Abstract
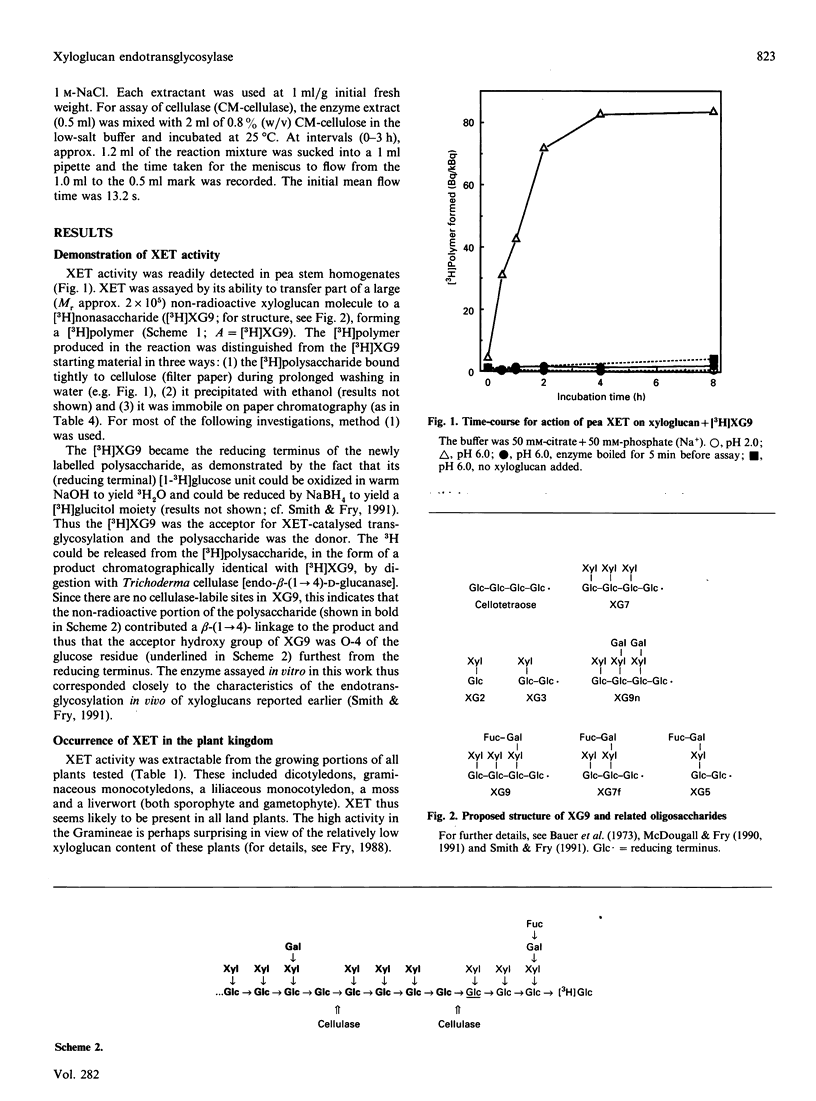
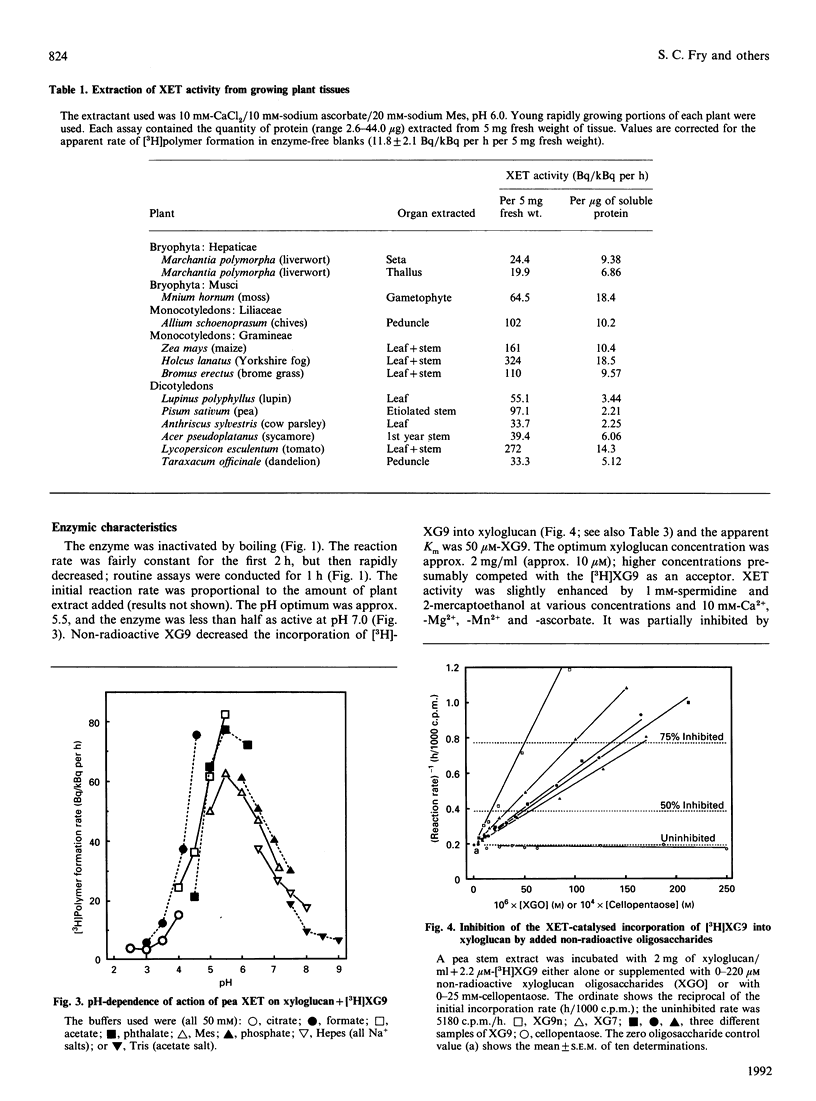
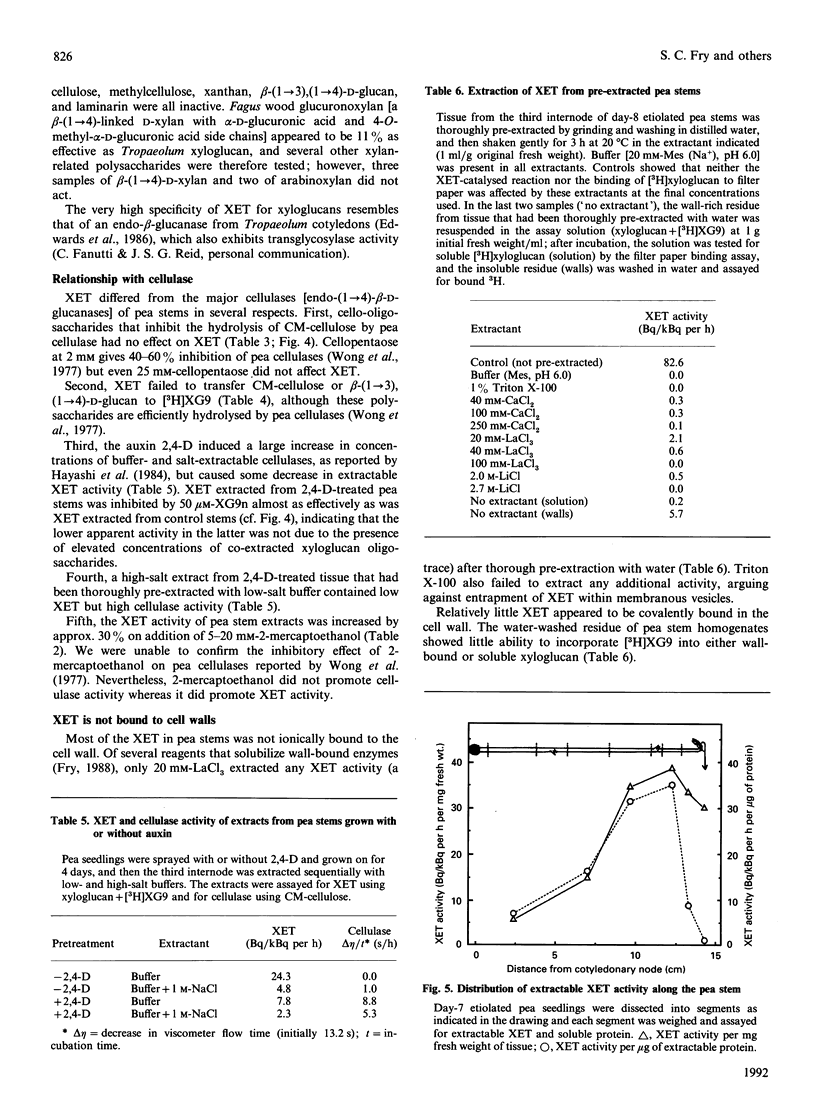
1. Cell-free extracts of all plants tested contained a novel enzyme activity (xyloglucan endotransglycosylase, XET) able to transfer a high-Mr portion from a donor xyloglucan to a suitable acceptor such as a xyloglucan-derived nonasaccharide (Glc4Xyl3GalFuc; XG9). 2. A simple assay for the enzyme, using [3H]XG9 and based on the ability of the [3H]polysaccharide product to bind to filter paper, is described. 3. The enzyme was highly specific for xyloglucan as the glycosyl donor, and showed negligible transglycosylation of other polysaccharides, including CM-cellulose. 4. The Km for XG9 was 50 microM; certain other 3H-labelled xyloglucan oligosaccharides also acted as acceptors, and certain non-radioactive xyloglucan oligosaccharides competed with [3H]XG9 as acceptor; the minimum acceptor structure was deduced to be: [formula: see text] 5. The pH optimum was approx. 5.5 and the enzyme was less than half as active at pH 7.0. The enzyme was slightly activated by Ca2+, Mg2+, Mn2+, spermidine, ascorbate and 2-mercaptoethanol, and inhibited by Ag+, Hg2+, Zn2+ and La3+. 6. XET activity was essentially completely extracted by aqueous solutions of low ionic strength; Triton X-100, Ca2+, La3+, and Li+ did not enhance extraction. Negligible activity was left in the unextractable (cell-wall-rich) residue. 7. The enzyme differed from the major cellulases (EC 3.2.1.4) of pea in: (a) susceptibility to inhibition by cello-oligosaccharides, (b) polysaccharide substrate specificity, (c) inducibility by auxin, (d) requirement for salt in the extraction buffer and (e) activation by 2-mercaptoethanol. XET is therefore concluded to be a new enzyme activity (xyloglucan: xyloglucan xyloglucanotransferase; EC 2.4.1.-). 8. XET was detected in extracts of the growing portions of dicotyledons, monocotyledons (graminaceous and liliaceous) and bryophytes. 9. The activity was positively correlated with growth rate in different zones of the pea stem. 10. We propose that XET is responsible for cutting and rejoining intermicrofibrillar xyloglucan chains and that it thus causes the wall-loosening required for plant cell expansion.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer W. D., Talmadge K. W., Keegstra K., Albersheim P. The Structure of Plant Cell Walls: II. The Hemicellulose of the Walls of Suspension-cultured Sycamore Cells. Plant Physiol. 1973 Jan;51(1):174–187. doi: 10.1104/pp.51.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvill A. G., McNeil M., Albersheim P. Structure of Plant Cell Walls: VIII. A New Pectic Polysaccharide. Plant Physiol. 1978 Sep;62(3):418–422. doi: 10.1104/pp.62.3.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M., Dea I. C., Bulpin P. V., Reid J. S. Purification and properties of a novel xyloglucan-specific endo-(1----4)-beta-D-glucanase from germinated nasturtium seeds (Tropaeolum majus L.). J Biol Chem. 1986 Jul 15;261(20):9489–9494. [PubMed] [Google Scholar]
- Hayashi T., Marsden M. P., Delmer D. P. Pea Xyloglucan and Cellulose: VI. Xyloglucan-Cellulose Interactions in Vitro and in Vivo. Plant Physiol. 1987 Feb;83(2):384–389. doi: 10.1104/pp.83.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Wong Y. S., Maclachlan G. Pea Xyloglucan and Cellulose : II. Hydrolysis by Pea Endo-1,4-beta-Glucanases. Plant Physiol. 1984 Jul;75(3):605–610. doi: 10.1104/pp.75.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labavitch J. M., Ray P. M. Relationship between Promotion of Xyloglucan Metabolism and Induction of Elongation by Indoleacetic Acid. Plant Physiol. 1974 Oct;54(4):499–502. doi: 10.1104/pp.54.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall G. J., Fry S. C. Purification and analysis of growth-regulating xyloglucan-derived oligosaccharides by high-pressure liquid chromatography. Carbohydr Res. 1991 Oct 14;219:123–132. doi: 10.1016/0008-6215(91)89047-j. [DOI] [PubMed] [Google Scholar]
- McDougall G. J., Fry S. C. Xyloglucan oligosaccharides promote growth and activate cellulase: evidence for a role of cellulase in cell expansion. Plant Physiol. 1990 Jul;93(3):1042–1048. doi: 10.1104/pp.93.3.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil M., Darvill A. G., Albersheim P. Structure of Plant Cell Walls: X. RHAMNOGALACTURONAN I, A STRUCTURALLY COMPLEX PECTIC POLYSACCHARIDE IN THE WALLS OF SUSPENSION-CULTURED SYCAMORE CELLS. Plant Physiol. 1980 Dec;66(6):1128–1134. doi: 10.1104/pp.66.6.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S. M., Northcote D. H. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal Biochem. 1981 Sep 1;116(1):53–64. doi: 10.1016/0003-2697(81)90321-3. [DOI] [PubMed] [Google Scholar]
- Smith R. C., Fry S. C. Endotransglycosylation of xyloglucans in plant cell suspension cultures. Biochem J. 1991 Oct 15;279(Pt 2):529–535. doi: 10.1042/bj2790529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K., Sakurai N., Kuraishi S. Differential Effect of Auxin on Molecular Weight Distributions of Xyloglucans in Cell Walls of Outer and Inner Tissues from Segments of Dark Grown Squash (Cucurbita maxima Duch.) Hypocotyls. Plant Physiol. 1991 Apr;95(4):1070–1076. doi: 10.1104/pp.95.4.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Y. S., Fincher G. B., Maclachlan G. A. Kinetic properties and substrate specificities of two cellulases from auxin-treated pea epicotyls. J Biol Chem. 1977 Feb 25;252(4):1402–1407. [PubMed] [Google Scholar]


