Abstract
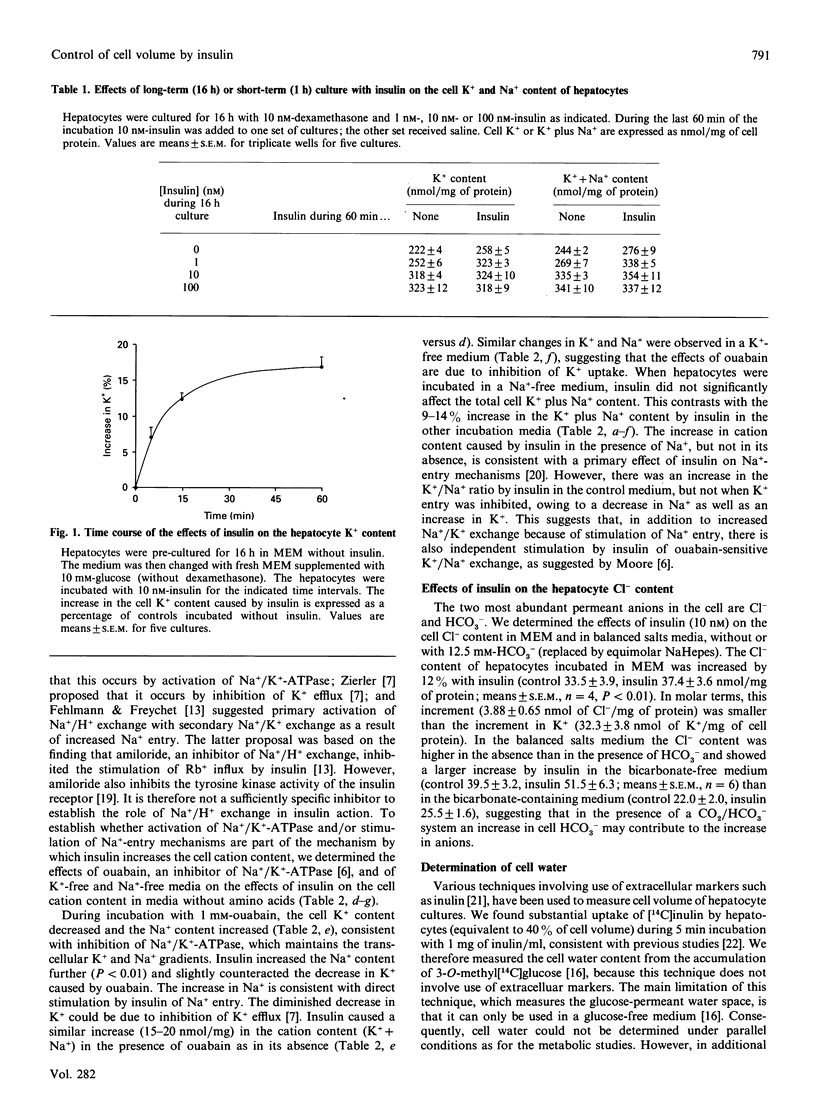
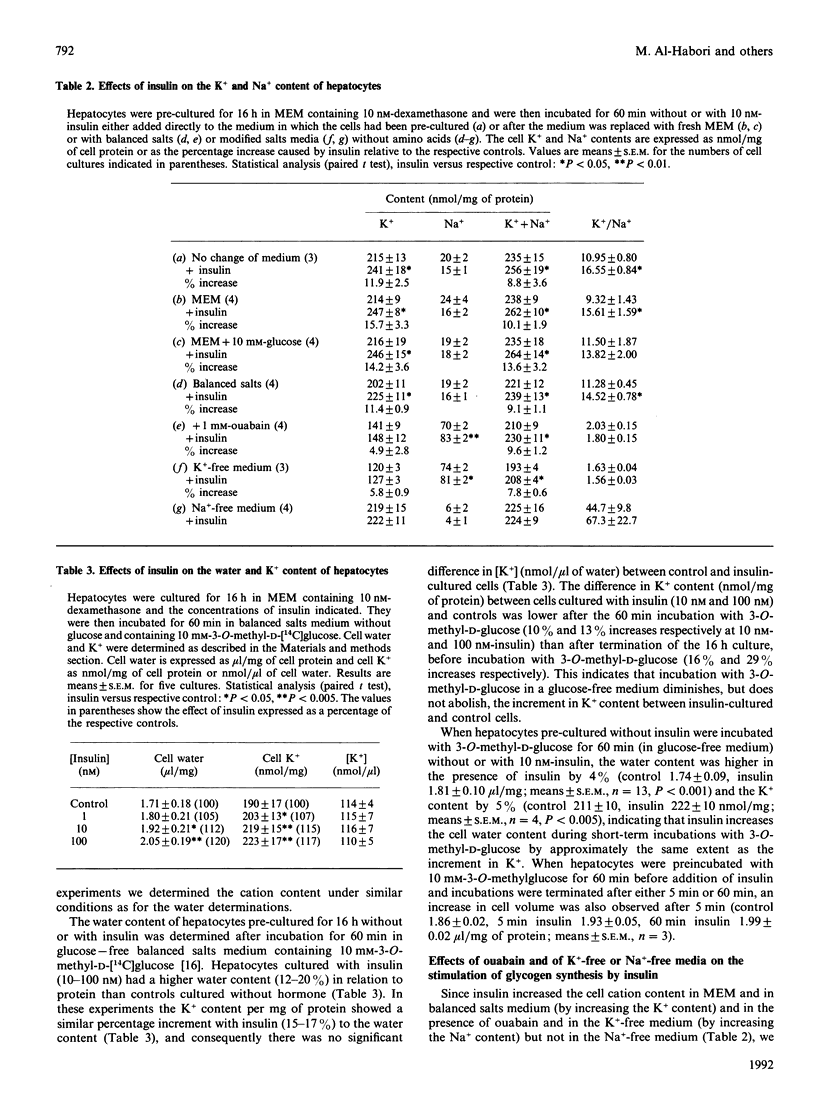
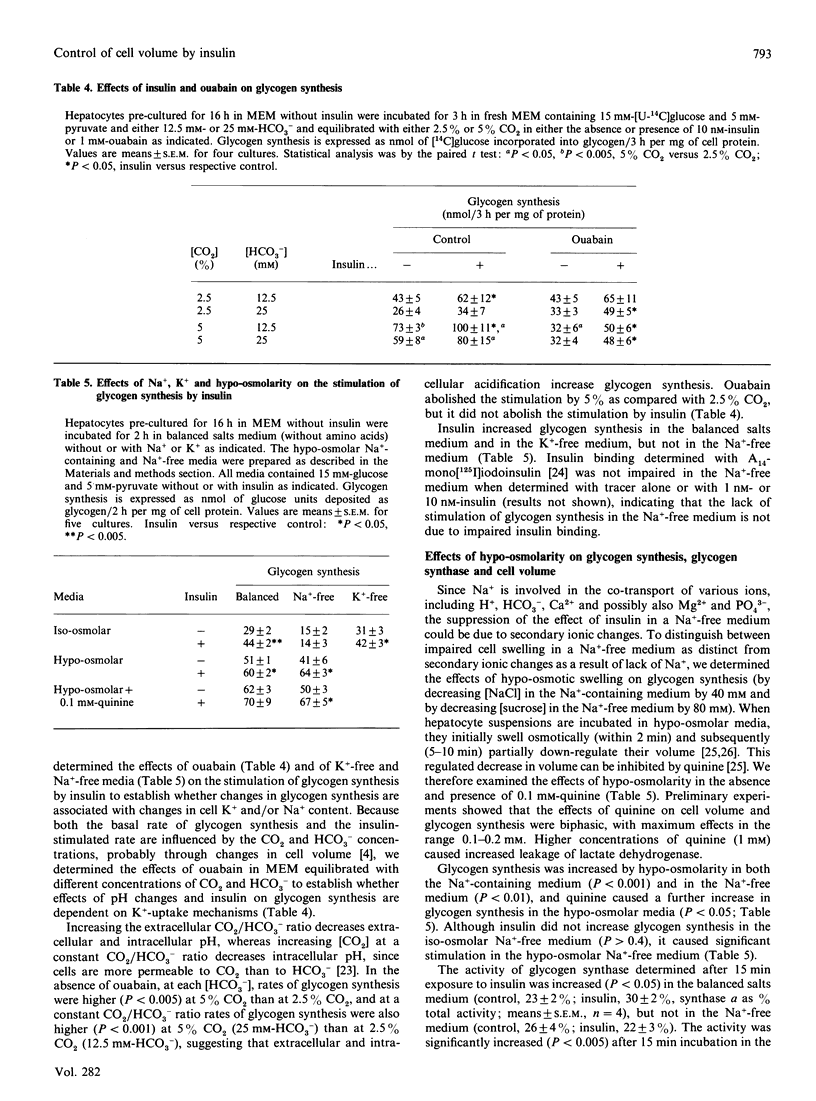
In hepatocyte cultures, insulin stimulates cellular accumulation of K+, partly (approximately 20%) by net replacement of cell Na+, but largely (approximately 80%) by increasing the cell K++Na+ content, with a consequent increase in cell volume. An increase in cation content occurred within 5 min of exposure to insulin and was not secondary to metabolic changes. Insulin also increased the cation content, by increasing the Na+ content, in a K(+)-free medium or when K+ uptake was inhibited with 1 mM-ouabain. However, insulin did not increase the cation content in a Na(+)-free medium. The stimulation of glycogen synthesis by insulin, like the increase in cation content, was blocked in a Na(+)-free medium, but not when K+ uptake was inhibited. Hypo-osmotic swelling restored the stimulation of glycogen synthesis in a Na(+)-free medium, indicating that the lack of effect of insulin in the iso-osmotic Na(+)-free medium was not due to a direct requirement for Na+ for glycogen synthesis, but to a secondary mechanism, dependent on Na+ entry, that can be mimicked by hypo-osmotic swelling. Quinine increased cell volume further and caused a further increase in glycogen synthesis. The hypothesis that cellular uptake of K+ may be part of the mechanism by which insulin controls metabolism was discounted, because inhibition of K+ uptake does not block the metabolic effects of insulin [Czech (1977) Annu. Rev. Biochem. 46, 359-384]. The present results support the hypothesis that an increase in cell cation content, and thereby cell volume, rather than K+ uptake, is part of the mechanism by which insulin stimulates glycogen synthesis in hepatocytes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDRES R., BALTZAN M. A., CADER G., ZIERLER K. L. Effect of insulin on carbohydrate metabolism and on potassium in the forearm of man. J Clin Invest. 1962 Jan;41:108–115. doi: 10.1172/JCI104452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agius L., Peak M., Alberti K. G. Regulation of glycogen synthesis from glucose and gluconeogenic precursors by insulin in periportal and perivenous rat hepatocytes. Biochem J. 1990 Feb 15;266(1):91–102. doi: 10.1042/bj2660091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agius L., Peak M., al-Habori M. What determines the increase in liver cell volume in the fasted-to-fed transition: glycogen or insulin? Biochem J. 1991 Jun 15;276(Pt 3):843–845. doi: 10.1042/bj2760843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoroso S., Di Renzo G., Taglialatela M., Canzoniero L. M., Cragoe E. J., Jr, Annunziato L. Cytoplasmic alkalinization induced by insulin through an activation of Na(+)-H+ antiporter inhibits tyrosine hydroxylase activity in striatal synaptosomes. Biochem Pharmacol. 1991 May 1;41(9):1279–1282. doi: 10.1016/0006-2952(91)90098-p. [DOI] [PubMed] [Google Scholar]
- Baquet A., Hue L., Meijer A. J., van Woerkom G. M., Plomp P. J. Swelling of rat hepatocytes stimulates glycogen synthesis. J Biol Chem. 1990 Jan 15;265(2):955–959. [PubMed] [Google Scholar]
- Baquet A., Meijer A. J., Hue L. Hepatocyte swelling increases inositol 1,4,5-trisphosphate, calcium and cyclic AMP concentration but antagonizes phosphorylase activation by Ca2(+)-dependent hormones. FEBS Lett. 1991 Jan 14;278(1):103–106. doi: 10.1016/0014-5793(91)80094-j. [DOI] [PubMed] [Google Scholar]
- Berg T., Iversen J. G. K+ transport in isolated rat liver cells stimulated by glucagon and insulin in vitro. Acta Physiol Scand. 1976 Jun;97(2):202–208. doi: 10.1111/j.1748-1716.1976.tb10253.x. [DOI] [PubMed] [Google Scholar]
- Clifton P. M., Chang L., Mackinnon A. M. Development of an automated Lowry protein assay for the Cobas-Bio centrifugal analyzer. Anal Biochem. 1988 Jul;172(1):165–168. doi: 10.1016/0003-2697(88)90426-5. [DOI] [PubMed] [Google Scholar]
- Corasanti J. G., Gleeson D., Boyer J. L. Effects of osmotic stresses on isolated rat hepatocytes. I. Ionic mechanisms of cell volume regulation. Am J Physiol. 1990 Feb;258(2 Pt 1):G290–G298. doi: 10.1152/ajpgi.1990.258.2.G290. [DOI] [PubMed] [Google Scholar]
- Czech M. P. Molecular basis of insulin action. Annu Rev Biochem. 1977;46:359–384. doi: 10.1146/annurev.bi.46.070177.002043. [DOI] [PubMed] [Google Scholar]
- Davis R. J., Czech M. P. Amiloride directly inhibits growth factor receptor tyrosine kinase activity. J Biol Chem. 1985 Feb 25;260(4):2543–2551. [PubMed] [Google Scholar]
- DeFronzo R. A., Felig P., Ferrannini E., Wahren J. Effect of graded doses of insulin on splanchnic and peripheral potassium metabolism in man. Am J Physiol. 1980 May;238(5):E421–E427. doi: 10.1152/ajpendo.1980.238.5.E421. [DOI] [PubMed] [Google Scholar]
- Doperé F., Vanstapel F., Stalmans W. Glycogen-synthase phosphatase activity in rat liver. Two protein components and their requirement for the activation of different types of substrate. Eur J Biochem. 1980 Feb;104(1):137–146. doi: 10.1111/j.1432-1033.1980.tb04409.x. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Some thoughts on the mechanism of action of insulin. Diabetes. 1991 May;40(5):521–526. doi: 10.2337/diab.40.5.521. [DOI] [PubMed] [Google Scholar]
- Fehlmann M., Freychet P. Insulin and glucagon stimulation of (Na+-K+)-ATPase transport activity in isolated rat hepatocytes. J Biol Chem. 1981 Jul 25;256(14):7449–7453. [PubMed] [Google Scholar]
- Frelin C., Vigne P., Ladoux A., Lazdunski M. The regulation of the intracellular pH in cells from vertebrates. Eur J Biochem. 1988 May 16;174(1):3–14. doi: 10.1111/j.1432-1033.1988.tb14055.x. [DOI] [PubMed] [Google Scholar]
- Gleeson D., Corasanti J. G., Boyer J. L. Effects of osmotic stresses on isolated rat hepatocytes. II. Modulation of intracellular pH. Am J Physiol. 1990 Feb;258(2 Pt 1):G299–G307. doi: 10.1152/ajpgi.1990.258.2.G299. [DOI] [PubMed] [Google Scholar]
- Goshima K., Masuda A., Owaribe K. Insulin-induced formation of ruffling membranes of KB cells and its correlation with enhancement of amino acid transport. J Cell Biol. 1984 Mar;98(3):801–809. doi: 10.1083/jcb.98.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk W. K. The pathway mediating insulin's effects on pyruvate dehydrogenase bypasses the insulin receptor tyrosine kinase. J Biol Chem. 1991 May 15;266(14):8814–8819. [PubMed] [Google Scholar]
- Katz J., McGarry J. D. The glucose paradox. Is glucose a substrate for liver metabolism? J Clin Invest. 1984 Dec;74(6):1901–1909. doi: 10.1172/JCI111610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kletzien R. F., Pariza M. W., Becker J. E., Potter V. R. A method using 3-O-methyl-D-glucose and phloretin for the determination of intracellular water space of cells in monolayer culture. Anal Biochem. 1975 Oct;68(2):537–544. doi: 10.1016/0003-2697(75)90649-1. [DOI] [PubMed] [Google Scholar]
- MORTIMORE G. E. Effect of insulin on potassium transfer in isolated rat liver. Am J Physiol. 1961 Jun;200:1315–1319. doi: 10.1152/ajplegacy.1961.200.6.1315. [DOI] [PubMed] [Google Scholar]
- Moore R. D. Effects of insulin upon ion transport. Biochim Biophys Acta. 1983 Mar 21;737(1):1–49. doi: 10.1016/0304-4157(83)90013-8. [DOI] [PubMed] [Google Scholar]
- Moule S. K., McGivan J. D. Epidermal growth factor and cyclic AMP stimulate Na+/H+ exchange in isolated rat hepatocytes. Eur J Biochem. 1990 Feb 14;187(3):677–682. doi: 10.1111/j.1432-1033.1990.tb15353.x. [DOI] [PubMed] [Google Scholar]
- Peak M., al-Habori M., Agius L. Regulation of glycogen synthesis and glycolysis by insulin, pH and cell volume. Interactions between swelling and alkalinization in mediating the effects of insulin. Biochem J. 1992 Mar 15;282(Pt 3):797–805. doi: 10.1042/bj2820797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebillard M., Leibovitch S., Jullien M., Talha S., Harel L. Early stimulation by EGF plus insulin of rRNA, c-fos, and actin mRNA expression: inhibition by cytochalasin D. Exp Cell Res. 1987 Oct;172(2):432–438. doi: 10.1016/0014-4827(87)90401-0. [DOI] [PubMed] [Google Scholar]
- Sardet C., Counillon L., Franchi A., Pouysségur J. Growth factors induce phosphorylation of the Na+/H+ antiporter, glycoprotein of 110 kD. Science. 1990 Feb 9;247(4943):723–726. doi: 10.1126/science.2154036. [DOI] [PubMed] [Google Scholar]
- Scharschmidt B. F., Lake J. R., Renner E. L., Licko V., Van Dyke R. W. Fluid phase endocytosis by cultured rat hepatocytes and perfused rat liver: implications for plasma membrane turnover and vesicular trafficking of fluid phase markers. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9488–9492. doi: 10.1073/pnas.83.24.9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart D. J. Monitoring of sodium:proton exchange in isolated hepatocytes by electronic cell sizing. Hepatology. 1989 Dec;10(6):986–994. doi: 10.1002/hep.1840100616. [DOI] [PubMed] [Google Scholar]
- Tomomura A., Nakamura T., Ichihara A. Role of the cytoskeleton in glycogenolysis stimulated by glucagon in primary cultures of adult rat hepatocytes. Biochem Biophys Res Commun. 1980 Dec 31;97(4):1276–1282. doi: 10.1016/s0006-291x(80)80004-0. [DOI] [PubMed] [Google Scholar]
- Zeuzem S., Taylor R., Agius L., Albisser A. M., Alberti K. G. Differential binding of sulphated insulin to adipocytes and hepatocytes. Diabetologia. 1984 Aug;27(2):184–188. doi: 10.1007/BF00273803. [DOI] [PubMed] [Google Scholar]
- Zierler K., Rogus E. M. Rapid hyperpolarization of rat skeletal muscle induced by insulin. Biochim Biophys Acta. 1981 Feb 6;640(3):687–692. doi: 10.1016/0005-2736(81)90098-5. [DOI] [PubMed] [Google Scholar]


