Abstract
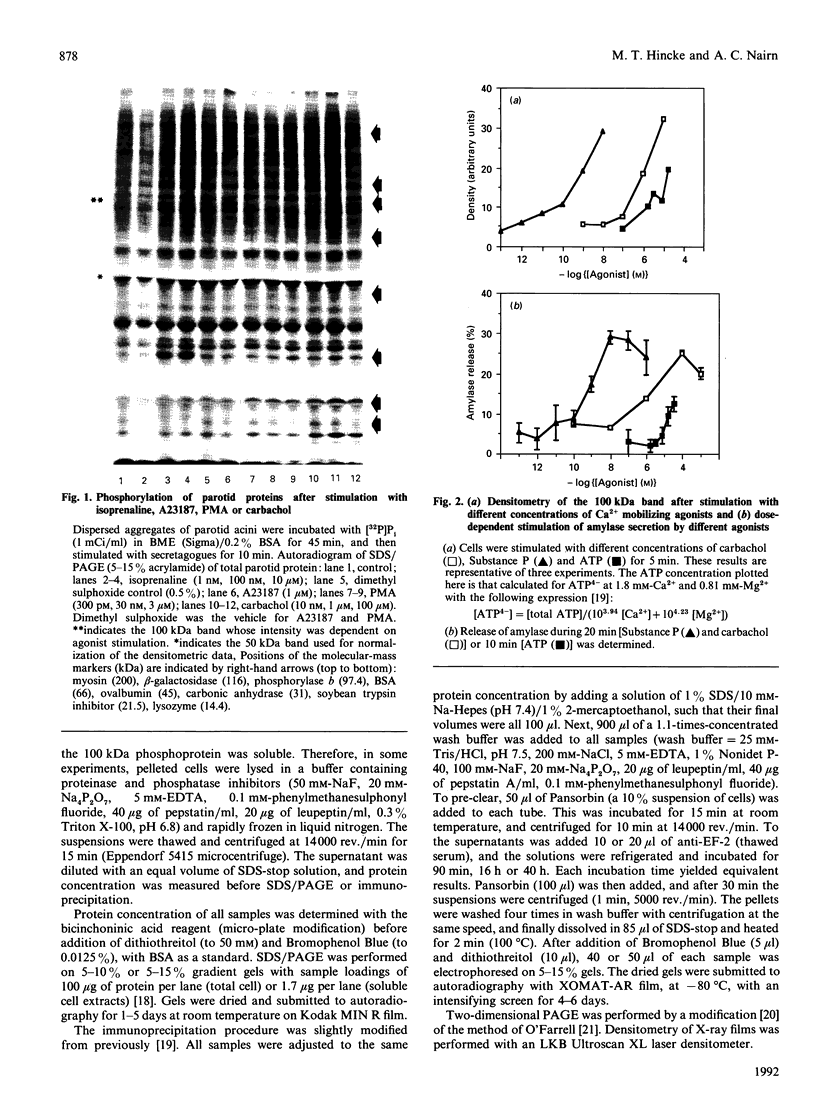
In this paper we report the rapid phosphorylation of a cytosolic 100 kDa protein during stimulation of secretion from dispersed aggregates of parotid acinar cells with Ca(2+)-mobilizing secretagogues (carbachol, Substance P, ATP and the Ca2+ ionophore A23187). Phosphorylation was inhibited by removal of extracellular Ca2+ but was not observed during stimulation with phorbol esters, suggesting that this protein is not a substrate for protein kinase C. Two-dimensional PAGE and immunoprecipitation with a specific antiserum indicated that this protein is elongation factor 2, whose Ca2+ calmodulin-dependent phosphorylation has been shown to inhibit protein synthesis [Nairn & Palfrey (1987) J. Biol. Chem. 262, 17299-17303]. These results suggest that phosphorylation of elongation factor 2 is the molecular mechanism for the inhibition of protein synthesis which has been previously observed in rat parotid cells during stimulation with Ca(2+)-mobilizing secretagogues.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Nikaido K. Two-dimensional gel electrophoresis of membrane proteins. Biochemistry. 1976 Feb 10;15(3):616–623. doi: 10.1021/bi00648a026. [DOI] [PubMed] [Google Scholar]
- Baum B. J., Freiberg J. M., Ito H., Roth G. S., Filburn C. R. beta-Adrenergic regulation of protein phosphorylation and its relationship to exocrine secretion in dispersed rat parotid gland acinar cells. J Biol Chem. 1981 Sep 25;256(18):9731–9736. [PubMed] [Google Scholar]
- Brostrom C. O., Brostrom M. A. Calcium-dependent regulation of protein synthesis in intact mammalian cells. Annu Rev Physiol. 1990;52:577–590. doi: 10.1146/annurev.ph.52.030190.003045. [DOI] [PubMed] [Google Scholar]
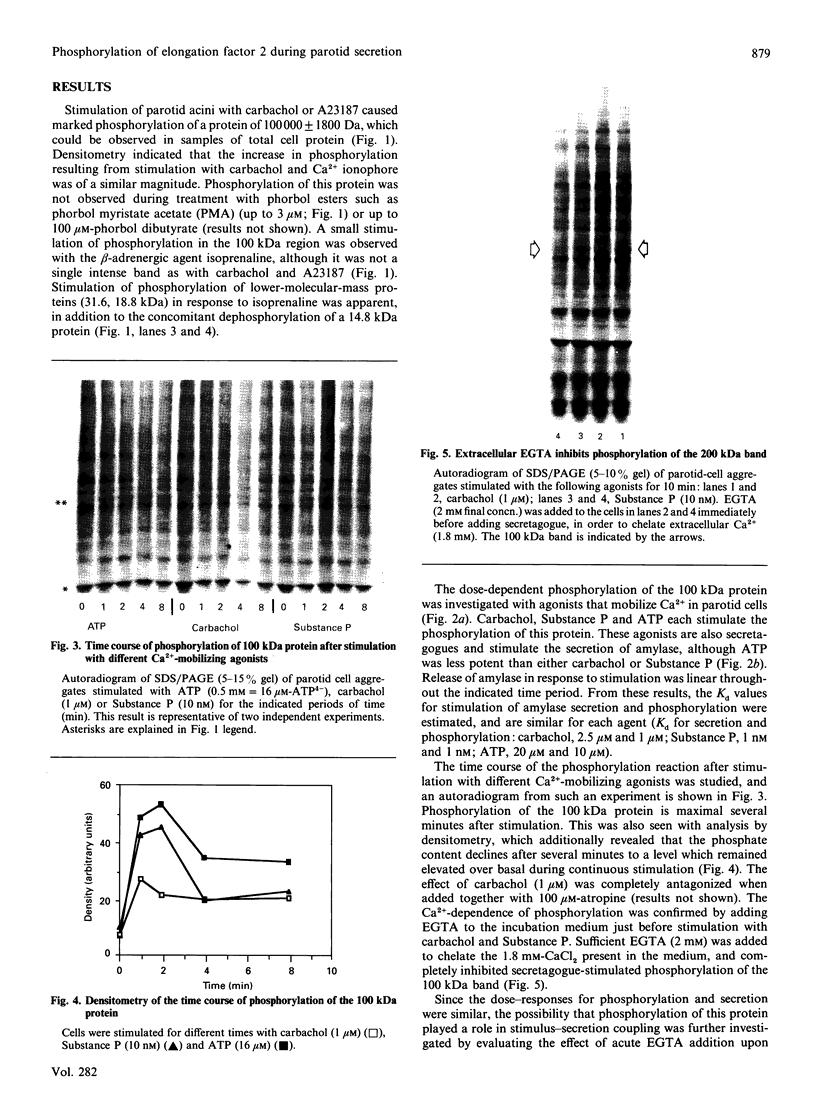
- Celis J. E., Madsen P., Ryazanov A. G. Increased phosphorylation of elongation factor 2 during mitosis in transformed human amnion cells correlates with a decreased rate of protein synthesis. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4231–4235. doi: 10.1073/pnas.87.11.4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denniss A. R., Schneyer L. H., Sucanthapree C., Young J. A. Actions of adrenergic agonists on isolated excretory ducts of submandibular glands. Am J Physiol. 1978 Dec;235(6):F548–F556. doi: 10.1152/ajprenal.1978.235.6.F548. [DOI] [PubMed] [Google Scholar]
- Freedman S. D., Jamieson J. D. Hormone-induced protein phosphorylation. I. Relationship between secretagogue action and endogenous protein phosphorylation in intact cells from the exocrine pancreas and parotid. J Cell Biol. 1982 Dec;95(3):903–908. doi: 10.1083/jcb.95.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman S. D., Jamieson J. D. Hormone-induced protein phosphorylation. II. Localization to the ribosomal fraction from rat exocrine pancreas and parotid of a 29,000-dalton protein phosphorylated in situ in response to secretagogues. J Cell Biol. 1982 Dec;95(3):909–917. doi: 10.1083/jcb.95.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallacher D. V., Petersen O. H. Stimulus-secretion coupling in mammalian salivary glands. Int Rev Physiol. 1983;28:1–52. [PubMed] [Google Scholar]
- Grand R. J. Amino acid pools in rat parotid gland during epinephrine-stimulated protein synthesis. Biochim Biophys Acta. 1969 Nov 19;195(1):252–254. doi: 10.1016/0005-2787(69)90624-8. [DOI] [PubMed] [Google Scholar]
- Greenberger L. M., Williams S. S., Georges E., Ling V., Horwitz S. B. Electrophoretic analysis of P-glycoproteins produced by mouse J774.2 and Chinese hamster ovary multidrug-resistant cells. J Natl Cancer Inst. 1988 Jun 1;80(7):506–510. doi: 10.1093/jnci/80.7.506. [DOI] [PubMed] [Google Scholar]
- Hincke M. T. The inhibition of cyclic AMP-mediated exocytosis from rat parotid cells by calcium chelation is due to depression of cellular ATP. Biochim Biophys Acta. 1988 Nov 17;967(2):204–210. doi: 10.1016/0304-4165(88)90010-4. [DOI] [PubMed] [Google Scholar]
- Horn V. J., Baum B. J., Ambudkar I. S. Beta-adrenergic receptor stimulation induces inositol trisphosphate production and Ca2+ mobilization in rat parotid acinar cells. J Biol Chem. 1988 Sep 5;263(25):12454–12460. [PubMed] [Google Scholar]
- Hughes A. R., Takemura H., Putney J. W., Jr Does beta-adrenoceptor activation stimulate Ca2+ mobilization and inositol trisphosphate formation in parotid acinar cells? Cell Calcium. 1989 Nov-Dec;10(8):519–525. doi: 10.1016/0143-4160(89)90013-4. [DOI] [PubMed] [Google Scholar]
- Jahn R., Söling H. D. Phosphorylation of the same specific protein during amylase release evoked by beta-adrenergic or cholinergic agonists in rat and mouse parotid glands. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6903–6906. doi: 10.1073/pnas.78.11.6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R., Unger C., Söling H. D. Specific protein phosphorylation during stimulation of amylase secretion by beta-agonists or dibutyryl adenosine 3',5'-monophosphate in the rat parotid gland. Eur J Biochem. 1980 Nov;112(2):345–352. doi: 10.1111/j.1432-1033.1980.tb07211.x. [DOI] [PubMed] [Google Scholar]
- Kanagasuntheram P., Lim S. C. Calcium-dependent inhibition of protein synthesis in rat parotid gland. Biochem J. 1978 Oct 15;176(1):23–29. doi: 10.1042/bj1760023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keryer G., Rossignol B. Effects of carbachol on extracellular Na-dependent AIB uptake in rat parotid gland. Am J Physiol. 1980 Sep;239(3):G183–G189. doi: 10.1152/ajpgi.1980.239.3.G183. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levenson R. M., Nairn A. C., Blackshear P. J. Insulin rapidly induces the biosynthesis of elongation factor 2. J Biol Chem. 1989 Jul 15;264(20):11904–11911. [PubMed] [Google Scholar]
- Mackie K. P., Nairn A. C., Hampel G., Lam G., Jaffe E. A. Thrombin and histamine stimulate the phosphorylation of elongation factor 2 in human umbilical vein endothelial cells. J Biol Chem. 1989 Jan 25;264(3):1748–1753. [PubMed] [Google Scholar]
- McMillian M. K., Soltoff S. P., Lechleiter J. D., Cantley L. C., Talamo B. R. Extracellular ATP increases free cytosolic calcium in rat parotid acinar cells. Differences from phospholipase C-linked receptor agonists. Biochem J. 1988 Oct 1;255(1):291–300. [PMC free article] [PubMed] [Google Scholar]
- McMillian M. K., Soltoff S. P., Talamo B. R. Rapid desensitization of substance P- but not carbachol-induced increases in inositol trisphosphate and intracellular Ca++ in rat parotid acinar cells. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1017–1024. doi: 10.1016/s0006-291x(87)80233-4. [DOI] [PubMed] [Google Scholar]
- McPherson M. A., Hales C. N. Control of amylase biosynthesis and release in the parotid gland of the rat. Biochem J. 1978 Dec 15;176(3):855–863. doi: 10.1042/bj1760855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A. P., Gallacher D. V., Irvine R. F., Petersen O. H. Synergism of inositol trisphosphate and tetrakisphosphate in activating Ca2+-dependent K+ channels. Nature. 1987 Dec 17;330(6149):653–655. doi: 10.1038/330653a0. [DOI] [PubMed] [Google Scholar]
- Nairn A. C., Bhagat B., Palfrey H. C. Identification of calmodulin-dependent protein kinase III and its major Mr 100,000 substrate in mammalian tissues. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7939–7943. doi: 10.1073/pnas.82.23.7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairn A. C., Palfrey H. C. Identification of the major Mr 100,000 substrate for calmodulin-dependent protein kinase III in mammalian cells as elongation factor-2. J Biol Chem. 1987 Dec 25;262(36):17299–17303. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov L. P., Motuz L. P., Natapov P. G., Averbuch L. J., Wettenhall R. E., Szyszka R., Kramer G., Hardesty B. Three phosphorylation sites in elongation factor 2. FEBS Lett. 1990 Nov 26;275(1-2):209–212. doi: 10.1016/0014-5793(90)81473-2. [DOI] [PubMed] [Google Scholar]
- Padel U., Kruppa J., Jahn R., Söling H. D. Phosphopeptide patterns of the ribosomal protein S6 following stimulation of guinea pig parotid glands by secretagogues involving either cAMP or calcium as second messenger. FEBS Lett. 1983 Aug 8;159(1-2):112–118. doi: 10.1016/0014-5793(83)80427-x. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Identification of cellular activation mechanisms associated with salivary secretion. Annu Rev Physiol. 1986;48:75–88. doi: 10.1146/annurev.ph.48.030186.000451. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr, Weiss S. J., Leslie B. A., Marier S. H. Is calcium the final mediator of exocytosis in the rat parotid gland? J Pharmacol Exp Ther. 1977 Oct;203(1):144–155. [PubMed] [Google Scholar]
- Quissell D. O., Deisher L. M., Barzen K. A. The rate-determining step in cAMP-mediated exocytosis in the rat parotid and submandibular glands appears to involve analogous 26-kDa integral membrane phosphoproteins. Proc Natl Acad Sci U S A. 1985 May;82(10):3237–3241. doi: 10.1073/pnas.82.10.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph S. A., Krueger B. K. Endogenous protein phosphorylation and dephosphorylation. Adv Cyclic Nucleotide Res. 1979;10:107–133. [PubMed] [Google Scholar]
- Ryazanov A. G., Shestakova E. A., Natapov P. G. Phosphorylation of elongation factor 2 by EF-2 kinase affects rate of translation. Nature. 1988 Jul 14;334(6178):170–173. doi: 10.1038/334170a0. [DOI] [PubMed] [Google Scholar]
- Scott J., Baum B. J. Involvement of cyclic AMP and calcium in exocrine protein secretion induced by vasoactive intestinal polypeptide in rat parotid cells. Biochim Biophys Acta. 1985 Nov 20;847(2):255–262. doi: 10.1016/0167-4889(85)90028-x. [DOI] [PubMed] [Google Scholar]
- Soltoff S. P., McMillian M. K., Cantley L. C., Cragoe E. J., Jr, Talamo B. R. Effects of muscarinic, alpha-adrenergic, and substance P agonists and ionomycin on ion transport mechanisms in the rat parotid acinar cell. The dependence of ion transport on intracellular calcium. J Gen Physiol. 1989 Feb;93(2):285–319. doi: 10.1085/jgp.93.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltoff S. P., McMillian M. K., Cragoe E. J., Jr, Cantley L. C., Talamo B. R. Effects of extracellular ATP on ion transport systems and [Ca2+]i in rat parotid acinar cells. Comparison with the muscarinic agonist carbachol. J Gen Physiol. 1990 Feb;95(2):319–346. doi: 10.1085/jgp.95.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soor S. K., Hincke M. T. Microplate reader-based kinetic determination of alpha-amylase activity: application to quantitation of secretion from rat parotid acini. Anal Biochem. 1990 Jul;188(1):187–191. doi: 10.1016/0003-2697(90)90550-s. [DOI] [PubMed] [Google Scholar]
- Spearman T. N., Hurley K. P., Olivas R., Ulrich R. G., Butcher F. R. Subcellular location of stimulus-affected endogenous phosphoproteins in the rat parotid gland. J Cell Biol. 1984 Oct;99(4 Pt 1):1354–1363. doi: 10.1083/jcb.99.4.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spät A., Lukács G. L., Eberhardt I., Kiesel L., Runnebaum B. Binding of inositol phosphates and induction of Ca2+ release from pituitary microsomal fractions. Biochem J. 1987 Jun 1;244(2):493–496. doi: 10.1042/bj2440493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura H. Changes in cytosolic free calcium concentration in isolated rat parotid cells by cholinergic and beta-adrenergic agonists. Biochem Biophys Res Commun. 1985 Sep 30;131(3):1048–1055. doi: 10.1016/0006-291x(85)90196-2. [DOI] [PubMed] [Google Scholar]
- Tanimura A., Matsumoto Y., Tojyo Y. Evidence that isoproterenol-induced Ca2(+)-mobilization in rat parotid acinar cells is not mediated by activation of beta-adrenoceptors. Biochim Biophys Acta. 1990 Dec 10;1055(3):273–277. doi: 10.1016/0167-4889(90)90043-d. [DOI] [PubMed] [Google Scholar]
- Yatani A., Imoto Y., Codina J., Hamilton S. L., Brown A. M., Birnbaumer L. The stimulatory G protein of adenylyl cyclase, Gs, also stimulates dihydropyridine-sensitive Ca2+ channels. Evidence for direct regulation independent of phosphorylation by cAMP-dependent protein kinase or stimulation by a dihydropyridine agonist. J Biol Chem. 1988 Jul 15;263(20):9887–9895. [PubMed] [Google Scholar]