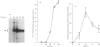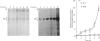Abstract
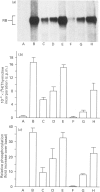
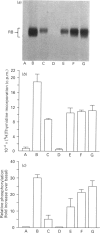
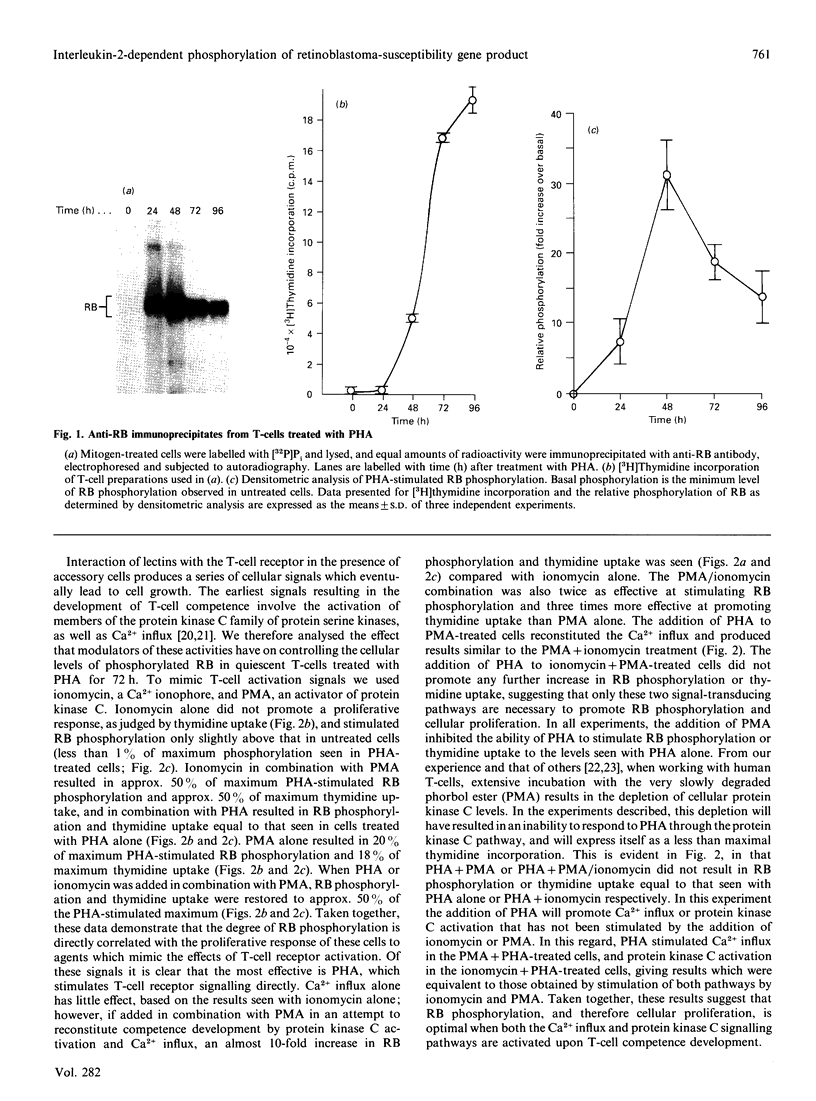
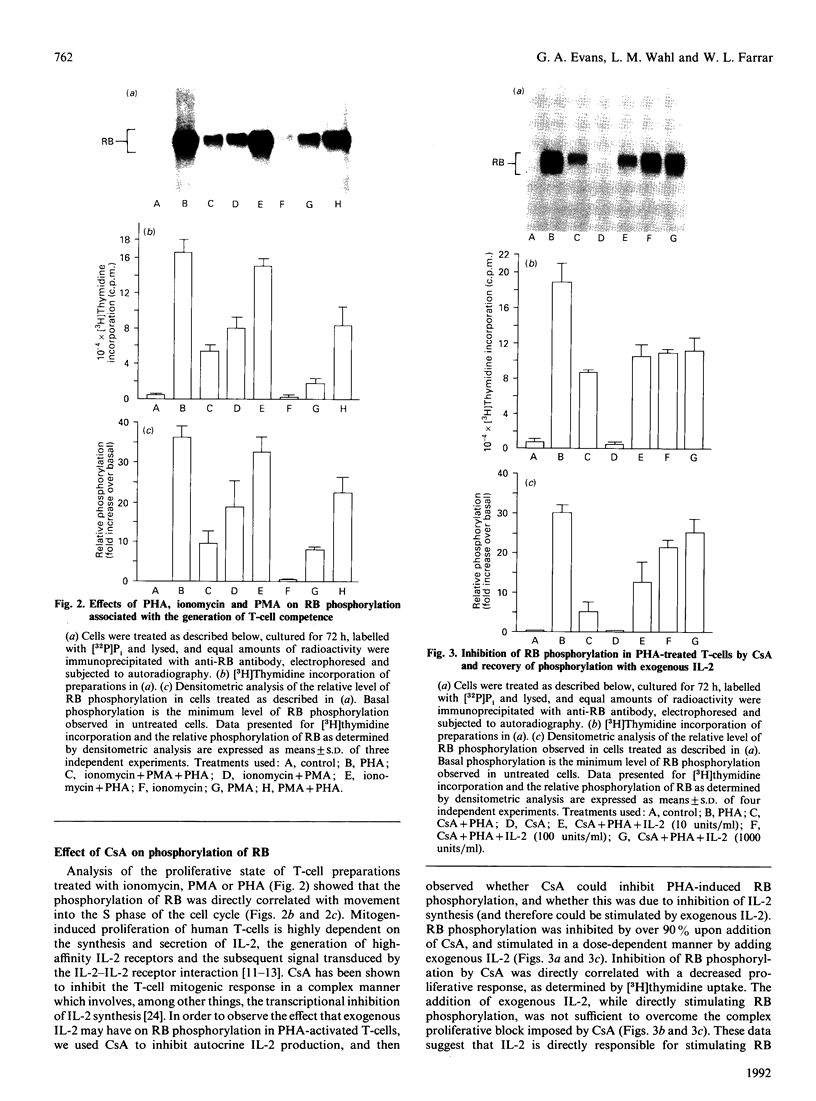
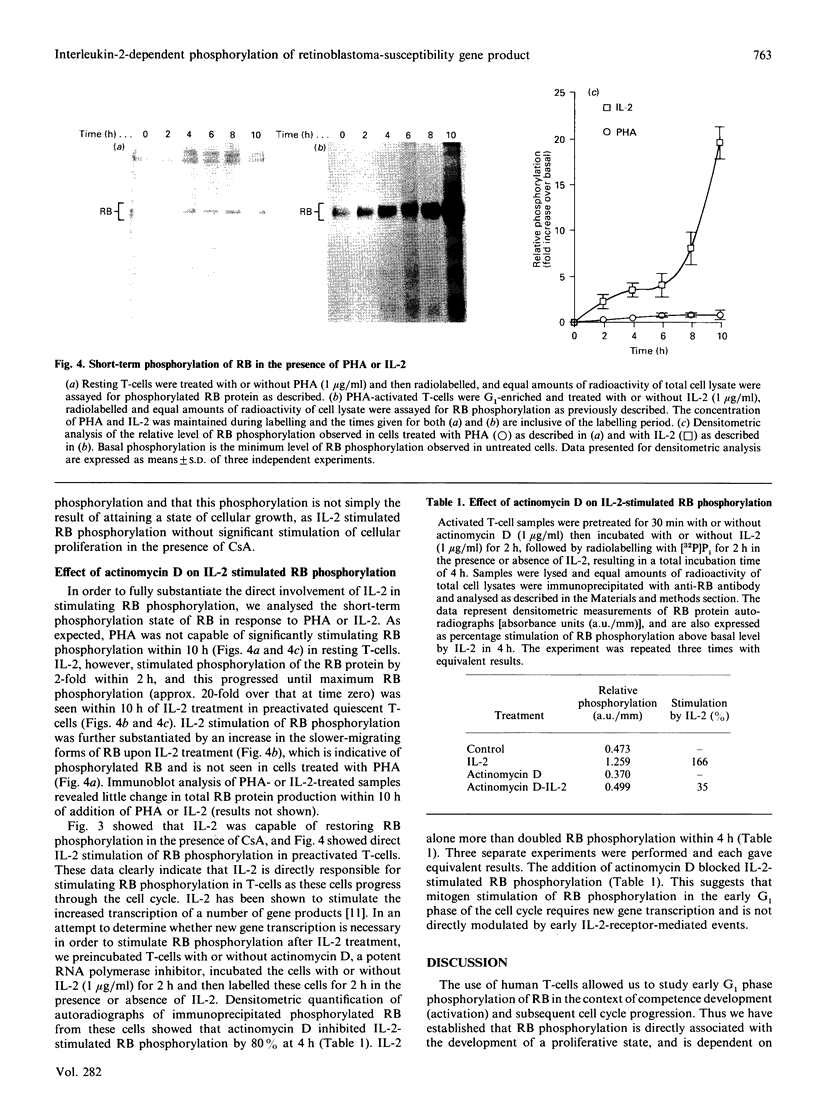
The state of phosphorylation of the retinoblastoma-susceptibility gene product, p110-115RB, is thought to have fundamental importance in controlling the progression of the cell through the cell cycle. We have studied RB phosphorylation in human T-cells in the context of T-cell activation, stimulated by phytohaemagglutinin (PHA) and interleukin-2 (IL-2). We show that, of the signals associated with T-cell activation, only signals that directly lead to movement into S phase of the cell cycle are capable of stimulating RB phosphorylation. Cyclosporin A (CsA), a potent inhibitor of IL-2 synthesis and cellular proliferation, blocked RB phosphorylation, and this was recovered with exogenous IL-2, indicating a direct involvement of IL-2 in controlling RB phosphorylation. We found that PHA did not stimulate RB phosphorylation within 10 h of treatment, but IL-2 could effectively stimulate RB phosphorylation within 2 h, and this approached a maximum within 8-10 h of IL-2 treatment. Further, by using actinomycin D to inhibit new gene transcription following IL-2 stimulation, we found that early-cell-cycle phosphorylation of RB required IL-2-stimulated gene transcription. From these data we conclude that, in human T-cells, RB phosphorylation is not directly associated with T-cell receptor-mediated events, but requires the interaction of IL-2 and new gene transcription following IL-2 stimulation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brizuela L., Draetta G., Beach D. Activation of human CDC2 protein as a histone H1 kinase is associated with complex formation with the p62 subunit. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4362–4366. doi: 10.1073/pnas.86.12.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchkovich K., Duffy L. A., Harlow E. The retinoblastoma protein is phosphorylated during specific phases of the cell cycle. Cell. 1989 Sep 22;58(6):1097–1105. doi: 10.1016/0092-8674(89)90508-4. [DOI] [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. The interleukin-2 T-cell system: a new cell growth model. Science. 1984 Jun 22;224(4655):1312–1316. doi: 10.1126/science.6427923. [DOI] [PubMed] [Google Scholar]
- Chen P. L., Scully P., Shew J. Y., Wang J. Y., Lee W. H. Phosphorylation of the retinoblastoma gene product is modulated during the cell cycle and cellular differentiation. Cell. 1989 Sep 22;58(6):1193–1198. doi: 10.1016/0092-8674(89)90517-5. [DOI] [PubMed] [Google Scholar]
- Crabtree G. R. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989 Jan 20;243(4889):355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Lynch D., Furukawa Y., Griffin J., Piwnica-Worms H., Huang C. M., Livingston D. M. The product of the retinoblastoma susceptibility gene has properties of a cell cycle regulatory element. Cell. 1989 Sep 22;58(6):1085–1095. doi: 10.1016/0092-8674(89)90507-2. [DOI] [PubMed] [Google Scholar]
- Draetta G., Beach D. Activation of cdc2 protein kinase during mitosis in human cells: cell cycle-dependent phosphorylation and subunit rearrangement. Cell. 1988 Jul 1;54(1):17–26. doi: 10.1016/0092-8674(88)90175-4. [DOI] [PubMed] [Google Scholar]
- Dyson N., Howley P. M., Münger K., Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989 Feb 17;243(4893):934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- Furukawa Y., DeCaprio J. A., Freedman A., Kanakura Y., Nakamura M., Ernst T. J., Livingston D. M., Griffin J. D. Expression and state of phosphorylation of the retinoblastoma susceptibility gene product in cycling and noncycling human hematopoietic cells. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2770–2774. doi: 10.1073/pnas.87.7.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano A., Whyte P., Harlow E., Franza B. R., Jr, Beach D., Draetta G. A 60 kd cdc2-associated polypeptide complexes with the E1A proteins in adenovirus-infected cells. Cell. 1989 Sep 8;58(5):981–990. doi: 10.1016/0092-8674(89)90949-5. [DOI] [PubMed] [Google Scholar]
- Greene W. C., Leonard W. J., Depper J. M. Growth of human T lymphocytes: an analysis of interleukin 2 and its cellular receptor. Prog Hematol. 1986;14:283–301. [PubMed] [Google Scholar]
- Gunter K. C., Irving S. G., Zipfel P. F., Siebenlist U., Kelly K. Cyclosporin A-mediated inhibition of mitogen-induced gene transcription is specific for the mitogenic stimulus and cell type. J Immunol. 1989 May 1;142(9):3286–3291. [PubMed] [Google Scholar]
- Huang H. J., Yee J. K., Shew J. Y., Chen P. L., Bookstein R., Friedmann T., Lee E. Y., Lee W. H. Suppression of the neoplastic phenotype by replacement of the RB gene in human cancer cells. Science. 1988 Dec 16;242(4885):1563–1566. doi: 10.1126/science.3201247. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Bookstein R., Lee E. Y. Studies on the human retinoblastoma susceptibility gene. J Cell Biochem. 1988 Nov;38(3):213–227. doi: 10.1002/jcb.240380309. [DOI] [PubMed] [Google Scholar]
- Lindsten T., June C. H., Thompson C. B. Transcription of T cell antigen receptor genes is induced by protein kinase C activation. J Immunol. 1988 Sep 1;141(5):1769–1774. [PubMed] [Google Scholar]
- Ludlow J. W., DeCaprio J. A., Huang C. M., Lee W. H., Paucha E., Livingston D. M. SV40 large T antigen binds preferentially to an underphosphorylated member of the retinoblastoma susceptibility gene product family. Cell. 1989 Jan 13;56(1):57–65. doi: 10.1016/0092-8674(89)90983-5. [DOI] [PubMed] [Google Scholar]
- Ludlow J. W., Shon J., Pipas J. M., Livingston D. M., DeCaprio J. A. The retinoblastoma susceptibility gene product undergoes cell cycle-dependent dephosphorylation and binding to and release from SV40 large T. Cell. 1990 Feb 9;60(3):387–396. doi: 10.1016/0092-8674(90)90590-b. [DOI] [PubMed] [Google Scholar]
- Mihara K., Cao X. R., Yen A., Chandler S., Driscoll B., Murphree A. L., T'Ang A., Fung Y. K. Cell cycle-dependent regulation of phosphorylation of the human retinoblastoma gene product. Science. 1989 Dec 8;246(4935):1300–1303. doi: 10.1126/science.2588006. [DOI] [PubMed] [Google Scholar]
- Mills G. B., Cragoe E. J., Jr, Gelfand E. W., Grinstein S. Interleukin 2 induces a rapid increase in intracellular pH through activation of a Na+/H+ antiport. Cytoplasmic alkalinization is not required for lymphocyte proliferation. J Biol Chem. 1985 Oct 15;260(23):12500–12507. [PubMed] [Google Scholar]
- Murphree A. L., Benedict W. F. Retinoblastoma: clues to human oncogenesis. Science. 1984 Mar 9;223(4640):1028–1033. doi: 10.1126/science.6320372. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Matsui H., Fujita T., Hatakeyama M., Kashima N., Fuse A., Hamuro J., Nishi-Takaoka C., Yamada G. Molecular analysis of the interleukin-2 system. Immunol Rev. 1986 Aug;92:121–133. doi: 10.1111/j.1600-065x.1986.tb01497.x. [DOI] [PubMed] [Google Scholar]
- Taya Y., Yasuda H., Kamijo M., Nakaya K., Nakamura Y., Ohba Y., Nishimura S. In vitro phosphorylation of the tumor suppressor gene RB protein by mitosis-specific histone H1 kinase. Biochem Biophys Res Commun. 1989 Oct 16;164(1):580–586. doi: 10.1016/0006-291x(89)91759-2. [DOI] [PubMed] [Google Scholar]
- Valge V. E., Wong J. G., Datlof B. M., Sinskey A. J., Rao A. Protein kinase C is required for responses to T cell receptor ligands but not to interleukin-2 in T cells. Cell. 1988 Oct 7;55(1):101–112. doi: 10.1016/0092-8674(88)90013-x. [DOI] [PubMed] [Google Scholar]
- Wahl L. M., Katona I. M., Wilder R. L., Winter C. C., Haraoui B., Scher I., Wahl S. M. Isolation of human mononuclear cell subsets by counterflow centrifugal elutriation (CCE). I. Characterization of B-lymphocyte-, T-lymphocyte-, and monocyte-enriched fractions by flow cytometric analysis. Cell Immunol. 1984 May;85(2):373–383. doi: 10.1016/0008-8749(84)90251-x. [DOI] [PubMed] [Google Scholar]
- Whyte P., Williamson N. M., Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989 Jan 13;56(1):67–75. doi: 10.1016/0092-8674(89)90984-7. [DOI] [PubMed] [Google Scholar]