captan
Short abstract
Based on field foliar captan concentrations measured, this work describes the captan kinetic dissipation curve and risk assessment via dermal exposure evaluation of apple growers during re-entry tasks and harvesting.
Introduction
Plant protection products have been extensively used for many decades in agriculture against pests, diseases, weeds, or other pathogenic plants to prevent yield losses and to guarantee high-quality products. However, in recent decades, many authors have suggested adverse health effects associated with long-term pesticide exposure.1−4 Serious concerns have been raised about the health risks resulting from occupational exposure when mixing and applying pesticides or working in treated fields,5 but knowledge of the occupational exposure levels and determinants is still limited. Previous studies underlined the need to account for exposure during re-entry tasks and during harvesting to assess pesticide exposure.6−9 Bureau et al. demonstrated that workers performing re-entry tasks had higher contamination than operators.10 Activities such as pruning or thinning are performed by workers who enter an agricultural plot that has been treated with pesticides.11 Dermal exposure is one of the most effective primary routes through which agricultural workers are exposed to pesticides.3,12 Dermal exposure is the outcome of direct contact with pesticide residues on, for example, leaf surfaces. The dermal route may represent more than 90% of the total daily exposure of workers performing re-entry tasks.8,13
The dislodgeable foliar residue (DFR) is the amount of pesticides that can be transferred from the two-sided leaf surface of the plant during a well-defined procedure.14 Many studies have demonstrated the link between pesticide residues on foliage and the dermal exposure of workers re-entering treated crops.7,15−18 DFR levels are mainly used to assess re-entry exposure. Dermal exposure due to contact with pesticide residues on foliage is estimated as the product of the DFR (μg·cm–2), the transfer coefficient (TC, expressed in cm2·h–1), and the duration of the task (h).11 The TC of the plant surface to the clothes or skin of the workers depends on the intensity of their contact with the foliage (nature of the re-entry activity, type of crop).11 Thus, the DFR and the way in which DFRs dissipate over time are key factors in estimating potential worker exposure and pesticide risk assessment. Both the form and nature of the dissipation curve are affected by different factors, including the chemical characteristics of the pesticide, the characteristics of the plant foliage, and the meteorological conditions (sunlight, rain, wind, temperature).19,20 As discussed by Fantke and Juraske,20 modeling the dissipation of pesticides in foliage and in the plant involves high uncertainty and relies to a great extent on experimental data. Furthermore, the processes that influence the kinetics of pesticide dissipation in foliage are still not fully understood. Fantke and Juraske20 presented several models (e.g., zero-order, first-order, second-order, biphasic kinetic) to fit residual pesticide concentration curves that demonstrate the variability of pesticide dissipation within plants or on the foliage. The foliar dissipation of many pesticides is mostly described by first-order kinetics.8,20,21 Nonetheless, DFR dissipation patterns are often more complex and need to be better understood and described in detail for the appropriate pesticide risk assessment. For example, Whitmyre et al.19 described biphasic dissipation kinetics for DFRs when estimating post-application occupational exposure to endosulfan. Previous studies also proposed biphasic dissipation kinetics for foliar pesticide residues.22,23
In 2019, apple production was 2 billion tons in France (on approximately 50 000 hectares (ha) of apple orchards).24 Pesticides are extensively used in fruit production to control several diseases, in particular apple scab and powdery mildew, to guarantee a good harvest and to obtain healthy fruits that both store well and are ≪flawless≫ for sale. Several insects, including codling moths and aphids, are also a concern in orchards. Control of fungal diseases in apples requires frequent applications of fungicides (10–15 applications per year in France). Captan [N-(trichloromethylthio)cyclohex-4-ene-1,2-dicarboximide, C9H8Cl3NO2S] is one of the most widely used fungicides to control apple scab. In France, captan is usually sprayed in apple orchards from May to August, when many re-entry tasks are carried out. Previous studies have been conducted on captan residues and also their dissipation behavior in different crops.25−29
In the present work, to improve the assessment of secondary exposure to captan residues by fruit-growing workers, dislodgeable captan foliar residues (captan DFR) were studied in an apple orchard on a farm in southwest France during the summer of 2018. For this purpose, DFR samples were collected from day 0 to day 48, after captan spraying, including three applications of captan during the sampling period, to (1) validate the sampling protocol, (2) monitor the DFR used for kinetic data, and (3) test the kinetic model. A method combining solid-phase microextraction (SPME) gas chromatography and high-resolution mass spectrometry (GC-QTOF-MS) was developed for the identification and quantification of captan residues from DFR aqueous extracts to investigate captan dissipation over time. Potential dermal exposure (PDE) values by workers during fruit thinning and harvesting were estimated from the representative experimental DFR values. This work provides further field data for improved estimation of occupational exposure and pesticide risk assessment. Finally, it is important to note that we also looked at the main degradation product of captan, tetrahydrophthalimide (THPI). Based on previous data from our research project (Bureau et al.),10 the exposure of workers in apple growing to THPI during re-entry and harvest was found to be negligible compared to captan exposure. THPI has also been analyzed in foliar samples in preliminary tests, and the results indicated that captan was predominant throughout all harvest seasons, even during harvesting (more than 40 days after spraying). Therefore, as two separate analytical methods were required to analyze THPI and captan, we decided to focus our study solely on captan residues.
Experimental Section
Study Site and Captan Applications
An apple farm located in the southwest of France (Occitanie Region) 2 h from the city of Bordeaux and 1 h from the city of Toulouse was selected for this study. The owners of the farm agreed to participate in the present study of captan dissipation on foliage in the summer of 2018. To ensure the privacy of the apple farmers, the exact location of the study fields cannot be shown. The farm selected for the study is located in the center-west of the Tarn-et-Garonne department, within a temperate climate zone. It comprises 6.3 ha of apple orchards, 4.5 ha of vineyards, and 4 ha of plum trees. A 5000 m2 plot of Pink Lady apples contains approximately 1350 trees (6 rows spaced 4 meters (m) apart, each row containing approximately 225 trees). Figure 1 shows pictures of the sampling plot. Half the terrain is flat, and the other half sloping. The trees were all approximately 3.7 m in height and spaced 1 m apart. The orchard was covered by anti-hail nets.
Figure 1.

Sampling plot. (a) Aerial view of the farm with the studied plot circled in red: zone with 6 rows of approximately 225 apple trees each. (b) Studied orchard. (c) Two rows spaced 4 m apart covered by an anti-hail net.
In 2018, only one captan formulation (MERPAN 80 WDG) was used on the farm. Due to spring meteorological conditions and the need to control fungal diseases of apples, seven applications of captan were sprayed at the sampling site from April to August 2018 (Figure 2). The application rate was always 1.9 kg·ha–1 (equivalent to 1.44 kg active ingredient a.i./ha) using a 15-year-old one-turbine-mounted sprayer with a 500 l tank (BALLESTE).
Figure 2.
Captan applications and sampling schedule in the apple orchard studied.
Sample Collection and Transport
Leaf Sampling
A leaf punch (Crealia) was systematically used to collect leaf punches 2.5 cm in diameter. The leaf sampling procedure was adapted from guidance for determination of dislodgeable foliar residue.14 The leaf disks were placed in a clean glass jar. Between each sample, the leaf punch was cleaned thoroughly with ethanol using paper towels to remove all plant admixtures. Particular care was paid to avoid touching the portion of the leaf to be punched out before the sample was collected or after it had been punched out of the leaf.
Study of Captan DFR Variability According to the Sampling Strategy
To study the variability of captan foliar residues in the apple orchard, a specific experimental design, presented in Table 1, was implemented on June 26, 2018 (Figure 2). Several parameters were studied: the sampling size (number of punches, number of replicates), the location of the tree in the row, and the height of the sampled leaves in the tree (top, middle, bottom).
Table 1. Experimental Design to Study the Variability of Captan Foliar Residues.
| height of sampling points | number of leaf punches | leaf surface area (cm2) | |
|---|---|---|---|
| sampling punches in a tree row | middle of the 1st treea | 10 | 98.2 |
| middle of the 50th treea | 10 | 98.2 | |
| middle of the 100th treea | 10 | 98.2 | |
| middle of the 150th treea | 10 | 98.2 | |
| middle of the 225th treea | 10 | 98.2 | |
| height of punched samples in 50 different trees | top | 50 | 490.9 |
| 50 | 490.9 | ||
| 50 | 490.9 | ||
| middle | 50 | 490.9 | |
| 50 | 490.9 | ||
| 50 | 490.9 | ||
| bottom | 50 | 490.9 | |
| 50 | 490.9 | ||
| 50 | 490.9 |
The row studied contained approximately 225 trees.
Sample Collection for the Captan Dissipation Kinetic Study
The DFR dissipation study started on July 5, 2018 (captan application no. 5, Figure 2) during the thinning period when the workers were largely in contact with the foliage. Each sample consisted of 50 leaf punches collected in triplicate from 50 different trees selected randomly among the 1350 trees (excluding the trees located at the end of the row). The leaves were collected in locations where contact with the workers’ body parts was the most probable, i.e., the outermost leaves at three different heights in the tree, since workers may work on an elevated platform, but avoiding the two extremes, i.e. disregarding leaves right at the top and right at the bottom of the tree. Only mature leaves were sampled in order to limit the loss of captan due to the dilution effect caused by new leaves in growth. Samples were collected on July 5, 2018, before and after application of captan (at, respectively, 11 am and 5 pm, captan being applied at 2 pm) and over a 2-week period included 8 sampling days (1, 4, 5, 6, 7, 8, 12, and 14 days after the spraying day, each at 11 am; Figure 2). No re-entry tasks (thinning, pruning, etc.) were performed by workers in the plot during the sampling period. With the exception of a light rain shower (0.6 mm) at the end of the captan application (on July 5, 2018) and an overnight irrigation event lasting 12 h on July 12, 2018, no rain fell during the sampling period. On each sampling day, the maximum temperature was recorded.
Collection of Other Samples
To obtain values of captan DFR outside the dissipation study period, samples were collected by using the same protocol as that described above. Figure 2 gives the collection dates, i.e., 48 days after the fourth captan application, 3 and 8 days after the sixth application, and 32 days after the seventh application. The values obtained were compared with those estimated from the kinetic equations of this work.
Sample Transport and Residue DFR Washing Technique
As recommended,14 the leaf samples were not frozen (freezing ruptures the leaf cell walls and may influence the measurement of DFR). The samples were stored at 4 °C during transport from the field to the laboratory, and they were treated within 4 h after collection. The sample leaves were washed in the same jar to avoid any loss of the pesticide. Organic solvents cannot be used to wash leaves, as they may cause the surface residue to penetrate the leaf tissues or extract previously penetrated residues.14 The samples were consequently washed twice with 100 mL of Milli-Q water and shaken on a platform shaker for 15 min at 100 rpm between each wash. The two washing extracts were then combined.
Sample Analysis and Validation
Sample Extraction
As previously described,30,31 preliminary experiments performed in the present study confirmed that captan and captan-d6 hydrolyzed at ambient temperature. Thus, to prevent underestimation of captan residues on foliage, captan-d6 (Techlab HPC, 98.9%) was added to each sample immediately after washing. Then, 200 μL of the washing extract, spiked with captan-d6 (25 μL of 5 μg·g–1, acetonitrile), were mixed with 9 mL of Milli-Q water in a 10 mL glass vial, sealed with an aluminum cap supplied with the PTFE-faced septa, and stored at −20 °C until SPME analysis. As hydrolysis rates were similar for both compounds, the loss of captan during the storage and the analysis could be corrected. No hydrolysis of captan and captan-d6 was observed at −20 °C during storage. Extraction was performed by immersing a DVB/CAR/PDMS fiber in the sample for 60 min at 50 °C and 250 rpm. The compounds were desorbed in the GC injector at 250 °C for 10 min.
NCI-GC-QTOF-MS Analysis
A 7200 accurate-mass GC-QTOF MS instrument (Agilent Technologies, Santa Clara, CA) operating in negative chemical ionization (NCI) mode was used to quantify captan residues in leaf washing water. GC separation was performed using an RTX-1614 (5% diphenyl, 95% dimethyl polysiloxane) capillary column (Restek, Beffefonte) with a 15 m × 0.25 mm inner diameter and a film thickness of 0.10 μm. The injector was operated at 250 °C, and helium (purity > 99.999%) was used as the carrier at a constant pressure of 6.5 psi. The GC oven temperature program was as follows: 50 °C (held for 2 min), then 5 °C·min–1 to 130 °C (held for 1 min), and 10 °C·min–1 to 300 °C (held for 1 min), resulting in a total run time of 37 min. The temperature of the transfer line was 300 °C, while the ion source was kept at 250 °C. TOF for MS was operated at 5 spectra·s–1, acquiring the mass range m/z of 30–300. Methane was used as the chemical ionization gas. Emission and electron energy were, respectively, fixed at 150 μA and 200 eV. The MS2 conditions were optimized for captan (Techlab HPC, 99.3%) and its internal standard with a quadrupole for isolation at a medium MS resolution, and the collision energy was selected as 25 eV. MassHunter qualitative analysis B.07 was used for data treatment.
Quality Control
For each analysis, a calibration solution was prepared with captan and captan-d6 solutions. The solution was injected before and after each sequence of analysis to calculate the response factor between captan and captan-d6. The response factor between captan and captan-d6 was 0.86 ± 0.03. The injections were carried out only if the conditions required for the analysis were fulfilled (good recovery yields and response factors consistent with control monitoring); the calibration solution was also used to check and monitor the condition of the chromatographic and detection systems. Field blanks and laboratory blanks were also extracted and analyzed using the same procedure to identify possible contamination during sampling, transport, storage, and the analytical protocol. No contamination was found in this work. The analytical method was carefully validated prior to the analysis of field samples. The analytical limit of quantification (LOQ) (S/N = 10) was 1.0 ng·cm–2 of leaf surface. Overall recoveries for the validation samples were 95 ± 4% for captan.
Study of Dissipation Kinetics
The dissipation kinetics of dislodgeable captan residues in foliage was determined by plotting the concentration of the captan residue as a function of time elapsed since spraying. An exponential relationship was found for the dislodgeable captan foliar residue dissipation in apple orchards, corresponding to a first-order kinetic equation (eq 1)
| 1 |
where DFRt represents the concentration of the captan residue (ng·cm–2) at time t (days), DFR0 is the initial concentration of the captan residue in foliage (ng·cm–2), and kdiss the first-order reaction constant (day–1).
From this equation, the dissipation half-life (t1/2) was calculated using eq 2
| 2 |
where t1/2 corresponds to the time at which DFRt equals half the initial value DFR0.
Evaluation of Dermal Exposure
Potential dermal exposure (PDE) from contact with pesticide residues on foliage was estimated as the product of DFR (μg·cm–2), the transfer coefficient (TC, expressed in cm2·h–1), and the duration (t, expressed in hour) of the task (eq 3)11
| 3 |
Three sets of TC values are usually provided for tasks performed in apple orchards:14 TC1 (22 500 cm2·h–1) is used for the evaluation of arms, hands, and legs that are not covered, TC2 (4500 cm2·h–1) assuming arms, body, and legs are covered (workwear but bare hands), and TC3 (2250 cm2·h–1) for to estimate covered body (workwear and gloves). Dermal absorption values for formulated captan (Merpan 80 WDG and Captan 80 WG) are 0.8% for the concentrate (800 g a.i./kg) and 12% for the spray dilution (1:1000).32 A value of 12% was thus selected to calculate actual dermal exposure (ADE) according to eq 4.
| 4 |
Results and Discussion
This article reports on a brief study of the variability of captan DFR (validation of sampling strategy) and the analysis of its dissipation after application on apple orchards. Another part of this work is dedicated to worker dermal exposure.
Study of Captan DFR Variability According to the Sampling Strategy
Figure 3 presents the captan residues on apple foliage according to the position of the tree in a row in the apple orchard with a sample size of n = 1 (98.2 cm2) (a) and according to the height of the sampled leaf in the tree, n = 3 (490.9 cm2) (b). Figure 3a shows that the leaves of trees situated at the end of the row (1st/225 tree and 225th/225 tree) were less contaminated than the other trees (respectively 247 and 384 ng·cm–2, compared to values ranging from 730 to 1450 ng·cm–2 in the middle of the row). This difference can be explained by the fact that the farmer stopped spraying pesticides just before turning (to move on to the following row). To avoid underestimating worker exposure, these end trees should consequently not be sampled. Based on data concerning a single sample (n = 1) comprising 10 leaf punches (98.2 cm2), it can be seen that the leaves in the middle part of the 100th and 150th trees (Figure 3a) had similar concentrations of captan (∼700 ng·cm–2) to those sampled at the same height with n = 3 comprising 50 leaf punches (490.9 cm2; Figure 3b), suggesting that height is an important parameter influencing the concentration of captan on foliage and that this sample size could be sufficient. However, the leaves on the 50th tree in the row were almost twice as contaminated as those on the 100th and 150th trees (1450–700 ng·cm–2). Several factors may cause uneven spraying in the apple orchards (wind speed, tractor speed, slope of the field, etc.). This suggests that the sample size, i.e., ten punches per sample in this experiment, is not large enough to smooth outliers. Figure 3b (n = 3, 490.9 cm2) clearly shows that the concentration of captan on the surface of the leaves was lower in the bottom part of the tree (around 200 ng·cm–2) than in the middle and top parts of the tree (∼700 ng·cm–2). This result suggests uneven spraying of captan onto the bottom part of the tree by the turbine and that no excess pesticides dripped on the bottom part of the tree. However, it should be noted that the results obtained for the top of the tree may be biased by the presence of the hail netting that may increase deposition on the upper leaves (caused by droplets falling from the net) instead of evaporation. These observations should thus be taken into account when analyzing orchard workers’ exposure to captan; in other words, workers may be mainly exposed to the bottom and middle parts of the tree (when standing on the ground) or to the middle and the top parts of the tree (when standing on a ladder or a platform), suggesting different occupational exposure conditions in these two cases. Workers who use a platform to reach the higher parts of the trees may thus risk higher occupational exposure. The repeatability of triplicate samples enabled the calculation of RSD < 30%, thereby confirming the need for triplicate sampling. Taken together, these results underline the importance of the method used to select leaves that are representative of worker exposure as well as of a sufficient sample size.
Figure 3.
Concentration of captan on the surface of leaves sampled from the selected apple orchard plot according to the position of a tree in a row (a) and according to the height of the sampled leaf in the tree (b).
Captan DFR Dissipation Kinetics
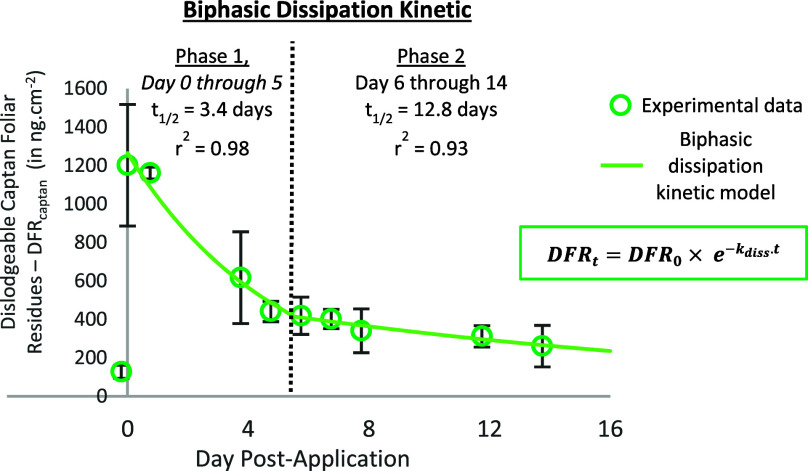
MERPAN 80 WDG (80% Captan) was applied at a dose of 1.9 kg·ha–1 on a Pink Lady apple orchard on July 5, 2018. Just after the application, the initial residue levels on foliage (DFR0) averaged 1201.6 ± 316.4 ng·cm–2. The DFR values, expressed in ng·cm–2, are listed in Table 2. The DFR values subsequently dropped from 1200 to 260 ng·cm–2, revealing a clear decrease in captan residues on apple foliage over the 2-week sampling period.
Table 2. Measured DFR of Captan in the Apple Orchard during the Kinetic Sampling Period.
| DFR
of captan (in ng·cm–2) |
|||||||
|---|---|---|---|---|---|---|---|
| sampling date | no. of days since previous treatment | maximum temperature (°C) | replicate 1 | replicate 2 | replicate 3 | mean values | RSD % |
| July 5, 2018 | captan application (1.9 kg·ha–1) at 2 pm | ||||||
| July 5, 2018 | 0 | 25.8 | 1294.5 | 849.2 | 1461.1 | 1,201.6 ± 316.4 | 26 |
| July 6, 2018 | 1 | 26.9 | 1191.4 | 1159.0 | 1130.9 | 1,160.5 ± 30.3 | 3 |
| July 9, 2018 | 4 | 31.3 | 405.6 | 569.9 | 875.3 | 616.9 ± 238.3 | 39 |
| July 10, 2018 | 5 | 30.8 | 436.6 | 496.2 | 390.3 | 441.0 ± 53.1 | 12 |
| July 11, 2018 | 6 | 30.1 | 525.8 | 393.7 | 336.8 | 418.8 ± 96.9 | 23 |
| July 12, 2018 | 7 | 29.6 | 456.5 | 356.7 | 393.0 | 402.1 ± 50.5 | 13 |
| July 13, 2018 | 8 | 30.5 | 299.4 | 252.9 | 468.8 | 340.4 ± 113.7 | 33 |
| July 17, 2018 | 12 | 28.5 | 269.1 | 292.1 | 375.1 | 312.1 ± 55.8 | 18 |
| July 19, 2018 | 14 | 32.9 | 136.1 | 330.7 | 315.3 | 260.7 ± 108.2 | 41 |
| July 23, 2018 | captan application (1.9 kg·ha–1) at 2 pm | ||||||
Figure 4 gives the DFR data plotted in a log–linear representation (i.e., in [DFR] vs time). The correlation coefficient r2 was 0.854 when the data were forced to fit a single log–linear regression over the entire 2-week sampling period. Several kinetic models can be used to fit foliar dissipation; for a review, see ref (20). In the present study, a clear break in the points constituting the plot between the two phases can be seen with an initial rapid decline (phase 1: from day 0 to day 5) followed by a slower decline (phase 2: from day 6 to day 14). This suggests that simple first-order kinetics may not be the most appropriate way to explain the dissipation of captan DFR in the samples. Following Whitmyre et al.19, biphasic dissipation kinetics was thus considered in our study. Hence, the kinetics of captan DFR was also evaluated by fitting the data set to a biphasic kinetic model (two successive linear first-order models). R2 values, i.e., 0.980 and 0.928 for phases 1 and 2, respectively, were obtained when the DFR data were fitted to two linear phases. Previous studies have also shown biphasic kinetics for dislodgeable foliar residues.19,22,23 For example, the biphasic dissipation behavior of endosulfan has been shown for melon, grape, and peach foliage19 and for tomato and pepper.23 Gunther et al.22 also demonstrated biphasic pesticide dissipation for parathion and azinphosmethyl on citrus leaves. Biphasic dissipation behaviors of pesticides have also been frequently observed in fruits (grape and pomegranate)33−35 and soil.36
Figure 4.
Dissipation kinetics of captan DFR.
The half-lives (t1/2) of captan were calculated for both kinetic models (Table 3). For the first-order kinetic model, t1/2 was 6.2 days, and for the biphasic kinetic model, t1/2 were 3.4 and 12.8 days for the initial rapid decline (day 0–day 5) and the slower decline phase (day 6–day 14), respectively. These differences underline the importance of describing the dissipation kinetics using an appropriate mathematical model to correctly predict workers’ exposure to pesticides. For instance, harvesting always takes place at least 30 days after the last treatment, so a simple first-order kinetic model may underestimate the occupational exposure of the harvesters.
Table 3. Regression Parameters Used to Fit the Captan DFR Data Using First-Order Dissipation Kinetic and Biphasic Dissipation Kinetic Models.
| case
description for regression of captan DFR data |
|||
|---|---|---|---|
| first-order kinetics | biphasic kinetic (phase 1, day 0–day 5) | biphasic kinetic (phase 2, day 6–day 14) | |
| k (day–1) | 0.1103 | 0.2031 | 0.0542 |
| t1/2 (day) | 6.2 | 3.4 | 12.8 |
| r2 | 0.85 | 0.98 | 0.93 |
It should be noted that longer half-lives of captan on leaf surfaces were found in a study conducted in 1991 and 1992 (t1/2 varied between 10 and 17 days).37 Many factors can influence pesticide dissipation kinetics including pesticide decomposition via chemical or microbial degradation mechanisms, the degree of uptake by the crop, or the meteorological conditions (sunlight, rain, wind, temperature).20,29 Increasing temperatures are known to accelerate several processes involved in pesticide dissipation (e.g., volatilization, pesticide solubility).20 In our experiments, meteorological conditions remained stable over the entire 2-week sampling period, from July 5 to 19, 2018, with high temperatures (maximum from 25.8 to 32.9 °C). Evaporation of the pesticide from the apple leaves, correlated with the vapor pressure of the chemical compound, may be a key factor explaining the biphasic dissipation kinetics of captan in the present study.20 Ntow et al.38 suggested that rapid volatilization of endosulfan in the first few days after the application compared to the subsequent decrease in volatilization may be responsible for the biphasic dissipation kinetics. Because temperatures are lower in spring, dissipation kinetics should be slower in spring, corresponding to a critical period when many of the cropping activities take place and pesticides are applied. Pesticide dissipation may also be due to wash off by rain, especially immediately after application. Xu et al.29 showed that around 50% of captan can be washed off by as little as 1 mm of rain following an application. Surprisingly, despite the overnight sprinkler of the orchards that lasted 12 h on July 12, 2018, no wash off of the captan on foliage was observed in our study. We thus suggest that the time that elapses between pesticide spraying and rainfall should be taken into account. The rapid phase 1 decline could represent more rapid loss processes on the leaf surface, thanks to the presence of more easily dislodgeable residues in poor contact with the leaf surface. Wash off caused by rainfall or dew during the slower decline phase may thus have less impact on captan residues that are not easily dislodgeable.
DFR values measured in independent samples collected outside the kinetic study period (indicated by the green square in Figure 2) were compared to the estimates of the same values taken from the kinetic study equations. The results are listed in Table 4. The estimated values were of the same order of magnitude as the measured values (factor between 0.33 and 0.74) but were systematically lower.
Table 4. Measured and Estimated DFR of Captan in the Apple Orchard during Sampling Conducted Outside the Kinetic Sampling Period.
| captan
DFR (ng·cm–2) |
||||
|---|---|---|---|---|
| sampling date | no. of days since the last treatment | maximum temperature (°C) | measured | estimated |
| May 18, 2018 | captan application (1.9 kg·ha–1) at 2 pm | |||
| July 5, 2018 | 48 | 25.8 | 127.4 ± 33.7 | 42.2b |
| July 23, 2018 | captan application (1.9 kg·ha–1) at 2 pm | |||
| July 26, 2018 | 3 | 36.2 | 1006.9 ± 37.6 | 709.1a |
| July 31, 2018 | 8 | 33.1 | 498.1 ± 155.2 | 368.6b |
| August 25, 2018 | captan application (1.9 kg·ha–1) at 2 pm | |||
| September 26, 2018 | 32 | 26.7 | 273.2 ± 17.1 | 100.4b |
Estimated from the phase 1 kinetic equation.
Estimated from the phase 2 kinetic equation.
Worker Exposure
Figure 5 presents the captan actual dermal exposure (ADE) values of workers during thinning and harvesting, depending on the TC presented in the Experimental Section. ADE was calculated using the experimental DFR (Table 4). More precisely, DFR determined 3 and 48 days after spraying in spring was used to calculate extreme ADE during thinning, and DFR measured 32 days after spraying in the fall was used to calculate ADE during harvesting. The AOEL (acceptable operator exposure level) was determined to be 0.1 mg·kg–1 bw (body weight)/day in 2009, revised to 0.25 mg·kg–1 bw in 2020.32 These values are presented in Figure 5 for a body weight of 65 kg.
Figure 5.
Captan ADE estimations for thinning and harvesting according to clothing conditions (for 8 h of work); TC1 (22 500 cm2·h–1) assuming arms, hands, and legs are not covered, TC2 (4500 cm2·h–1) assuming arms, body, and legs covered (workwear but bare hands), and TC3 (2250 cm2·h–1) for the covered body (workwear and gloves). The number of days indicates the delay after captan application before the estimation.
For thinning, worker ADE was estimated to range from 0.3 to 21.7 mg/day (8 h of work) depending on the number of days since the previous application of captan (i.e., 48 days and 3 days, respectively) and on the type of protective clothing (TC3 and TC1, respectively). Compared to the recently defined AOEL (16.25 mg/day), exposure was below the AOEL (1.7–16.9%) after a delay of 48 days, irrespective of the clothing. After a delay of 3 days after captan application, the AOEL was also respected when the worker’s arms, body, and legs were covered (13.4–26.8% of AOEL), but the value was exceeded when this was not the case (133.8% of AOEL with TC1). The re-entry interval (REI) is a regulatory value corresponding to the minimum amount of time that must pass between the time a pesticide is applied to an area or crop and the time that people are allowed to enter the plot. In France, the REI value for captan is 48 h. Thanks to the kinetic equation determined in this work, the delay calculated at which the ADE corresponds to 100% of the AOEL is 8.2 days, considering the strictest AOEL of 0.1 mg·kg–1 bw. This result is in line with results obtained in a worker field study that reported 107% of this AOEL 7 days after application.32 Considering the actual AOEL of 0.25 mg·kg–1 bw, the calculated delay for which the exposure reaches this value is 2.6 days (62 h), which is still longer than the REI (48 h). Thus, based on our results, this delay appears to be not long enough to protect workers whose arms, hands, and legs are not covered. In a noncontrolled field study of 42 workers involved in thinning, 83, 95, and 60% of the workers had bare forearms, bare hands, and bare lower legs, respectively.10 In that study, the ADE calculated from the measured median PDE was 4.55 mg of captan (mean of 30 days since the previous application), in agreement with the results of the present study.
For harvesting, the ADE estimations were made using a DFR value measured in the fall 32 days after an application of captan. The values ranged from 0.6 to 5.9 mg/day (8 h of work) depending on the clothing. Whatever the protection, exposure was lower than the AOEL (3.6–36.3%). The corresponding PDE values (5.0–49.2 mg) are globally in line with those determined experimentally in the field with a median of daily contamination for harvesters of 5.82 mg of captan (2.1–14.48 mg, 25th percentile and 75th percentile, respectively, n = 54).10 In addition, although this was not the aim of the work, captan and tetrahydrophthalimide (THPI: transformation product of captan) levels on apples have also been determined by apple surface wiping during re-entry and during harvest (data not shown); the results showed that apple surface was systematically less concentrated in captan than foliar one and that THPI levels on both were systematically lower than captan levels (article in progress). Thus, by considering foliar concentration to estimate dermal worker exposure, even during harvest, we consider the worst case, which is coherent with a prevention strategy.
Thus, this study provides valuable DFR data that enable a model that may be useful for risk assessment. In fact, the model can be adapted to estimate dermal exposure of workers linked to their working conditions, in particular, the re-entry interval and the use of protective clothing, thanks to biphasic kinetic equations and TC. In this way, the dermal exposure estimations based on DFR result in values that are in agreement with experimental values. It is important to emphasize that workers whose arms, hands, and legs are not covered should not enter in fields within 2.6 days after captan spraying. This result suggests the need to document occupational exposure to pesticides during re-entry tasks and harvesting as well as during treatment itself.
Acknowledgments
The authors would like to thank the French National Research Agency (ANR) for financial support for the research project CANEPA as part of COTE cluster of excellence (ANR-10-LABX-45) and SIRIC BRIO cluster of excellence and Bordeaux Idex (ANR-10-IDEX-03-02). The authors are also grateful to the owners of the apple orchard for their kind cooperation throughout the course of this study.
The authors declare no competing financial interest.
References
- Bonner M. R.; Freeman L. E. B.; Hoppin J. A.; Koutros S.; Sandler D. P.; Lynch C. F.; Hines C. J.; Thomas K.; Blair A.; Alavanja M. C. Occupational Exposure to Pesticides and the Incidence of Lung Cancer in the Agricultural Health Study. Environ. Health Perspect. 2017, 125 (4), 544–551. 10.1289/EHP456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair A.; Zahm S. H. Agricultural Exposures and Cancer. Environ. Health Perspect. 1995, 103 (8), 205–208. 10.1289/ehp.95103s8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.-H.; Kabir E.; Jahan S. A. Exposure to Pesticides and the Associated Human Health Effects. Sci. Total Environ. 2017, 575, 525–535. 10.1016/j.scitotenv.2016.09.009. [DOI] [PubMed] [Google Scholar]
- Alavanja M. C.; Hoppin J. A.; Kamel F. Health Effects of Chronic Pesticide Exposure: Cancer and Neurotoxicity. Annu. Rev. Public Health 2004, 25, 155–197. 10.1146/annurev.publhealth.25.101802.123020. [DOI] [PubMed] [Google Scholar]
- Damalas C. A.; Eleftherohorinos I. G. Pesticide Exposure, Safety Issues, and Risk Assessment Indicators. Int. J. Environ. Res. Public Health 2011, 8 (5), 1402–1419. 10.3390/ijerph8051402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi I.; Lebailly P.; Bouvier G.; Rondeau V.; Kientz-Bouchart V.; Canal-Raffin M.; Garrigou A. Levels and Determinants of Pesticide Exposure in Re-Entry Workers in Vineyards: Results of the PESTEXPO Study. Environ. Res. 2014, 132, 360–369. 10.1016/j.envres.2014.04.035. [DOI] [PubMed] [Google Scholar]
- Kasiotis K. M.; Tsakirakis A. N.; Glass C. R.; Charistou A. N.; Anastassiadou P.; Gerritsen-Ebben R.; Machera K. Assessment of Field Re-Entry Exposure to Pesticides: A Dislodgeable Foliar Residue Study. Sci. Total Environ. 2017, 596, 178–186. 10.1016/j.scitotenv.2017.04.016. [DOI] [PubMed] [Google Scholar]
- Dong M. H.; Beauvais S. Assessment of Field Reentry Exposure to Pesticides: Limitations, Uncertainties, and Alternatives. Hum. Ecol. Risk Assess.: Int. J. 2013, 19 (3), 579–600. 10.1080/10807039.2012.762848. [DOI] [Google Scholar]
- Beauvais S. L.; Silva M. H.; Powell S. Human Health Risk Assessment of Endosulfan. Part IV: Occupational Reentry and Public Non-Dietary Exposure and Risk. Regul. Toxicol. Pharmacol. 2010, 56 (1), 38–50. 10.1016/j.yrtph.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Bureau M.; Béziat B.; Duporté G.; Bouchart V.; Lecluse Y.; Barron E.; Garrigou A.; Dévier M.-H.; Budzinski H.; Lebailly P.; Baldi I. Pesticide Exposure of Workers in Apple Growing in France. Int. Arch. Occup. Environ. Health 2022, 95 (4), 811–823. 10.1007/s00420-021-01810-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Authority E. F. S. Guidance on the Assessment of Exposure of Operators, Workers, Residents and Bystanders in Risk Assessment for Plant Protection Products. EFSA J. 2014, 12 (10), 3874 10.2903/j.efsa.2014.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S. E.; Meade B. J. Potential Health Effects Associated with Dermal Exposure to Occupational Chemicals. Environ. Health Insights 2014, 8 (1), 51–62. 10.4137/EHI.S15258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J. H.; Dong M. H.; Krieger R. I. Conservatism in Pesticide Exposure Assessment. Regul. Toxicol. Pharmacol. 2000, 31 (1), 53–58. 10.1006/rtph.1999.1363. [DOI] [PubMed] [Google Scholar]
- Edmiston S.; Powell S.; Spencer J.; Curtis C.. Guidance for Determination of Dislodgeable Foliar Residue, Health & Safety Report, Worker Health and Safety Branch, HS-1600; 2002.
- Belsey N. A.; Cordery S. F.; Bunge A. L.; Guy R. H. Assessment of Dermal Exposure to Pesticide Residues during Re-Entry. Environ. Sci. Technol. 2011, 45 (10), 4609–4615. 10.1021/es200172q. [DOI] [PubMed] [Google Scholar]
- Simcox N. J.; Camp J.; Kalman D.; Stebbins A.; Bellamy G.; Lee I.-C.; Fenske R. Farmworker Exposure to Organophosphorus Pesticide Residues during Apple Thinning in Central Washington State. Am. Ind. Hyg. Assoc. J. 1999, 60 (6), 752–761. 10.1080/00028899908984498. [DOI] [PubMed] [Google Scholar]
- Doran E. M.; Fenske R. A.; Kissel J. C.; Curl C. L.; Simcox N. J. Impact of Dermal Absorption Factors in Occupational Exposure Assessment: Comparison of Two Models for Agricultural Reentry Workers Exposed to Azinphosmethyl. Appl. Occup. Environ. Hyg. 2003, 18 (9), 669–677. 10.1080/10473220301383. [DOI] [PubMed] [Google Scholar]
- Fenske R. A.; Curl C. L.; Kissel J. C. The Effect of the 14-Day Agricultural Restricted Entry Interval on Azinphosmethyl Exposures in a Group of Apple Thinners in Washington State. Regul. Toxicol. Pharmacol. 2003, 38 (1), 91–97. 10.1016/S0273-2300(03)00073-4. [DOI] [PubMed] [Google Scholar]
- Whitmyre G. K.; Ross J. H.; Lunchick C.; Volger B.; Singer S. Biphasic Dissipation Kinetics for Dislodgeable Foliar Residues in Estimating Postapplication Occupational Exposures to Endosulfan. Arch. Environ. Contam. Toxicol. 2004, 46 (1), 17–23. 10.1007/s00244-003-2166-y. [DOI] [PubMed] [Google Scholar]
- Fantke P.; Juraske R. Variability of Pesticide Dissipation Half-Lives in Plants. Environ. Sci. Technol. 2013, 47 (8), 3548–3562. 10.1021/es303525x. [DOI] [PubMed] [Google Scholar]
- Korpalski S.; Bruce E.; Holden L.; Klonne D. Dislodgeable Foliar Residues Are Lognormally Distributed for Agricultural Re-Entry Studies. J. Expo. Sci. Environ. Epidemiol. 2005, 15 (2), 160–163. 10.1038/sj.jea.7500383. [DOI] [PubMed] [Google Scholar]
- Gunther F. A.; Iwata Y.; Carman G. E.; Smith C. A.. The Citrus Reentry Problem: Research on Its Causes and Effects, and Approaches to Its Minimization. In Residue Reviews; Springer, 1977; pp 1–132. 10.1007/978-1-4684-7062-8_1. [DOI] [PubMed] [Google Scholar]
- Antonious G. F.; Byers M. E.; Snyder J. C. Residues and Fate of Endosulfan on Field-grown Pepper and Tomato. Pestic. Sci. 1998, 54 (1), 61–67. . [DOI] [Google Scholar]
- United Nations Food and Agriculture Organization Statistics Division . Production Quantities by Crops; United Nations Food and Agriculture Organization: Rome, 2018. [Google Scholar]
- Smith F. D.; MacHardy W. E. The Retention and Redistribution of Captan on Apple Foliage. Phytopathology 1984, 74 (8), 894–899. 10.1094/Phyto-74-894. [DOI] [Google Scholar]
- Stamper J. H.; Nigg H. N.; Queen R. M. Dislodgeable Captan Residues at Florida Strawberry Farms. Chemosphere 1987, 16 (6), 1257–1271. 10.1016/0045-6535(87)90062-2. [DOI] [Google Scholar]
- Ritcey G.; Frank R.; McEwen F. L.; Braun H. E. Captan Residues on Strawberries and Estimates of Exposure to Pickers. Bull. Environ. Contam. Toxicol. 1987, 38 (5), 840–846. 10.1007/BF01616710. [DOI] [PubMed] [Google Scholar]
- Ripley B. D.; Ritcey G. M.; Harris C. R.; Denommé M. A.; Lissemore L. I. Comparative Persistence of Pesticides on Selected Cultivars of Specialty Vegetables. J. Agric. Food Chem. 2003, 51 (5), 1328–1335. 10.1021/jf020139o. [DOI] [PubMed] [Google Scholar]
- Xu X.-M.; Murray R. A.; Salazar J. D.; Hyder K. The Effects of Temperature, Humidity and Rainfall on Captan Decline on Apple Leaves and Fruit in Controlled Environment Conditions. Pest Manage. Sci. 2008, 64 (3), 296–307. 10.1002/ps.1520. [DOI] [PubMed] [Google Scholar]
- Barreda M.; Lpez F. J.; Villarroya M.; Beltran J.; Garca-Baudn J. M.; Hernndez F. Residue Determination of Captan and Folpet in Vegetable Samples by Gas Chromatography/Negative Chemical Ionizationmass Spectrometry. J. AOAC Int. 2006, 89 (4), 1080–1087. 10.1093/jaoac/89.4.1080. [DOI] [PubMed] [Google Scholar]
- Wolfe N. L.; Zepp R. G.; Doster J. C.; Hollis R. C. Captan Hydrolysis. J. Agric. Food Chem. 1976, 24 (5), 1041–1045. 10.1021/jf60207a004. [DOI] [PubMed] [Google Scholar]
- Anastassiadou M.; Arena M.; Auteri D.; Brancato A.; Bura L.; Cabrera L. C.; Chaideftou E.; Chiusolo A.; Crivellente F.; De Lentdecker C.; Egsmose M.; Fait G.; Greco L.; Ippolito A.; Istace F.; Jarrah S.; Kardassi D.; Leuschner R.; Lostia A.; Lythgo C.; Magrans O.; Mangas I.; Miron I.; Molnar T.; Padovani L.; Morte J. M. P.; Pedersen R.; Reich H.; Santos M.; Sharp R.; Sturma J.; Szentes C.; Terron A.; Tiramani M.; Vagenende B.; Villamar-Bouza L.; Peer Review of the Pesticide Risk Assessment of the Active Substance Captan. EFSA J. 2020, 18 (9), e06230 10.2903/j.efsa.2020.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utture S. C.; Banerjee K.; Kolekar S. S.; Dasgupta S.; Oulkar D. P.; Patil S. H.; Wagh S. S.; Adsule P. G.; Anuse M. A. Food Safety Evaluation of Buprofezin, Dimethoate and Imidacloprid Residues in Pomegranate. Food Chem. 2012, 131 (3), 787–795. 10.1016/j.foodchem.2011.09.044. [DOI] [Google Scholar]
- Utture S. C.; Banerjee K.; Dasgupta S.; Patil S. H.; Jadhav M. R.; Wagh S. S.; Kolekar S. S.; Anuse M. A.; Adsule P. G. Dissipation and Distribution Behavior of Azoxystrobin, Carbendazim, and Difenoconazole in Pomegranate Fruits. J. Agric. Food Chem. 2011, 59 (14), 7866–7873. 10.1021/jf200525d. [DOI] [PubMed] [Google Scholar]
- Banerjee K.; Oulkar D. P.; Patil S. H.; Dasgupta S.; Adsule P. G. Degradation Kinetics and Safety Evaluation of Tetraconazole and Difenoconazole Residues in Grape. Pest Manag. Sci. 2008, 64 (3), 283–289. 10.1002/ps.1524. [DOI] [PubMed] [Google Scholar]
- Yu Y.; Wu J.; Stahler M.; Pestemer W. Residual Dynamics of Thiacloprid in Medical Herbs Marjoram, Thyme, and Camomile in Soil. J. Environ. Sci. 2007, 19 (2), 205–209. 10.1016/S1001-0742(07)60033-3. [DOI] [PubMed] [Google Scholar]
- de Cock J.; Heederik D.; Boleij J. S. M.; Kromhout H.; Hoek F.; Wegh H.; Ny E. T. Determinants of Exposure to Captan in Fruit Growing. Am. Ind. Hyg. Assoc. J. 1998, 59 (3), 166–172. 10.1080/15428119891010424. [DOI] [PubMed] [Google Scholar]
- Ntow W. J.; Ameyibor J.; Kelderman P.; Drechsel P.; Gijzen H. J. Dissipation of Endosulfan in Field-Grown Tomato (Lycopersicon esculentum) and Cropped Soil at Akumadan, Ghana. J. Agric. Food Chem. 2007, 55 (26), 10864–10871. 10.1021/jf0718648. [DOI] [PubMed] [Google Scholar]